Extracellular Vesicle Abundance, but Not a High Aggregation-Prone Peptide Cargo, Is Associated with Dihydroartemisinin Exposure in Plasmodium falciparum
Abstract
:1. Introduction
2. Results
2.1. Determination of Parasite Sensitivity to Artemisinin
2.2. Size Distribution of EVs Is Similar Among Parasite Lines for All Conditions
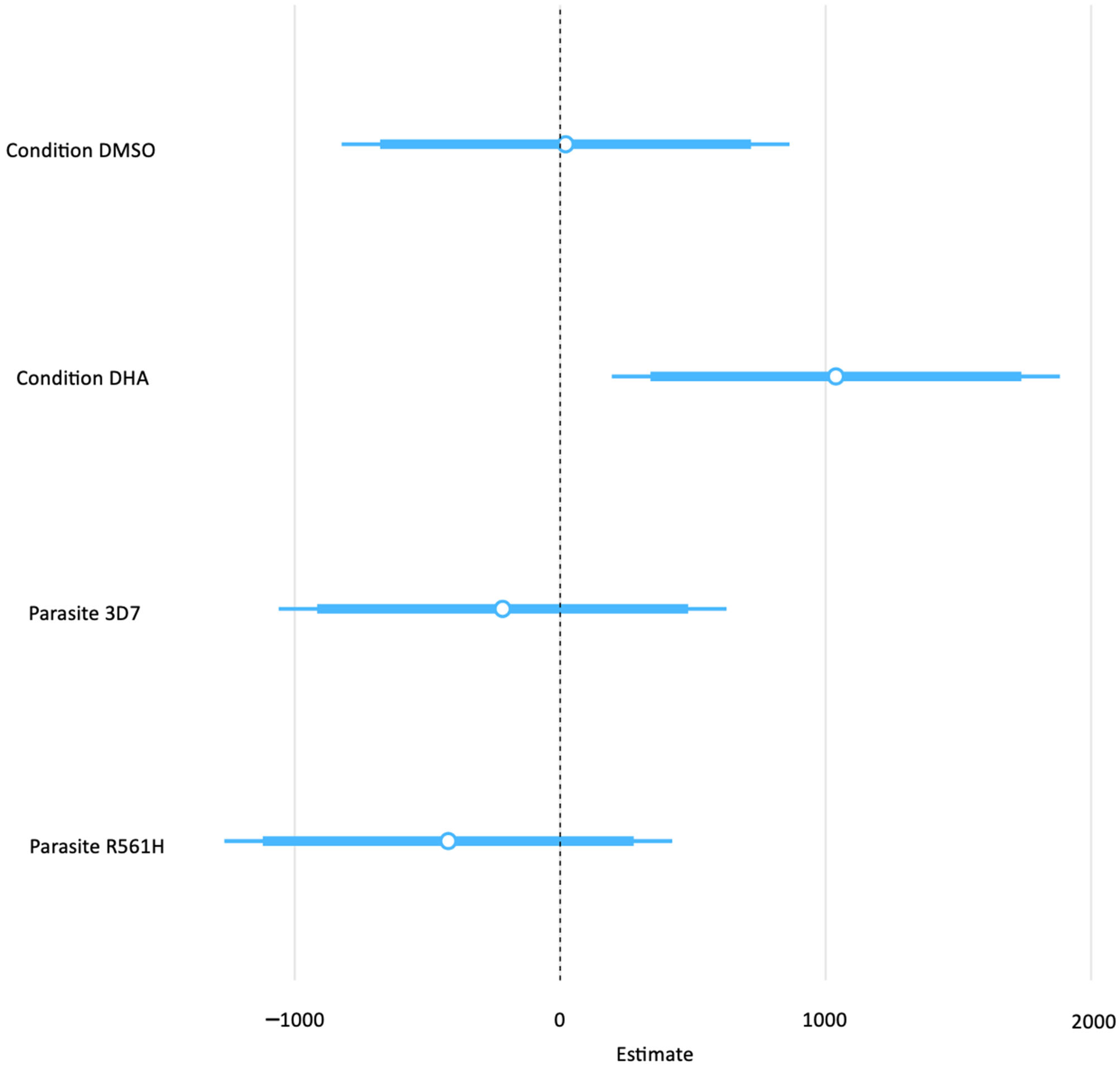
2.3. DHA Treatment Condition Is the Best Predictor of EV Abundance
2.4. One Aggregation-Prone Peptide out of Nine Identified Peptides Was Found in the Proteome Cargo of EVs from DHA-Exposed ART-Susceptible and Resistant Parasites
3. Discussion
4. Methods
4.1. P. falciparum In Vitro Parasite Culture and Treatment Conditions
4.2. Ring Stage Survival Analysis
4.3. EV Extraction and Purification
4.4. EV Size and Abundance Measurements
4.5. Sample Preparation for Mass Spectrometry
4.6. Analytical Procedure and Equipment Used for LC–MS/MS
4.7. Data Analysis for LC–MS/MS Data
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dondorp, A.M.; Nosten, F.; Yi, P.; Das, D.; Phyo, A.P.; Tarning, J.; Lwin, K.M.; Ariey, F.; Hanpithakpong, W.; Lee, S.J.; et al. Artemisinin resistance in Plasmodium falciparum malaria. N. Engl. J. Med. 2009, 361, 455–467. [Google Scholar] [CrossRef] [PubMed]
- Noedl, H.; Se, Y.; Schaecher, K.; Smith, B.L.; Socheat, D.; Fukuda, M.M. Evidence of artemisinin-resistant malaria in western Cambodia. N. Engl. J. Med. 2008, 359, 2619–2620. [Google Scholar] [CrossRef] [PubMed]
- WHO. World Malaria Report 2023. 2023. Available online: https://www.who.int/teams/global-malaria-programme/reports/world-malaria-report-2023 (accessed on 26 November 2024).
- Balikagala, B.; Fukuda, N.; Ikeda, M.; Katuro, O.T.; Tachibana, S.I.; Yamauchi, M.; Opio, W.; Emoto, S.; Anywar, D.A.; Kimura, E.; et al. Evidence of Artemisinin-Resistant Malaria in Africa. N. Engl. J. Med. 2021, 385, 1163–1171. [Google Scholar] [CrossRef]
- Uwimana, A.; Legrand, E.; Stokes, B.H.; Ndikumana, J.M.; Warsame, M.; Umulisa, N.; Ngamije, D.; Munyaneza, T.; Mazarati, J.B.; Munguti, K.; et al. Emergence and clonal expansion of in vitro artemisinin-resistant Plasmodium falciparum kelch13 R561H mutant parasites in Rwanda. Nat. Med. 2020, 26, 1602–1608. [Google Scholar] [CrossRef]
- Siddiqui, F.A.; Liang, X.; Cui, L. Plasmodium falciparum resistance to ACTs: Emergence, mechanisms, and outlook. Int. J. Parasitol. Drugs Drug Resist. 2021, 16, 102–118. [Google Scholar] [CrossRef] [PubMed]
- Nsanzabana, C. Time to scale up molecular surveillance for anti-malarial drug resistance in sub-Saharan Africa. Malar. J. 2021, 20, 401. [Google Scholar] [CrossRef]
- Nsanzabana, C.; Djalle, D.; Guérin, P.J.; Ménard, D.; González, I.J. Tools for surveillance of anti-malarial drug resistance: An assessment of the current landscape. Malar. J. 2018, 17, 75. [Google Scholar] [CrossRef]
- Couch, Y.; Buzàs, E.I.; Di Vizio, D.; Gho, Y.S.; Harrison, P.; Hill, A.F.; Lötvall, J.; Raposo, G.; Stahl, P.D.; Théry, C.; et al. A brief history of nearly EV-erything—The rise and rise of extracellular vesicles. J. Extracell. Vesicles 2021, 10, e12144. [Google Scholar] [CrossRef]
- Avalos-Padilla, Y.; Georgiev, V.N.; Lantero, E.; Pujals, S.; Verhoef, R.; Borgheti-Cardoso, L.N.; Albertazzi, L.; Dimova, R.; Fernàndez-Busquets, X. The ESCRT-III machinery participates in the production of extracellular vesicles and protein export during Plasmodium falciparum infection. PLoS Pathog. 2021, 17, e1009455. [Google Scholar] [CrossRef]
- Regev-Rudzki, N.; Wilson, D.W.; Carvalho, T.G.; Sisquella, X.; Coleman, B.M.; Rug, M.; Bursac, D.; Angrisano, F.; Gee, M.; Hill, A.F. Cell-cell communication between malaria-infected red blood cells via exosome-like vesicles. Cell 2013, 153, 1120–1133. [Google Scholar] [CrossRef]
- Tandoh, K.Z.; Morang’a, C.M.; Wilson, M.; Quashie, N.B.; Duah-Quashie, N.O. Malaria artemisinin resistance: An extracellular vesicles export hypothesis. Trends Parasitol. 2022, 38, 614–617. [Google Scholar] [CrossRef]
- Lancaster, A.K.; Nutter-Upham, A.; Lindquist, S.; King, O.D. PLAAC: A web and command-line application to identify proteins with prion-like amino acid composition. Bioinformatics 2014, 30, 2501–2502. [Google Scholar] [CrossRef] [PubMed]
- Biosca, A.; Bouzón-Arnáiz, I.; Spanos, L.; Siden-Kiamos, I.; Iglesias, V.; Ventura, S.; Fernàndez-Busquets, X. Detection of Protein Aggregation in Live Plasmodium Parasites. Antimicrob. Agents Chemother. 2020, 64, e02135-19. [Google Scholar] [CrossRef]
- Blow, F.; Buck, A.H. Extracellular vesicles from malaria-infected red blood cells: Not all are secreted equal. EMBO Rep. 2022, 23, e55499. [Google Scholar] [CrossRef] [PubMed]
- Abou Karam, P.; Rosenhek-Goldian, I.; Ziv, T.; Ben Ami Pilo, H.; Azuri, I.; Rivkin, A.; Kiper, E.; Rotkopf, R.; Cohen, S.R.; Torrecilhas, A.C.; et al. Malaria parasites release vesicle subpopulations with signatures of different destinations. EMBO Rep. 2022, 23, e54755. [Google Scholar] [CrossRef]
- Nantakomol, D.; Dondorp, A.M.; Krudsood, S.; Udomsangpetch, R.; Pattanapanyasat, K.; Combes, V.; Grau, G.E.; White, N.J.; Viriyavejakul, P.; Day, N.P.; et al. Circulating red cell-derived microparticles in human malaria. J. Infect. Dis. 2011, 203, 700–706. [Google Scholar] [CrossRef] [PubMed]
- Cheng, I.S.; Sealy, B.C.; Tiberti, N.; Combes, V. Extracellular vesicles, from pathogenesis to biomarkers: The case for cerebral malaria. Vessel. Plus 2020, 4, 17. [Google Scholar] [CrossRef]
- Antwi-Baffour, S.; Malibha-Pinchbeck, M.; Stratton, D.; Jorfi, S.; Lange, S.; Inal, J. Plasma mEV levels in Ghanain malaria patients with low parasitaemia are higher than those of healthy controls, raising the potential for parasite markers in mEVs as diagnostic targets. J. Extracell. Vesicles 2020, 9, 1697124. [Google Scholar] [CrossRef]
- Pallarès, I.; de Groot, N.S.; Iglesias, V.; Sant’Anna, R.; Biosca, A.; Fernàndez-Busquets, X.; Ventura, S. Discovering Putative Prion-Like Proteins in Plasmodium falciparum: A Computational and Experimental Analysis. Front. Microbiol. 2018, 9, 1737. [Google Scholar] [CrossRef]
- Bouzón-Arnáiz, I.; Avalos-Padilla, Y.; Biosca, A.; Caño-Prades, O.; Román-Álamo, L.; Valle, J.; Andreu, D.; Moita, D.; Prudêncio, M.; Arce, E.M.; et al. The protein aggregation inhibitor YAT2150 has potent antimalarial activity in Plasmodium falciparum in vitro cultures. BMC Biol. 2022, 20, 197. [Google Scholar] [CrossRef]
- Lambros, C.; Vanderberg, J.P. Synchronization of Plasmodium falciparum erythrocytic stages in culture. J. Parasitol. 1979, 65, 418–420. [Google Scholar] [CrossRef] [PubMed]
- Ariey, F.; Witkowski, B.; Amaratunga, C.; Beghain, J.; Langlois, A.-C.; Khim, N.; Kim, S.; Duru, V.; Bouchier, C.; Ma, L. A molecular marker of artemisinin-resistant Plasmodium falciparum malaria. Nature 2014, 505, 50. [Google Scholar] [CrossRef] [PubMed]
- Witkowski, B.; Amaratunga, C.; Khim, N.; Sreng, S.; Chim, P.; Kim, S.; Lim, P.; Mao, S.; Sopha, C.; Sam, B. Novel phenotypic assays for the detection of artemisinin-resistant Plasmodium falciparum malaria in Cambodia: In-vitro and ex-vivo drug-response studies. Lancet Infect. Dis. 2013, 13, 1043–1049. [Google Scholar] [CrossRef] [PubMed]
- Borgheti-Cardoso, L.N.; Fernàndez-Busquets, X. Turning Plasmodium survival strategies against itself. Future Med. Chem. 2018, 10, 2245–2248. [Google Scholar] [CrossRef]
- Hubert, A.; Subra, C.; Jenabian, M.A.; Tremblay Labrecque, P.F.; Tremblay, C.; Laffont, B.; Provost, P.; Routy, J.P.; Gilbert, C. Elevated Abundance, Size, and MicroRNA Content of Plasma Extracellular Vesicles in Viremic HIV-1+ Patients: Correlations With Known Markers of Disease Progression. J. Acquir. Immune Defic. Syndr. 2015, 70, 219–227. [Google Scholar] [CrossRef]
- Core Team, R. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021. [Google Scholar]


| (A) | |||
|---|---|---|---|
| Parasite Line | RSA0–3 % (Standard Deviation) | p-Value (Kruskal–Wallis Test) | Interpretation |
| 3D7 | 0.7% (0.08) | 4 × 10−5 | ART sensitive |
| R561H | 12.7% (2.4) | ART resistant | |
| PfVps60KO | 0.4% (0.09) | ART sensitive | |
| (B) | |||
| Comparison with Dunn’s Post Hoc Test | Z Value | p-Value Unadjusted | p-Value Adjusted |
| 3D7 versus R561H | −2.302104 | 2.132930 × 10−2 | 3.199395 × 10−2 |
| 3D7 versus PfVps60KO | 2.195853 | 2.810245 × 10−2 | 2.810245 × 10−2 |
| R561H versus PfVps60KO | 4.497957 | 6.860944 × 10−2 | 2.058283 × 10−2 |
| EV Abundance (Mega Counts) | |||||
|---|---|---|---|---|---|
| Untreated (n = 9) | DMSO (n = 9) | DHA (n = 9) | KW Chi-Square | p-Value | |
| PfVps60KO (n = 9) | 321 (241, 338) | 93 (91, 114) | 1572 (1222, 3254) | 7.2 | 0.03 |
| 3D7 (n = 9) | 414 (358, 563) | 400 (369, 1030) | 700 (692, 1037) | 1.16 | 0.56 |
| R561H (n = 9) | 376 (314, 462) | 245 (191, 389) | 652 (628, 1024) | 5.96 | 0.05 |
| KW chi-square | 1.87 | 5.96 | 3.47 | ||
| p-value | 0.39 | 0.05 | 0.18 | ||
| EV Abundance (Mega Counts) | |||
|---|---|---|---|
| Predictors | Estimates | Confidence Interval | p-Value |
| (Intercept) | 595.23 | −174.98–1365.44 | 0.123 |
| Condition DMSO | 20.45 | −823.27–864.18 | 0.960 |
| Condition DHA | 1038.58 | 194.86–1882.30 | 0.018 |
| Parasite 3D7 | −216.71 | −1060.43–627.01 | 0.600 |
| Parasite R561H | −421.42 | −1265.14–422.30 | 0.312 |
| Observations | 27 | ||
| R2/R2 adjusted | 0.304/0.177 | ||
| PlasmoDB ID | Protein Name | PrLD Score (PLAAC) |
|---|---|---|
| PF3D7_1035300 | Glutamate-rich protein GLURP | Undefined |
| PF3D7_1401400 | Early transcribed membrane protein | Undefined |
| PF3D7_1353200 | Membrane-associated histidine-rich protein 2 | Undefined |
| PF3D7_1444800 | Fructose-bisphosphate aldolase | Undefined |
| PF3D7_1149000 | Antigen 332, DBL-like protein | Undefined |
| PF3D7_0727400 | Proteasome subunit alpha type | Undefined |
| PF3D7_1355300 | Putative histone-lysine N-methyltransferase 1 | Undefined |
| PF3D7_1021700 | Chorein N-terminal domain-containing protein | 26.5 |
| PF3D7_1234000 | Thioredoxin domain-containing protein | Undefined |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tandoh, K.Z.; Avalos-Padilla, Y.; Ameyaw, P.; Laryea-Akrong, E.K.; Awandare, G.A.; Wilson, M.D.; Quashie, N.B.; Fernàndez-Busquets, X.; Duah-Quashie, N.O. Extracellular Vesicle Abundance, but Not a High Aggregation-Prone Peptide Cargo, Is Associated with Dihydroartemisinin Exposure in Plasmodium falciparum. Int. J. Mol. Sci. 2025, 26, 3962. https://doi.org/10.3390/ijms26093962
Tandoh KZ, Avalos-Padilla Y, Ameyaw P, Laryea-Akrong EK, Awandare GA, Wilson MD, Quashie NB, Fernàndez-Busquets X, Duah-Quashie NO. Extracellular Vesicle Abundance, but Not a High Aggregation-Prone Peptide Cargo, Is Associated with Dihydroartemisinin Exposure in Plasmodium falciparum. International Journal of Molecular Sciences. 2025; 26(9):3962. https://doi.org/10.3390/ijms26093962
Chicago/Turabian StyleTandoh, Kwesi Z., Yunuen Avalos-Padilla, Prince Ameyaw, Elisabeth K. Laryea-Akrong, Gordon A. Awandare, Michael David Wilson, Neils B. Quashie, Xavier Fernàndez-Busquets, and Nancy O. Duah-Quashie. 2025. "Extracellular Vesicle Abundance, but Not a High Aggregation-Prone Peptide Cargo, Is Associated with Dihydroartemisinin Exposure in Plasmodium falciparum" International Journal of Molecular Sciences 26, no. 9: 3962. https://doi.org/10.3390/ijms26093962
APA StyleTandoh, K. Z., Avalos-Padilla, Y., Ameyaw, P., Laryea-Akrong, E. K., Awandare, G. A., Wilson, M. D., Quashie, N. B., Fernàndez-Busquets, X., & Duah-Quashie, N. O. (2025). Extracellular Vesicle Abundance, but Not a High Aggregation-Prone Peptide Cargo, Is Associated with Dihydroartemisinin Exposure in Plasmodium falciparum. International Journal of Molecular Sciences, 26(9), 3962. https://doi.org/10.3390/ijms26093962







