Exosomes in Breast Milk: Their Impact on the Intestinal Microbiota of the Newborn and Therapeutic Perspectives for High-Risk Neonates
Abstract
1. Introduction
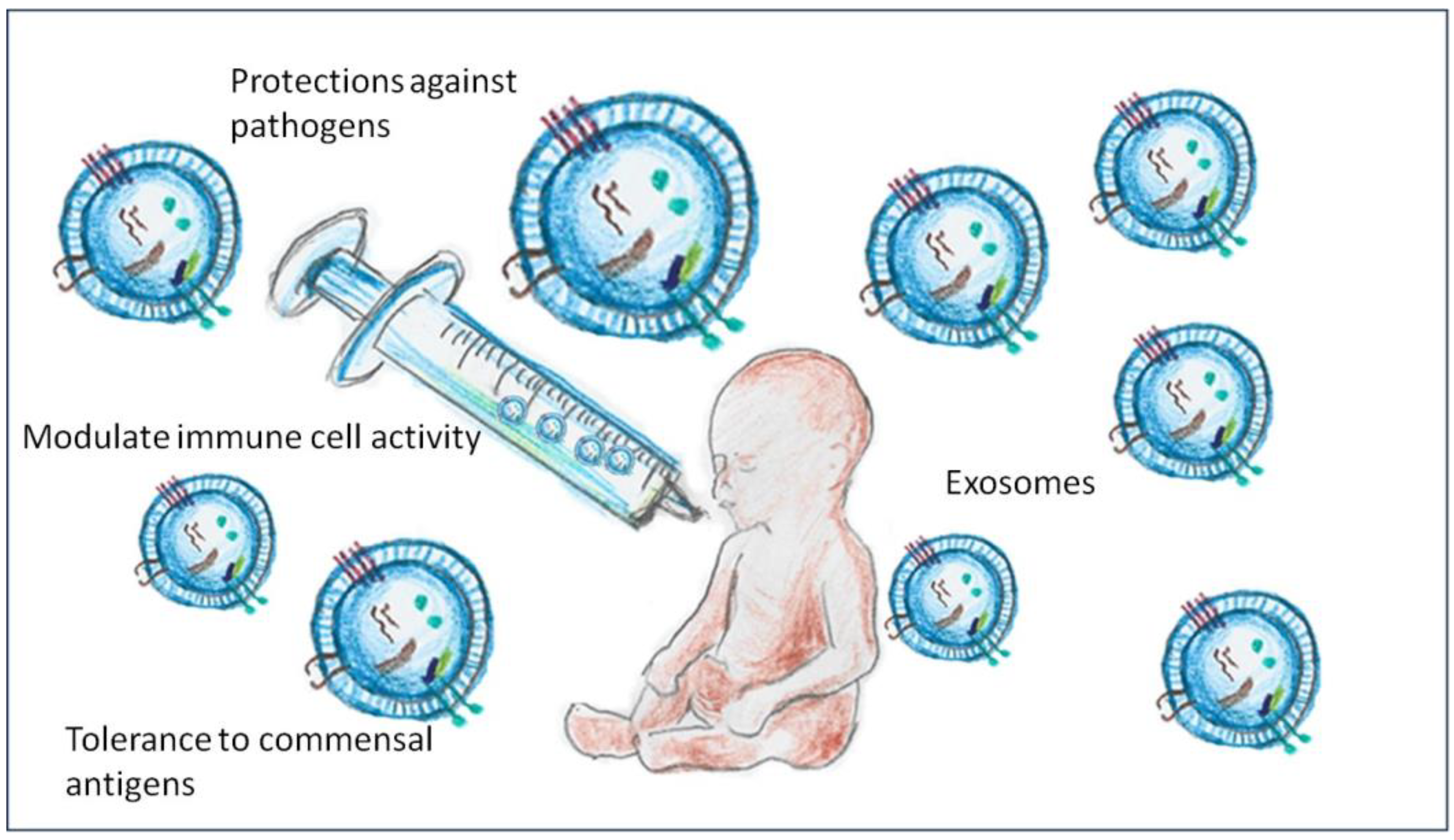
2. Compositions and Functions of Exosomes in Breast Milk
3. Impact on Newborn Intestinal Microbiota
4. Exosomes and Protection Against Necrotising Enterocolitis (NEC)
5. Techniques for the Isolation and Sequencing of Exosomes in Breast Milk
6. Future Therapeutic Applications of Breast Milk Exosomes
7. Challenges and Ethical Considerations
8. Conclusions and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bzikowska-Jura, A.; Sobieraj, P.; Szostak-Węgierek, D.; Wesołowska, A. Impact of infant and maternal factors on energy and macronutrient composition of human milk. Nutrients 2020, 12, 2591. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Golan-Gerstl, R.; Elbaum Shiff, Y.; Moshayoff, V.; Schecter, D.; Leshkowitz, D.; Reif, S. Characterization and biological function of milk-derived miRNAs. Mol. Nutr. Food Res. 2017, 61, 10. [Google Scholar] [CrossRef] [PubMed]
- Le Doare, K.; Holder, B.; Bassett, A.; Pannaraj, P.S. Mother’s milk: A purposeful contribution to the development of the infant microbiota and immunity. Front. Immunol. 2018, 9, 361. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Brockway, M.M.; Daniel, A.I.; Reyes, S.M.; Gauglitz, J.M.; Granger, M.; McDermid, J.M.; Chan, D.; Refvik, R.; Sidhu, K.K.; Musse, S.; et al. Human milk bioactive components and child growth and body composition in the first 2 years: A systematic review. Adv. Nutr. 2024, 15, 100127. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Reniker, L.N.; Frazer, L.C.; Good, M. Key biologically active components of breast milk and their beneficial effects. Semin. Pediatr. Surg. 2023, 32, 151306. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Alsaweed, M.; Hartmann, P.E.; Geddes, D.T.; Kakulas, F. MicroRNAs in breastmilk and the lactating breast: Potential immunoprotectors and developmental regulators for the infant and the mother. Int. J. Environ. Res. Public Health 2015, 12, 13981–14020. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Weber, J.A.; Baxter, D.H.; Zhang, S.; Huang, D.Y.; Huang, K.H.; Lee, M.J.; Galas, D.J.; Wang, K. The microRNA spectrum in 12 body fluids. Clin. Chem. 2010, 56, 1733–1741. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Munch, E.M.; Harris, R.A.; Mohammad, M.; Benham, A.L.; Pejerrey, S.M.; Showalter, L.; Hu, M.; Shope, C.D.; Maningat, P.D.; Gunaratne, P.H.; et al. Transcriptome profiling of microRNA by next-gen deep sequencing reveals known and novel miRNA species in the lipid fraction of human breast milk. PLoS ONE 2013, 8, e50564. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Carney, M.C.; Tarasiuk, A.; DiAngelo, S.L.; Silveyra, P.; Podany, A.; Birch, L.L.; Paul, I.M.; Kelleher, S.; Hicks, S.D. Metabolism-related microRNAs in maternal breast milk are influenced by premature delivery. Pediatr. Res. 2017, 82, 226–236. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jiang, M.; Sang, X.; Hong, Z. Beyond nutrients: Food-derived microRNAs provide cross-kingdom regulation. Bioessays 2012, 34, 280–284. [Google Scholar] [CrossRef] [PubMed]
- Mirza, A.H.; Kaur, S.; Nielsen, L.B.; Størling, J.; Yarani, R.; Roursgaard, M.; Mathiesen, E.R.; Damm, P.; Svare, J.; Mortensen, H.B.; et al. Breast milk-derived extracellular vesicles enriched in exosomes from mothers with type 1 diabetes contain aberrant levels of microRNAs. Front. Immunol. 2019, 10, 2543. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cui, Z.; Amevor, F.K.; Zhao, X.; Mou, C.; Pang, J.; Peng, X.; Liu, A.; Lan, X.; Liu, L. Potential therapeutic effects of milk-derived exosomes on intestinal diseases. J. Nanobiotechnol. 2023, 21, 496. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kim, S.Y.; Yi, D.Y. Analysis of the human breast milk microbiome and bacterial extracellular vesicles in healthy mothers. Exp. Mol. Med. 2020, 52, 1288–1297. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yang, M.; Song, D.; Cao, X.; Wu, R.; Liu, B.; Ye, W.; Wu, J.; Yue, X. Comparative proteomic analysis of milk-derived exosomes in human and bovine colostrum and mature milk samples by iTRAQ-coupled LC-MS/MS. Food Res. Int. 2017, 92, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Li, M.; Wang, X.; Li, Q.; Wang, T.; Zhu, Q.; Zhou, X.; Wang, X.; Gao, X.; Li, X. Immune-related microRNAs are abundant in breast milk exosomes. Int. J. Biol. Sci. 2012, 8, 118–123. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Andres, S.F.; Scottoline, B.; Good, M. Shaping infant development from the inside out: Bioactive factors in human milk. Semin. Perinatol. 2023, 47, 151690. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- van Herwijnen, M.J.; Zonneveld, M.I.; Goerdayal, S.; Nolte-’t Hoen, E.N.; Garssen, J.; Stahl, B.; Maarten Altelaar, A.F.; Redegeld, F.A.; Wauben, M.H. Comprehensive proteomic analysis of human milk-derived extracellular vesicles unveils a novel functional proteome distinct from other milk components. Mol. Cell. Proteom. 2016, 15, 3412–3423. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tlaskalová-Hogenová, H.; Stepánková, R.; Hudcovic, T.; Tucková, L.; Cukrowska, B.; Lodinová-Zádníková, R.; Kozáková, H.; Rossmann, P.; Bártová, J.; Sokol, D.; et al. Commensal bacteria (normal microflora), mucosal immunity and chronic inflammatory and autoimmune diseases. Immunol. Lett. 2004, 93, 97–108. [Google Scholar] [CrossRef] [PubMed]
- Nowrouzian, F.L.; Ljung, A.; Hesselmar, B.; Nilsson, S.; Adlerberth, I.; Wold, A.E. Bacterial carriage of genes encoding fibronectin-binding proteins is associated with long-term persistence of staphylococcus aureus in the nasal and gut microbiota of infants. Appl. Environ. Microbiol. 2021, 87, e0067121. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Garg, P.M.; Garg, P.P.; Lal, C.V. Necrotizing enterocolitis (NEC): A devastating disease of prematurity. J. Neonatal Biol. 2015, 4, 1000202. [Google Scholar] [PubMed] [PubMed Central]
- Moubareck, C.A. Human milk microbiota and oligosaccharides: A glimpse into benefits, diversity, and correlations. Nutrients 2021, 13, 1123. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ganji, N.; Li, B.; Lee, C.; Pierro, A. Necrotizing enterocolitis: Recent advances in treatment with translational potential. Pediatr. Surg. Int. 2023, 39, 205. [Google Scholar] [CrossRef] [PubMed]
- Fu, C.; Sun, W.; Wang, X.; Zhu, X. Human breast milk: A promising treatment for necrotizing enterocolitis. Early Hum. Dev. 2023, 184, 105833. [Google Scholar] [CrossRef] [PubMed]
- Madden, J.W. Human breast milk exosomes may protect against necrotizing enterocolitis in preterm infants. Pediatr. Res. 2021, 90, 244–245. [Google Scholar] [CrossRef] [PubMed]
- García-Martínez, J.; Pérez-Castillo, Í.M.; Salto, R.; López-Pedrosa, J.M.; Rueda, R.; Girón, M.D. Beneficial effects of bovine milk exosomes in metabolic interorgan cross-talk. Nutrients 2022, 14, 1442. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, S.; Friedberg, I.; Ivanov, I.V.; Davidson, L.A.; Goldsby, J.S.; Dahl, D.B.; Herman, D.; Wang, M.; Donovan, S.M.; Chapkin, R.S. A metagenomic study of diet-dependent interaction between gut microbiota and host in infants reveals differences in immune response. Genome Biol. 2012, 13, 1–16. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zozaya, C.; García González, I.; Avila-Alvarez, A.; Oikonomopoulou, N.; Sánchez Tamayo, T.; Salguero, E.; Saenz de Pipaón, M.; García-Muñoz Rodrigo, F.; Couce, M.L. Incidence, treatment, and outcome trends of necrotizing enterocolitis in preterm infants: A multicenter cohort study. Front. Pediatr. 2020, 8, 188. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pammi, M.; Cope, J.; Tarr, P.I.; Warner, B.B.; Morrow, A.L.; Mai, V.; Gregory, K.E.; Kroll, J.S.; McMurtry, V.; Ferris, M.J.; et al. Intestinal dysbiosis in preterm infants preceding necrotizing enterocolitis: A systematic review and meta-analysis. Microbiome 2017, 5, 31. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- He, S.; Liu, G.; Zhu, X. Human breast milk-derived exosomes may help maintain intestinal epithelial barrier integrity. Pediatr. Res. 2021, 90, 366–372. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Hock, A.; Wu, R.Y.; Minich, A.; Botts, S.R.; Lee, C.; Antounians, L.; Miyake, H.; Koike, Y.; Chen, Y.; et al. Bovine milk-derived exosomes enhance goblet cell activity and prevent the development of experimental necrotizing enterocolitis. PLoS ONE 2019, 14, e0211431. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hock, A.; Miyake, H.; Li, B.; Lee, C.; Ermini, L.; Koike, Y.; Chen, Y.; Määttänen, P.; Zani, A.; Pierro, A. Breast milk-derived exosomes promote intestinal epithelial cell growth. J. Pediatr. Surg. 2017, 52, 755–759. [Google Scholar] [CrossRef] [PubMed]
- Pisano, C.; Galley, J.; Elbahrawy, M.; Wang, Y.; Farrell, A.; Brigstock, D.; Besner, G.E. Human breast milk-derived extracellular vesicles in the protection against experimental necrotizing enterocolitis. J. Pediatr. Surg. 2020, 55, 54–58. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Çelik, E.; Cemali, Ö.; Şahin, T.Ö.; Deveci, G.; Biçer, N.Ç.; Hirfanoğlu, İ.M.; Ağagündüz, D.; Budán, F. Human breast milk exosomes: Affecting factors, their possible health outcomes, and future directions in dietetics. Nutrients 2024, 16, 3519. [Google Scholar] [CrossRef] [PubMed]
- Melnik, B.C.; Stremmel, W.; Weiskirchen, R.; John, S.M.; Schmitz, G. Exosome-derived MicroRNAs of human milk and their effects on infant health and development. Biomolecules 2021, 11, 851. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lin, C.; Guo, J.; Jia, R. Roles of regulatory T cell-derived extracellular vesicles in human diseases. Int. J. Mol. Sci. 2022, 23, 11206. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, L.; Gao, R.; Li, B.; Alganabi, M.; He, W.; Shen, C.; Zhu, H.; Pierro, A. Human breast milk-derived exosomes protect against intestinal ischemia and reperfusion injury in neonatal rats. J. Pediatr. Surg. 2022, 57, 1264–1268. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Wang, X.; Yan, X.; Yu, Z.; Zhang, J.; Han, S. The emerging role of exosomes in the pathogenesis, prognosis and treatment of necrotizing enterocolitis. Am. J. Transl. Res. 2020, 12, 7020–7033. [Google Scholar] [PubMed] [PubMed Central]
- Tian, C.M.; Yang, M.F.; Xu, H.M.; Zhu, M.Z.; Zhang, Y.; Yao, J.; Wang, L.S.; Liang, Y.J.; Li, D.F. Mesenchymal stem cell-derived exosomes: Novel therapeutic approach for inflammatory bowel diseases. Stem Cells Int. 2023, 2023, 4245704. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Khatun, Z.; Bhat, A.; Sharma, S.; Sharma, A. Elucidating diversity of exosomes: Biophysical and molecular characterization methods. Nanomedicine 2016, 11, 2359–2377. [Google Scholar] [CrossRef] [PubMed]
- Medel-Martinez, A.; Redrado-Osta, A.; Crespo-Barreda, A.; Sancho-Albero, M.; Sánchez, L.; Sebastián, V.; Pardo, M.; de la Vieja, A.; Martín-Duque, P. Isolation and characterization of milk exosomes for use in advanced therapies. Biomolecules 2024, 14, 810. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zonneveld, M.I.; Brisson, A.R.; van Herwijnen, M.J.; Tan, S.; van de Lest, C.H.; Redegeld, F.A.; Garssen, J.; Wauben, M.H.; Nolte-’t Hoen, E.N. Recovery of extracellular vesicles from human breast milk is influenced by sample collection and vesicle isolation procedures. J. Extracell. Vesicles 2014, 14, 3. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rashidi, M.; Bijari, S.; Khazaei, A.H.; Shojaei-Ghahrizjani, F.; Rezakhani, L. The role of milk-derived exosomes in the treatment of diseases. Front. Genet. 2022, 13, 1009338. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Barathan, M.; Ng, S.L.; Lokanathan, Y.; Ng, M.H.; Law, J.X. Milk-derived extracellular vesicles: A novel perspective on comparative therapeutics and targeted nanocarrier application. Vaccines 2024, 12, 1282. [Google Scholar] [CrossRef] [PubMed]
- Quintana, J.F.; Makepeace, B.L.; Babayan, S.A.; Ivens, A.; Pfarr, K.M.; Blaxter, M.; Debrah, A.; Wanji, S.; Ngangyung, H.F.; Bah, G.S.; et al. Extracellular Onchocerca-derived small RNAs in host nodules and blood. Parasit. Vectors 2015, 8, 58. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yun, B.; Kim, Y.; Park, D.J.; Oh, S. Comparative analysis of dietary exosome-derived microRNAs from human, bovine and caprine colostrum and mature milk. J. Anim. Sci. Technol. 2021, 63, 593–602. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, H.; Wu, D.; Sukreet, S.; Delaney, A.; Belfort, M.B.; Zempleni, J. Quantitation of exosomes and their MicroRNA cargos in frozen human milk. JPGN Rep. 2022, 3, e172. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Verma, P.; Mohanty, N.; Pruseth, B.; Sahoo, S.; Katiyar, A.; Singh, H.; Jena, S.K.; Das, R.R.; Som, T.K.; Sahoo, S.K.; et al. Identification of candidate immune system MicroRNAs differentially found in colostrum and milk exosomes. Microrna 2022, 11, 216–226. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.V.E.; Walsh, V.; McGuire, W. Formula versus maternal breast milk for feeding preterm or low birth weight infants. Cochrane Database Syst. Rev. 2019, 8, CD002972. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Galley, J.D.; Besner, G.E. The therapeutic potential of breast milk-derived extracellular vesicles. Nutrients 2020, 12, 745. [Google Scholar] [CrossRef]
- Kahn, S.; Liao, Y.; Du, X.; Xu, W.; Li, J.; Lönnerdal, B. Exosomal MicroRNAs in milk from mothers delivering preterm infants survive in vitro digestion and are taken up by human intestinal cells. Mol. Nutr. Food. Res. 2018, 62, e1701050. [Google Scholar] [CrossRef] [PubMed]
- McCulloh, C.J.; Olson, J.K.; Wang, Y.; Zhou, Y.; Tengberg, N.H.; Deshpande, S.; Besner, G.E. Treatment of experimental necrotizing enterocolitis with stem cell-derived exosomes. J. Pediatr. Surg. 2018, 53, 1215–1220. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rager, T.M.; Olson, J.K.; Zhou, Y.; Wang, Y.; Besner, G.E. Exosomes secreted from bone marrow-derived mesenchymal stem cells protect the intestines from experimental necrotizing enterocolitis. J. Pediatr. Surg. 2016, 51, 942–947. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Olson, J.K.; Navarro, J.B.; Allen, J.M.; McCulloh, C.J.; Mashburn-Warren, L.; Wang, Y.; Varaljay, V.A.; Bailey, M.T.; Goodman, S.D.; Besner, G.E. An enhanced Lactobacillus reuteri biofilm formulation that increases protection against experimental necrotizing enterocolitis. Am. J. Physiol. Gastrointest. Liver Physiol. 2018, 315, G408–G419. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Feng, X.; Chen, X.; Zheng, X.; Zhu, H.; Qi, Q.; Liu, S.; Zhang, H.; Che, J. Latest trend of milk derived exosomes: Cargos, functions, and applications. Front. Nutr. 2021, 8, 747294. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Carr, L.E.; Virmani, M.D.; Rosa, F.; Munblit, D.; Matazel, K.S.; Elolimy, A.A.; Yeruva, L. Role of human milk bioactives on infants’ gut and immune health. Front. Immunol. 2021, 12, 604080. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Słyk-Gulewska, P.; Kondracka, A.; Kwaśniewska, A. MicroRNA as a new bioactive component in breast milk. Noncoding RNA Res. 2023, 8, 520–526. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sonbhadra, S.; Mehak; Pandey, L.M. Biogenesis, isolation, and detection of exosomes and their potential in therapeutics and diagnostics. Biosensors 2023, 13, 802. [Google Scholar] [CrossRef]
- Chen, T.; Xie, M.Y.; Sun, J.J.; Ye, R.S.; Cheng, X.; Sun, R.P.; Wei, L.M.; Li, M.; Lin, D.L.; Jiang, Q.Y.; et al. Porcine milk-derived exosomes promote proliferation of intestinal epithelial cells. Sci. Rep. 2016, 6, 33862. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cristóbal-Cañadas, D.; Parrón-Carrillo, R.; Parrón-Carreño, T. Exosomes in Breast Milk: Their Impact on the Intestinal Microbiota of the Newborn and Therapeutic Perspectives for High-Risk Neonates. Int. J. Mol. Sci. 2025, 26, 3421. https://doi.org/10.3390/ijms26073421
Cristóbal-Cañadas D, Parrón-Carrillo R, Parrón-Carreño T. Exosomes in Breast Milk: Their Impact on the Intestinal Microbiota of the Newborn and Therapeutic Perspectives for High-Risk Neonates. International Journal of Molecular Sciences. 2025; 26(7):3421. https://doi.org/10.3390/ijms26073421
Chicago/Turabian StyleCristóbal-Cañadas, Delia, Rocio Parrón-Carrillo, and Tesifón Parrón-Carreño. 2025. "Exosomes in Breast Milk: Their Impact on the Intestinal Microbiota of the Newborn and Therapeutic Perspectives for High-Risk Neonates" International Journal of Molecular Sciences 26, no. 7: 3421. https://doi.org/10.3390/ijms26073421
APA StyleCristóbal-Cañadas, D., Parrón-Carrillo, R., & Parrón-Carreño, T. (2025). Exosomes in Breast Milk: Their Impact on the Intestinal Microbiota of the Newborn and Therapeutic Perspectives for High-Risk Neonates. International Journal of Molecular Sciences, 26(7), 3421. https://doi.org/10.3390/ijms26073421





