Synergistic Activity of Gloeophyllum striatum-Derived AgNPs with Ciprofloxacin and Gentamicin Against Human Pathogenic Bacteria
Abstract
1. Introduction
2. Results
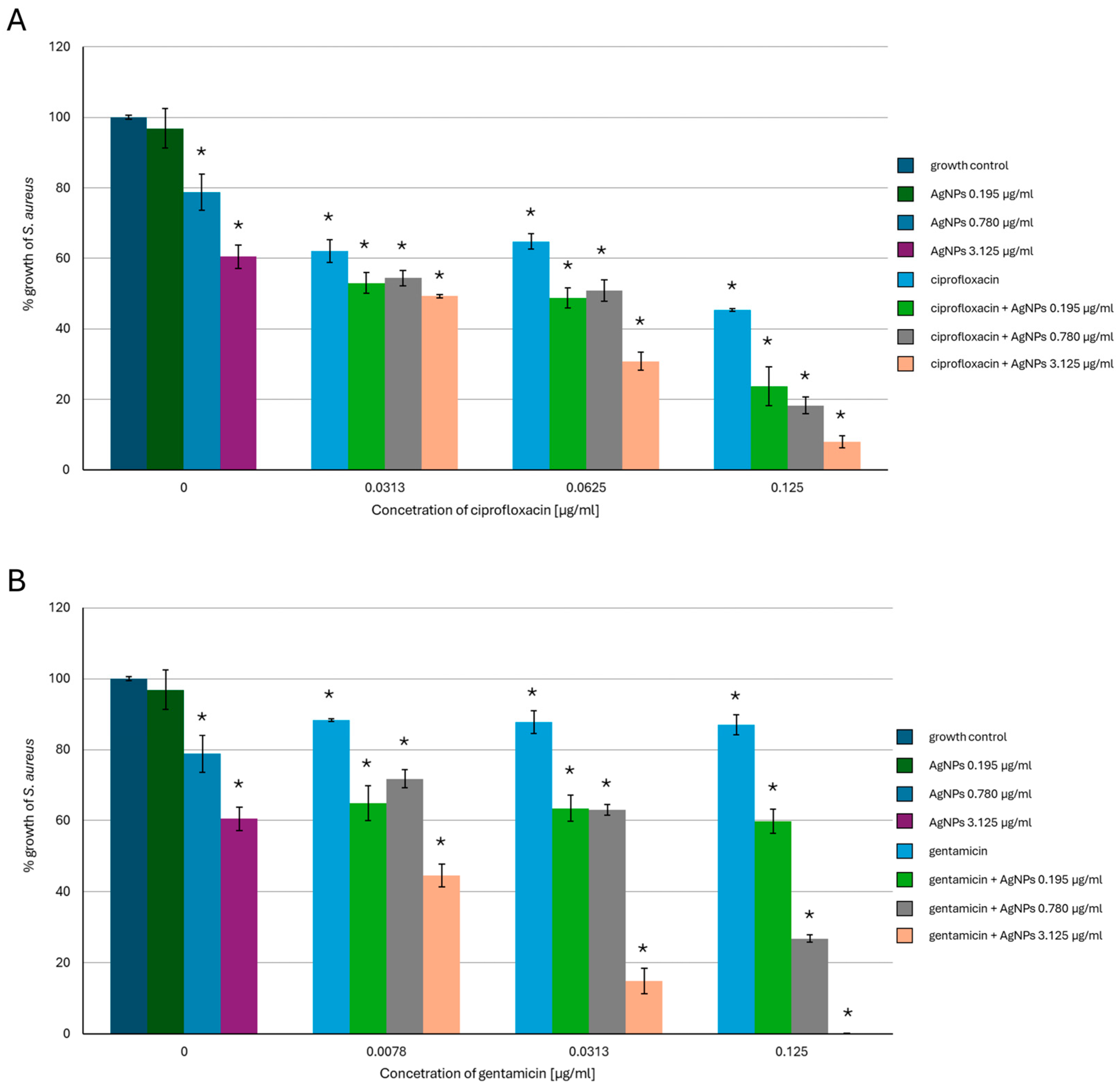
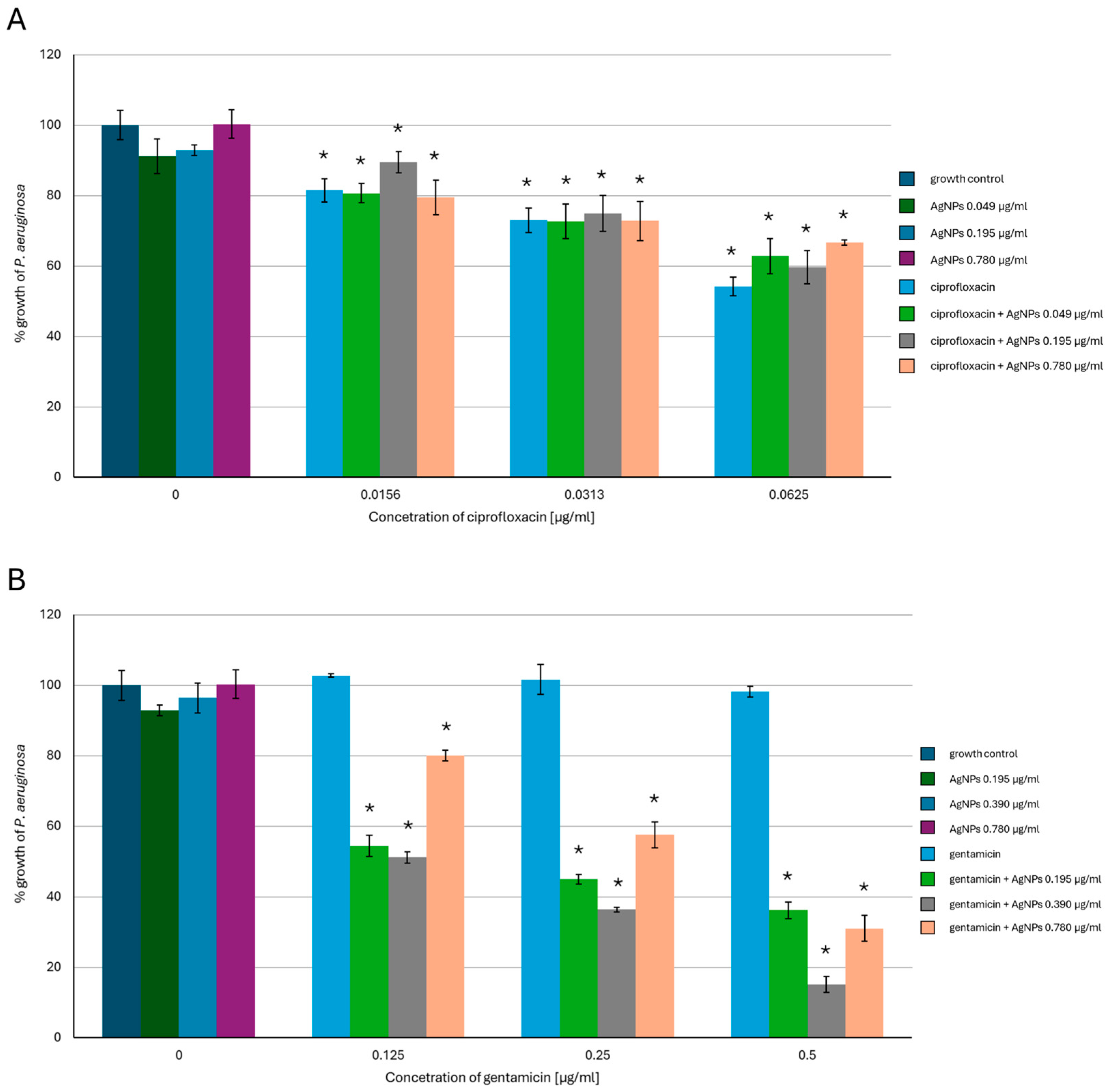
2.1. Antimicrobial Activity of AgNPs, Antibiotics, or a Combination of the Agents
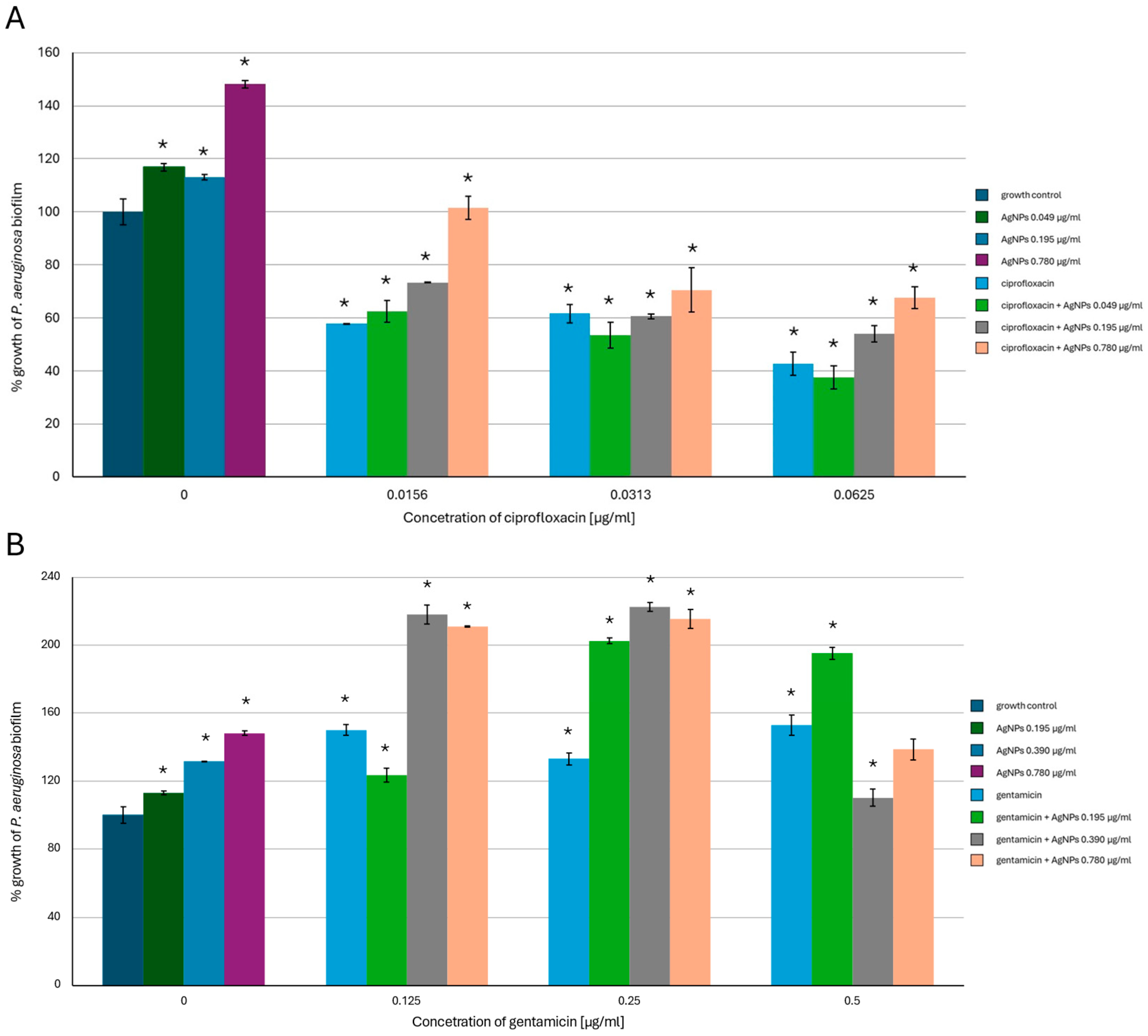
2.2. Antibiofilm-Forming Activity of AgNPs, the Antibiotics and a Combination of the Agents
2.3. Changes in the Phospholipid Profiles of the Tested Bacteria in the Presence of AgNPs, the Antibiotics or a Combination of Agents
2.4. Changes in the Fatty Acid Content in the Tested Bacterial Strains in the Presence of AgNPs, the Antibiotics or a Combination of the Agents
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Antimicrobial Activity of AgNPs, Antibiotics and Agents Combined
4.3. Antibiofilm-Forming Activity of AgNPs, Antibiotics and Agents Combined
4.4. Changes in the Phospholipid Profiles and Fatty Acid Contents in the Tested Bacteria in the Presence of AgNPs, the Antibiotics, or a Combination of the Agents
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hadi, A.A.; Malek, N.A.N.N.; Matmin, J.; Asraf, M.H.; Susanto, H.; Din, S.M.; Shamsuddin, M. Synergistic antibacterial effect of Persicaria odorata synthesized silver nanoparticles with antibiotics on drug-resistant bacteria. Inorg. Chem. Commun. 2024, 159, 111725. [Google Scholar] [CrossRef]
- Yassin, M.T.; Mostafa, A.A.-F.; Al-Askar, A.A.; Al-Otibi, F.O. Synergistic Antibacterial Activity of Green Synthesized Silver Nanomaterials with Colistin Antibiotic Against Multidrug-Resistant Bacterial Pathogens. Crystals 2022, 12, 1057. [Google Scholar] [CrossRef]
- Rodrigues, A.S.; Batista, J.G.S.; Rodrigues, M.A.V.; Thipe, V.C.; Minarini, L.A.R.; Lopes, P.S.; Lugão, A.B. Advances in silver nanoparticles: A comprehensive review on their potential as antimicrobial agents and their mechanisms of action elucidated by proteomics. Front. Microbiol. 2024, 15, 1440065. [Google Scholar] [CrossRef]
- Ribeiro, A.I.; Vieira, B.; Dantas, D.; Silva, B.; Pinto, E.; Cerqueira, F.; Silva, R.; Remiãe, F.; Padrão, J.; Dias, A.M.; et al. Synergistic Antimicrobial Activity of Silver Nanoparticles with an Emergent Class of Azoimidazoles. Pharmaceutics 2023, 15, 926. [Google Scholar] [CrossRef]
- Luceri, A.; Francese, R.; Lembo, D.; Ferraris, M.; Balagna, C. Silver Nanoparticles: A Review of Antiviral Properties, Mechanisms of Action and Applications. Microorganisms 2023, 11, 629. [Google Scholar] [CrossRef] [PubMed]
- Ong, W.T.J.; Nyam, K.L. Evaluation of silver nanoparticles in cosmeceutical and potential biosafety complications. Saudi J. Biol. Sci. 2022, 29, 2085–2094. [Google Scholar] [CrossRef]
- Meher, A.; Tandi, A.; Moharana, S.; Chakroborty, S.; Mohapatra, S.S.; Mondal, A.; Dey, S.; Chandra, P. Silver nanoparticle for biomedical applications: A review. Hybrid Adv. 2024, 6, 100184. [Google Scholar] [CrossRef]
- Tończyk, A.; Niedziałkowska, K.; Lisowska, K. Optimizing the microbial synthesis of silver nanoparticles using Gloeophyllum striatum and their antimicrobial potential evaluation. Sci. Rep. 2023, 13, 21124. [Google Scholar] [CrossRef]
- Zawadzka, K.; Felczak, A.; Nowak, M.; Kowalczyk, A.; Piwoński, I.; Lisowska, K. Antimicrobial activity and toxicological risk assessment of silver nanoparticles synthesized using an eco-friendly method with Gloeophyllum striatum. J. Hazard. Mater. 2021, 418, 126316. [Google Scholar] [CrossRef]
- Khan, T.; Yasmin, A.; Townley, H.E. An evaluation of the activity of biologically synthesized silver nanoparticles against bacteria, fungi and mammalian cell lines. Colloids Surf. B Biointerfaces 2020, 194, 111156. [Google Scholar] [CrossRef]
- Apreja, M.; Sharma, A.; Balda, S.; Kataria, K.; Capalash, N.; Sharma, P. Antibiotic residues in environment: Antimicrobial resistance development, ecological risks, and bioremediation. Environ. Sci. Pollut. Res. 2022, 29, 3355–3371. [Google Scholar] [CrossRef]
- Grenni, P.; Ancona, V.; Caracciolo, A.B. Ecological effects of antibiotics on natural ecosystems: A review. Microchem. J. 2018, 136, 25–39. [Google Scholar] [CrossRef]
- Barathe, P.; Kaur, K.; Reddy, S.; Shriram, V.; Kumar, V. Antibiotic pollution and associated antimicrobial resistance in the environment. J. Hazard. Mater. 2024, 5, 100105. [Google Scholar] [CrossRef]
- Franci, G.; Falanga, A.; Galdiero, S.; Palomba, L.; Rai, M.; Morelli, G.; Galdiero, M. Silver Nanoparticles as Potential Antibacterial Agents. Molecules 2015, 20, 8856–8874. [Google Scholar] [CrossRef] [PubMed]
- Dove, A.S.; Dzurny, D.I.; Dees, W.R.; Qin, N.; Rodriguez, C.C.N.; Alt, L.A.; Ellward, G.L.; Best, J.A.; Rudawski, N.G.; Fujii, K.; et al. Silver nanoparticles enhance the efficacy of aminoglycosides against antibiotic-resistant bacteria. Front. Microbiol. 2023, 13, 1064095. [Google Scholar] [CrossRef]
- Merghini, A.; Lassoued, M.A.; Noumi, E.; Lajimi, R.H.; Adnan, M.; Mastouri, M.; Snoussi, M. Cytotoxic Activity and Antibiofilm Efficacy of Biosynthesized Silver Nanoparticles Against Methicillin-Resistant Staphylococcus aureus Strains Colonizing Cell Phones. Can. J. Infect. Dis. 2022, 25, 9410024. [Google Scholar] [CrossRef] [PubMed]
- Tawre, M.S.; Shiledar, A.; Satpute, S.K.; Ahire, K.; Ghosh, S.; Pardesi, K. Synergistic and antibiofilm potential of Curcuma aromatica derived silver nanoparticles in combination with antibiotics against multidrug-resistant pathogens. Front. Chem. 2022, 10, 1029056. [Google Scholar] [CrossRef]
- LewisOscar, F.; Nithya, C.; Vismaya, S.; Arunkumar, M.; Pugazhendhi, A.; Nguyen-Tri, P.; Alharbi, S.A.; Alharbi, N.S.; Thajuddin, N. In vitro analysis of green fabricated silver nanoparticles (AgNPs) against Pseudomonas aeruginosa PA14 biofilm formation, their application on urinary catheter. Prog. Org. Coat. 2021, 151, 106058. [Google Scholar] [CrossRef]
- Mah, T.-H. Biofilm-specific antibiotic resistance. Future Microbiol. 2012, 7, 1061–1072. [Google Scholar] [CrossRef]
- Hosnedlova, B.; Kabanov, D.; Kepinska, M.; Narayanan, V.H.B.; Parikesit, A.A.; Fernandez, C.; Bjørklund, G.; Nguyen, H.V.; Farid, A.; Sochor, J.; et al. Effect of Biosynthesized Silver Nanoparticles on Bacterial Biofilm Changes in S. aureus E. coli. Nanomaterials 2022, 12, 2183. [Google Scholar] [CrossRef]
- Farioli, A.S.; Martinez, M.V.; Barbero, C.A.; Acevedo, D.F.; Yslas, E.I. Antimicrobial Activity of Gentamicin-Loaded Biocomposites Synthesized through Inverse Vulcanization from Soybean and Sunflower Oils. Sustain. Chem. 2024, 5, 229–243. [Google Scholar] [CrossRef]
- Shariati, A.; Arshadi, M.; Khosrojerdi, M.A.; Abedinzadeh, M.; Ganjalishahi, M.; Maleki, A.; Heidary, M.; Khoshnood, S. The resistance mechanisms of bacteria against ciprofloxacin and new approaches for enhancing the efficacy of this antibiotic. Front. Public Health 2022, 10, 1025633. [Google Scholar] [CrossRef]
- More, P.R.; Pandit, S.; De Filippis, A.; Franci, G.; Mijakovic, I.; Galdiero, M. Silver Nanoparticles: Bactericidal and Mechanistic Approach Against Drug Resistant Patogens. Microorganisms 2023, 11, 369. [Google Scholar] [CrossRef]
- Yin, I.X.; Zhang, J.; Zhao, I.S.; Mei, M.L.; Li, Q.; Chu, C.H. The Antibacterial Mechanism of Silver Nanoparticles and Its Application in Dentistry. Int. J. Nanomed. 2020, 15, 2555–2562. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, L.G.; Roque, G.S.C.; Conrado, R.; De Souza, A.O. Antifungal Activity of Mycogenic Silver Nanoparticles on Clinical Yeasts and Phytopathogens. Antibiotics 2023, 12, 91. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Carrizales, M.; Velasco, K.I.; Castillo, C.; Flores, A.; Magaña, M.; Martinez-Castanon, G.A.; Martinez-Gutierrez, M. In Vitro Synergism of Silver Nanoparticles with Antibiotics as an Alternative Treatment in Multiresistant Uropathogens. Antibiotics 2018, 7, 50. [Google Scholar] [CrossRef]
- Ghaffar, N.; Javad, S.; Shah, A.A.; Ilyas, S.; Hashe, A.; Avila-Quezada, G.D.; Abd-Allah, E.F.; Tariq, A. Restoration of Antibacterial Activity of Inactive Antibiotics via Combined Treatment with AgNPs. ASC Omega 2024, 9, 13621–13635. [Google Scholar] [CrossRef]
- Ipe, D.S.; Kumar, P.T.S.; Love, R.M.; Hamlet, S.M. Silver Nanoparticles at Biocompatible Dosage Synergistically Increases Bacterial Susceptibility to Antibiotics. Front. Microbiol. 2020, 11, 1074. [Google Scholar] [CrossRef]
- Wang, Y.-W.; Tang, H.; Wu, D.; Liu, D.; Liu, Y.; Cao, A.; Wang, H. Enhanced bactericidal toxicity of silver nanoparticles by antibiotic gentamicin. Environ. Sci. Nano 2016, 3, 788–798. [Google Scholar] [CrossRef]
- Bhat, M.A.; Nayak, B.K.; Nanda, A. Evaluation of bactericidal activity of biologically synthesized Silver Nanoparticles from Candida albicans in combination with Ciprofloxacin. Mater. Today Proc. 2015, 2, 4395–4401. [Google Scholar] [CrossRef]
- Nikparast, Y.; Saliani, M. Synergistic Effect between Phyto-Synthesized Silver Nanoparticles and Ciprofloxacin Antibiotic on some Pathogenic Bacterial Strains. J. Med. Bacteriol. 2018, 7, 36043. [Google Scholar]
- Chatterjee, S.; Paul, P.; Chakraborty, P.; Das, S.; Gupta, A.D.; Roy, R.; Malik, M.; Sarkar, S.; Sarker, R.K.; Tribedi, P. Combinational application of cuminaldehyde and gentamicin shows enhanced antimicrobial and antibiofilm action on Pseudomonas aeruginosa. Folia Microbiol. 2024, 69, 823–834. [Google Scholar] [CrossRef]
- De Lacerda Coriolano, D.; de Souza, J.B.; Bueno, E.V.; de Fatima, S.M.; dos Santos Medeiros, R.; Cavalcanti, I.D.L.; Cavalcanti, I.M.F. Antibacterial and antibiofilm potential of silver nanoparticles against antibiotic-sensitive and multidrug-resistant Pseudomonas aeruginosa strains. Braz. J. Microbiol. 2021, 52, 267–278. [Google Scholar] [CrossRef]
- Tabassum, N.; Khan, F.; Jeong, G.-J.; Jo, D.-M.; Kim, Y.-M. Silver nanoparticles synthesized from Pseudomonas aeruginosa pyoverdine: Antibiofilm and antivirulence agents. Biofilm 2024, 7, 100192. [Google Scholar] [CrossRef] [PubMed]
- Salman, M.; Rizwana, R.; Khan, H.; Munir, I.; Hamayun, M.; Iqbal, A.; Rehman, A.; Amin, K.; Ahmed, G.; Khan, M.; et al. Synergistic effect of silver nanoparticles and polymyxin B against biofilm produced by Pseudomonas aeruginosa isolates of pus samples in vitro. Artif. Cells Nanomed. Biotechnol. 2019, 47, 2465–2472. [Google Scholar] [CrossRef]
- Vadakkan, K.; Jose, B.; Mapranathukaran, V.O.; Sathishkumar, K.; Ngangba, A.K.; Rumjit, N.P. Biofilm suppression of Pseudomonas aeruginosa by bio-engineered silver nanoparticles from Hellenia speciosa rhizome extract. Microb. Pathog. 2025, 198, 107105. [Google Scholar] [CrossRef]
- Leoney, A.; Karthigeyan, S.; Asharaf, A.S.; Felix, A.J.W. Detection and categorization of biofilm-forming Staphylococcus aureus, Viridans streptococcus, Klebsiella pneumoniae, and Escherichia coliIsolated from complete denture patients and visualization using scanning electron microscopy. J. Int. Soc. Prev. Community Dent. 2020, 10, 627–633. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Alvarez, J.J. Sublethal Concentrations of Silver Nanoparticles Stimulate Biofilm Development. Environ. Sci. Technol. Lett. 2015, 2, 221–226. [Google Scholar] [CrossRef]
- Kumar, A.; Saha, S.K.; Banerjee, P.; Sengupta, T.K. Antibiotic induced biofilm formations in pseudomonas aeruginosa strains KPW.1-S1 and HRW.1-S3 are associated with increased production of eDNA and exoproteins, increased ROS generation, and in-creased cell surface hydrophobicity. Curr. Microbiol. 2024, 81, 11. [Google Scholar] [CrossRef]
- Swidan, N.S.; Hashem, Y.A.; Elkhatib, W.F.; Yassien, M.A. Antibiofilm activity of green synthesized silver nanoparticles against biofilm associated enterococcal urinary pathogens. Sci. Rep. 2022, 12, 3869. [Google Scholar] [CrossRef]
- Luo, Z.-X.; Li, Y.; Liu, M.-F.; Zhao, R. Ciprofloxacin enhances the biofilm formation of Staphylococcus aureus via an agrC-dependent mechanism. Front. Microbiol. 2023, 14, 1328947. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, S.; Slavetinsky, C.J.; Peschel, A. Synthesis and function of phospholipids in Staphylococcus aureus. Int. J. Med. Microbiol. 2015, 305, 196–202. [Google Scholar] [CrossRef]
- Felczak, A.; Zawadzka, K.; Bernat, P.; Nowak-Lange, M.; Lisowska, K. Effect of Quinoline on the Phospholipid Profile of Curvularia lunata and Its Microbial Detoxification. Molecules 2022, 27, 2081. [Google Scholar] [CrossRef]
- Lyon, R.; Jones, R.A.; Shropshire, H.; Aberdeen, I.; Scanlan, D.J.; Millard, A.; Chen, Y. Membrane lipid renovation in pseudomonas aeruginosa–implications for phage therapy? Environ. Microbiol. 2022, 24, 4533–4546. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, K.B.; Gatto, C.; Wilkinson, B.J. Interrelationships Among Fatty Acid Composition, Staphyloxanthin Content, Fluidity, and Carbon Flow on the Staphylococcus aureus Membrane. Molecules 2018, 23, 1201. [Google Scholar] [CrossRef] [PubMed]
- Boudjemaa, R.; Cabriel, C.; Dubois-Brissonnet, F.; Bourg, N.; Dupuls, G.; Gruss, A.; Lévêque-Fort, S.; Briandet, R.; Fontaine-Aupart, M.-P.; Steenkeste, K. Impact of Bacterial Membrane Fatty Acid Composition on the Failure of Daptomycin To Kill Staphylococcus aureus. Antimicrob. Agents Chemother. 2018, 62, e00023-18. [Google Scholar] [CrossRef]
- Dubois-Brisonnet, F.; Trotier, E.; Briandet, R. The Biofilm Lifestyle Involves an Increase in Bacterial Membrane Saturated Fatty Acids. Front. Microbiol. 2016, 7, 1673. [Google Scholar] [CrossRef]
- Yoon, Y.; Lee, H.; Lee, S.; Kim, S.; Choi, K.-H. Membrane fluidity-related adaptive response mechanisms of foodborne bacterial pathogens under environmental stresses. Food Res. Int. 2015, 72, 25–36. [Google Scholar] [CrossRef]
- Perez-Lopez, M.I.; Mendez-Reina, R.; Trier, S.; Herrfurth, C.; Feussmer, I.; Bernal, A.; Forero-Shelton, M.; Leidy, C. Variations in carotenoid content and acyl chain composition in exponential, stationary and biofilm states of Staphylococcus aureus, and their influence on membrane biophysical properties. BBA–Biomembr. 2019, 1861, 978–987. [Google Scholar] [CrossRef] [PubMed]
- Rode, D.K.H.; Singh, P.K.; Drescher, K. Multicellular and unicellular responses of microbial biofilm to stress. Biol. Chem. 2020, 401, 1365–1374. [Google Scholar] [CrossRef]
- Mozaheb, N.; Van Der Smissen, P.; Opsomer, T.; Mignolet, E.; Terrasi, R.; Paquot, A.; Larondelle, Y.; Dehaen, W.; Muccioli, G.G.; Mingeot-Leclercq, M.-P. Contribution of membrane vesicle to reprogramming of bacterial membrane fluidity in Pseudomonas aeruginosa. mSphere 2022, 7, e00187-22. [Google Scholar] [CrossRef] [PubMed]
- Desirac, F.; Cmalens, T.; Rosay, T.; Rodrigues, S.; Tahrioui, A.; Enault, J.; Roquigny, L.; Racine, P.-J.; Taupin, L.; Bazire, A.; et al. Different Dose-Dependent Modes of Action of C-Type Natruretic Peptide on Pseudomonas aeruginosa Biofilm Formation. Pathogens 2018, 7, 47. [Google Scholar] [CrossRef]
- Bernat, P.; Jasińska, A.; Niedziałkowska, K.; Słaba, M.; Różalska, S.; Paraszkiewicz, K.; Sas-Paszt, L.; Heipieper, H.J. Adaptation of the metolachlor-degrading fungus Trichoderma harzianum to the simultaneous presence of low-density polyethylene (LDPE) microplastics. Ecotoxicol. Environ. Saf. 2023, 267, 115656. [Google Scholar] [CrossRef]
- Bernat, P.; Nykiel-Szymańska, J.; Stolarek, P.; Słaba, M.; Szewczyk, R.; Różalska, S. 2,4-dichlorophenoxyacetic acid-induced oxidative stress: Metabolome and membrane modifications in Umbelopsis isabellina, a herbicide degrader. PLoS ONE 2018, 13, e0199677. [Google Scholar] [CrossRef] [PubMed]
- Ichihara, K.; Fukabayashi, Y. Preparation of fatty acid methyl esters for gas-liquid chromatography. J. Lipid Res. 2010, 51, 635–640. [Google Scholar] [CrossRef]

| Phospholipid Species | Growth Control | AgNPs | Ciprofloxacin | Gentamicin | AgNPs + C | AgNPs + G |
|---|---|---|---|---|---|---|
| PE 28:0 | 0.03 ± 0.028 | 0.01 ± 0.000 | 0.03 ± 0.007 | 0.02 ± 0.007 | 0.02 ± 0.007 | 0.01 ± 0.000 |
| PE 32:2 | 0.24 ± 0.014 | 0.11 ± 0.007 * | 0.36 ± 0.092 | 0.23 ± 0.134 | 0.3 ± 0.000 * | 0.16 ± 0.198 |
| PE 28:0 | 0.03 ± 0.028 | 0.01 ± 0.000 | 0.03 ± 0.007 | 0.02 ± 0.007 | 0.02 ± 0.007 | 0.01 ± 0.000 |
| PE 30:0 | 0.02 ± 0.007 | 0.04 ± 0.035 | 0.02 ± 0.007 | 0.04 ± 0.035 | 0.01 ± 0.014 | 0.01 ± 0.007 |
| PE 30:1 | 0.01 ± 0.000 | 0.01 ± 0.007 | 0.02 ± 0.000 | 0.01 ± 0.007 | 0.01 ± 0.007 | 0.03 ± 0.007 |
| PE 31:0 | 0.03 ± 0.000 | 0.02 ± 0.007 | 0.04 ± 0.021 | 0.02 ± 0.007 | 0.04 ± 0.021 | 0.01 ± 0.000 |
| PE 31:1 | 0.02 ± 0.007 | 0.02 ± 0.028 | 0.01 ± 0.000 | 0.02 ± 0.007 | 0.03 ± 0.014 | 0.02 ± 0.014 |
| PE 32:0 | 0.15 ± 0.106 | 0.20 ± 0.226 | 0.06 ± 0.014 | 0.11 ± 0.042 | 0.05 ± 0.035 | 0.10 ± 0.049 |
| PE 32:1 | 0.17 ± 0.007 | 0.10 ± 0.085 | 0.04 ± 0.028 * | 0.02 ± 0.000 * | 0.04 ± 0.021 * | 0.03 ± 0.000 * |
| PE 32:2 | 0.24 ± 0.014 | 0.11 ± 0.007 * | 0.36 ± 0.092 | 0.23 ± 0.134 | 0.3 ± 0.000 * | 0.16 ± 0.198 |
| PE 33:2 | 0.13 ± 0.007 | 0.09 ± 0.099 | 0.02 ± 0.007 * | 0.02 ± 0.007 * | 0.03 ± 0.007 * | 0.00 ± 0.000 * |
| PG 30:1 | 4.76 ± 0.06 | 1.93 ± 0.219 * | 5.37 ± 0.516 | 2.80 ± 0.184 * | 3.20 ± 1.195 | 3.85 ± 2.171 |
| PG 31:1 | 17.57 ± 2.638 | 8.48 ± 0.976 | 19.43 ± 2.857 | 13.02 ± 3.330 | 11.15 ± 1.853 | 18.34 ± 6.824 |
| PG 32:0 | 6.36 ± 1.513 | 3.59 ± 0.042 | 5.90 ± 1.584 | 6.36 ± 2.305 | 4.66 ± 0.530 | 6.79 ± 0.552 |
| PG 32:1 | 0.33 ± 0.028 | 0.22 ± 0.057 | 0.33 ± 0.085 | 0.25 ± 0.049 | 0.28 ± 0.198 | 0.33 ± 0.148 |
| PG 33:1 | 0.40 ± 0.085 | 0.15 ± 0.014 | 0.29 ± 0.177 | 0.31 ± 0.106 | 0.31 ± 0.028 | 0.32 ± 0.134 |
| PG 32:2 | 0.06 ± 0.042 | 0.03 ± 0.014 | 0.08 ± 0.064 | 0.04 ± 0.049 | 0.04 ± 0.021 | 0.05 ± 0.007 |
| PG 34:1 | 2.30 ± 0.417 | 1.62 ± 0.580 | 2.55 ± 0.834 | 2.71 ± 0.502 | 1.49 ± 0.156 | 2.09 ± 0.078 |
| LysylPG 31:0 | 18.99 ± 2.058 | 14.31 ± 4.045 * | 22.96 ± 1.846 | 20.76 ± 2.927 | 26.86 ± 8.577 | 18.44 ± 1.669 |
| LysylPG 32:0 | 27.15 ± 6.314 | 22.96 ± 8.061 | 15.09 ± 1.032 | 22.01 ± 3.366 | 9.73 ± 1.768 | 28.00 ± 10.628 |
| LysylPG 32:1 | 21.32 ± 3.776 | 46.13 ± 1.930 | 27.47 ± 8.902 | 31.31 ± 5.148 | 41.82 ± 14.121 | 21.47 ± 1.103 |
| Phospholipid Species | Growth Control | AgNPs | Ciprofloxacin | Gentamicin | AgNPs + C | AgNPs + G |
|---|---|---|---|---|---|---|
| PC 34:0 | 0.47 ± 0.059 | 0.50 ± 0.003 | 0.27 ± 0.0176 | 0.27 ± 0.004 | 0.45 ± 0.076 | 0.27 ± 0.092 |
| PC 34:1 | 1.55 ± 0.178 | 1.61 ± 0.010 | 1.28 ± 0.001 | 1.39 ± 1.588 | 1.46 ± 0.267 | 0.74 ± 0.272 |
| PC 34:1 | 0.23 ± 0.022 | 0.10 ± 0.000 * | 0.12 ± 0.000 * | 0.09 ± 0.001 * | 0.16 ± 0.055 | 0.11 ± 0.024 * |
| PC 36:2 | 0.10 ± 0.014 | 0.06 ± 5.034 | 0.04 ± 0.004 | 0.09 ± 0.000 | 0.07 ± 0.016 | 0.07 ± 0.010 |
| PE 28:0 | 0.01 ± 0.010 | 0.02 ± 4.155 | 0.01 ± 2.663 | 0.01 ± 2.510 | 0.01 ± 0.002 | 0.02 ± 0.001 |
| PE 30:0 | 0.37 ± 0.017 | 0.38 ± 0.002 | 0.34 ± 6.527 | 0.37 ± 0.000 | 0.38 ± 0.019 | 0.42 ± 0.007 |
| PE 30:1 | 0.07 ± 0.009 | 0.08 ± 0.001 | 0.05 ± 4.326 | 0.07 ± 7.344 | 0.07 ± 0.011 | 0.09 ± 0.004 |
| PE 31:0 | 0.06 ± 0.001 | 0.07 ± 1.933 | 0.07 ± 0.000 | 0.07 ± 7.763 | 0.06 ± 0.003 | 0.06 ± 4.997 |
| PE 31:1 | 0.02 ± 0.004 | 0.02 ± 0.000 | 0.01 ± 2.730 | 0.02 ± 5.417 | 0.02 ± 0.001 | 0.02 ± 0.003 |
| PE 32:0 | 4.93 ± 0.047 | 4.70 ± 0.204 | 5.05 ± 0.104 | 5.07 ± 0.195 | 4.66 ± 0.191 | 5.23 ± 0.022 * |
| PE 32:1 | 4.51 ± 0.068 | 4.28 ± 0.810 | 4.30 ± 0.215 | 4.66 ± 0.005 | 4.73 ± 0.376 | 5.54 ± 0.305 * |
| PE 32:2 | 0.47 ± 0.009 | 0.50 ± 0.016 | 0.33 ± 8.678 * | 0.41 ± 0.000 | 0.48 ± 0.061 | 0.46 ± 0.007 |
| PE 33:1 | 0.13 ± 0.004 | 0.13 ± 1.358 | 0.14 ± 0.000 | 0.13 ± 2.713 | 0.13 ± 0.012 | 0.13 ± 0.011 |
| PE 33:2 | 0.18 ± 0.002 | 0.18 ± 0.000 | 0.181 ± 0.000 | 0.17 ± 1.125 | 0.23 ± 0.022 | 0.16 ± 0.000 * |
| PE 34:1 | 41.41 ± 0.681 | 39.54 ± 0.500 | 44.36 ± 2.384 | 40.62 ± 0.886 | 40.73 ± 1.352 | 35.64 ± 0.914 * |
| PE 34:2 | 16.01 ± 0.934 | 15.47 ± 0.010 | 15.58 ± 0.024 | 15.64 ± 0.000 | 16.27 ± 0.089 | 14.97 ± 0.849 |
| PE 35:1 | 0.16 ± 0.009 | 0.15 ± 2.215 | 0.15 ± 0.000 | 0.16 ± 4.219 | 0.11 ± 0.046 | 0.13 ± 0.013 |
| PE 35:2 | 1.02 ± 0.086 | 0.98 ± 0.061 | 1.19 ± 2.239 | 1.05 ± 0.000 | 0.97 ± 0.094 | 0.78 ± 0.026 |
| PE 36:2 | 1.81 ± 0.101 | 1.67 ± 0.008 | 1.74 ± 0.031 | 1.69 ± 0.004 | 1.82 ± 0.011 | 1.53 ± 0.145 |
| PG 31:1 | 0.01 ± 0.003 | 0.01 ± 3.656 | 0.01 ± 1.0389 | 0.01 ± 1.228 | 0.01 ± 0.000 | 0.01 ± 0.004 |
| PG 32:0 | 2.10 ± 0.047 | 2.18 ± 0.001 | 2.07 ± 0.012 | 2.43 ± 0.037 | 2.06 ± 0.005 | 3.19 ± 0.053 * |
| PG 32:1 | 2.21 ± 0.050 | 2.26 ± 0.188 | 1.86 ± 0.039 | 2.30 ± 0.015 | 2.23 ± 0.186 | 3.32 ± 0.296 * |
| PG 32:2 | 0.27 ± 0.007 | 0.24 ± 0.002 | 0.19 ± 0.004 | 0.23 ± 6.767 | 0.24 ± 0.043 * | 0.27 ± 0.037 |
| PG 33:1 | 0.07 ± 0.004 | 0.09 ± 9.740 | 0.07 ± 2.156 | 0.07 ± 1.903 | 0.07 ± 0.007 | 0.09 ± 0.002 * |
| PG 34:1 | 14.09 ± 0.792 | 16.32 ± 6.014 | 14.10 ± 0.392 | 15.66 ± 1.571 | 14.69 ± 0.834 | 17.50 ± 1.027 |
| PG 34:2 | 6.95 ± 0.495 | 7.61 ± 0.051 | 5.75 ± 0.003 | 6.53 ± 0.036 | 7.06 ± 0.078 | 8.37 ± 1.129 |
| PG 36:2 | 0.75 ± 0.012 | 0.86 ± 3.481 | 0.75 ± 0.000 | 0.81 ± 4.572 | 0.83 ± 0.054 | 0.87 ± 0.093 |
| Fatty Acid Species | Growth Control | AgNPs | Ciprofloxacin | Gentamicin | AgNPs + C | AgNPs + G |
|---|---|---|---|---|---|---|
| i-14:0 | 1.13 ± 0.003 | 1.22 ± 0.007 | 0.60 ± 0.138 | 1.41 ± 0.820 | 1.31 ± 0.108 | 1.23 ± 0.091 |
| 14:0 | 0.94 ± 0.016 | 1.59 ± 0.374 | 1.29 ± 0.400 | 1.99 ± 0.398 * | 1.53 ± 0.086 * | 1.33 ± 0.338 |
| i-15:0 | 8.760.301 | 8.16 ± 0.206 | 7.66 ± 0.921 | 7.68 ± 0.198 | 7.89 ± 0.003 | 8.80 ± 1.218 |
| a-15:0 | 40.98 ± 2.523 | 35.51 ± 1.320 | 37.66 ± 1.482 | 34.27 ± 4.277 * | 36.81 ± 0.543 | 37.34 ± 3.455 |
| 15:0 | 0.82 ± 0.003 | 0.78 ± 0.057 | 0.85 ± 0.020 | 0.92 ± 0.006 | 0.86 ± 0.019 | 0.83 ± 0.031 |
| 16:0 | 1.08 ± 0.007 | 1.21 ± 0.081 | 1.08 ± 0.077 | 1.12 ± 0.014 | 1.15 ± 0.093 | 1.15 ± 0.021 |
| i-17:0 | 3.44 ± 0.064 | 3.42 ± 0.357 | 2.98 ± 0.186 | 2.92 ± 0.029 | 3.14 ± 0.180 | 3.67 ± 0.494 |
| a-17:0 | 9.80 ± 0.434 | 8.83 ± 0.433 | 8.99 ± 0.113 | 8.29 ± 0.554 | 8.67 ± 0.679 | 9.58 ± 0.880 |
| 17:0 | 2.08 ± 0.001 | 1.97 ± 0.261 | 2.06 ± 0.204 | 2.08 ± 0.021 | 2.00 ± 0.010 | 1.95 ± 0.065 |
| 18:0 | 15.68 ± 7.019 | 25.78 ± 0.903 * | 22.66 ± 0.100 | 28.44 ± 5.714 * | 24.15 ± 1.237 | 21.10 ± 6.358 |
| i-19:0 | 2.18 ± 0.004 | 1.82 ± 0.361 | 1.88 ± 0.275 | 1.58 ± 0.186 * | 1.87 ± 0.175 | 2.08 ± 0.189 |
| a-19:0 | 4.95 ± 1.998 | 3.85 ± 0.609 | 4.53 ± 0.848 | 3.63 ± 0.482 * | 4.01 ± 0.359 | 4.34 ± 0.300 |
| 19:0 | 4.13 ± 0.032 | 3.07 ± 0.478 | 3.90 ± 0.793 | 2.89 ± 0.696 * | 3.45 ± 0.278 | 3.28 ± 0.095 |
| 20:0 | 4.05 ± 0.044 | 2.78 ± 0.289 * | 3.87 ± 1.089 | 2.76 ± 0.523 * | 3.16 ± 0.391 | 3.34 ± 0.168 |
| Fatty Acid Species | Growth Control | AgNPs | Ciprofloxacin | Gentamicin | AgNPs + C | AgNPs + G |
|---|---|---|---|---|---|---|
| 8:0 | 0.24 ± 0.093 | 0.17 ± 0.007 | 0.35 ± 0.058 | 0.20 ± 0.036 | 0.219 ± 4.276 | 0.24 ± 0.048 |
| i-12:0 | 0,00 ± 0.000 | 0.06 ± 0.088 | 0.14 ± 0.191 | 0.16 ± 0.027 * | 0.17 ± 0.003 * | 0.00 ± 0.000 |
| 14:0 | 1.43 ± 0.217 | 1.23 ± 0.191 | 1.27 ± 0.160 | 1.12 ± 0.044 | 1.24 ± 0.122 | 1.34 ± 0.033 |
| i-15:0 | 0.46 ± 0.014 | 0.46 ± 0.033 | 0.41 ± 0.015 | 0.41 ± 0.025 | 0.43 ± 0.033 | 0.45 ± 0.012 |
| 16:0 | 40.84 ± 0.0460 | 40.26 ± 1.345 | 42.40 ± 1.046 | 41.65 ± 0.586 | 40.27 ± 0.496 | 40.16 ± 0.570 |
| 16:1 | 8.83 ± 0.273 | 9.62 ± 1.071 | 7.78 ± 0.861 | 8.87 ± 0.604 | 9.25 ± 0.410 | 10.32 ± 0.508 |
| 17:0 | 0.61 ± 0.143 | 0.52 ± 0.006 | 0.61 ± 0.081 | 0.48 ± 0.014 | 0.55 ± 0.021 | 0.57 ± 0.029 |
| 18:0 | 11.39 ± 2.360 | 7.10 ± 0.414 | 11.48 ± 2.075 | 7.82 ± 1.3120 | 9.02 ± 0.374 | 8.87 ± 0.573 |
| 18:1 | 36.19 ± 2.600 | 39.69 ± 0.274 | 35.57 ± 2.765 | 39.29 ± 1.390 | 38.86 ± 0.466 | 38.06 ± 0.757 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tończyk, A.; Niedziałkowska, K.; Bernat, P.; Lisowska, K. Synergistic Activity of Gloeophyllum striatum-Derived AgNPs with Ciprofloxacin and Gentamicin Against Human Pathogenic Bacteria. Int. J. Mol. Sci. 2025, 26, 3529. https://doi.org/10.3390/ijms26083529
Tończyk A, Niedziałkowska K, Bernat P, Lisowska K. Synergistic Activity of Gloeophyllum striatum-Derived AgNPs with Ciprofloxacin and Gentamicin Against Human Pathogenic Bacteria. International Journal of Molecular Sciences. 2025; 26(8):3529. https://doi.org/10.3390/ijms26083529
Chicago/Turabian StyleTończyk, Aleksandra, Katarzyna Niedziałkowska, Przemysław Bernat, and Katarzyna Lisowska. 2025. "Synergistic Activity of Gloeophyllum striatum-Derived AgNPs with Ciprofloxacin and Gentamicin Against Human Pathogenic Bacteria" International Journal of Molecular Sciences 26, no. 8: 3529. https://doi.org/10.3390/ijms26083529
APA StyleTończyk, A., Niedziałkowska, K., Bernat, P., & Lisowska, K. (2025). Synergistic Activity of Gloeophyllum striatum-Derived AgNPs with Ciprofloxacin and Gentamicin Against Human Pathogenic Bacteria. International Journal of Molecular Sciences, 26(8), 3529. https://doi.org/10.3390/ijms26083529





