Decreased Cdk2 Activity Hindered Embryonic Development and Parthenogenesis Induction in Silkworm, Bombyx mori L.
Abstract
:1. Introduction
2. Results
2.1. Protein Structure and Phylogenetic Identification of Cdk2
2.2. Differences in Cdk2 mRNA Expression and Activity Between PLs and ALs
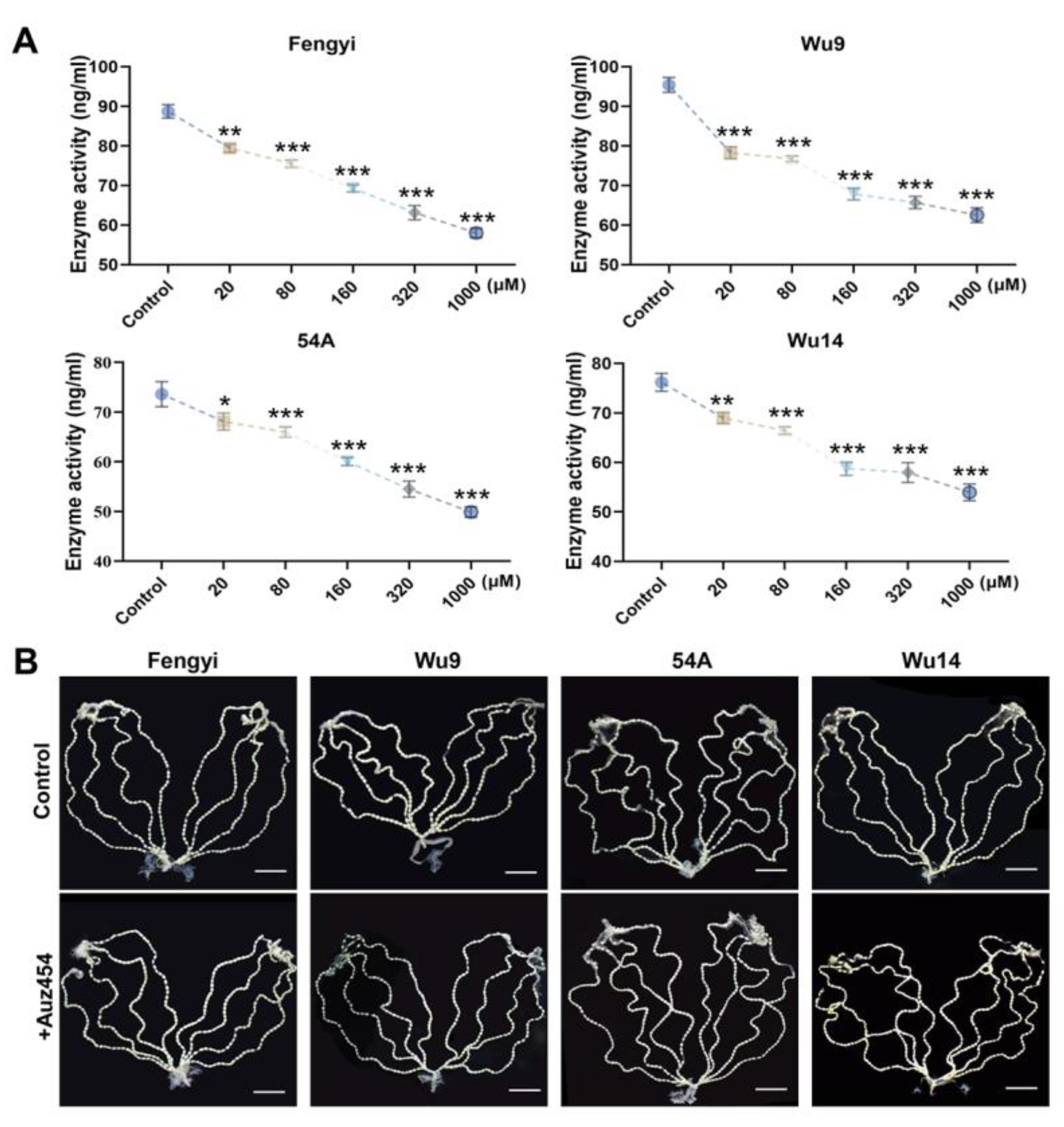
2.3. AUZ454 Effectively Inhibited Cdk2 Activity and Was Non-Toxic
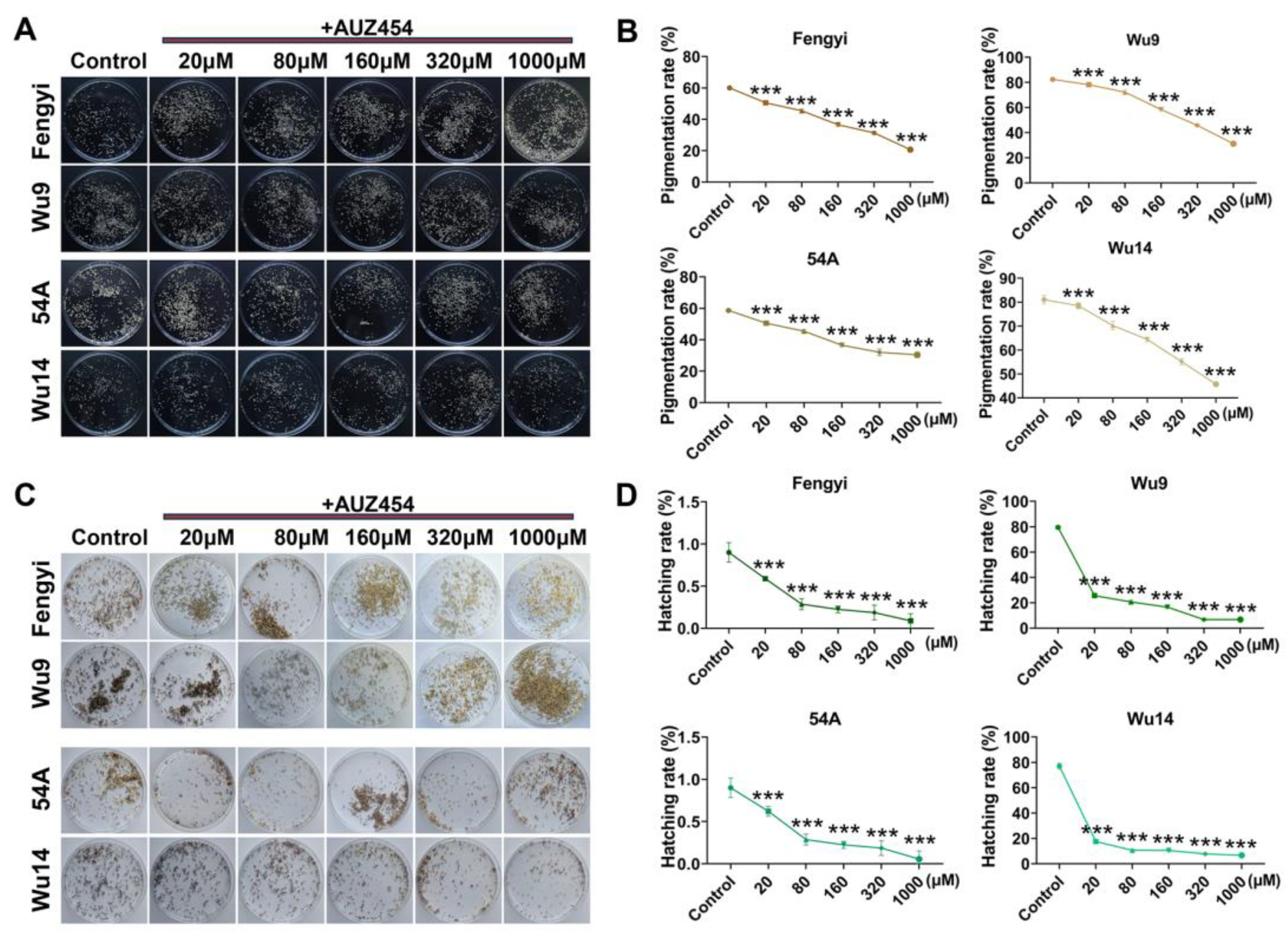
2.4. Cdk2 Activity Affected Parthenogenesis Induction
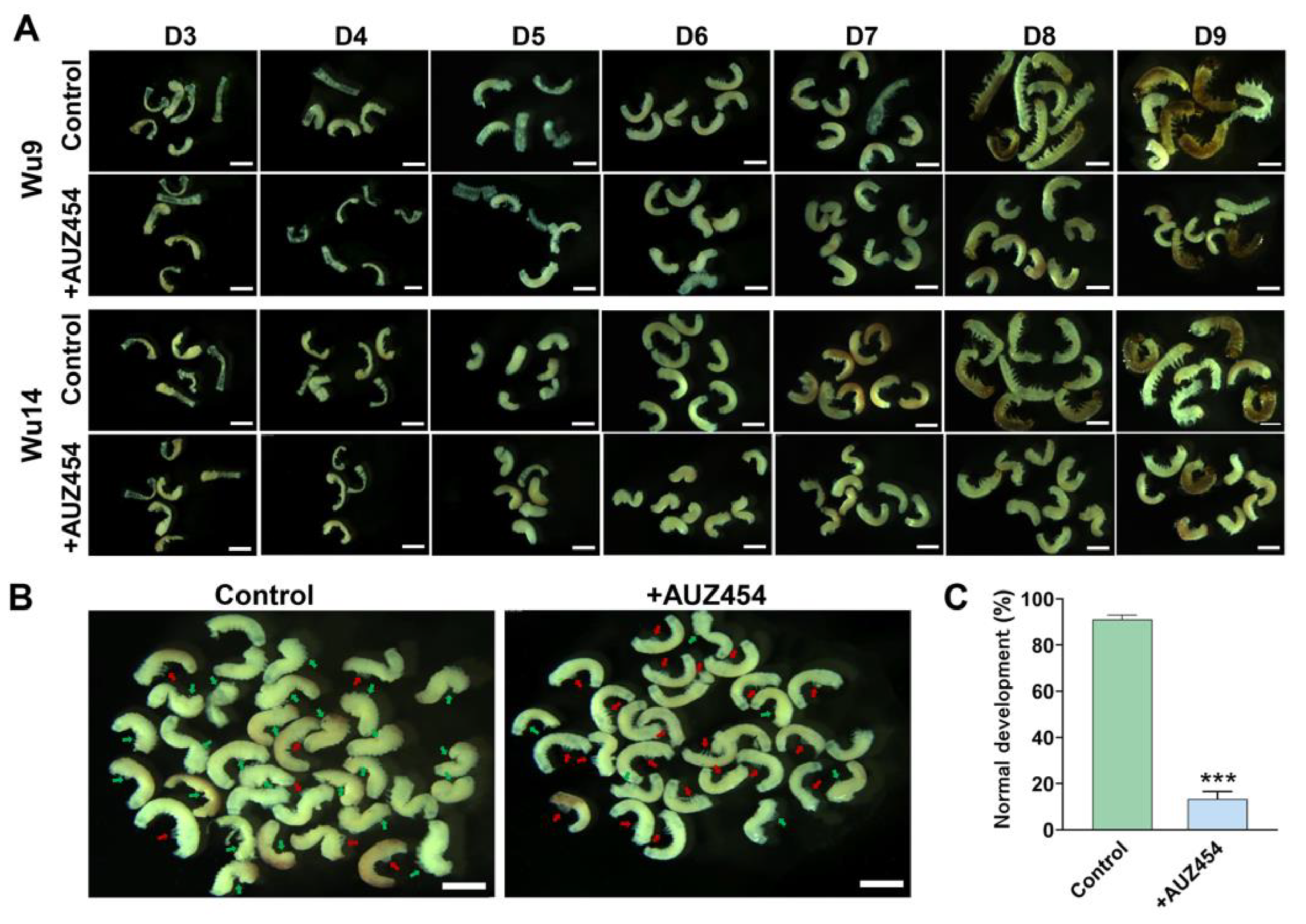
2.5. Inhibition of Cdk2 Activity Hindered Embryonic Development
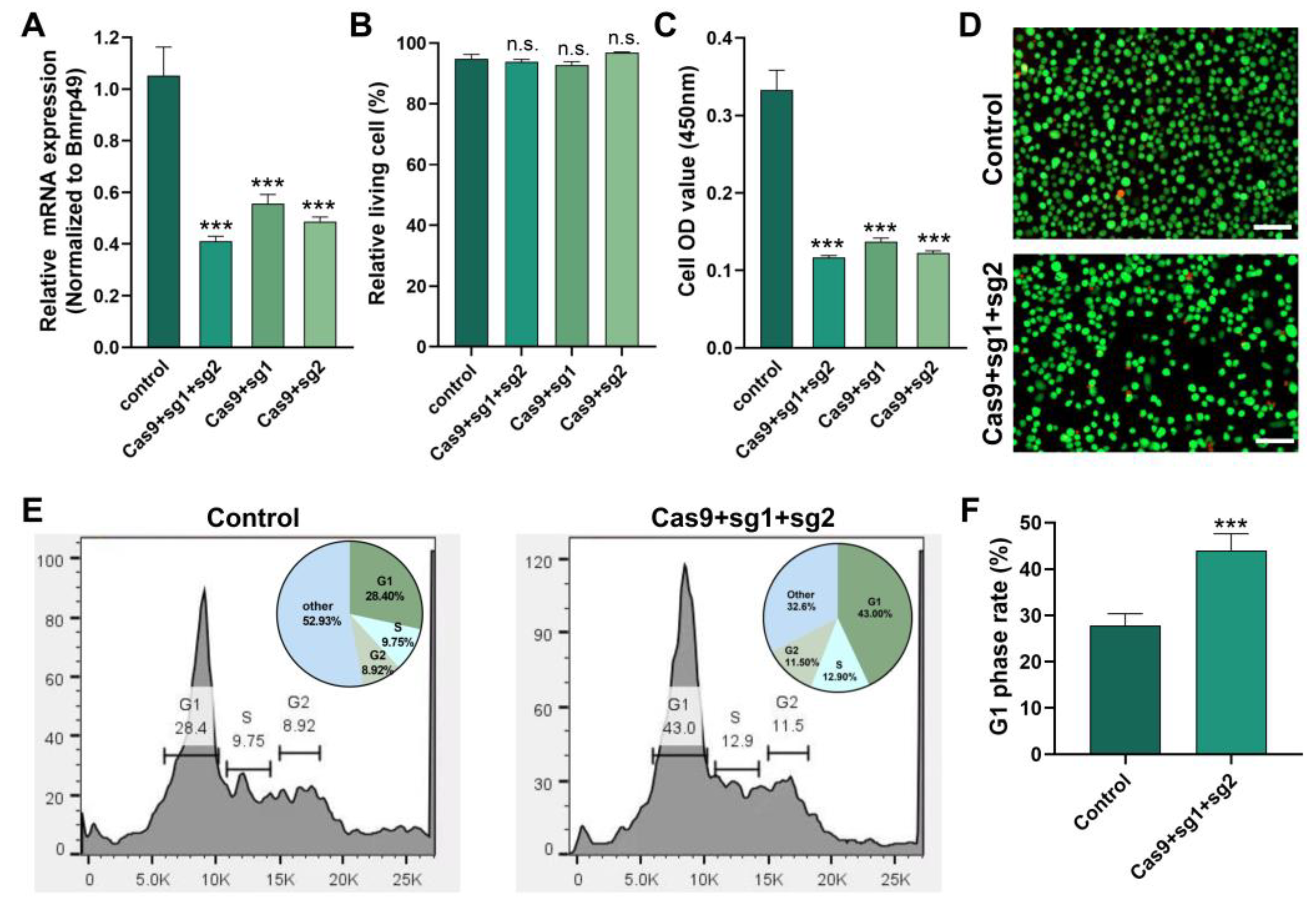
2.6. Knockdown of Cdk2 Gene Affected G1/S Cell Cycle Transition
3. Discussion
4. Materials and Methods
4.1. Silkworm and Cell Lines
4.2. Artificially Induced Parthenogenesis
4.3. Protein Structure and Phylogenetics Analysis
4.4. RNA Isolation, cDNA Synthesis, and qPCR Analysis
4.5. Enzyme Inhibition and Activity Detection
4.6. Ovariole and Embryo Observation
4.7. mRNA Synthesis and Cell Transfection
4.8. Cell Viability and Cycle Assay
4.9. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rabeling, C.; Kronauer, D.J. Thelytokous parthenogenesis in eusocial Hymenoptera. Annu. Rev. Entomol. 2013, 58, 273–292. [Google Scholar]
- Etges, W.J.; Chang, C.-C.; Ting, C.-T.; Chang, C.-H.; Fang, S.; Chang, H.-Y. The persistence of facultative parthenogenesis in Drosophila albomicans. PLoS ONE 2014, 9, e113275. [Google Scholar]
- Kearney, M.R.; Jasper, M.E.; White, V.L.; Aitkenhead, I.J.; Blacket, M.J.; Kong, J.D.; Chown, S.L.; Hoffmann, A.A. Parthenogenesis without costs in a grasshopper with hybrid origins. Science 2022, 376, 1110–1114. [Google Scholar] [PubMed]
- Moreira, M.O.; Fonseca, C.; Rojas, D. Parthenogenesis is self-destructive for scaled reptiles. Biol. Lett. 2021, 17, 20210006. [Google Scholar]
- Becks, L.; Agrawal, A.F. The evolution of sex is favoured during adaptation to new environments. PLos Biol. 2012, 10, e1001317. [Google Scholar]
- Snyman, M.; Xu, S. Transcriptomics and the origin of obligate parthenogenesis. Heredity 2023, 131, 119–129. [Google Scholar]
- Overton, J.B. Artificial parthenogenesis in fucus. Science 1913, 37, 841–844. [Google Scholar] [PubMed]
- Wyffels, J.T.; Adams, L.M.; Bulman, F.; Fustukjian, A.; Hyatt, M.W.; Feldheim, K.A.; Penfold, L.M. Artificial insemination and parthenogenesis in the whitespotted bamboo shark Chiloscyllium plagiosum. Sci. Rep. 2021, 11, 9966. [Google Scholar]
- Li, X.; Zou, C.; Li, M.; Fang, C.; Li, K.; Liu, Z.; Li, C. Transcriptome analysis of in vitro fertilization and parthenogenesis activation during early embryonic development in pigs. Genes 2021, 12, 1461. [Google Scholar] [CrossRef]
- Grenier, A.M.; Da Rocha, M.; Jalabert, A.; Royer, C.; Mauchamp, B.; Chavancy, G. Artificial parthenogenesis and control of voltinism to manage transgenic populations in Bombyx mori. J. Insect Physiol. 2004, 50, 751–760. [Google Scholar]
- Goldsmith, M.R.; Shimada, T.; Abe, H. The genetics and genomics of the silkworm. Bombyx mori. Annu. Rev. Entomol. 2005, 50, 71–100. [Google Scholar]
- Liu, P.; Wang, Y.; Du, X.; Yao, L.; Li, F.; Meng, Z. Transcriptome analysis of thermal parthenogenesis of the domesticated silkworm. PLoS ONE 2015, 10, e0135215. [Google Scholar]
- Strunnikov, V.A. Nature of heterosis and combining ability in the silkworm. Theor. Appl. Genet. 1986, 72, 503–512. [Google Scholar] [PubMed]
- Astaurov, B.L.; Vereiskaia, V.N. Long-term reproduction of triploid and tetraploid parthenogenetic clones of silkworms during artificial thermal parthenogenesis. Ontogenez 1977, 8, 3–10. [Google Scholar] [PubMed]
- Kato, M.; Hiruta, C.; Tochinai, S. The behavior of chromosomes during parthenogenetic oogenesis in marmorkrebs Procambarus fallax f. virginalis. Zool. Sci. 2016, 33, 426–430. [Google Scholar]
- Zabelina, V.; Yonemura, N.; Uchino, K.; Iizuka, T.; Mochida, Y.; Takemura, Y.; Klymenko, V.; Sezutsu, H.; Sehnal, F.; Tamura, T. Production of cloned transgenic silkworms by breeding non-diapausing parthenogenetic strains. J. Insect Physiol. 2021, 132, 104265. [Google Scholar]
- Sperling, A.L.; Glover, D.M. Parthenogenesis in dipterans: A genetic perspective. Proc. Biol. Sci. 2023, 290, 20230261. [Google Scholar]
- Zabelina, V.; Klymenko, V.; Tamura, T.; Doroshenko, K.; Liang, H.; Sezutsu, H.; Sehnal, F. Genome engineering and parthenocloning in the silkworm, Bombyx mori. J. Biomed. Sci. 2015, 40, 645–655. [Google Scholar]
- Strunnikov, V.A. Research on the artificial regulation of sex in animals in the USSR. Ontogenez 1978, 9, 3–19. [Google Scholar]
- Vereĭskaia, V.N. Cytology of egg maturation in the silkworm (Bombyx mori L.) in artificial parthenogenesis. Ontogenez 1979, 10, 244–252. [Google Scholar]
- Malumbres, M.; Harlow, E.; Hunt, T.; Hunter, T.; Lahti, J.M.; Manning, G.; Morgan, D.O.; Tsai, L.-H.; Wolgemuth, D.J. Cyclin-dependent kinases: A family portrait. Nat. Cell Biol. 2009, 11, 1275–1276. [Google Scholar]
- Hydbring, P.; Malumbres, M.; Sicinski, P. Non-canonical functions of cell cycle cyclins and cyclin-dependent kinases. Nat. Rev. Mol. Cell Biol. 2016, 17, 280–292. [Google Scholar]
- Malumbres, M. Cyclin-dependent kinases. Genome Biol. 2014, 15, 122. [Google Scholar]
- Schmidt, A.; Rauh, N.R.; Nigg, E.A.; Mayer, T.U. Cytostatic factor: An activity that puts the cell cycle on hold. J. Cell Sci. 2006, 119, 1213–1218. [Google Scholar] [PubMed]
- Berthet, C.; Aleem, E.; Coppola, V.; Tessarollo, L.; Kaldis, P. Cdk2 knockout mice are viable. Curr. Biol. 2003, 13, 1775–1785. [Google Scholar] [PubMed]
- Sugiura, K.; Naito, K.; Tojo, H. Cdk2 activity is essential for the first to second meiosis transition in porcine oocytes. J. Reprod. Dev. 2005, 51, 143–149. [Google Scholar] [PubMed]
- Zhu, X.N.; Kim, D.H.; Lin, H.R.; Budhavarapu, V.N.; Rosenbaum, H.B.; Mueller, P.R.; Yew, P.R. Proteolysis of Xenopus Cip-type CDK inhibitor, p16Xic2, is regulated by PCNA binding and CDK2 phosphorylation. Cell Div. 2013, 8, 5. [Google Scholar]
- Chauhan, S.; Diril, M.K.; Lee, J.H.; Bisteau, X.; Manoharan, V.; Adhikari, D.; Ratnacaram, C.K.; Janela, B.; Noffke, J.; Ginhoux, F.; et al. Cdk2 catalytic activity is essential for meiotic cell division in vivo. Biochem. J. 2016, 473, 2783–2798. [Google Scholar] [CrossRef]
- Li, J.; Chang, H.Y.; Yi, Z.Y.; Zhang, C.H.; Sun, Q.Y.; Qian, W.P. Transient inhibition of CDK2 activity prevents oocyte meiosis I completion and egg activation in mouse. J. Cell. Physiol. 2022, 237, 4317–4325. [Google Scholar]
- Gabrielli, B.G.; Roy, L.M.; Maller, J.L. Requirement for Cdk2 in cytostatic factor-mediated metaphase II arrest. Science 1993, 259, 1766–1769. [Google Scholar]
- Jones, K.T. Anaphase-promoting complex control in female mouse meiosis. Results Probl. Cell Differ. 2011, 53, 343–363. [Google Scholar] [PubMed]
- Grimison, B.; Liu, J.; Lewellyn, A.L.; Maller, J.L. Metaphase arrest by cyclin E-Cdk2 requires the spindle-checkpoint kinase Mps1. Curr. Biol. 2006, 16, 1968–1973. [Google Scholar]
- Boutros, R.; Mondesert, O.; Lorenzo, C.; Astuti, P.; McArthur, G.; Chircop, M.; Ducommun, B.; Gabrielli, B. CDC25B overexpression stabilises centrin 2 and promotes the formation of excess centriolar foci. PLoS ONE 2013, 8, e67822. [Google Scholar] [CrossRef]
- Matsumoto, Y.; Hayashi, K.; Nishida, E. Cyclin-dependent kinase 2 (Cdk2) is required for centrosome duplication in mammalian cells. Curr. Biol. 1999, 9, 429–432. [Google Scholar] [PubMed]
- Arora, M.; Moser, J.; Hoffman, T.E.; Watts, L.P.; Min, M.; Musteanu, M.; Rong, Y.; Ill, C.R.; Nangia, V.; Schneider, J.; et al. Rapid adaptation to CDK2 inhibition exposes intrinsic cell-cycle plasticity. Cell 2023, 186, 2628–2643.e21. [Google Scholar]
- Tripathi, S.K.; Muttineni, R.; Singh, S.K. Extra precision docking, free energy calculation and molecular dynamics simulation studies of CDK2 inhibitors. J. Theor. Biol. 2013, 334, 87–100. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Ma, Z.; Li, Y.; Zhu, S.; Xiao, Z.; Zhang, H.; Wang, Y. Development of in silico models for pyrazoles and pyrimidine derivatives as cyclin-dependent kinase 2 inhibitors. J. Mol. Graph. Model. 2011, 30, 67–81. [Google Scholar] [CrossRef]
- Faber, E.B.; Wang, N.; Georg, G.I. Review of rationale and progress toward targeting cyclin-dependent kinase 2 (CDK2) for male contraceptiondagger. Biol. Reprod. 2020, 103, 357–367. [Google Scholar]
- Greiss, H.; Vassilieva, J.; Petkov, N.; Petkov, Z. Comparative study of biological and technological characters in three generations of silkworm Bombyx mori L. ameiotic, parthenogenetically cloned lines. J. Assist. Reprod. Genet. 2004, 21, 415–420. [Google Scholar] [CrossRef]
- Neganova, I.; Vilella, F.; Atkinson, S.P.; Lloret, M.; Passos, J.F.; von Zglinicki, T.; O’Connor, J.-E.; Burks, D.; Jones, R.; Armstrong, L.; et al. An important role for CDK2 in G1 to S checkpoint activation and DNA damage response in human embryonic stem cells. Stem Cells 2011, 29, 651–659. [Google Scholar] [CrossRef]
- Chen, J.; Du, X.; Xu, X.; Zhang, S.; Yao, L.; He, X.; Wang, Y. Comparative Proteomic Analysis Provides New Insights into the Molecular Basis of Thermal-Induced Parthenogenesis in Silkworm (Bombyx mori). Insects 2023, 14, 134. [Google Scholar] [CrossRef]
- Sherr, C.J.; Roberts, J.M. Living with or without cyclins and cyclin-dependent kinases. Genes. Dev. 2004, 18, 2699–2711. [Google Scholar] [CrossRef] [PubMed]
- Sherr, C.J. G1 phase progression: Cycling on cue. Cell 1994, 79, 551–555. [Google Scholar] [CrossRef] [PubMed]
- Pan, M.H.; Cai, X.J.; Liu, M.; Lv, J.; Tang, H.; Tan, J.; Lu, C. Establishment and characterization of an ovarian cell line of the silkworm, Bombyx mori. Tissue Cell 2010, 42, 42–46. [Google Scholar] [CrossRef]
- Strunnikova, L.V. Biological method of determining the capacity of mulberry silkworm eggs which remain unpigmented for development. Ontogenez 1979, 10, 516–519. [Google Scholar]
- Waterhouse, A.; Bertoni, M.; Bienert, S.; Studer, G.; Tauriello, G.; Gumienny, R.; Heer, F.T.; De Beer, T.A.P.; Rempfer, C.; Bordoli, L.; et al. SWISS-MODEL: Homology modelling of protein structures and complexes. Nucleic Acids Res. 2018, 46, W296–W303. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Teng, X.; Zhang, Z.; He, G.; Yang, L.; Li, F. Validation of reference genes for quantitative expression analysis by real-time rt-PCR in four lepidopteran insects. J. Insect Sci. 2012, 12, 60. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Z.; Xu, J.; Zeng, B.; Ling, L.; You, L.; Chen, Y.; Huang, Y.; Tan, A. The CRISPR/Cas system mediates efficient genome engineering in Bombyx mori. Cell Res. 2013, 23, 1414–1416. [Google Scholar] [CrossRef]


| Primer Name | Primer Sequence (5′-3′) |
|---|---|
| qRT-PCR | |
| BmCdk2-F | TACCGTGCACCAGAAATCCT |
| BmCdk2-R | GCTCGGAAGTCAGGAAGTCT |
| BmRP49-F | TCAATCGGATCGCTATGACA |
| BmRP49-R | ATGACGGGTCTTCTTGTTGG |
| Plasmid construction | |
| sgRNA-F1 | TAATACGACTCACTATAGGTCTCTGTGGAGAACACGTGTTTTAGAGCTAGAAATAGCAA |
| sgRNA-F2 | TAATACGACTCACTATAGGTCTATTTCACTGTCACCGGTTTTAGAGCTAGAAATAGCAA |
| sgRNA-R | AGCACCGACTCGGTGCCACTTTTTCAAGTTGATAACGGACTAGCCTTATTTTAACTTGCTATTTCTAGCT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, C.; Jiang, Y.; Ma, C.; Xu, F.; Cui, C.; Du, X.; Chen, J.; Zhu, L.; Yu, S.; He, X.; et al. Decreased Cdk2 Activity Hindered Embryonic Development and Parthenogenesis Induction in Silkworm, Bombyx mori L. Int. J. Mol. Sci. 2025, 26, 3341. https://doi.org/10.3390/ijms26073341
Hu C, Jiang Y, Ma C, Xu F, Cui C, Du X, Chen J, Zhu L, Yu S, He X, et al. Decreased Cdk2 Activity Hindered Embryonic Development and Parthenogenesis Induction in Silkworm, Bombyx mori L. International Journal of Molecular Sciences. 2025; 26(7):3341. https://doi.org/10.3390/ijms26073341
Chicago/Turabian StyleHu, Chengjie, Yonghou Jiang, Chenkai Ma, Fang Xu, Chunguang Cui, Xin Du, Jine Chen, Linbao Zhu, Shaofang Yu, Xingjian He, and et al. 2025. "Decreased Cdk2 Activity Hindered Embryonic Development and Parthenogenesis Induction in Silkworm, Bombyx mori L." International Journal of Molecular Sciences 26, no. 7: 3341. https://doi.org/10.3390/ijms26073341
APA StyleHu, C., Jiang, Y., Ma, C., Xu, F., Cui, C., Du, X., Chen, J., Zhu, L., Yu, S., He, X., Yu, W., Wang, Y., & Xu, X. (2025). Decreased Cdk2 Activity Hindered Embryonic Development and Parthenogenesis Induction in Silkworm, Bombyx mori L. International Journal of Molecular Sciences, 26(7), 3341. https://doi.org/10.3390/ijms26073341




