Distinct Hepatic Metabolic Reprogramming in Acute and Chronic Sleep Deprivation and the Protective Effects of the Chalcone Analogue TAK
Abstract
:1. Introduction
2. Results
2.1. Body Weight Changes, Hormonal Disorders, and Rhythm Disturbances in SD and the Effects of TAK
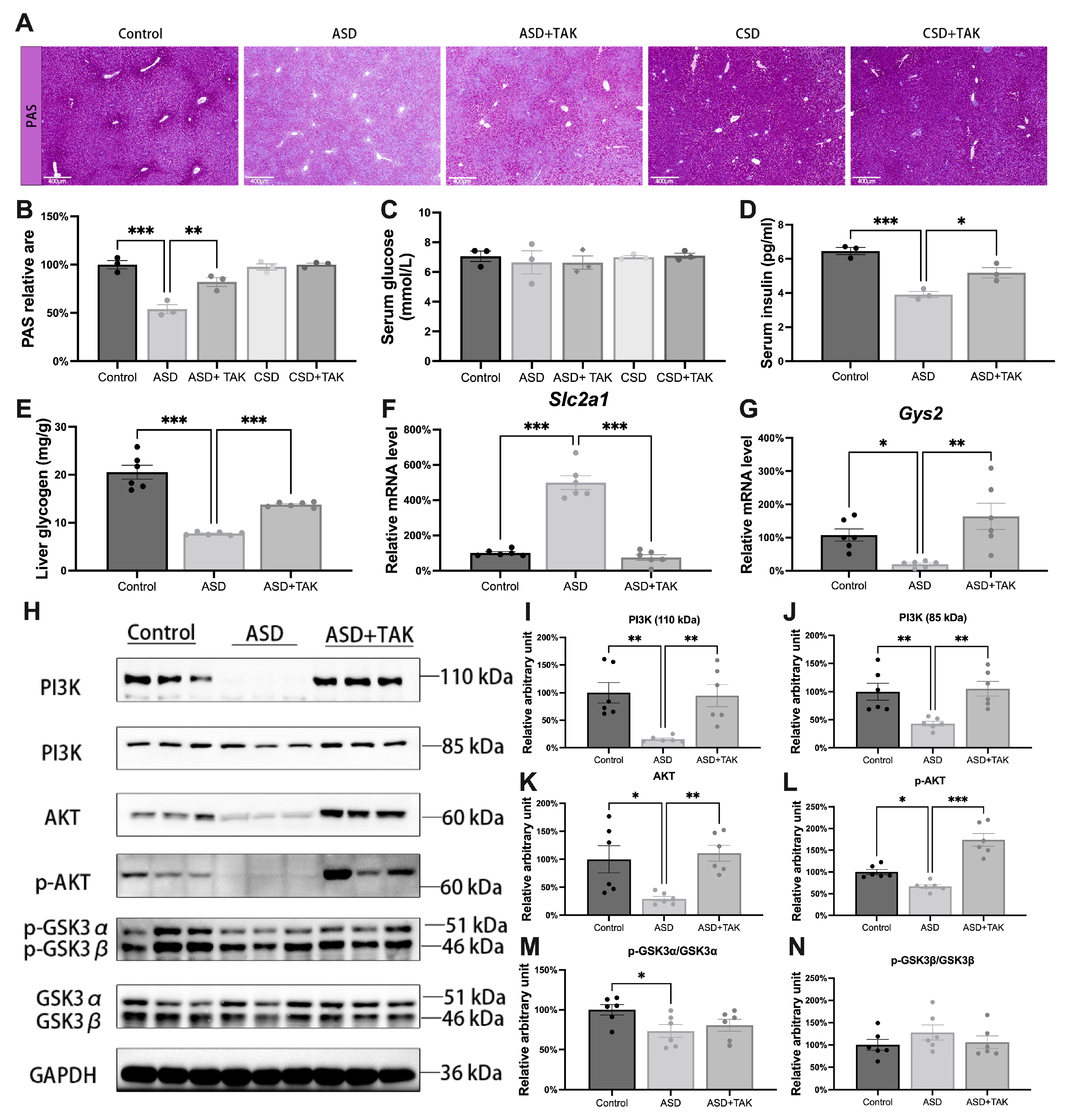
2.2. Glycogen Synthesis Was Inhibited Following ASD and Was Rescued by TAK Possibly via the PI3K/AKT/GSK3/GYS2 Pathway
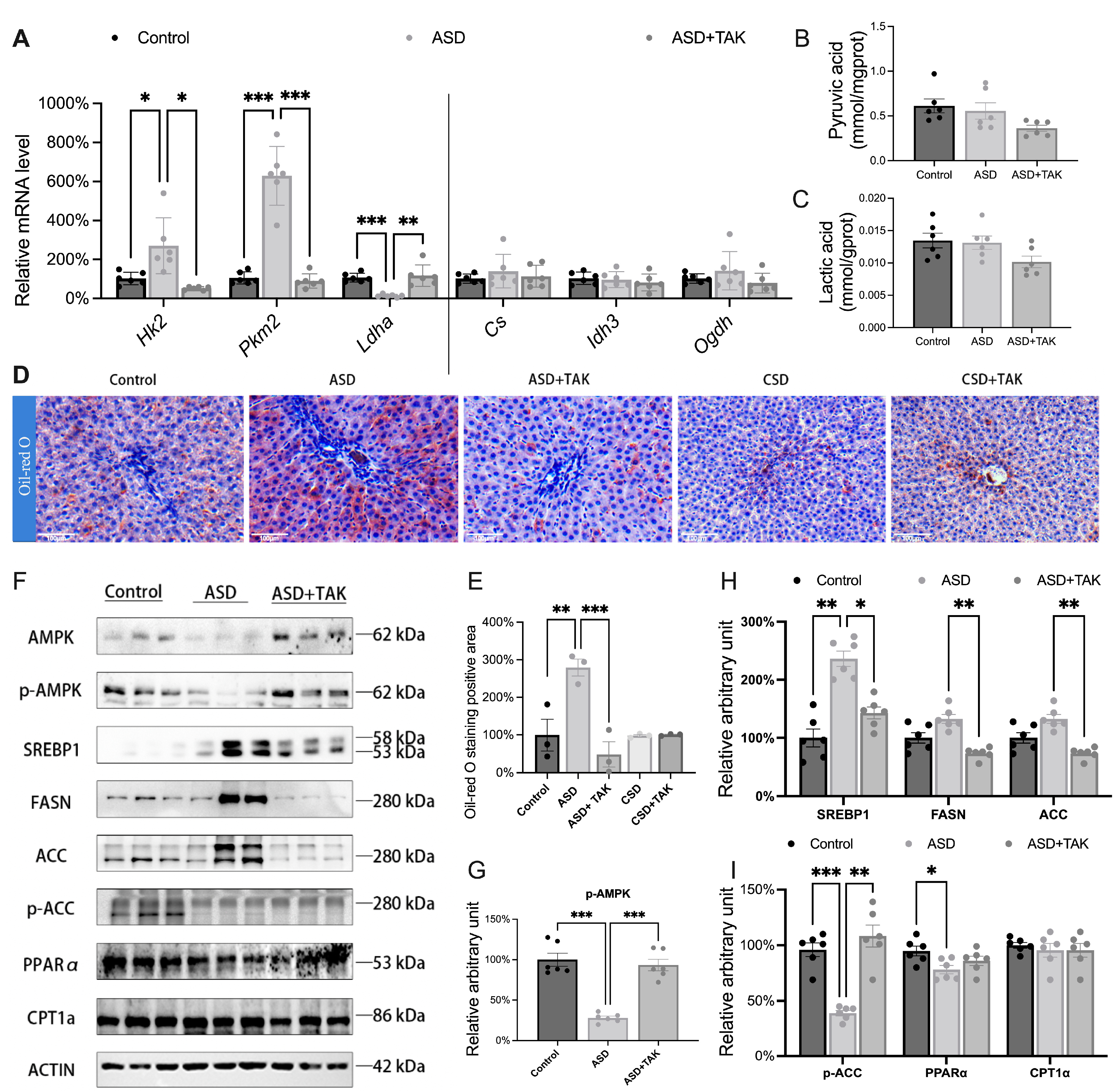
2.3. Glucose Converted to Lipid Accumulation After ASD: Hindered by TAK Treatment
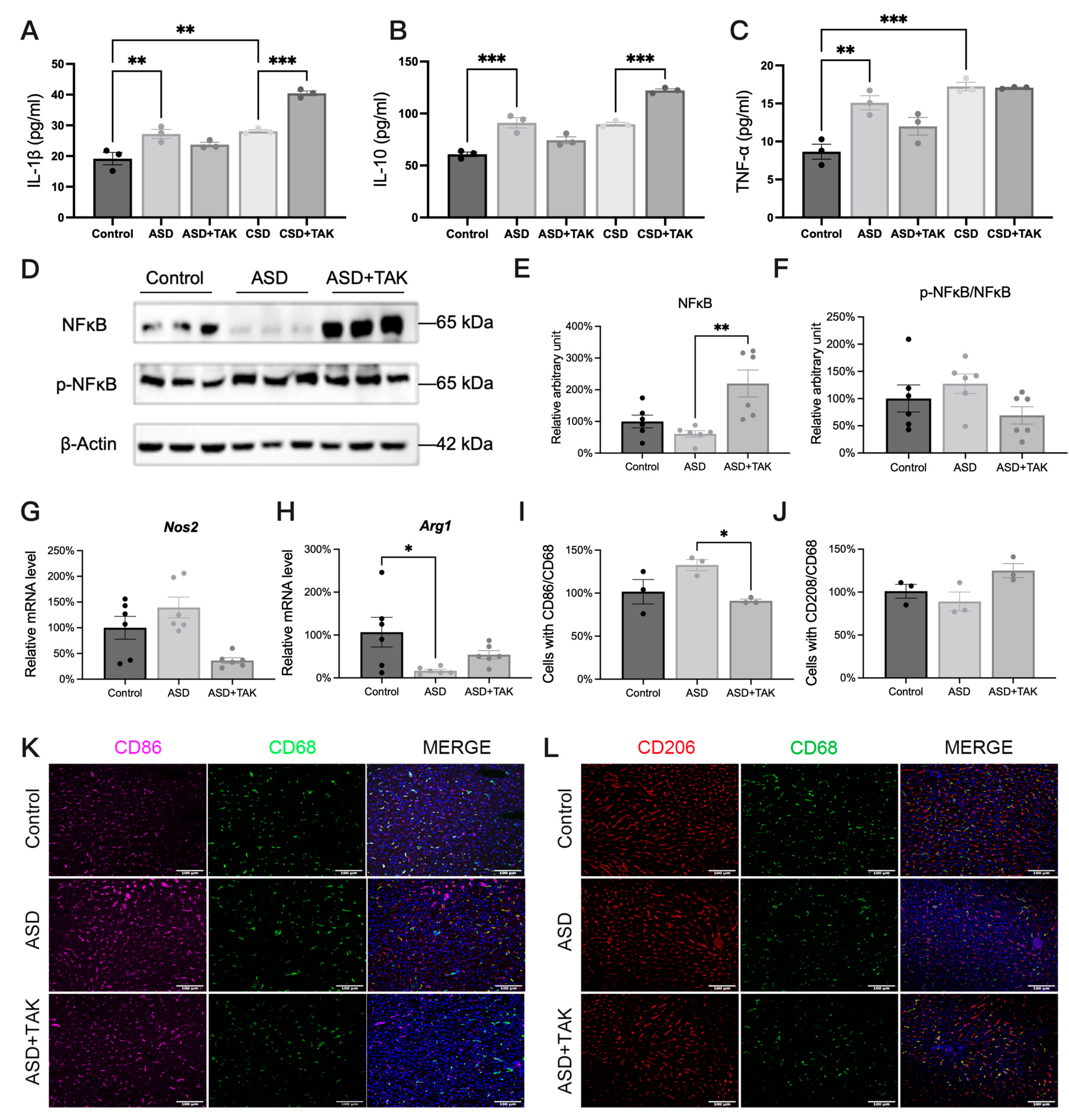
2.4. ASD-Induced Inflammation and Possible Anti-Inflammatory Effects of TAK
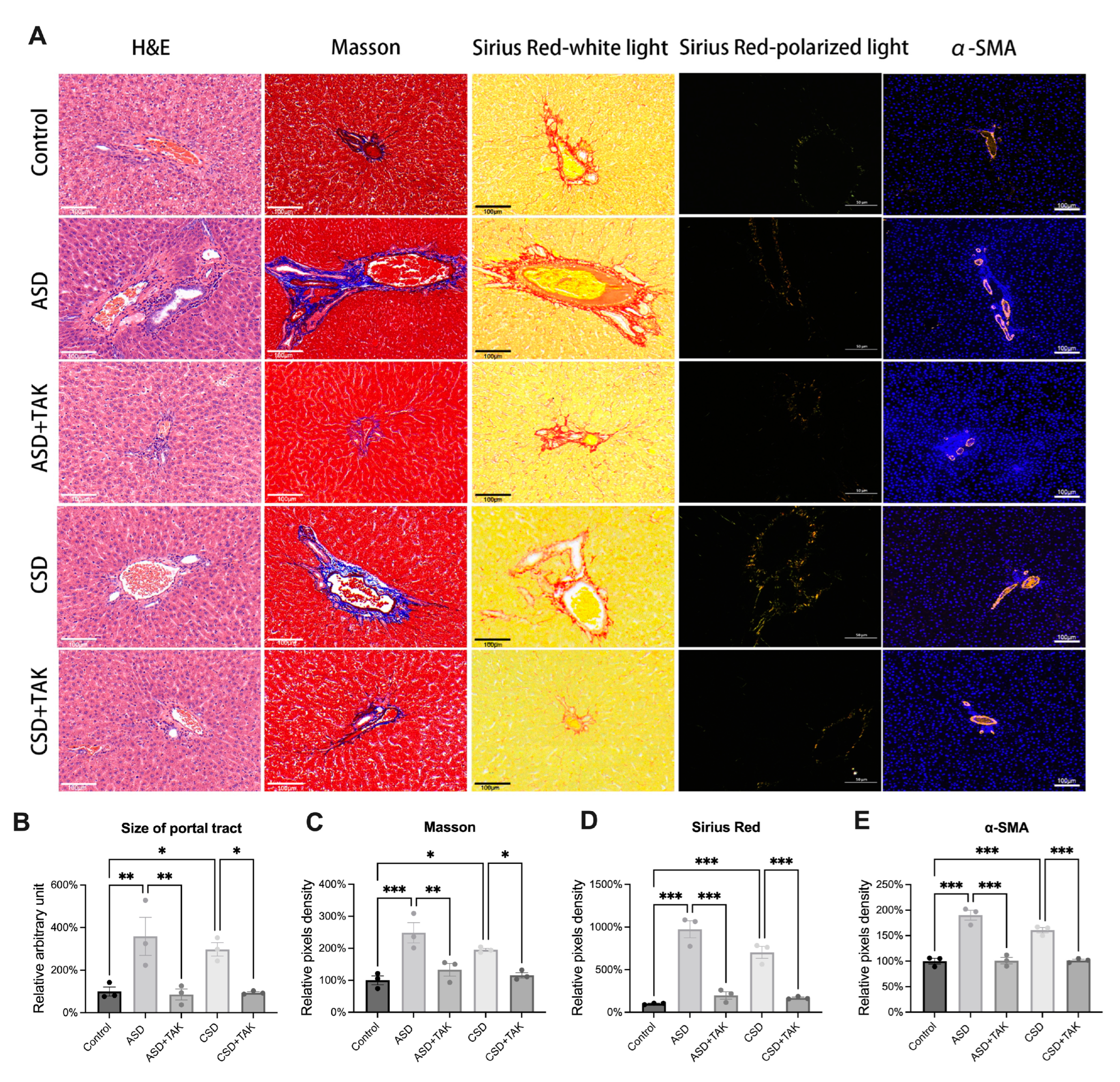
2.5. SD Stimulated Hepatic Portal Area Expansion and Fibrosis, Which Were Rescued by TAK
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. ASD and CSD Models
4.3. Serum Test
4.4. Liver Glycogen and Cholesterol Test
4.5. Extraction of RNA and Quantitative Real-Time Quantitative PCR (qRT-PCR)
4.6. Protein Extraction and Western Blot
4.7. Histology and Immunostaining
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ACC | Acetyl CoA Carboxylase |
| ASD | Acute Sleep Deprivation |
| CPT1A | Carnitine Palmitoyl Transferase 1A |
| CSD | Chronic Sleep Deprivation |
| CS | Citrate Synthase |
| FASN | Fatty Acid Synthase |
| GLUT1 | Glucose Transporter 1 |
| GYS2 | Glycogen Synthase 2 |
| GSK3 | Glycogen Synthase Kinase-3 |
| H&E | Hematoxylin and Eosin |
| HSCs | Hepatic Stellate Cells |
| HK2 | Hexokinase 2 |
| IDH | Isocitrate Dehydrogenase |
| KCs | Kupffer Cells |
| LDHA | Lactate Dehydrogenase A |
| MMPM | Modified Multi-Platform Method |
| NREM | Non-Rapid Eye Movement |
| Nrf2 | Nuclear Factor E2-Related Factor 2 |
| PAS staining | Periodic Acid-Schiff Staining |
| PI3K | Phosphoinositide 3-kinase |
| AKT | Protein Kinase B |
| PKM2 | Pyruvate Kinase M2 |
| qRT-PCR | Quantitative real-time polymerase chain reaction |
| REM | Rapid Eye Movement |
| SREBP | S1P Mediated Cholesterol Regulatory Element Binding Protein |
| UDPG | Uridine Diphosphate Glucose |
| α-SMA | α-Smooth Muscle Actin |
| α-KGDH | α-Ketoglutarate Dehydrogenase |
References
- Wang, J.; Zhang, Y.; Liu, N. Annual Sleep Report of China 2024; Social Sciences Academic Press: Beijing, China, 2024. [Google Scholar]
- Liu, Y.; Wheaton, A.G.; Chapman, D.P.; Cunningham, T.J.; Lu, H.; Croft, J.B. Prevalence of Healthy Sleep Duration among Adults--United States, 2014. MMWR. Morb. Mortal. Wkly. Rep. 2016, 65, 137–141. [Google Scholar] [PubMed]
- Marjot, T.; Ray, D.W.; Williams, F.R.; Tomlinson, J.W.; Armstrong, M.J. Sleep and liver disease: A bidirectional relationship. Lancet Gastroenterol. Hepatol. 2021, 6, 850–863. [Google Scholar] [PubMed]
- Shigiyama, F.; Kumashiro, N.; Tsuneoka, Y.; Igarashi, H.; Yoshikawa, F.; Kakehi, S.; Funato, H.; Hirose, T. Mechanisms of sleep deprivation-induced hepatic steatosis and insulin resistance in mice. Am. J. Physiol. Endocrinol. Metab. 2018, 315, E848–E858. [Google Scholar] [PubMed]
- Domínguez, F.; Fuster, V.; Fernández-Alvira, J.M.; Fernández-Friera, L.; López-Melgar, B.; Blanco-Rojo, R.; Fernández-Ortiz, A.; García-Pavía, P.; Sanz, J.; Mendiguren, J.M.; et al. Association of Sleep Duration and Quality with Subclinical Atherosclerosis. J. Am. Coll. Cardiol. 2019, 73, 134–144. [Google Scholar]
- Tobaldini, E.; Costantino, G.; Solbiati, M.; Cogliati, C.; Kara, T.; Nobili, L.; Montano, N. Sleep, sleep deprivation, autonomic nervous system and cardiovascular diseases. Neurosci. Biobehav. Rev. 2017, 74 Pt B, 321–329. [Google Scholar]
- Irwin, M.R.; Olmstead, R.; Carroll, J.E. Sleep Disturbance, Sleep Duration, and Inflammation: A Systematic Review and Meta-Analysis of Cohort Studies and Experimental Sleep Deprivation. Biol. Psychiatry 2016, 80, 40–52. [Google Scholar]
- Vaccaro, A.; Kaplan Dor, Y.; Nambara, K.; Pollina, E.A.; Lin, C.; Greenberg, M.E.; Rogulja, D. Sleep Loss Can Cause Death through Accumulation of Reactive Oxygen Species in the Gut. Cell 2020, 181, 1307–1328.e15. [Google Scholar]
- Trefts, E.; Gannon, M.; Wasserman, D.H. The liver. Curr. Biol. 2017, 27, R1147–R1151. [Google Scholar]
- Collins, K.P.; Geller, D.A.; Antoni, M.; Donnell, D.M.; Tsung, A.; Marsh, J.W.; Burke, L.; Penedo, F.; Terhorst, L.; Kamarck, T.W.; et al. Sleep duration is associated with survival in advanced cancer patients. Sleep. Med. 2017, 32, 208–212. [Google Scholar]
- Hu, S.; Li, P.; Zhang, R.; Liu, X.; Wei, S. Integrated metabolomics and proteomics analysis reveals energy metabolism disorders in the livers of sleep-deprived mice. J. Proteom. 2021, 245, 104290. [Google Scholar]
- Christoffersson, G.; Vågesjö, E.; Pettersson, U.S.; Massena, S.; Nilsson, E.K.; Broman, J.-E.; Schiöth, H.B.; Benedict, C.; Phillipson, M. Acute sleep deprivation in healthy young men: Impact on population diversity and function of circulating neutrophils. Brain Behav. Immun. 2014, 41, 162–172. [Google Scholar] [CrossRef] [PubMed]
- Zager, A.; Ruiz, F.S.; Tufik, S.; Andersen, M.L. Immune outcomes of paradoxical sleep deprivation on cellular distribution in naive and lipopolysaccharide-stimulated mice. Neuroimmunomodulation 2012, 19, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Song, P.; Hang, K.; Chen, Z.; Zhu, Z.; Zhang, Y.; Xu, J.; Qin, J.; Wang, B.; Qu, W.; et al. Sleep Deprivation Disturbs Immune Surveillance and Promotes the Progression of Hepatocellular Carcinoma. Front. Immunol. 2021, 12, 727959. [Google Scholar] [CrossRef]
- De Lorenzo, B.H.P.; Novaes E Brito, R.R.; Paslar Leal, T.; Piqueira Garcia, N.; Martins Dos Santos, R.M.; Alvares-Saraiva, A.M.; Perez Hurtado, E.C.; Braga Dos Reis, T.C.; Duarte Palma, B. Chronic Sleep Restriction Impairs the Antitumor Immune Response in Mice. Neuroimmunomodulation 2018, 25, 59–67. [Google Scholar] [CrossRef]
- Rao, Y.K.; Fang, S.-H.; Tzeng, Y.-M. Synthesis and biological evaluation of 3’,4’,5’-Trimethoxychalcone analogues as inhibitors of nitric oxide production and tumor cell proliferation. Bioorg. Med. Chem. 2009, 17, 7909–7914. [Google Scholar] [CrossRef]
- Cui, Y.; Xiong, Y.; Li, H.; Zeng, M.; Wang, Y.; Li, Y.; Zou, X.; Lv, W.; Gao, J.; Cao, R.; et al. Chalcone-Derived Nrf2 Activator Protects Cognitive Function via Maintaining Neuronal Redox Status. Antioxidants 2021, 10, 1811. [Google Scholar] [CrossRef]
- Welsh, G.I.; Wilson, C.; Proud, C.G. GSK3: A SHAGGY frog story. Trends Cell Biol. 1996, 6, 274–279. [Google Scholar] [CrossRef]
- Rechtschaffen, A. Current perspectives on the function of sleep. Perspect. Biol. Med. 1998, 41, 359–390. [Google Scholar] [CrossRef]
- Wright, K.P.; Drake, A.L.; Frey, D.J.; Fleshner, M.; Desouza, C.A.; Gronfier, C.; Czeisler, C.A. Influence of sleep deprivation and circadian misalignment on cortisol, inflammatory markers, and cytokine balance. Brain Behav. Immun. 2015, 47, 24–34. [Google Scholar] [CrossRef]
- Qi, J.; Wang, W.; Zhu, Q.; He, Y.; Lu, Y.; Wang, Y.; Li, X.; Chen, Z.-J.; Sun, Y. Local Cortisol Elevation Contributes to Endometrial Insulin Resistance in Polycystic Ovary Syndrome. J. Clin. Endocrinol. Metab. 2018, 103, 2457–2467. [Google Scholar] [CrossRef]
- Chen, J.; Zhou, Y.; Liu, Z.; Lu, Y.; Jiang, Y.; Cao, K.; Zhou, N.; Wang, D.; Zhang, C.; Zhou, N.; et al. Hepatic glycogenesis antagonizes lipogenesis by blocking S1P via UDPG. Science 2024, 383, eadi3332. [Google Scholar] [PubMed]
- Pawlak, M.; Lefebvre, P.; Staels, B. Molecular mechanism of PPARα action and its impact on lipid metabolism, inflammation and fibrosis in non-alcoholic fatty liver disease. J. Hepatol. 2015, 62, 720–733. [Google Scholar] [PubMed]
- Heymann, F.; Tacke, F. Immunology in the liver—From homeostasis to disease. Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 88–110. [Google Scholar] [PubMed]
- Irwin, M.R.; Carrillo, C.; Olmstead, R. Sleep loss activates cellular markers of inflammation: Sex differences. Brain Behav. Immun. 2010, 24, 54–57. [Google Scholar] [CrossRef]
- Chen, S.; Cai, X.; Liu, Y.; Shen, Y.; Guillot, A.; Tacke, F.; Tang, L.; Liu, H. The macrophage-associated microRNA-4715-3p/Gasdermin D axis potentially indicates fibrosis progression in nonalcoholic fatty liver disease: Evidence from transcriptome and biological data. Bioengineered 2022, 13, 11740–11751. [Google Scholar]
- Borish, L.C.; Steinke, J.W. 2. Cytokines and chemokines. J. Allergy Clin. Immunol. 2003, 111 (Suppl. S2), S460–S475. [Google Scholar]
- Zhao, X.; Kwan, J.Y.Y.; Yip, K.; Liu, P.P.; Liu, F.-F. Targeting metabolic dysregulation for fibrosis therapy. Nat. Rev. Drug Discov. 2020, 19, 57–75. [Google Scholar]
- Hammerich, L.; Tacke, F. Hepatic inflammatory responses in liver fibrosis. Nat. Rev. Gastroenterol. Hepatol. 2023, 20, 633–646. [Google Scholar] [CrossRef]
- Lin, Y.; Dong, M.-Q.; Liu, Z.-M.; Xu, M.; Huang, Z.-H.; Liu, H.-J.; Gao, Y.; Zhou, W.-J. A strategy of vascular-targeted therapy for liver fibrosis. Hepatology 2022, 76, 660–675. [Google Scholar] [CrossRef]
- Kisseleva, T.; Brenner, D. Molecular and cellular mechanisms of liver fibrosis and its regression. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 151–166. [Google Scholar]
- Tsuchida, T.; Friedman, S.L. Mechanisms of hepatic stellate cell activation. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 397–411. [Google Scholar] [PubMed]
- Trivedi, P.; Wang, S.; Friedman, S.L. The Power of Plasticity-Metabolic Regulation of Hepatic Stellate Cells. Cell Metab. 2021, 33, 242–257. [Google Scholar] [PubMed]
- Li, Y.; Wang, J.; Song, K.; Liu, S.; Zhang, H.; Wang, F.; Ni, C.; Zhai, W.; Liang, J.; Qin, Z.; et al. S100A4 promotes hepatocellular carcinogenesis by intensifying fibrosis-associated cancer cell stemness. OncoImmunology 2020, 9, 1725355. [Google Scholar] [PubMed]

| Gene Name | Primer Sequence | |
|---|---|---|
| Per1 | Forward | TACCAGCCATTCCGCCTAAC |
| Per1 | Reverse | CCGGGGAGCTTCATAACCAG |
| Per2 | Forward | CGAAGCGCCTCATTCCAGAG |
| Per2 | Reverse | TGCTCATGTCCACGTCTTCC |
| Gys2 | Forward | GTGTGACTACGAACCTCTC |
| Gys2 | Reverse | CCTCCTCTTCCTCATCATATC |
| Slc2a1 | Forward | TGGCCAAGGACACACGAATACTGA |
| Slc2a1 | Reverse | TGGAAGAGACAGGAATGGGCGAAT |
| Hk2 | Forward | CGAGAGCATCCTCCTCAAGTG |
| Hk2 | Reverse | AGCCACAGGTCATCATAGTTCC |
| Pkm2 | Forward | TGGGAGAGAAGGGAAAGAACATC |
| Pkm2 | Reverse | GCACCGTCCAATCATCATCTTC |
| Ldha | Forward | ATGAGTTGGACTGTGCCTGTTGTG |
| Ldha | Reverse | GTGAAGAGCCAGGTGCCGTTG |
| Cs | Forward | CATACTGAGCAATCTGATACC |
| Cs | Reverse | GAGCCAAGAGACCTGTTC |
| Idh3 | Forward | ACCAATACAGAGCAACAGAT |
| Idh3 | Reverse | GCACATCACCATCATAGTTC |
| Ogdh | Forward | ATGATGGGCAGCAAGATG |
| Ogdh | Reverse | TTCCTCCTGAGACATAGGTT |
| Nos2 | Forward | GCATCCCAAGTACGAGTGGT |
| Nos2 | Reverse | GAAGGCGTAGCTGAACAAGG |
| Arg1 | Forward | GGACATCGTGTACATCGGCT |
| Arg1 | Reverse | GTAGCCGGGGTGAATACTGG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Hu, Y.; Wang, P.; Hu, R.; Chen, Z.; Zhang, T.; Liu, J.; Noda, M.; Long, J.; Peng, Y. Distinct Hepatic Metabolic Reprogramming in Acute and Chronic Sleep Deprivation and the Protective Effects of the Chalcone Analogue TAK. Int. J. Mol. Sci. 2025, 26, 3485. https://doi.org/10.3390/ijms26083485
Wang Y, Hu Y, Wang P, Hu R, Chen Z, Zhang T, Liu J, Noda M, Long J, Peng Y. Distinct Hepatic Metabolic Reprogramming in Acute and Chronic Sleep Deprivation and the Protective Effects of the Chalcone Analogue TAK. International Journal of Molecular Sciences. 2025; 26(8):3485. https://doi.org/10.3390/ijms26083485
Chicago/Turabian StyleWang, Yifang, Yachong Hu, Pengxiao Wang, Ranrui Hu, Zhongqi Chen, Tiantian Zhang, Jiankang Liu, Mami Noda, Jiangang Long, and Yunhua Peng. 2025. "Distinct Hepatic Metabolic Reprogramming in Acute and Chronic Sleep Deprivation and the Protective Effects of the Chalcone Analogue TAK" International Journal of Molecular Sciences 26, no. 8: 3485. https://doi.org/10.3390/ijms26083485
APA StyleWang, Y., Hu, Y., Wang, P., Hu, R., Chen, Z., Zhang, T., Liu, J., Noda, M., Long, J., & Peng, Y. (2025). Distinct Hepatic Metabolic Reprogramming in Acute and Chronic Sleep Deprivation and the Protective Effects of the Chalcone Analogue TAK. International Journal of Molecular Sciences, 26(8), 3485. https://doi.org/10.3390/ijms26083485









