Cyclosporine A Accelerates Neurorecovery Transcriptional Trajectory in a Swine Model of Diffuse Traumatic Brain Injury
Abstract
1. Introduction
2. Results
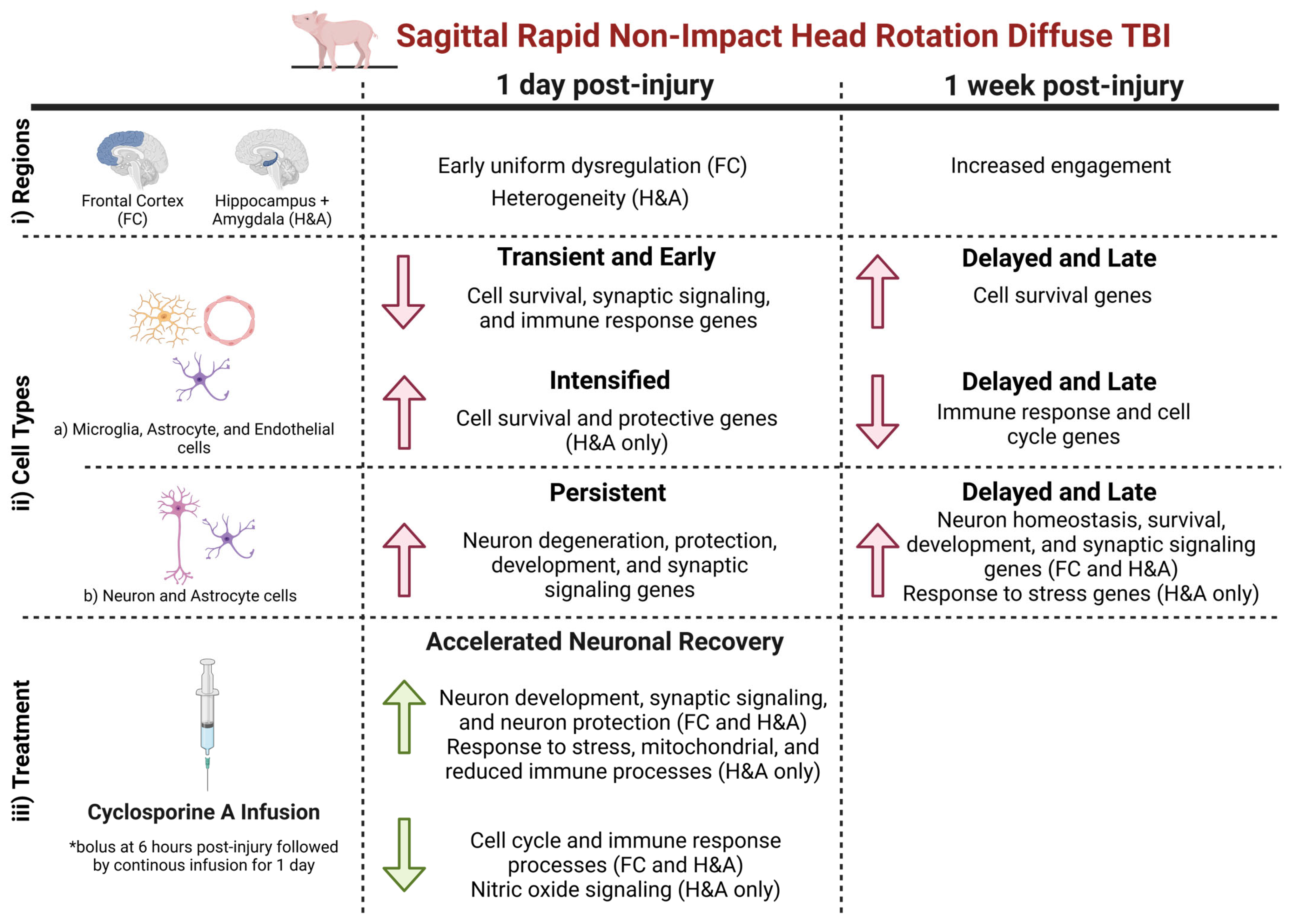
2.1. Brain Transcriptional Alterations Follow Six Temporal Patterns Between 1 Day and 1 Week Post-Injury
- Early: DEG only at 1 day compared to Sham.
- Transient: DEG only at 1 day compared to both Sham and 1 week post-injury.
- Persistent: DEG at 1 day and remained significantly altered by 1 week post-injury, both compared to Sham.
- Intensified: DEG at 1 day and significantly altered further at 1 week post-injury relative to the 1-day level.
- Delayed: DEG only at 1 week post-injury, when compared to both Sham and 1 day post-injury.
- Late: DEG only at 1 week post-injury compared to Sham.
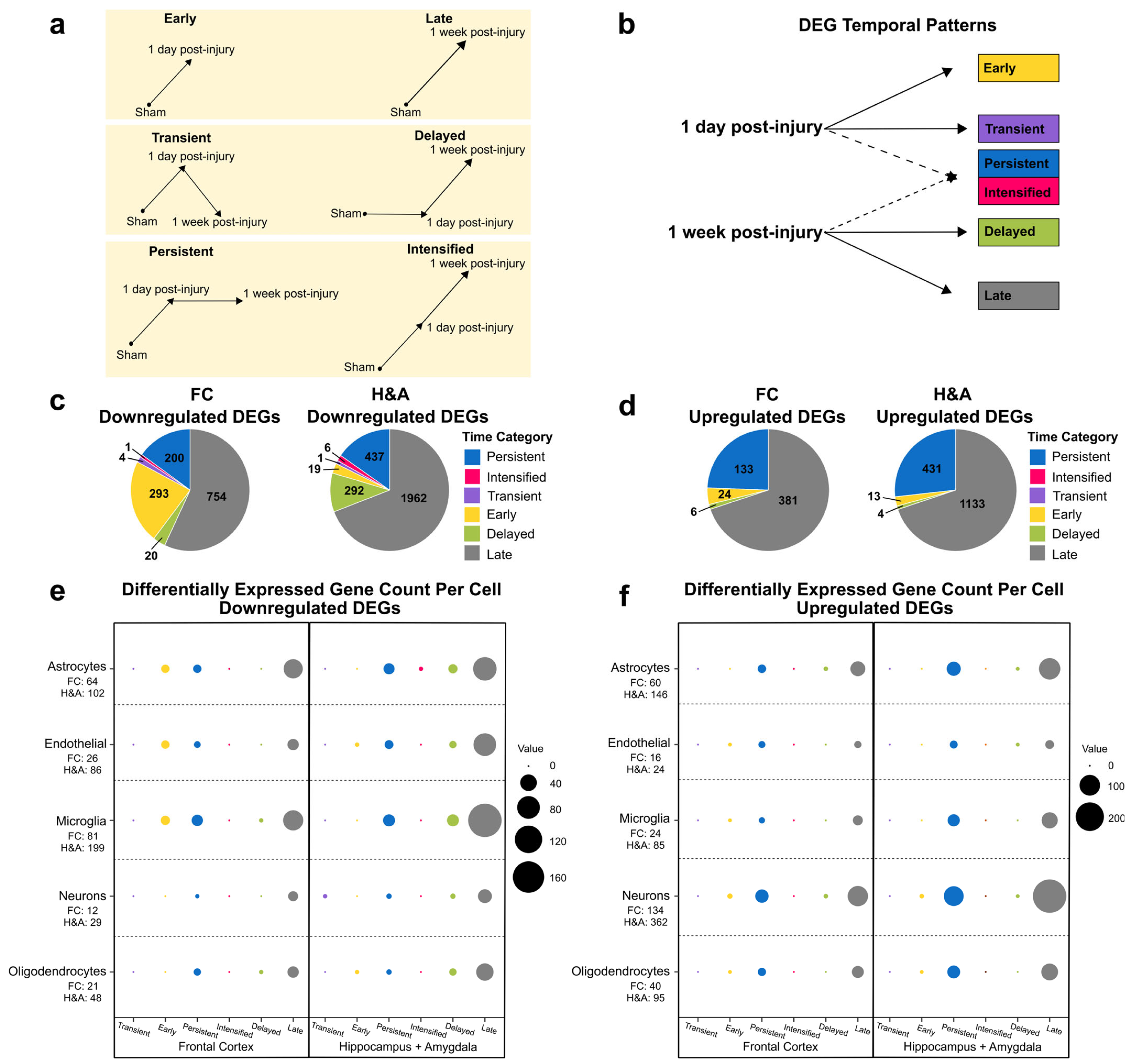
2.2. Genes Associated with Neurodegeneration, Immune Signaling, Synaptic Signaling, and Development Are Altered at 1 Day Post-Injury
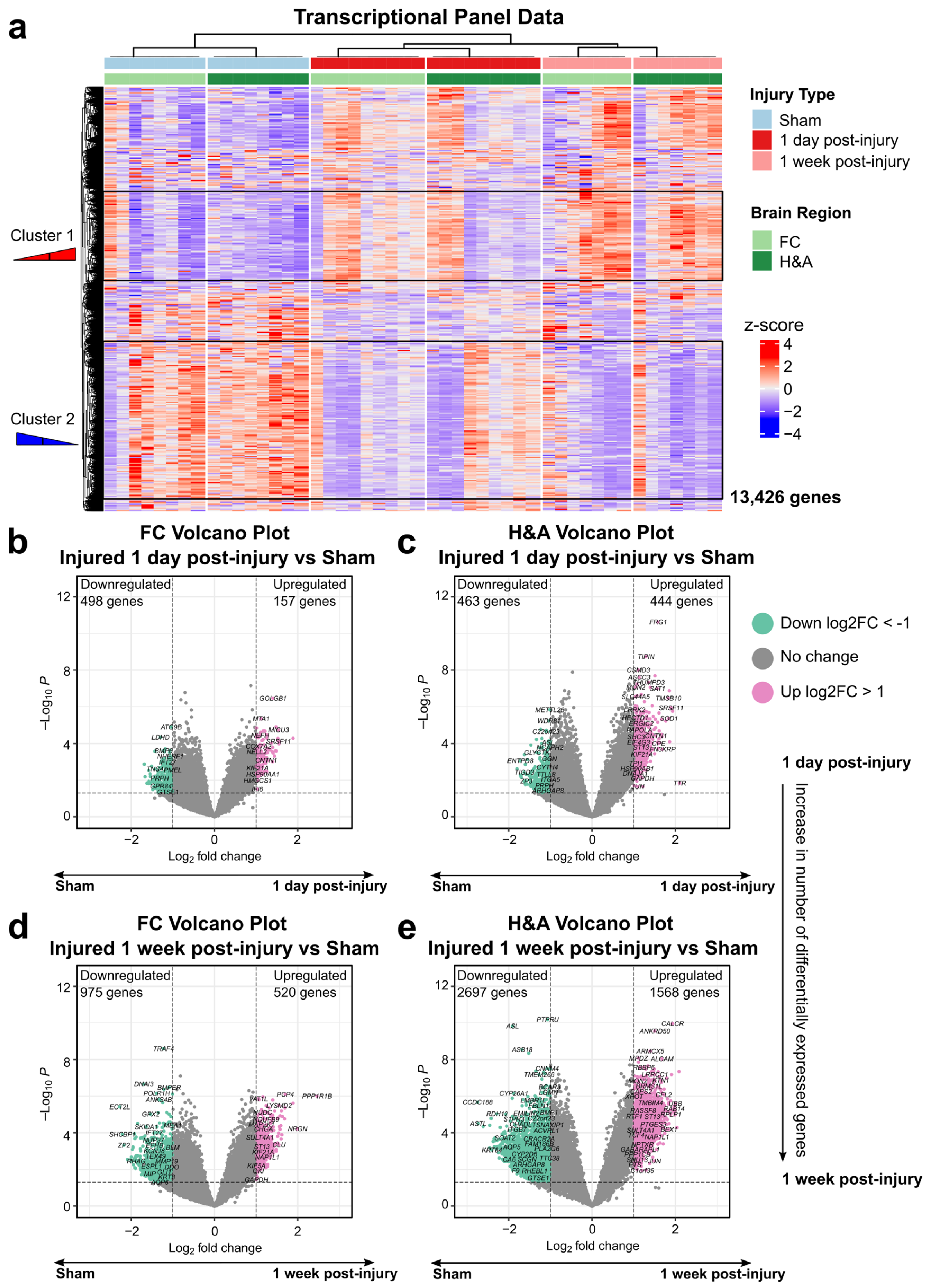
2.3. Homeostatic, Synaptic, and Neurodevelopment Genes Were Increased, While Immune and Cell Cycle Genes Were Decreased, at 1 Week After Injury
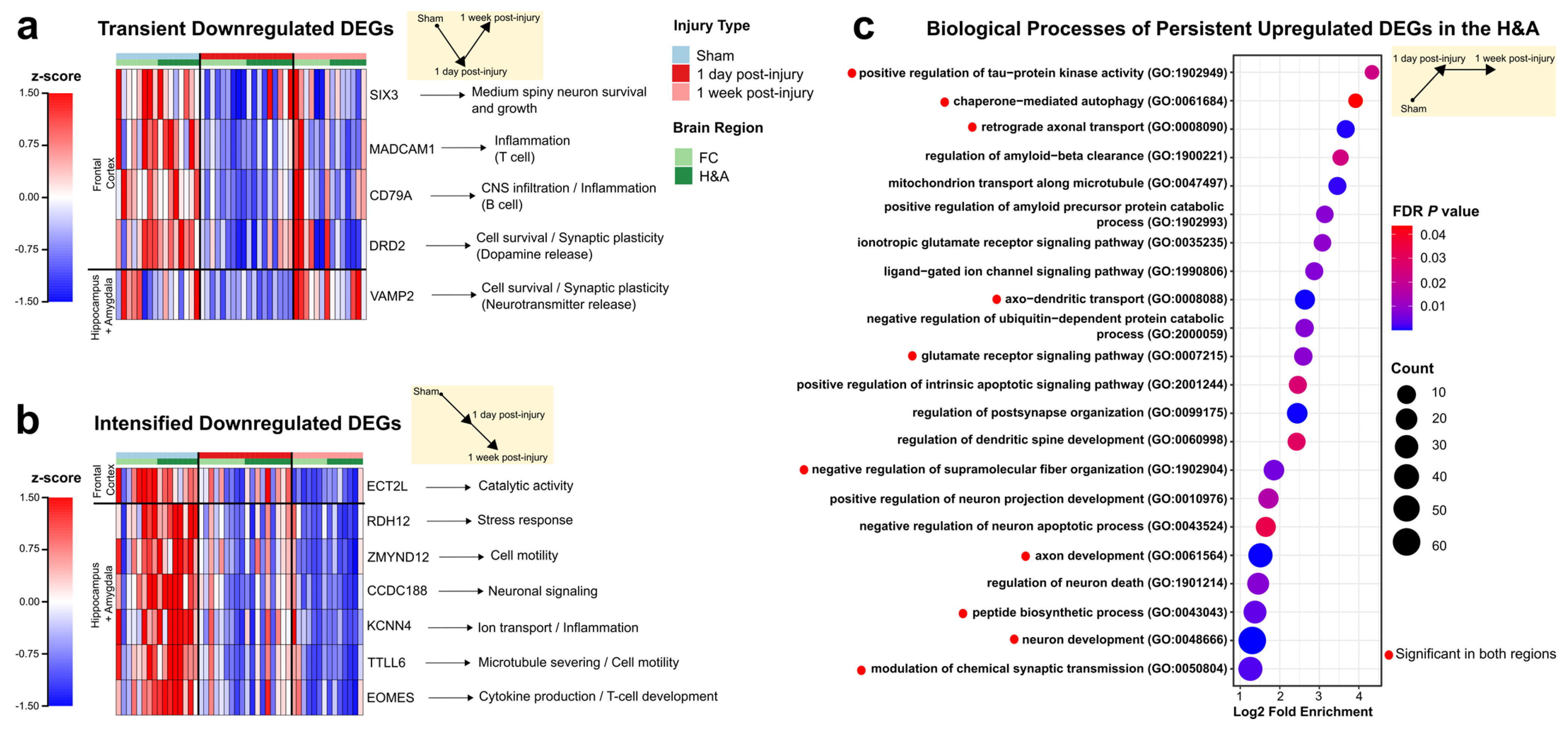
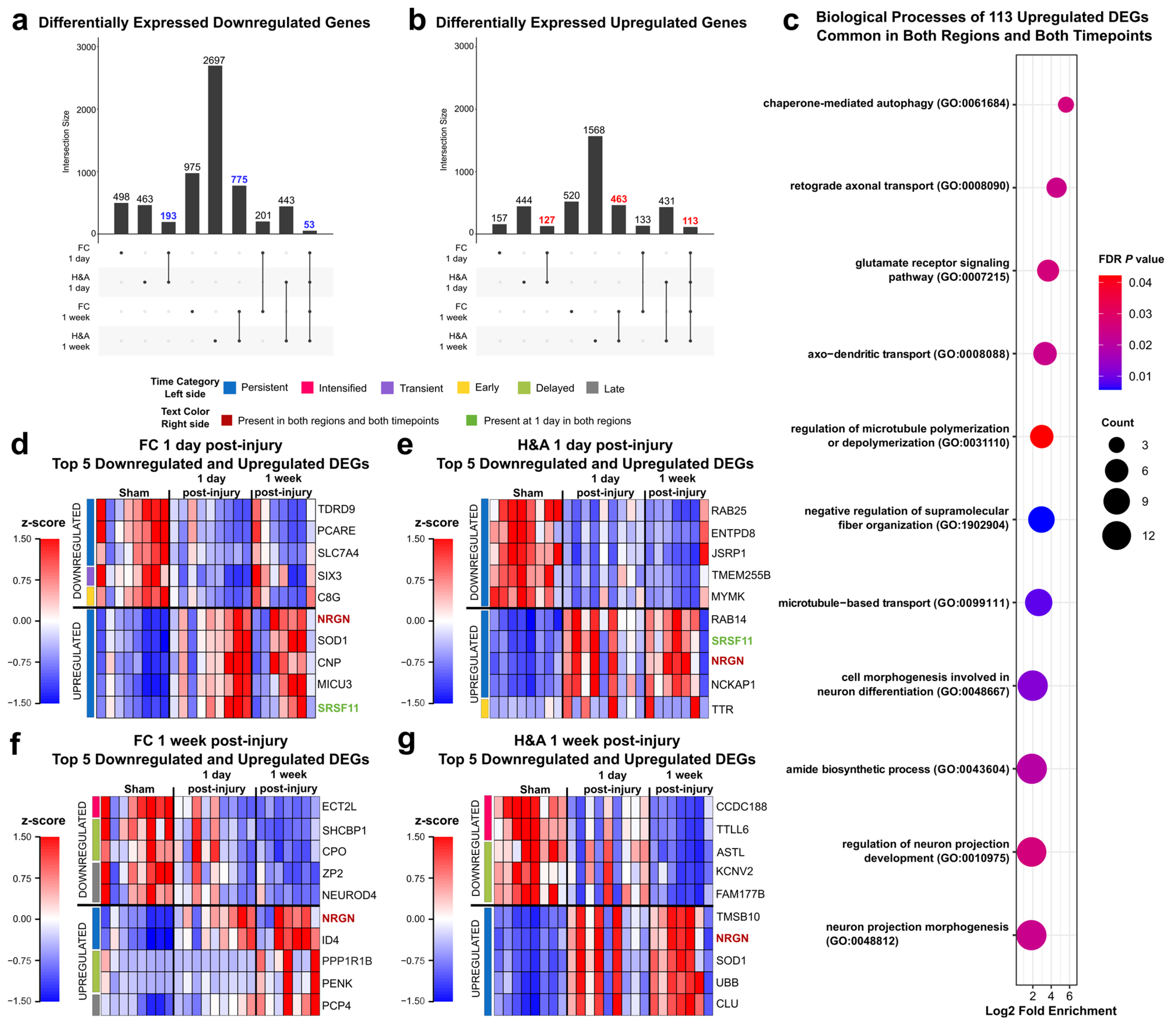
2.4. Emerging Trends: Commonalities Across Region and Time
2.5. Emerging Trends: Largest Fold Changes
2.6. Cyclosporine A (CSA) Treatment Accelerates Neurorecovery Processes
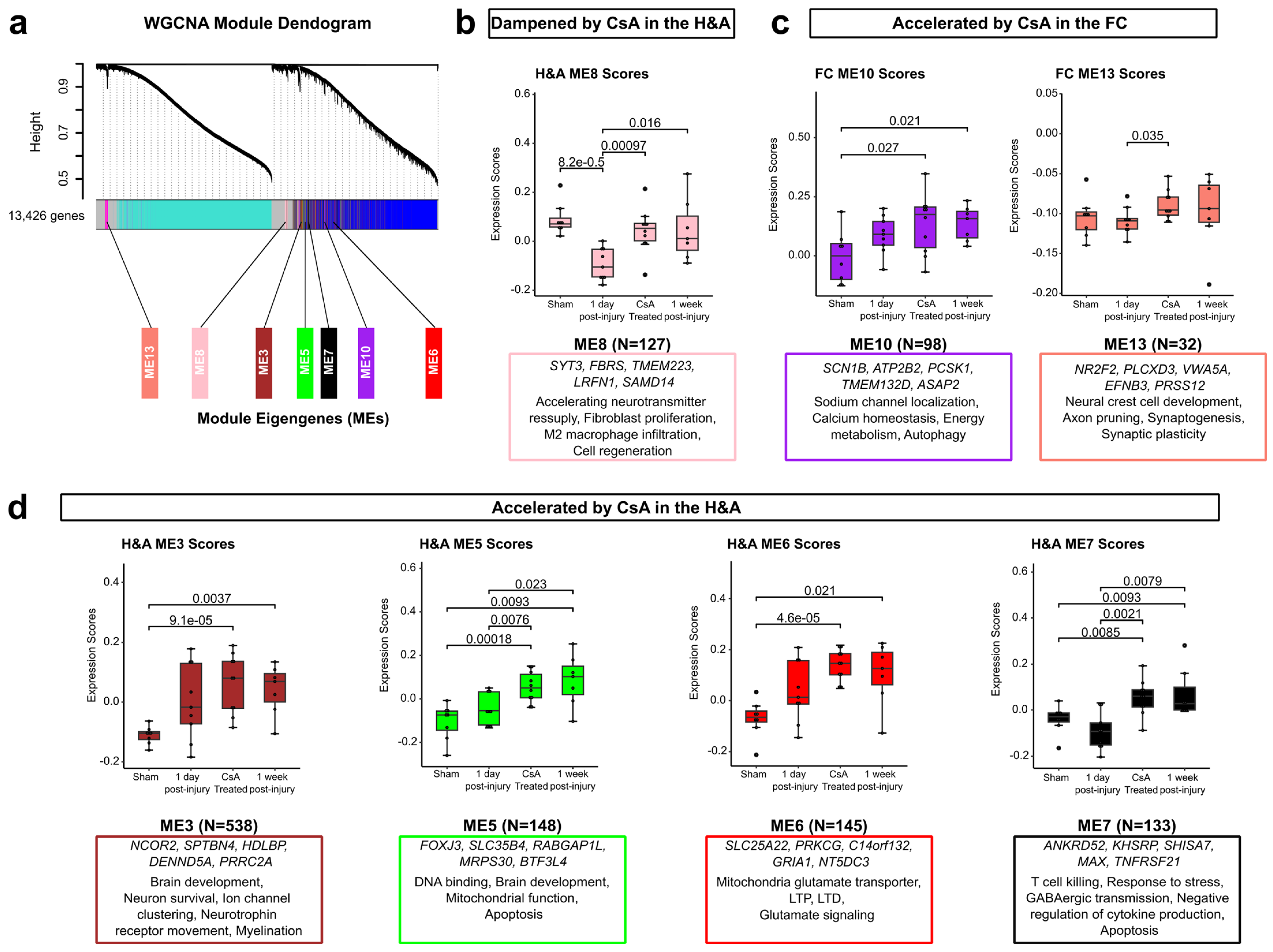
- Dampened DEGs: These were CsA-responsive DEGs that normalized to Sham levels upon CsA treatment; DEGs that were ameliorated (not significant in treated vs Sham) in injured subjects after 1 day of CsA treatment to levels similar to uninjured Shams; Transient, Early, Persistent, and Intensified DEGs whose expressions became similar to Sham following CsA treatment (Figure 2).
- Accelerated DEGs: These were CsA-fast-responsive DEGs that changed to levels similar to the untreated 1 week post-injury group within 1 day of CsA treatment; DEGs in injured subjects that were assessed just 1 day after CsA treatment that were increased or decreased to levels similar to untreated injured subjects at a 1 week post-injury timepoint; Late or Delayed DEGs now altered at 1 day following CsA treatment (Figure 2).
2.7. WGCNA Highlights Modules of Genes Dampened or Accelerated by CsA Treatment
3. Discussion
3.1. Early Alteration of Genes in the Frontal Cortex (FC) at 1 Day Post-Injury
3.2. Increased and Uniform Engagement of Hippocampus + Amygdala (H&A) at 1 Week Post-Injury Following Initial Heterogeneity
3.3. Increased Neuron Degenerative and Protective DEGs After 1 Day
3.4. Delayed Emergence of Neuronal Recovery Processes Until 1 Week
3.5. Cyclosporine A Accelerates the Expression of Genes Associated with Improved Outcome
3.6. Limitations and Future Work
4. Materials and Methods
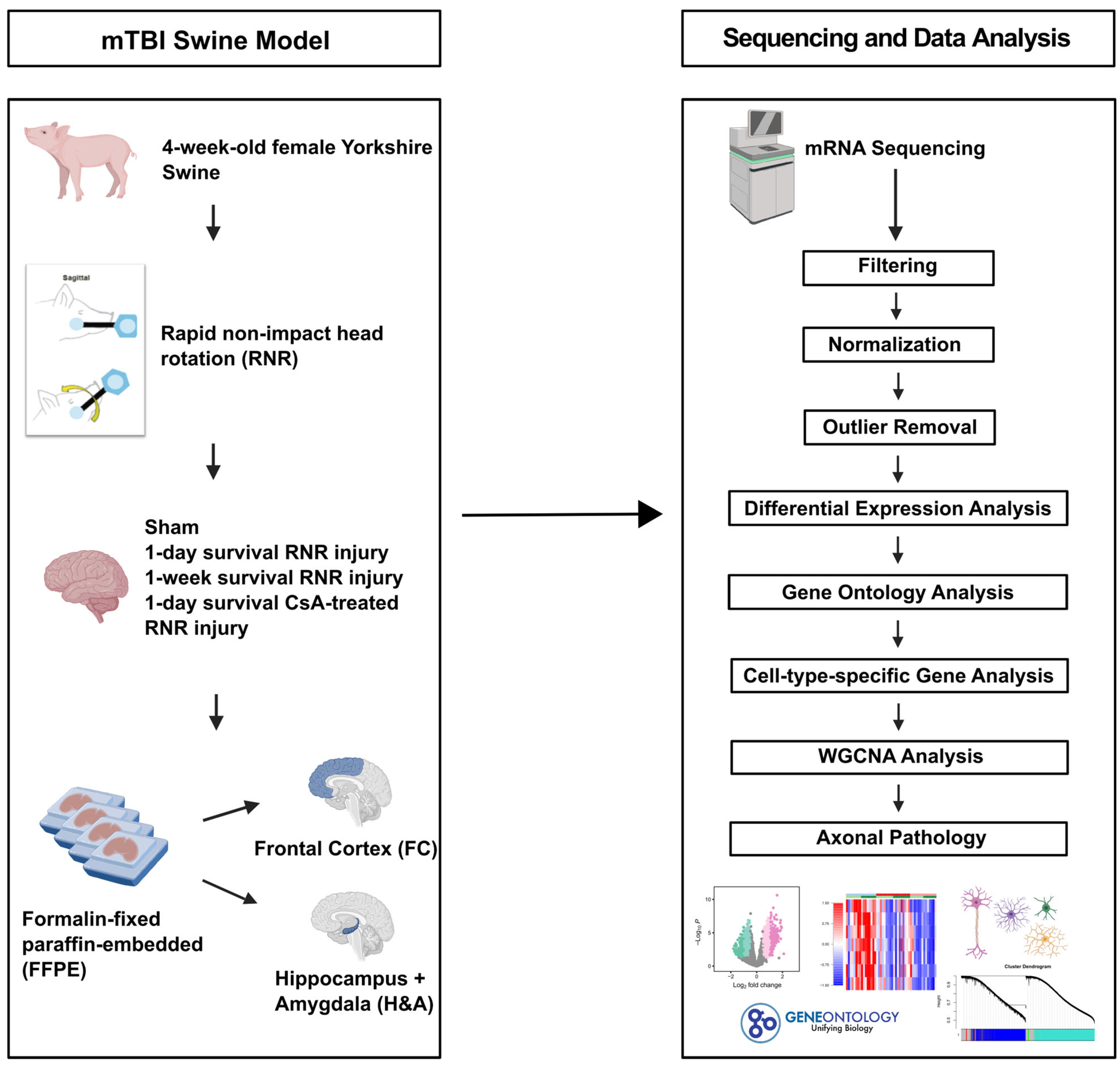
4.1. Animals
4.2. mTBI Model and Surgical Procedures
4.3. mRNA Sequencing Protocol
4.3.1. Total RNA Isolation and Quality Assessment
4.3.2. Library Preparation and mRNA Sequencing
4.3.3. Filtering, Normalization, Outlier Removal, Differential Expression Analysis, and Visualization
4.4. Gene Ontology Analysis
4.5. Cell Type-Specific Gene Analysis
4.6. Weighted Gene Co-Expression Network Analysis (WGCNA)
4.7. Axonal Pathology (Percent Axonal Injury)
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| mTBI | mild traumatic brain injury |
| FFPE | formalin-fixed paraffin-embedded |
| FC | frontal cortex |
| H&A | hippocampus + amygdala |
| RNR | rapid non-impact head rotation |
| CsA | cyclosporine A |
| DEGs | differentially expressed genes |
| Log2FC | log2 fold change |
| FDR | false discovery rate |
| RNA-seq | RNA sequencing |
| GO | gene ontology |
| WGCNA | weighted gene co-expression network analysis |
| ME | module eigengene |
References
- Kureshi, N.; Erdogan, M.; Thibault-Halman, G.; Fenerty, L.; Green, R.S.; Clarke, D.B. Long-Term Trends in the Epidemiology of Major Traumatic Brain Injury. J. Community Health 2021, 46, 1197–1203. [Google Scholar] [CrossRef] [PubMed]
- Theodorou, C.M.; Nuño, M.; Yamashiro, K.J.; Brown, E.G. Increased Mortality in Very Young Children with Traumatic Brain Injury Due to Abuse: A Nationwide Analysis of 10,965 Patients. J. Pediatr. Surg. 2021, 56, 1174–1179. [Google Scholar] [CrossRef] [PubMed]
- Dewan, M.C.; Mummareddy, N.; Wellons, J.C.; Bonfield, C.M. Epidemiology of Global Pediatric Traumatic Brain Injury: Qualitative Review. World Neurosurg. 2016, 91, 497–509.e1. [Google Scholar] [CrossRef]
- Georges, A.; M Das, J. Traumatic Brain Injury. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Røe, C.; Sveen, U.; Alvsåker, K.; Bautz-Holter, E. Post-Concussion Symptoms after Mild Traumatic Brain Injury: Influence of Demographic Factors and Injury Severity in a 1-Year Cohort Study. Disabil. Rehabil. 2009, 31, 1235–1243. [Google Scholar] [CrossRef]
- McCrea, M.A.; Nelson, L.D.; Guskiewicz, K. Diagnosis and Management of Acute Concussion. Phys. Med. Rehabil. Clin. N. Am. 2017, 28, 271–286. [Google Scholar] [CrossRef]
- Yeates, K.O.; Swift, E.; Taylor, H.G.; Wade, S.L.; Drotar, D.; Stancin, T.; Minich, N. Short- and Long-Term Social Outcomes Following Pediatric Traumatic Brain Injury. J. Int. Neuropsychol. Soc. 2004, 10, 412–426. [Google Scholar] [CrossRef]
- Hessen, E.; Nestvold, K.; Sundet, K. Neuropsychological Function in a Group of Patients 25 Years after Sustaining Minor Head Injuries as Children and Adolescents. Scand. J. Psychol. 2006, 47, 245–251. [Google Scholar] [CrossRef]
- Weil, Z.M.; Karelina, K. Lifelong Consequences of Brain Injuries during Development: From Risk to Resilience. Front. Neuroendocrinol. 2019, 55, 100793. [Google Scholar] [CrossRef]
- Hayes, R.L.; Yang, K.; Raghupathi, R.; McINTOSH, T.K. Changes in Gene Expression Following Traumatic Brain Injury in the Rat. J. Neurotrauma 1995, 12, 779–790. [Google Scholar] [CrossRef]
- White, T.E.; Ford, G.D.; Surles-Zeigler, M.C.; Gates, A.S.; LaPlaca, M.C.; Ford, B.D. Gene Expression Patterns Following Unilateral Traumatic Brain Injury Reveals a Local Pro-Inflammatory and Remote Anti-Inflammatory Response. BMC Genom. 2013, 14, 282. [Google Scholar] [CrossRef]
- Lipponen, A.; Paananen, J.; Puhakka, N.; Pitkänen, A. Analysis of Post-Traumatic Brain Injury Gene Expression Signature Reveals Tubulins, Nfe2l2, Nfkb, Cd44 and S100a4 as Treatment Targets. Sci. Rep. 2016, 6, 31570. [Google Scholar] [CrossRef] [PubMed]
- Meng, Q.; Zhuang, Y.; Ying, Z.; Agrawal, R.; Yang, X.; Gomez-Pinilla, F. Traumatic Brain Injury Induces Genome-Wide Transcriptomic, Methylomic, and Network Perturbations in Brain and Blood Predicting Neurological Disorders. EBioMedicine 2017, 16, 184–194. [Google Scholar] [CrossRef] [PubMed]
- Boone, D.R.; Weisz, H.A.; Willey, H.E.; Torres, K.E.O.; Falduto, M.T.; Sinha, M.; Spratt, H.; Bolding, I.J.; Johnson, K.M.; Parsley, M.A.; et al. Traumatic Brain Injury Induces Long-Lasting Changes in Immune and Regenerative Signaling. PLoS ONE 2019, 14, e0214741. [Google Scholar] [CrossRef]
- Ma, Y.; Liu, Y.; Ruan, X.; Liu, X.; Zheng, J.; Teng, H.; Shao, L.; Yang, C.; Wang, D.; Xue, Y. Gene Expression Signature of Traumatic Brain Injury. Front. Genet. 2021, 12, 646436. [Google Scholar] [CrossRef]
- Tang, Q.; Song, M.; Zhao, R.; Han, X.; Deng, L.; Xue, H.; Li, W.; Li, G. Comprehensive RNA Expression Analysis Revealed Biological Functions of Key Gene Sets and Identified Disease-Associated Cell Types Involved in Rat Traumatic Brain Injury. J. Clin. Med. 2022, 11, 3437. [Google Scholar] [CrossRef]
- Bolte, A.C.; Shapiro, D.A.; Dutta, A.B.; Ma, W.F.; Bruch, K.R.; Kovacs, M.A.; Royo Marco, A.; Ennerfelt, H.E.; Lukens, J.R. The Meningeal Transcriptional Response to Traumatic Brain Injury and Aging. eLife 2023, 12, e81154. [Google Scholar] [CrossRef]
- Butler, M.L.M.D.; Pervaiz, N.; Ypsilantis, P.; Wang, Y.; Cammasola Breda, J.; Mazzilli, S.; Nicks, R.; Spurlock, E.; Hefti, M.M.; Huber, B.R.; et al. Repetitive Head Impacts Induce Neuronal Loss and Neuroinflammation in Young Athletes. bioRxiv 2024, 2024.03.26.586815. [Google Scholar] [CrossRef]
- Shin, S.S.; Gottschalk, A.C.; Mazandi, V.M.; Kilbaugh, T.J.; Hefti, M.M. Transcriptional Profiling in a Novel Swine Model of Traumatic Brain Injury. Neurotrauma Rep. 2022, 3, 178–184. [Google Scholar] [CrossRef]
- Mychasiuk, R.; Hehar, H.; Ma, I.; Esser, M.J. Dietary Intake Alters Behavioral Recovery and Gene Expression Profiles in the Brain of Juvenile Rats That Have Experienced a Concussion. Front. Behav. Neurosci. 2015, 9, 17. [Google Scholar] [CrossRef]
- Zamani, A.; Powell, K.L.; May, A.; Semple, B.D. Validation of Reference Genes for Gene Expression Analysis Following Experimental Traumatic Brain Injury in a Pediatric Mouse Model. Brain Res. Bull. 2020, 156, 43–49. [Google Scholar] [CrossRef]
- Sun, D.; Colello, R.J.; Daugherty, W.P.; Kwon, T.H.; McGinn, M.J.; Harvey, H.B.; Bullock, M.R. Cell Proliferation and Neuronal Differentiation in the Dentate Gyrus in Juvenile and Adult Rats Following Traumatic Brain Injury. J. Neurotrauma 2005, 22, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Kilbaugh, T.J.; Bhandare, S.; Lorom, D.H.; Saraswati, M.; Robertson, C.L.; Margulies, S.S. Cyclosporin A Preserves Mitochondrial Function after Traumatic Brain Injury in the Immature Rat and Piglet. J. Neurotrauma 2011, 28, 763–774. [Google Scholar] [CrossRef]
- Margulies, S.S.; Kilbaugh, T.; Sullivan, S.; Smith, C.; Propert, K.; Byro, M.; Saliga, K.; Costine, B.A.; Duhaime, A. Establishing a Clinically Relevant Large Animal Model Platform for TBI Therapy Development: Using Cyclosporin A as a Case Study. Brain Pathol. 2015, 25, 289–303. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, M.; Pukenas, B.; Chawla, S.; Ehinger, J.K.; Plyler, R.; Stolow, M.; Gabello, M.; Hugerth, M.; Elmér, E.; Hansson, M.J.; et al. Neuroprotective Effects of Cyclosporine in a Porcine Pre-Clinical Trial of Focal Traumatic Brain Injury. J. Neurotrauma 2019, 36, 14–24. [Google Scholar] [CrossRef] [PubMed]
- Halestrap, A.P.; Clarke, S.J.; Javadov, S.A. Mitochondrial Permeability Transition Pore Opening during Myocardial Reperfusion—A Target for Cardioprotection. Cardiovasc. Res. 2004, 61, 372–385. [Google Scholar] [CrossRef]
- Leshnower, B.G.; Kanemoto, S.; Matsubara, M.; Sakamoto, H.; Hinmon, R.; Gorman, J.H.; Gorman, R.C. Cyclosporine Preserves Mitochondrial Morphology After Myocardial Ischemia/Reperfusion Independent of Calcineurin Inhibition. Ann. Thorac. Surg. 2008, 86, 1286–1292. [Google Scholar] [CrossRef]
- Periman, L.M.; Mah, F.S.; Karpecki, P.M. A Review of the Mechanism of Action of Cyclosporine A: The Role of Cyclosporine A in Dry Eye Disease and Recent Formulation Developments. Clin. Ophthalmol. 2020, 14, 4187–4200. [Google Scholar] [CrossRef]
- Steiner, S.; Daniel, C.; Fischer, A.; Atreya, I.; Hirschmann, S.; Waldner, M.; Neumann, H.; Neurath, M.; Atreya, R.; Weigmann, B. Cyclosporine A Regulates Pro-Inflammatory Cytokine Production in Ulcerative Colitis. Arch. Immunol. Ther. Exp. 2015, 63, 53–63. [Google Scholar] [CrossRef]
- Matsuda, S.; Moriguchi, T.; Koyasu, S.; Nishida, E. T Lymphocyte Activation Signals for Interleukin-2 Production Involve Activation of MKK6-P38 and MKK7-SAPK/JNK Signaling Pathways Sensitive to Cyclosporin A. J. Biol. Chem. 1998, 273, 12378–12382. [Google Scholar] [CrossRef]
- Matsuda, S.; Koyasu, S. Mechanisms of Action of Cyclosporine. Immunopharmacology 2000, 47, 119–125. [Google Scholar] [CrossRef]
- Hatton, J.; Rosbolt, B.; Empey, P.; Kryscio, R.; Young, B. Dosing and Safety of Cyclosporine in Patients with Severe Brain Injury. J. Neurosurg. 2008, 109, 699–707. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, P.G.; Sebastian, A.H.; Hall, E.D. Therapeutic Window Analysis of the Neuroprotective Effects of Cyclosporine A after Traumatic Brain Injury. J. Neurotrauma 2011, 28, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Aminmansour, B.; Fard, S.A.; Habibabadi, M.R.; Moein, P.; Norouzi, R.; Naderan, M. The Efficacy of Cyclosporine-A on Diffuse Axonal Injury after Traumatic Brain Injury. Adv. Biomed. Res. 2014, 3, 35. [Google Scholar] [CrossRef]
- Pischiutta, F.; D’Amico, G.; Dander, E.; Biondi, A.; Biagi, E.; Citerio, G.; De Simoni, M.G.; Zanier, E.R. Immunosuppression Does Not Affect Human Bone Marrow Mesenchymal Stromal Cell Efficacy after Transplantation in Traumatized Mice Brain. Neuropharmacology 2014, 79, 119–126. [Google Scholar] [CrossRef]
- Ishikawa, Y.; Uchino, H.; Morota, S.; Li, C.; Takahashi, T.; Ikeda, Y.; Ishii, N.; Shibasaki, F. Search for Novel Gene Markers of Traumatic Brain Injury by Time Differential Microarray Analysis. Acta Neurochir. Suppl. 2006, 96, 163–167. [Google Scholar] [CrossRef]
- Poon, W.; Matula, C.; Vos, P.E.; Muresanu, D.F.; von Steinbüchel, N.; von Wild, K.; Hömberg, V.; Wang, E.; Lee, T.M.C.; Strilciuc, S.; et al. Safety and Efficacy of Cerebrolysin in Acute Brain Injury and Neurorecovery: CAPTAIN I—A Randomized, Placebo-Controlled, Double-Blind, Asian-Pacific Trial. Neurol. Sci. 2020, 41, 281–293. [Google Scholar] [CrossRef]
- Muresanu, D.F.; Florian, S.; Hömberg, V.; Matula, C.; von Steinbüchel, N.; Vos, P.E.; von Wild, K.; Birle, C.; Muresanu, I.; Slavoaca, D.; et al. Efficacy and Safety of Cerebrolysin in Neurorecovery after Moderate-Severe Traumatic Brain Injury: Results from the CAPTAIN II Trial. Neurol. Sci. 2020, 41, 1171–1181. [Google Scholar] [CrossRef]
- Fortin, N.J.; Agster, K.L.; Eichenbaum, H.B. Critical Role of the Hippocampus in Memory for Sequences of Events. Nat. Neurosci. 2002, 5, 458–462. [Google Scholar] [CrossRef]
- Arciniegas, D.B.; Held, K.; Wagner, P. Cognitive Impairment Following Traumatic Brain Injury. Curr. Treat. Options Neurol. 2002, 4, 43–57. [Google Scholar] [CrossRef]
- Tottenham, N.; Sheridan, M.A. A Review of Adversity, The Amygdala and the Hippocampus: A Consideration of Developmental Timing. Front. Hum. Neurosci. 2010, 3, 68. [Google Scholar] [CrossRef]
- Badre, D.; Nee, D.E. Frontal Cortex and the Hierarchical Control of Behavior. Trends Cogn. Sci. 2018, 22, 170–188. [Google Scholar] [CrossRef] [PubMed]
- Eucker, S.A.; Smith, C.; Ralston, J.; Friess, S.H.; Margulies, S.S. Physiological and Histopathological Responses Following Closed Rotational Head Injury Depend on Direction of Head Motion. Exp. Neurol. 2011, 227, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Galea, E.; Weinstock, L.D.; Larramona-Arcas, R.; Pybus, A.F.; Giménez-Llort, L.; Escartin, C.; Wood, L.B. Multi-Transcriptomic Analysis Points to Early Organelle Dysfunction in Human Astrocytes in Alzheimer’s Disease. Neurobiol. Dis. 2022, 166, 105655. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Su, Z.; Wang, Z.; Li, Z.; Shang, Z.; Du, H.; Liu, G.; Qi, D.; Yang, Z.; Xu, Z.; et al. Transcriptional Profiling Reveals the Transcription Factor Networks Regulating the Survival of Striatal Neurons. Cell Death Dis. 2021, 12, 262. [Google Scholar] [CrossRef]
- Xu, Z.; Liang, Q.; Song, X.; Zhang, Z.; Lindtner, S.; Li, Z.; Wen, Y.; Liu, G.; Guo, T.; Qi, D.; et al. SP8 and SP9 Coordinately Promote D2-Type Medium Spiny Neuron Production by Activating Six3 Expression. Development 2018, 145, dev165456. [Google Scholar] [CrossRef]
- Meadows, J.D.; Breuer, J.A.; Lavalle, S.N.; Hirschenberger, M.R.; Patel, M.M.; Nguyen, D.; Kim, A.; Cassin, J.; Gorman, M.R.; Welsh, D.K.; et al. Deletion of Six3 in Post-Proliferative Neurons Produces Weakened SCN Circadian Output, Improved Metabolic Function, and Dwarfism in Male Mice. Mol. Metab. 2022, 57, 101431. [Google Scholar] [CrossRef]
- Kuhbandner, K.; Hammer, A.; Haase, S.; Terbrack, E.; Hoffmann, A.; Schippers, A.; Wagner, N.; Hussain, R.Z.; Miller-Little, W.A.; Koh, A.Y.; et al. MAdCAM-1-Mediated Intestinal Lymphocyte Homing Is Critical for the Development of Active Experimental Autoimmune Encephalomyelitis. Front. Immunol. 2019, 10, 903. [Google Scholar] [CrossRef]
- Nakache, M.; Lakey Berg, E.; Streeter, P.R.; Butcher, E.C. The Mucosal Vascular Addressin Is a Tissue-Specific Endothelial Cell Adhesion Molecule for Circulating Lymphocytes. Nature 1989, 337, 179–181. [Google Scholar] [CrossRef]
- Lenk, L.; Carlet, M.; Vogiatzi, F.; Spory, L.; Winterberg, D.; Cousins, A.; Vossen-Gajcy, M.; Ibruli, O.; Vokuhl, C.; Cario, G.; et al. CD79a Promotes CNS-Infiltration and Leukemia Engraftment in Pediatric B-Cell Precursor Acute Lymphoblastic Leukemia. Commun. Biol. 2021, 4, 73. [Google Scholar] [CrossRef]
- Ford, C.P. The Role of D2-Autoreceptors in Regulating Dopamine Neuron Activity and Transmission. Neuroscience 2014, 282, 13–22. [Google Scholar] [CrossRef]
- Chiang, C.-W.; Chang, C.-W.; Jackson, M.B. The Transmembrane Domain of Synaptobrevin Influences Neurotransmitter Flux through Synaptic Fusion Pores. J. Neurosci. 2018, 38, 7179–7191. [Google Scholar] [CrossRef] [PubMed]
- Kokotos, A.C.; Harper, C.B.; Marland, J.R.K.; Smillie, K.J.; Cousin, M.A.; Gordon, S.L. Synaptophysin Sustains Presynaptic Performance by Preserving Vesicular Synaptobrevin-II Levels. J. Neurochem. 2019, 151, 28–37. [Google Scholar] [CrossRef] [PubMed]
- Yan, C.; Jiang, J.; Yang, Y.; Geng, X.; Dong, W. The Function of VAMP2 in Mediating Membrane Fusion: An Overview. Front. Mol. Neurosci. 2022, 15, 948160. [Google Scholar] [CrossRef] [PubMed]
- Chaillou, T.; Zhang, X.; McCarthy, J.J. Expression of Muscle-Specific Ribosomal Protein L3-like Impairs Myotube Growth. J. Cell Physiol. 2016, 231, 1894–1902. [Google Scholar] [CrossRef]
- Reich, E.-P.; Cui, L.; Yang, L.; Pugliese-Sivo, C.; Golovko, A.; Petro, M.; Vassileva, G.; Chu, I.; Nomeir, A.A.; Zhang, L.-K.; et al. Blocking Ion Channel KCNN4 Alleviates the Symptoms of Experimental Autoimmune Encephalomyelitis in Mice. Eur. J. Immunol. 2005, 35, 1027–1036. [Google Scholar] [CrossRef]
- Bouhy, D.; Ghasemlou, N.; Lively, S.; Redensek, A.; Rathore, K.I.; Schlichter, L.C.; David, S. Inhibition of the Ca2+-Dependent K+ Channel, KCNN4/KCa3.1, Improves Tissue Protection and Locomotor Recovery after Spinal Cord Injury. J. Neurosci. 2011, 31, 16298–16308. [Google Scholar] [CrossRef]
- Vega, G.; Guequén, A.; Philp, A.R.; Gianotti, A.; Arzola, L.; Villalón, M.; Zegarra-Moran, O.; Galietta, L.J.V.; Mall, M.A.; Flores, C.A. Lack of Kcnn4 Improves Mucociliary Clearance in Muco-Obstructive Lung Disease. JCI Insight 2020, 5, e140076. [Google Scholar] [CrossRef]
- Zempel, H.; Luedtke, J.; Kumar, Y.; Biernat, J.; Dawson, H.; Mandelkow, E.; Mandelkow, E.-M. Amyloid-β Oligomers Induce Synaptic Damage via Tau-Dependent Microtubule Severing by TTLL6 and Spastin. EMBO J. 2013, 32, 2920–2937. [Google Scholar] [CrossRef]
- Hirschhorn-Cymerman, D.; Budhu, S.; Kitano, S.; Liu, C.; Zhao, F.; Zhong, H.; Lesokhin, A.M.; Avogadri-Connors, F.; Yuan, J.; Li, Y.; et al. Induction of Tumoricidal Function in CD4+ T Cells Is Associated with Concomitant Memory and Terminally Differentiated Phenotype. J. Exp. Med. 2012, 209, 2113–2126. [Google Scholar] [CrossRef]
- Raveney, B.J.E.; Oki, S.; Hohjoh, H.; Nakamura, M.; Sato, W.; Murata, M.; Yamamura, T. Eomesodermin-Expressing T-Helper Cells Are Essential for Chronic Neuroinflammation. Nat. Commun. 2015, 6, 8437. [Google Scholar] [CrossRef]
- Thelen, B.; Schipperges, V.; Knörlein, P.; Hummel, J.F.; Arnold, F.; Kupferschmid, L.; Klose, C.S.N.; Arnold, S.J.; Boerries, M.; Tanriver, Y. Eomes Is Sufficient to Regulate IL-10 Expression and Cytotoxic Effector Molecules in Murine CD4+ T Cells. Front. Immunol. 2023, 14, 1058267. [Google Scholar] [CrossRef] [PubMed]
- Stanco, A.; Pla, R.; Vogt, D.; Chen, Y.; Mandal, S.; Walker, J.; Hunt, R.F.; Lindtner, S.; Erdman, C.A.; Pieper, A.A.; et al. NPAS1 Represses the Generation of Specific Subtypes of Cortical Interneurons. Neuron 2014, 84, 940–953. [Google Scholar] [CrossRef] [PubMed]
- Naef, V.; De Sarlo, M.; Testa, G.; Corsinovi, D.; Azzarelli, R.; Borello, U.; Ori, M. The Stemness Gene Mex3A Is a Key Regulator of Neuroblast Proliferation During Neurogenesis. Front. Cell Dev. Biol. 2020, 8, 549533. [Google Scholar] [CrossRef]
- Ruzha, Y.; Ni, J.; Quan, Z.; Li, H.; Qing, H. Role of Vitronectin and Its Receptors in Neuronal Function and Neurodegenerative Diseases. Int. J. Mol. Sci. 2022, 23, 12387. [Google Scholar] [CrossRef]
- Su, D.; Cheng, Y.; Li, S.; Dai, D.; Zhang, W.; Lv, M. Sphk1 Mediates Neuroinflammation and Neuronal Injury via TRAF2/NF-κB Pathways in Activated Microglia in Cerebral Ischemia Reperfusion. J. Neuroimmunol. 2017, 305, 35–41. [Google Scholar] [CrossRef]
- Zhang, G.-Y.; Ma, Z.-J.; Wang, L.; Sun, R.-F.; Jiang, X.-Y.; Yang, X.-J.; Long, B.; Ye, H.-L.; Zhang, S.-Z.; Yu, Z.-Y.; et al. The Role of Shcbp1 in Signaling and Disease. Curr. Cancer Drug Targets 2019, 19, 854–862. [Google Scholar] [CrossRef]
- Tanenbaum, M.E.; Macůrek, L.; Janssen, A.; Geers, E.F.; Alvarez-Fernández, M.; Medema, R.H. Kif15 Cooperates with Eg5 to Promote Bipolar Spindle Assembly. Curr. Biol. 2009, 19, 1703–1711. [Google Scholar] [CrossRef]
- Nosratpour, S.; Ndiaye, K. Ankyrin-Repeat and SOCS Box-Containing Protein 9 (ASB9) Regulates Ovarian Granulosa Cells Function and MAPK Signaling. Mol. Reprod. Dev. 2021, 88, 830–843. [Google Scholar] [CrossRef]
- Lisachev, P.D.; Shtark, M.B.; Sokolova, O.O.; Pustylnyak, V.O.; Salakhutdinova, M.Y.; Epstein, O.I. A Comparison of the Dynamics of S100B, S100A1, and S100A6 mRNA Expression in Hippocampal CA1 Area of Rats during Long-Term Potentiation and after Low-Frequency Stimulation. Cardiovasc. Psychiatry Neurol. 2010, 2010, 720958. [Google Scholar] [CrossRef]
- Leclerc, E.; Fritz, G.; Vetter, S.W.; Heizmann, C.W. Binding of S100 Proteins to RAGE: An Update. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2009, 1793, 993–1007. [Google Scholar] [CrossRef]
- Patel, N.; Klassert, T.E.; Greco, S.J.; Patel, S.A.; Munoz, J.L.; Reddy, B.Y.; Bryan, M.; Campbell, N.; Kokorina, N.; Sabaawy, H.E.; et al. Developmental Regulation of TAC1 in Peptidergic-Induced Human Mesenchymal Stem Cells: Implication for Spinal Cord Injury in Zebrafish. Stem Cells Dev. 2012, 21, 308–320. [Google Scholar] [CrossRef] [PubMed]
- Straccia, M.; Barriga, G.G.-D.; Sanders, P.; Bombau, G.; Carrere, J.; Mairal, P.B.; Vinh, N.-N.; Yung, S.; Kelly, C.M.; Svendsen, C.N.; et al. Quantitative High-Throughput Gene Expression Profiling of Human Striatal Development to Screen Stem Cell–Derived Medium Spiny Neurons. Mol. Ther.-Methods Clin. Dev. 2015, 2, 15030. [Google Scholar] [CrossRef] [PubMed]
- Mencacci, N.E.; Reynolds, R.; Ruiz, S.G.; Vandrovcova, J.; Forabosco, P.; Consortium, U.B.E.; Consortium, I.P.D.G.; Weale, M.E.; Bhatia, K.P.; Hardy, J.; et al. Transcriptomic Analysis of Dystonia-Associated Genes Reveals Functional Convergence within Specific Cell Types and Shared Neurobiology with Psychiatric Disorders. bioRxiv 2020. [Google Scholar] [CrossRef]
- Ma, H.; Qiu, R.; Zhang, W.; Chen, X.; Zhang, L.; Wang, M. Association of PPP1R1B Polymorphisms with Working Memory in Healthy Han Chinese Adults. Front. Neurosci. 2022, 16, 989046. [Google Scholar] [CrossRef]
- Marazzi, G.; Buckley, K.M. Accumulation of mRNAs Encoding Synaptic Vesicle-Specific Proteins Precedes Neurite Extension during Early Neuronal Development. Dev. Dyn. 1993, 197, 115–124. [Google Scholar] [CrossRef]
- Alder, J.; Kanki, H.; Valtorta, F.; Greengard, P.; Poo, M.M. Overexpression of Synaptophysin Enhances Neurotransmitter Secretion at Xenopus Neuromuscular Synapses. J. Neurosci. 1995, 15, 511–519. [Google Scholar] [CrossRef]
- Daly, C.; Sugimori, M.; Moreira, J.E.; Ziff, E.B.; Llinás, R. Synaptophysin Regulates Clathrin-Independent Endocytosis of Synaptic Vesicles. Proc. Natl. Acad. Sci. USA 2000, 97, 6120–6125. [Google Scholar] [CrossRef]
- Shen, Y.-C.; Tsai, H.-M.; Ruan, J.-W.; Liao, Y.-C.; Chen, S.-F.; Chen, C.-H. Genetic and Functional Analyses of the Gene Encoding Synaptophysin in Schizophrenia. Schizophr. Res. 2012, 137, 14–19. [Google Scholar] [CrossRef]
- Sterky, F.H.; Trotter, J.H.; Lee, S.-J.; Recktenwald, C.V.; Du, X.; Zhou, B.; Zhou, P.; Schwenk, J.; Fakler, B.; Südhof, T.C. Carbonic Anhydrase-Related Protein CA10 Is an Evolutionarily Conserved Pan-Neurexin Ligand. Proc. Natl. Acad. Sci. USA 2017, 114, E1253–E1262. [Google Scholar] [CrossRef]
- Wang, P.-Y.; Kuo, T.-Y.; Wang, L.-H.; Liang, W.-H.; Wang, G.-S. Loss of MBNL1-Mediated Retrograde BDNF Signaling in the Myotonic Dystrophy Brain. Acta Neuropathol. Commun. 2023, 11, 44. [Google Scholar] [CrossRef]
- Di Giovanni, S.; Movsesyan, V.; Ahmed, F.; Cernak, I.; Schinelli, S.; Stoica, B.; Faden, A.I. Cell Cycle Inhibition Provides Neuroprotection and Reduces Glial Proliferation and Scar Formation after Traumatic Brain Injury. Proc. Natl. Acad. Sci. USA 2005, 102, 8333–8338. [Google Scholar] [CrossRef] [PubMed]
- Naim, M.Y.; Friess, S.; Smith, C.; Ralston, J.; Ryall, K.; Helfaer, M.A.; Margulies, S.S. Folic Acid Enhances Early Functional Recovery in a Piglet Model of Pediatric Head Injury. Dev. Neurosci. 2011, 32, 466–479. [Google Scholar] [CrossRef]
- Weeks, D.; Sullivan, S.; Kilbaugh, T.; Smith, C.; Margulies, S.S. Influences of Developmental Age on the Resolution of Diffuse Traumatic Intracranial Hemorrhage and Axonal Injury. J. Neurotrauma 2014, 31, 206–214. [Google Scholar] [CrossRef] [PubMed]
- Uryu, K.; Chen, X.-H.; Martinez, D.; Browne, K.D.; Johnson, V.E.; Graham, D.I.; Lee, V.M.-Y.; Q. Trojanowski, J.; Smith, D.H. Multiple Proteins Implicated in Neurodegenerative Diseases Accumulate in Axons After Brain Trauma in Humans. Exp. Neurol. 2007, 208, 185–192. [Google Scholar] [CrossRef]
- Li, X.; Zhang, X.; Ladiwala, A.R.A.; Du, D.; Yadav, J.K.; Tessier, P.M.; Wright, P.E.; Kelly, J.W.; Buxbaum, J.N. Mechanisms of Transthyretin Inhibition of β-Amyloid Aggregation In Vitro. J. Neurosci. 2013, 33, 19423–19433. [Google Scholar] [CrossRef]
- Dumoulin, A.; Dagane, A.; Dittmar, G.; Rathjen, F.G. S-Palmitoylation Is Required for the Control of Growth Cone Morphology of DRG Neurons by CNP-Induced cGMP Signaling. Front. Mol. Neurosci. 2018, 11, 345. [Google Scholar] [CrossRef]
- Guo, H.; Zhang, Q.; Dai, R.; Yu, B.; Hoekzema, K.; Tan, J.; Tan, S.; Jia, X.; Chung, W.K.; Hernan, R.; et al. NCKAP1 Disruptive Variants Lead to a Neurodevelopmental Disorder with Core Features of Autism. Am. J. Hum. Genet. 2020, 107, 963–976. [Google Scholar] [CrossRef]
- Nelson, A.R.; Sagare, A.P.; Zlokovic, B.V. Role of Clusterin in the Brain Vascular Clearance of Amyloid-β. Proc. Natl. Acad. Sci. USA 2017, 114, 8681–8682. [Google Scholar] [CrossRef]
- Ma, Q.; Zhang, L. C-Type Natriuretic Peptide Functions as an Innate Neuroprotectant in Neonatal Hypoxic-Ischemic Brain Injury in Mouse via Natriuretic Peptide Receptor 2. Exp. Neurol. 2018, 304, 58–66. [Google Scholar] [CrossRef]
- Decker, J.M.; Wójtowicz, A.M.; Bartsch, J.C.; Liotta, A.; Braunewell, K.H.; Heinemann, U.; Behrens, C.J. C-Type Natriuretic Peptide Modulates Bidirectional Plasticity in Hippocampal Area CA1 in Vitro. Neuroscience 2010, 169, 8–22. [Google Scholar] [CrossRef]
- Patron, M.; Granatiero, V.; Espino, J.; Rizzuto, R.; De Stefani, D. MICU3 Is a Tissue-Specific Enhancer of Mitochondrial Calcium Uptake. Cell Death Differ. 2019, 26, 179–195. [Google Scholar] [CrossRef] [PubMed]
- Perez-Ternero, C.; Pallier, P.N.; Tremoleda, J.L.; Delogu, A.; Fernandes, C.; Michael-Titus, A.T.; Hobbs, A.J. C-Type Natriuretic Peptide Preserves Central Neurological Function by Maintaining Blood-Brain Barrier Integrity. Front. Mol. Neurosci. 2022, 15, 991112. [Google Scholar] [CrossRef]
- Wang, H.; Pun, P.; Kwee, L.; Craig, D.; Hanynes, C.; Hauser, E.; Gregory, S.; Pollak, M.; Svetkey, L.; Patel, U.; et al. Abstract 18788: Genetic Modifiers of Renal Dysfunction in African-American APOL1 Carriers Identified Through a Genome-Wide Screen. Circulation 2016, 134, A18788. [Google Scholar]
- Zhang, Y.; Sloan, S.A.; Clarke, L.E.; Caneda, C.; Plaza, C.A.; Blumenthal, P.D.; Vogel, H.; Steinberg, G.K.; Edwards, M.S.B.; Li, G.; et al. Purification and Characterization of Progenitor and Mature Human Astrocytes Reveals Transcriptional and Functional Differences with Mouse. Neuron 2016, 89, 37–53. [Google Scholar] [CrossRef]
- Dong, Y.-D.; Yuan, Y.-L.; Yu, H.-B.; Tian, G.-J.; Li, D.-Y. SHCBP1 Is a Novel Target and Exhibits Tumor-Promoting Effects in Gastric Cancer. Oncol. Rep. 2019, 41, 1649–1657. [Google Scholar] [CrossRef]
- Burke, L.C.; Ezeribe, H.O.; Kwon, A.Y.; Dockery, D.; Lyons, P.J. Carboxypeptidase O Is a Lipid Droplet-Associated Enzyme Able to Cleave Both Acidic and Polar C-Terminal Amino Acids. PLoS ONE 2018, 13, e0206824. [Google Scholar] [CrossRef]
- Jorge, B.S.; Campbell, C.M.; Miller, A.R.; Rutter, E.D.; Gurnett, C.A.; Vanoye, C.G.; George, A.L.; Kearney, J.A. Voltage-Gated Potassium Channel KCNV2 (Kv8.2) Contributes to Epilepsy Susceptibility. Proc. Natl. Acad. Sci. USA 2011, 108, 5443–5448. [Google Scholar] [CrossRef]
- Jorstad, N.L.; Song, J.H.T.; Exposito-Alonso, D.; Suresh, H.; Castro-Pacheco, N.; Krienen, F.M.; Yanny, A.M.; Close, J.; Gelfand, E.; Long, B.; et al. Comparative Transcriptomics Reveals Human-Specific Cortical Features. Science 2023, 382, eade9516. [Google Scholar] [CrossRef]
- Fukuoka, T.; Kato, A.; Hirano, M.; Ohka, F.; Aoki, K.; Awaya, T.; Adilijiang, A.; Sachi, M.; Tanahashi, K.; Yamaguchi, J.; et al. Neurod4 Converts Endogenous Neural Stem Cells to Neurons with Synaptic Formation after Spinal Cord Injury. iScience 2021, 24, 102074. [Google Scholar] [CrossRef]
- Harashima, S.; Wang, Y.; Horiuchi, T.; Seino, Y.; Inagaki, N. Purkinje Cell Protein 4 Positively Regulates Neurite Outgrowth and Neurotransmitter Release. J. Neurosci. Res. 2011, 89, 1519–1530. [Google Scholar] [CrossRef]
- Kim, E.E.; Shekhar, A.; Lu, J.; Lin, X.; Liu, F.-Y.; Zhang, J.; Delmar, M.; Fishman, G.I. PCP4 Regulates Purkinje Cell Excitability and Cardiac Rhythmicity. J. Clin. Investig. 2014, 124, 5027–5036. [Google Scholar] [CrossRef] [PubMed]
- Raihan, O.; Brishti, A.; Li, Q.; Zhang, Q.; Li, D.; Li, X.; Zhang, Q.; Xie, Z.; Li, J.; Zhang, J.; et al. SRSF11 Loss Leads to Aging-Associated Cognitive Decline by Modulating LRP8 and ApoE. Cell Rep. 2019, 28, 78–90.e6. [Google Scholar] [CrossRef]
- Zhong, L.; Cherry, T.; Bies, C.E.; Florence, M.A.; Gerges, N.Z. Neurogranin Enhances Synaptic Strength through Its Interaction with Calmodulin. EMBO J. 2009, 28, 3027–3039. [Google Scholar] [CrossRef]
- Zhong, L.; Gerges, N.Z. Neurogranin and Synaptic Plasticity Balance. Commun. Integr. Biol. 2010, 3, 340–342. [Google Scholar] [CrossRef]
- Zhong, L.; Brown, J.; Kramer, A.; Kaleka, K.; Petersen, A.; Krueger, J.N.; Florence, M.; Muelbl, M.J.; Battle, M.; Murphy, G.G.; et al. Increased Prefrontal Cortex Neurogranin Enhances Plasticity and Extinction Learning. J. Neurosci. 2015, 35, 7503–7508. [Google Scholar] [CrossRef]
- Gao, Y.; Tatavarty, V.; Korza, G.; Levin, M.K.; Carson, J.H. Multiplexed Dendritic Targeting of α Calcium Calmodulin-Dependent Protein Kinase II, Neurogranin, and Activity-Regulated Cytoskeleton-Associated Protein RNAs by the A2 Pathway. MBoC 2008, 19, 2311–2327. [Google Scholar] [CrossRef]
- Yang, J.; Korley, F.K.; Dai, M.; Everett, A.D. Serum Neurogranin Measurement as a Biomarker of Acute Traumatic Brain Injury. Clin. Biochem. 2015, 48, 843–848. [Google Scholar] [CrossRef]
- Iqbal, J. Transthyretin—A Key Gene Involved in Regulating Learning and Memory in Brain, and Providing Neuroprotection in Alzheimer Disease via Neuronal Synthesis of Transthyretin Protein. J. Behav. Brain Sci. 2018, 8, 77–92. [Google Scholar] [CrossRef][Green Version]
- Batsukh, T.; Schulz, Y.; Wolf, S.; Rabe, T.I.; Oellerich, T.; Urlaub, H.; Schaefer, I.-M.; Pauli, S. Identification and Characterization of FAM124B as a Novel Component of a CHD7 and CHD8 Containing Complex. PLoS ONE 2012, 7, e52640. [Google Scholar] [CrossRef]
- Weingarten, D.J.; Shrestha, A.; Juda-Nelson, K.; Kissiwaa, S.A.; Spruston, E.; Jackman, S.L. Fast Resupply of Synaptic Vesicles Requires Synaptotagmin-3. Nature 2022, 611, 320–325. [Google Scholar] [CrossRef]
- Hirano, S.; Bless, D.M.; Hartig, G.K.; Massey, R.J.; Ford, C.N. Morphological and Functional Changes of Human Vocal Fold Fibroblasts with Hepatocyte Growth Factor. Ann. Otol. Rhinol. Laryngol. 2003, 112, 1026–1033. [Google Scholar] [CrossRef] [PubMed]
- Prakash, S.; Paul, W.E.; Robbins, P.W. Fibrosin, a Novel Fibrogenic Cytokine, Modulates Expression of Myofibroblasts. Exp. Mol. Pathol. 2007, 82, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Gurtner, G.C.; Werner, S.; Barrandon, Y.; Longaker, M.T. Wound Repair and Regeneration. Nature 2008, 453, 314–321. [Google Scholar] [CrossRef] [PubMed]
- Dennerlein, S.; Poerschke, S.; Oeljeklaus, S.; Wang, C.; Richter-Dennerlein, R.; Sattmann, J.; Bauermeister, D.; Hanitsch, E.; Stoldt, S.; Langer, T.; et al. Defining the Interactome of the Human Mitochondrial Ribosome Identifies SMIM4 and TMEM223 as Respiratory Chain Assembly Factors. eLife 2021, 10, e68213. [Google Scholar] [CrossRef]
- Xu, W.; Liu, W.; Anwaier, A.; Tian, X.; Su, J.; Shi, G.; Wei, S.; Qu, Y.; Zhang, H.; Ye, D. Deciphering the Role of miR-187-3p/LRFN1 Axis in Modulating Progression, Aerobic Glycolysis and Immune Microenvironment of Clear Cell Renal Cell Carcinoma. Discov. Oncol. 2022, 13, 59. [Google Scholar] [CrossRef]
- Hewitt, K.J.; Katsumura, K.R.; Matson, D.R.; Devadas, P.; Tanimura, N.; Hebert, A.S.; Coon, J.J.; Kim, J.-S.; Dewey, C.N.; Keles, S.; et al. GATA Factor-Regulated Samd14 Enhancer Confers Red Blood Cell Regeneration and Survival in Severe Anemia. Dev. Cell 2017, 42, 213–225.e4. [Google Scholar] [CrossRef]
- Gong, J.-E.; Liao, H.-M.; Long, H.-Y.; Li, X.-M.; Long, L.-L.; Zhou, L.; Gu, W.-P.; Lu, S.-H.; Qu, Q.; Yang, L.-M.; et al. SCN1B and SCN2B Gene Variants Analysis in Dravet Syndrome Patients: Analysis of 22 Cases. Medicine 2019, 98, e14974. [Google Scholar] [CrossRef]
- Taheri, M.; Pourtavakoli, A.; Eslami, S.; Ghafouri-Fard, S.; Sayad, A. Assessment of Expression of Calcium Signaling Related lncRNAs in Epilepsy. Sci. Rep. 2023, 13, 17993. [Google Scholar] [CrossRef]
- Löffler, D.; Behrendt, S.; Creemers, J.W.M.; Klammt, J.; Aust, G.; Stanik, J.; Kiess, W.; Kovacs, P.; Körner, A. Functional and Clinical Relevance of Novel and Known PCSK1 Variants for Childhood Obesity and Glucose Metabolism. Mol. Metab. 2016, 6, 295–305. [Google Scholar] [CrossRef]
- Chen, Q.; Fang, J.; Shen, H.; Chen, L.; Shi, M.; Huang, X.; Miao, Z.; Gong, Y. Roles, Molecular Mechanisms, and Signaling Pathways of TMEMs in Neurological Diseases. Am. J. Transl. Res. 2021, 13, 13273–13297. [Google Scholar]
- Seuter, S.; Ryynänen, J.; Carlberg, C. The ASAP2 Gene Is a Primary Target of 1,25-Dihydroxyvitamin D3 in Human Monocytes and Macrophages. J. Steroid Biochem. Mol. Biol. 2014, 144, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Rada-Iglesias, A.; Bajpai, R.; Prescott, S.; Brugmann, S.A.; Swigut, T.; Wysocka, J. Epigenomic Annotation of Enhancers Predicts Transcriptional Regulators of Human Neural Crest. Cell Stem Cell 2012, 11, 633–648. [Google Scholar] [CrossRef] [PubMed]
- Xu, N.-J.; Henkemeyer, M. Ephrin-B3 Reverse Signaling through Grb4 and Cytoskeletal Regulators Mediates Axon Pruning. Nat. Neurosci. 2009, 12, 268–276. [Google Scholar] [CrossRef] [PubMed]
- Aoto, J.; Ting, P.; Maghsoodi, B.; Xu, N.; Henkemeyer, M.; Chen, L. Postsynaptic EphrinB3 Promotes Shaft Glutamatergic Synapse Formation. J. Neurosci. 2007, 27, 7508–7519. [Google Scholar] [CrossRef]
- Mitsui, S.; Hidaka, C.; Furihata, M.; Osako, Y.; Yuri, K. A Mental Retardation Gene, Motopsin/Prss12, Modulates Cell Morphology by Interaction with Seizure-Related Gene 6. Biochem. Biophys. Res. Commun. 2013, 436, 638–644. [Google Scholar] [CrossRef]
- Jepsen, K.; Solum, D.; Zhou, T.; McEvilly, R.J.; Kim, H.-J.; Glass, C.K.; Hermanson, O.; Rosenfeld, M.G. SMRT-Mediated Repression of an H3K27 Demethylase in Progression from Neural Stem Cell to Neuron. Nature 2007, 450, 415–419. [Google Scholar] [CrossRef]
- Nuottamo, M.E.; Häppölä, P.; Artto, V.; Hautakangas, H.; Pirinen, M.; Hiekkalinna, T.; Ellonen, P.; Lepistö, M.; Hämäläinen, E.; Siren, A.; et al. NCOR2 Is a Novel Candidate Gene for Migraine-Epilepsy Phenotype. Cephalalgia 2022, 42, 631–644. [Google Scholar] [CrossRef]
- Nip, K.; Kashiwagura, S.; Kim, J.H. Loss of Β4-Spectrin Impairs Nav Channel Clustering at the Heminode and Temporal Fidelity of Presynaptic Spikes in Developing Auditory Brain. Sci. Rep. 2022, 12, 5854. [Google Scholar] [CrossRef]
- Feicht, J.; Jansen, R.-P. The High-Density Lipoprotein Binding Protein HDLBP Is an Unusual RNA-Binding Protein with Multiple Roles in Cancer and Disease. RNA Biol. 2024, 21, 312–321. [Google Scholar] [CrossRef]
- Han, C.; Alkhater, R.; Froukh, T.; Minassian, A.G.; Galati, M.; Liu, R.H.; Fotouhi, M.; Sommerfeld, J.; Alfrook, A.J.; Marshall, C.; et al. Epileptic Encephalopathy Caused by Mutations in the Guanine Nucleotide Exchange Factor DENND5A. Am. J. Hum. Genet. 2016, 99, 1359–1367. [Google Scholar] [CrossRef][Green Version]
- Wu, R.; Li, A.; Sun, B.; Sun, J.-G.; Zhang, J.; Zhang, T.; Chen, Y.; Xiao, Y.; Gao, Y.; Zhang, Q.; et al. A Novel m6A Reader Prrc2a Controls Oligodendroglial Specification and Myelination. Cell Res. 2019, 29, 23–41. [Google Scholar] [CrossRef] [PubMed]
- Landgren, H.; Carlsson, P. Foxj3, a Novel Mammalian Forkhead Gene Expressed in Neuroectoderm, Neural Crest, and Myotome. Dev. Dyn. 2004, 231, 396–401. [Google Scholar] [CrossRef] [PubMed]
- Anderson, D.; Neri, J.I.C.F.; Souza, C.R.M.; Valverde, J.G.; De Araújo, J.M.G.; Nascimento, M.D.S.B.; Branco, R.C.C.; Arrais, N.M.R.; Lassmann, T.; Blackwell, J.M.; et al. Zika Virus Changes Methylation of Genes Involved in Immune Response and Neural Development in Brazilian Babies Born With Congenital Microcephaly. J. Infect. Dis. 2021, 223, 435–440. [Google Scholar] [CrossRef]
- Huang, G.; Li, H.; Zhang, H. Abnormal Expression of Mitochondrial Ribosomal Proteins and Their Encoding Genes with Cell Apoptosis and Diseases. Int. J. Mol. Sci. 2020, 21, 8879. [Google Scholar] [CrossRef]
- Poduri, A.; Heinzen, E.L.; Chitsazzadeh, V.; Lasorsa, F.M.; Elhosary, P.C.; LaCoursiere, C.M.; Martin, E.; Yuskaitis, C.J.; Hill, R.S.; Atabay, K.D.; et al. SLC25A22 Is a Novel Gene for Migrating Partial Seizures in Infancy. Ann. Neurol. 2013, 74, 873–882. [Google Scholar] [CrossRef]
- Saito, N.; Shirai, Y. Protein Kinase Cγ (PKCγ): Function of Neuron Specific Isotype. J. Biochem. 2002, 132, 683–687. [Google Scholar] [CrossRef]
- Ismail, V.; Zachariassen, L.G.; Godwin, A.; Sahakian, M.; Ellard, S.; Stals, K.L.; Baple, E.; Brown, K.T.; Foulds, N.; Wheway, G.; et al. Identification and Functional Evaluation of GRIA1 Missense and Truncation Variants in Individuals with ID: An Emerging Neurodevelopmental Syndrome. Am. J. Hum. Genet. 2022, 109, 1217–1241. [Google Scholar] [CrossRef]
- Song, T.-Y.; Long, M.; Zhao, H.-X.; Zou, M.-W.; Fan, H.-J.; Liu, Y.; Geng, C.-L.; Song, M.-F.; Liu, Y.-F.; Chen, J.-Y.; et al. Tumor Evolution Selectively Inactivates the Core microRNA Machinery for Immune Evasion. Nat. Commun. 2021, 12, 7003. [Google Scholar] [CrossRef]
- Käfer, R.; Schmidtke, L.; Schrick, K.; Montermann, E.; Bros, M.; Kleinert, H.; Pautz, A. The RNA-Binding Protein KSRP Modulates Cytokine Expression of CD4+ T Cells. J. Immunol. Res. 2019, 2019, 4726532. [Google Scholar] [CrossRef]
- Morishita, K.; Watanabe, K.; Naguro, I.; Ichijo, H. Sodium Ion Influx Regulates Liquidity of Biomolecular Condensates in Hyperosmotic Stress Response. Cell Rep. 2023, 42, 112315. [Google Scholar] [CrossRef]
- Wu, K.; Shepard, R.D.; Castellano, D.; Han, W.; Tian, Q.; Dong, L.; Lu, W. Shisa7 Phosphorylation Regulates GABAergic Transmission and Neurodevelopmental Behaviors. Neuropsychopharmacol. 2022, 47, 2160–2170. [Google Scholar] [CrossRef]
- Zhang, H.; Fan, S.; Prochownik, E.V. Distinct Roles for MAX Protein Isoforms in Proliferation and Apoptosis. J. Biol. Chem. 1997, 272, 17416–17424. [Google Scholar] [CrossRef]
- Benschop, R.; Wei, T.; Na, S. Tumor Necrosis Factor Receptor Superfamily Member 21: TNFR-Related Death Receptor-6, DR6. In Therapeutic Targets of the TNF Superfamily; Advances in Experimental Medicine and Biology; Grewal, I.S., Ed.; Springer: New York, NY, USA, 2009; pp. 186–194. ISBN 978-0-387-89520-8. [Google Scholar]
- Hofman, M.A. Size and Shape of the Cerebral Cortex in Mammals. I. The Cortical Surface. Brain Behav. Evol. 1985, 27, 28–40. [Google Scholar] [CrossRef]
- Lind, N.M.; Moustgaard, A.; Jelsing, J.; Vajta, G.; Cumming, P.; Hansen, A.K. The Use of Pigs in Neuroscience: Modeling Brain Disorders. Neurosci. Biobehav. Rev. 2007, 31, 728–751. [Google Scholar] [CrossRef]
- Dickerson, J.W.; Dobbing, J. Prenatal and Postnatal Growth and Development of the Central Nervous System of the Pig. Proc. R. Soc. Lond. B Biol. Sci. 1967, 166, 384–395. [Google Scholar] [CrossRef]
- Flynn, T.J. Developmental Changes of Myelin-Related Lipids in Brain of Miniature Swine. Neurochem. Res. 1984, 9, 935–945. [Google Scholar] [CrossRef]
- Thibault, K.L.; Margulies, S.S. Age-Dependent Material Properties of the Porcine Cerebrum: Effect on Pediatric Inertial Head Injury Criteria. J. Biomech. 1998, 31, 1119–1126. [Google Scholar] [CrossRef]
- Araki, T.; Yokota, H.; Morita, A. Pediatric Traumatic Brain Injury: Characteristic Features, Diagnosis, and Management. Neurol. Med. Chir. 2017, 57, 82–93. [Google Scholar] [CrossRef]
- Figaji, A.A. Anatomical and Physiological Differences between Children and Adults Relevant to Traumatic Brain Injury and the Implications for Clinical Assessment and Care. Front. Neurol. 2017, 8, 685. [Google Scholar]
- Serpa, R.O.; Ferguson, L.; Larson, C.; Bailard, J.; Cooke, S.; Greco, T.; Prins, M.L. Pathophysiology of Pediatric Traumatic Brain Injury. Front. Neurol. 2021, 12, 696510. [Google Scholar] [CrossRef]
- Finnie, J. Animal Models of Traumatic Brain Injury: A Review. Aust. Vet. J. 2001, 79, 628–633. [Google Scholar] [CrossRef] [PubMed]
- Cernak, I. Animal Models of Head Trauma. NeuroRX 2005, 2, 410–422. [Google Scholar] [CrossRef] [PubMed]
- Vink, R. Large Animal Models of Traumatic Brain Injury. J. Neurosci. Res. 2018, 96, 527–535. [Google Scholar] [CrossRef] [PubMed]
- Dahnke, R.; Gaser, C. Surface and Shape Analysis. In Brain Morphometry; Neuromethods; Spalletta, G., Piras, F., Gili, T., Eds.; Springer: New York, NY, USA, 2018; pp. 51–73. ISBN 978-1-4939-7647-8. [Google Scholar]
- Hajiaghamemar, M.; Seidi, M.; Oeur, R.A.; Margulies, S.S. Toward Development of Clinically Translatable Diagnostic and Prognostic Metrics of Traumatic Brain Injury Using Animal Models: A Review and a Look Forward. Exp. Neurol. 2019, 318, 101–123. [Google Scholar] [CrossRef]
- Duhaime, A.-C. Large Animal Models of Traumatic Injury to the Immature Brain. Dev. Neurosci. 2006, 28, 380–387. [Google Scholar] [CrossRef]
- Morganti-Kossmann, M.C.; Yan, E.; Bye, N. Animal Models of Traumatic Brain Injury: Is There an Optimal Model to Reproduce Human Brain Injury in the Laboratory? Injury 2010, 41, S10–S13. [Google Scholar] [CrossRef]
- Xiong, Y.; Mahmood, A.; Chopp, M. Animal Models of Traumatic Brain Injury. Nat. Rev. Neurosci. 2013, 14, 128–142. [Google Scholar] [CrossRef]
- Mull, M.; Aderibigbe, O.; Hajiaghamemar, M.; Oeur, R.A.; Margulies, S.S. Multiple Head Rotations Result in Persistent Gait Alterations in Piglets. Biomedicines 2022, 10, 2976. [Google Scholar] [CrossRef]
- Teo, L.; Rosenfeld, J.V.; Bourne, J.A. Models of CNS Injury in the Nonhuman Primate: A New Era for Treatment Strategies. Translat.Neurosci. 2012, 3, 181–195. [Google Scholar] [CrossRef]
- Dilberović, F.; Sećerov, D.; Tomić, V. Morphological Characteristics of the Gyrus Dentatus in Some Animal Species and in Man. Anat. Anz. 1986, 161, 231–238. [Google Scholar]
- Pampiglione, G. Some Aspects of Development of Cerebral Function in Mammals. Proc. R. Soc. Med. 1971, 64, 429–435. [Google Scholar] [CrossRef] [PubMed]
- Pond, W.G.; Boleman, S.L.; Fiorotto, M.L.; Ho, H.; Knabe, D.A.; Mersmann, H.J.; Savell, J.W.; Su, D.R. Perinatal Ontogeny of Brain Growth in the Domestic Pig (44469). Proc. Soc. Exp. Biol. Med. 2000, 223, 102–108. [Google Scholar] [PubMed]
- Jelsing, J.; Nielsen, R.; Olsen, A.K.; Grand, N.; Hemmingsen, R.; Pakkenberg, B. The Postnatal Development of Neocortical Neurons and Glial Cells in the Göttingen Minipig and the Domestic Pig Brain. J. Exp. Biol. 2006, 209, 1454–1462. [Google Scholar] [CrossRef] [PubMed]
- Duhaime, A.-C.; Margulies, S.; Durham, S.; O’Rourke, M.; Golden, J.; Marwaha, S.; Raghupathi, R. Maturation-Dependent Response of the Piglet Brain to Scaled Cortical Impact. J. Neurosurg. 2000, 93, 455–462. [Google Scholar] [CrossRef]
- Hagberg, H.; Ichord, R.; Palmer, C.; Yager, J.Y.; Vannucci, S.J. Animal Models of Developmental Brain Injury: Relevance to Human Disease: A Summary of the Panel Discussion from the Third Hershey Conference on Developmental Cerebral Blood Flow and Metabolism. Dev. Neurosci. 2002, 24, 364–366. [Google Scholar] [CrossRef]
- Ibrahim, N.G.; Ralston, J.; Smith, C.; Margulies, S.S. Physiological and Pathological Responses to Head Rotations in Toddler Piglets. J. Neurotrauma 2010, 27, 1021–1035. [Google Scholar] [CrossRef]
- Kochanek, P.M.; Wallisch, J.S.; Bayır, H.; Clark, R.S.B. Pre-Clinical Models in Pediatric Traumatic Brain Injury—Challenges and Lessons Learned. Childs Nerv. Syst. 2017, 33, 1693–1701. [Google Scholar] [CrossRef]
- Ryan, M.C.; Kochunov, P.; Sherman, P.M.; Rowland, L.M.; Wijtenburg, S.A.; Acheson, A.; Hong, L.E.; Sladky, J.; McGuire, S. Miniature Pig Magnetic Resonance Spectroscopy Model of Normal Adolescent Brain Development. J. Neurosci. Methods 2018, 308, 173–182. [Google Scholar] [CrossRef]
- Ryan, M.C.; Sherman, P.; Rowland, L.M.; Wijtenburg, S.A.; Acheson, A.; Fieremans, E.; Veraart, J.; Novikov, D.S.; Hong, L.E.; Sladky, J.; et al. Miniature Pig Model of Human Adolescent Brain White Matter Development. J. Neurosci. Methods 2018, 296, 99–108. [Google Scholar] [CrossRef]
- Cullen, D.K.; Harris, J.P.; Browne, K.D.; Wolf, J.A.; Duda, J.E.; Meaney, D.F.; Margulies, S.S.; Smith, D.H. A Porcine Model of Traumatic Brain Injury via Head Rotational Acceleration. Methods Mol. Biol. 2016, 1462, 289–324. [Google Scholar] [CrossRef]
- Arulsamy, A.; Teng, J.; Colton, H.; Corrigan, F.; Collins-Praino, L. Evaluation of Early Chronic Functional Outcomes and Their Relationship to Pre-Frontal Cortex and Hippocampal Pathology Following Moderate-Severe Traumatic Brain Injury. Behav. Brain Res. 2018, 348, 127–138. [Google Scholar] [CrossRef] [PubMed]
- Holm, I.E.; West, M.J. Hippocampus of the Domestic Pig: A Stereological Study of Subdivisional Volumes and Neuron Numbers. Hippocampus 1994, 4, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Kinder, H.A.; Baker, E.W.; West, F.D. The Pig as a Preclinical Traumatic Brain Injury Model: Current Models, Functional Outcome Measures, and Translational Detection Strategies. Neural Regen. Res. 2019, 14, 413–424. [Google Scholar] [CrossRef]
- Wakade, C.; Sukumari-Ramesh, S.; Laird, M.D.; Dhandapani, K.M.; Vender, J.R. Delayed Reduction in Hippocampal Postsynaptic Density Protein-95 Expression Temporally Correlates with Cognitive Dysfunction Following Controlled Cortical Impact in Mice: Laboratory Investigation. J. Neurosurg. 2010, 113, 1195–1201. [Google Scholar] [CrossRef]
- Komoltsev, I.G.; Tret’yakova, L.V.; Frankevich, S.O.; Shirobokova, N.I.; Volkova, A.A.; Butuzov, A.V.; Novikova, M.R.; Kvichansky, A.A.; Moiseeva, Y.V.; Onufriev, M.V.; et al. Neuroinflammatory Cytokine Response, Neuronal Death, and Microglial Proliferation in the Hippocampus of Rats During the Early Period After Lateral Fluid Percussion-Induced Traumatic Injury of the Neocortex. Mol. Neurobiol. 2022, 59, 1151–1167. [Google Scholar] [CrossRef] [PubMed]
- Komoltsev, I.; Shalneva, D.; Kostyunina, O.; Volkova, A.; Frankevich, S.; Shirobokova, N.; Belikova, A.; Balan, S.; Chizhova, O.; Salyp, O.; et al. Delayed TBI-Induced Neuronal Death in the Ipsilateral Hippocampus and Behavioral Deficits in Rats: Influence of Corticosterone-Dependent Survivorship Bias? Int. J. Mol. Sci. 2023, 24, 4542. [Google Scholar] [CrossRef]
- Petito, C.K.; Pulsinelli, W.A. Delayed Neuronal Recovery and Neuronal Death in Rat Hippocampus Following Severe Cerebral Ischemia: Possible Relationship to Abnormalities in Neuronal Processes. J. Cereb. Blood Flow. Metab. 1984, 4, 194–205. [Google Scholar] [CrossRef]
- Scheepens, A.; Wassink, G.; Piersma, M.J.; Van de Berg, W.D.J.; Blanco, C.E. A Delayed Increase in Hippocampal Proliferation Following Global Asphyxia in the Neonatal Rat. Dev. Brain Res. 2003, 142, 67–76. [Google Scholar] [CrossRef]
- Dash, P.K.; Mach, S.A.; Moore, A.N. Enhanced Neurogenesis in the Rodent Hippocampus Following Traumatic Brain Injury. J. Neurosci. Res. 2001, 63, 313–319. [Google Scholar] [CrossRef]
- Ibrahim, S.; Hu, W.; Wang, X.; Gao, X.; He, C.; Chen, J. Traumatic Brain Injury Causes Aberrant Migration of Adult-Born Neurons in the Hippocampus. Sci. Rep. 2016, 6, 21793. [Google Scholar] [CrossRef]
- Petito, C.K.; Feldmann, E.; Pulsinelli, W.A.; Plum, F. Delayed Hippocampal Damage in Humans Following Cardiorespiratory Arrest. Neurology 1987, 37, 1281. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Morrison, B. Experimental Mild Traumatic Brain Injury Induces Functional Alteration of the Developing Hippocampus. J. Neurophysiol. 2010, 103, 499–510. [Google Scholar] [CrossRef]
- Johnson, V.E.; Stewart, J.E.; Begbie, F.D.; Trojanowski, J.Q.; Smith, D.H.; Stewart, W. Inflammation and White Matter Degeneration Persist for Years after a Single Traumatic Brain Injury. Brain 2013, 136, 28–42. [Google Scholar] [CrossRef]
- Faden, A.I.; Loane, D.J. Chronic Neurodegeneration After Traumatic Brain Injury: Alzheimer Disease, Chronic Traumatic Encephalopathy, or Persistent Neuroinflammation? Neurotherapeutics 2015, 12, 143–150. [Google Scholar] [CrossRef]
- Stocchetti, N.; Zanier, E.R. Chronic Impact of Traumatic Brain Injury on Outcome and Quality of Life: A Narrative Review. Crit. Care 2016, 20, 148. [Google Scholar] [CrossRef]
- Dunn, C.S.; Ferreira, L.A.; Venier, S.M.; Ali, S.F.; Wolchok, J.C.; Balachandran, K. Functional Analysis of the Cortical Transcriptome and Proteome Reveal Neurogenesis, Inflammation, and Cell Death after Repeated Traumatic Brain Injury In Vivo. Neurotrauma Rep. 2022, 3, 224–239. [Google Scholar] [CrossRef]
- Maria, N.S.S.; Sargolzaei, S.; Prins, M.L.; Dennis, E.L.; Asarnow, R.F.; Hovda, D.A.; Harris, N.G.; Giza, C.C. Bridging the Gap: Mechanisms of Plasticity and Repair after Pediatric TBI. Exp. Neurol. 2019, 318, 78–91. [Google Scholar] [CrossRef]
- Zotey, V.; Andhale, A.; Shegekar, T.; Juganavar, A. Adaptive Neuroplasticity in Brain Injury Recovery: Strategies and Insights. Cureus 2023, 15, e45873. [Google Scholar] [CrossRef]
- Sophie Su, Y.; Veeravagu, A.; Grant, G. Neuroplasticity after Traumatic Brain Injury. In Translational Research in Traumatic Brain Injury; Frontiers in Neuroscience; Laskowitz, D., Grant, G., Eds.; CRC Press/Taylor and Francis Group: Boca Raton, FL, USA, 2016; ISBN 978-1-4665-8491-4. [Google Scholar]
- Brown, C.E.; Li, P.; Boyd, J.D.; Delaney, K.R.; Murphy, T.H. Extensive Turnover of Dendritic Spines and Vascular Remodeling in Cortical Tissues Recovering from Stroke. J. Neurosci. 2007, 27, 4101–4109. [Google Scholar] [CrossRef]
- Nudo, R.J. Recovery after Brain Injury: Mechanisms and Principles. Front. Hum. Neurosci. 2013, 7, 887. [Google Scholar] [CrossRef]
- Eroglu, C.; Barres, B.A. Regulation of Synaptic Connectivity by Glia. Nature 2010, 468, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Park, J.; Choi, Y.K. The Role of Astrocytes in the Central Nervous System Focused on BK Channel and Heme Oxygenase Metabolites: A Review. Antioxidants 2019, 8, 121. [Google Scholar] [CrossRef] [PubMed]
- Albensi, B.C.; Sullivan, P.G.; Thompson, M.B.; Scheff, S.W.; Mattson, M.P. Cyclosporin Ameliorates Traumatic Brain-Injury-Induced Alterations of Hippocampal Synaptic Plasticity. Exp. Neurol. 2000, 162, 385–389. [Google Scholar] [CrossRef] [PubMed]
- McEwen, M.L.; Sullivan, P.G.; Springer, J.E. Pretreatment with the Cyclosporin Derivative, NIM811, Improves the Function of Synaptic Mitochondria Following Spinal Cord Contusion in Rats. J. Neurotrauma 2007, 24, 613–624. [Google Scholar] [CrossRef]
- Kulbe, J.R.; Hill, R.L.; Singh, I.N.; Wang, J.A.; Hall, E.D. Synaptic Mitochondria Sustain More Damage than Non-Synaptic Mitochondria after Traumatic Brain Injury and Are Protected by Cyclosporine A. J. Neurotrauma 2017, 34, 1291–1301. [Google Scholar] [CrossRef]
- Wakita, H.; Tomimoto, H.; Akiguchi, I.; Kimura, J. Protective Effect of Cyclosporin A on White Matter Changes in the Rat Brain After Chronic Cerebral Hypoperfusion. Stroke 1995, 26, 1415–1422. [Google Scholar] [CrossRef]
- Lendvai-Emmert, D.; Magyar-Sumegi, Z.D.; Hegedus, E.; Szarka, N.; Fazekas, B.; Amrein, K.; Czeiter, E.; Buki, A.; Ungvari, Z.; Toth, P. Mild Traumatic Brain Injury-Induced Persistent Blood–Brain Barrier Disruption Is Prevented by Cyclosporine A Treatment in Hypertension. Front. Neurol. 2023, 14, 1252796. [Google Scholar] [CrossRef]
- Evers, D.L.; He, J.; Kim, Y.H.; Mason, J.T.; O’Leary, T.J. Paraffin Embedding Contributes to RNA Aggregation, Reduced RNA Yield, and Low RNA Quality. J. Mol. Diagn. 2011, 13, 687–694. [Google Scholar] [CrossRef]
- Yang, S.; Tang, J.; Nie, B.; Zhou, Q. Assessment of Brain Injury Characterization and Influence of Modeling Approaches. Sci. Rep. 2022, 12, 13597. [Google Scholar] [CrossRef]
- Vester, M.E.M.; Bilo, R.A.C.; Loeve, A.J.; van Rijn, R.R.; van Zandwijk, J.P. Modeling of Inflicted Head Injury by Shaking Trauma in Children: What Can We Learn? Forensic Sci. Med. Pathol. 2019, 15, 408–422. [Google Scholar] [CrossRef]
- Song, H.; Tomasevich, A.; Paolini, A.; Browne, K.D.; Wofford, K.L.; Kelley, B.; Kantemneni, E.; Kennedy, J.; Qiu, Y.; Schneider, A.L.C.; et al. Sex Differences in the Extent of Acute Axonal Pathologies after Experimental Concussion. Acta Neuropathol. 2024, 147, 79. [Google Scholar] [CrossRef] [PubMed]
- Farace, E.; Alves, W.M. Do Women Fare Worse: A Metaanalysis of Gender Differences in Traumatic Brain Injury Outcome. J. Neurosurg. 2000, 93, 539–545. [Google Scholar] [CrossRef] [PubMed]
- Biswas, R.K.; Kabir, E.; King, R. Effect of Sex and Age on Traumatic Brain Injury: A Geographical Comparative Study. Arch. Public Health 2017, 75, 43. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Wu, X.; Shen, X.; Yang, Y.; Chen, Z.; Sun, X.; Wang, Z. Sex Differences in Traumatic Brain Injury: A Multi-Dimensional Exploration in Genes, Hormones, Cells, Individuals, and Society. Chin. Neurosurg. J. 2019, 5, 24. [Google Scholar] [CrossRef]
- Robertson, C.L.; Puskar, A.; Hoffman, G.E.; Murphy, A.Z.; Saraswati, M.; Fiskum, G. Physiologic Progesterone Reduces Mitochondrial Dysfunction and Hippocampal Cell Loss after Traumatic Brain Injury in Female Rats. Exp. Neurol. 2006, 197, 235–243. [Google Scholar] [CrossRef]
- Wei, J.; Xiao, G. The Neuroprotective Effects of Progesterone on Traumatic Brain Injury: Current Status and Future Prospects. Acta Pharmacol. Sin. 2013, 34, 1485–1490. [Google Scholar] [CrossRef]
- Lengel, D.; Huh, J.W.; Barson, J.R.; Raghupathi, R. Progesterone Treatment Following Traumatic Brain Injury in the 11-Day-Old Rat Attenuates Cognitive Deficits and Neuronal Hyperexcitability in Adolescence. Exp. Neurol. 2020, 330, 113329. [Google Scholar] [CrossRef]
- Jacobsen, S.B.; Tfelt-Hansen, J.; Smerup, M.H.; Andersen, J.D.; Morling, N. Comparison of Whole Transcriptome Sequencing of Fresh, Frozen, and Formalin-Fixed, Paraffin-Embedded Cardiac Tissue. PLoS ONE 2023, 18, e0283159. [Google Scholar] [CrossRef]
- Bossel Ben-Moshe, N.; Gilad, S.; Perry, G.; Benjamin, S.; Balint-Lahat, N.; Pavlovsky, A.; Halperin, S.; Markus, B.; Yosepovich, A.; Barshack, I.; et al. mRNA-Seq Whole Transcriptome Profiling of Fresh Frozen versus Archived Fixed Tissues. BMC Genom. 2018, 19, 419. [Google Scholar] [CrossRef]
- Ewels, P.; Magnusson, M.; Lundin, S.; Käller, M. MultiQC: Summarize Analysis Results for Multiple Tools and Samples in a Single Report. Bioinformatics 2016, 32, 3047–3048. [Google Scholar] [CrossRef]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A Flexible Trimmer for Illumina Sequence Data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef] [PubMed]
- Martin, M. Cutadapt Removes Adapter Sequences from High-Throughput Sequencing Reads. EMBnet. J. 2011, 17, 10–12. [Google Scholar] [CrossRef]
- Dobin, A.; Davis, C.A.; Schlesinger, F.; Drenkow, J.; Zaleski, C.; Jha, S.; Batut, P.; Chaisson, M.; Gingeras, T.R. STAR: Ultrafast Universal RNA-Seq Aligner. Bioinformatics 2013, 29, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Handsaker, B.; Wysoker, A.; Fennell, T.; Ruan, J.; Homer, N.; Marth, G.; Abecasis, G.; Durbin, R. 1000 Genome Project Data Processing Subgroup The Sequence Alignment/Map Format and SAMtools. Bioinformatics 2009, 25, 2078–2079. [Google Scholar] [CrossRef]
- Anders, S.; Pyl, P.T.; Huber, W. HTSeq—A Python Framework to Work with High-Throughput Sequencing Data. Bioinformatics 2015, 31, 166–169. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated Estimation of Fold Change and Dispersion for RNA-Seq Data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Leys, C.; Klein, O.; Dominicy, Y.; Ley, C. Detecting Multivariate Outliers: Use a Robust Variant of the Mahalanobis Distance. J. Exp. Soc. Psychol. 2018, 74, 150–156. [Google Scholar] [CrossRef]
- Ashburner, M.; Ball, C.A.; Blake, J.A.; Botstein, D.; Butler, H.; Cherry, J.M.; Davis, A.P.; Dolinski, K.; Dwight, S.S.; Eppig, J.T.; et al. Gene Ontology: Tool for the Unification of Biology. Nat. Genet. 2000, 25, 25–29. [Google Scholar] [CrossRef]
- Mi, H.; Muruganujan, A.; Huang, X.; Ebert, D.; Mills, C.; Guo, X.; Thomas, P.D. Protocol Update for Large-Scale Genome and Gene Function Analysis with the PANTHER Classification System (v.14.0). Nat. Protoc. 2019, 14, 703–721. [Google Scholar] [CrossRef]
- Zhang, B.; Horvath, S. A General Framework for Weighted Gene Co-Expression Network Analysis. Stat. Appl. Genet. Mol. Biol. 2005, 4. [Google Scholar] [CrossRef]
- Langfelder, P.; Horvath, S. WGCNA: An R Package for Weighted Correlation Network Analysis. BMC Bioinform. 2008, 9, 559. [Google Scholar] [CrossRef]


| Time Post-Injury | FC Log2FC | H&A Log2FC | |
|---|---|---|---|
| Downregulated | 1 day | −1.68 to −1.58 | −1.80 to −1.70 |
| 1 week | −2.28 to −2.00 | −2.75 to −2.46 | |
| Upregulated | 1 day | 1.53 to 1.89 | 1.85 to 2.10 |
| 1 week | 1.69 to 2.46 | 2.00 to 2.09 |
| Group | Body Weight (kg) | Velocity (rad/s) | Acceleration (krad/s2) |
|---|---|---|---|
| Anesthesia-only Sham | 9.3 ± 1.5 | - | - |
| Naïve | 8.6 ± 1.4 | - | - |
| Injured 1-day survival | 8.3 ± 0.7 | 124.3 ± 1.7 | 52.0 ± 8.1 |
| Injured 1-week survival | 9.4 ± 1.5 | 124.7 ± 3.3 | 40.8 ± 6.9 |
| Injured 1-day survival CsA-treated | 8.2 ± 0.8 | 124.8 ± 2.5 | 59.4 ± 9.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aderibigbe, O.; Wood, L.B.; Margulies, S.S. Cyclosporine A Accelerates Neurorecovery Transcriptional Trajectory in a Swine Model of Diffuse Traumatic Brain Injury. Int. J. Mol. Sci. 2025, 26, 3531. https://doi.org/10.3390/ijms26083531
Aderibigbe O, Wood LB, Margulies SS. Cyclosporine A Accelerates Neurorecovery Transcriptional Trajectory in a Swine Model of Diffuse Traumatic Brain Injury. International Journal of Molecular Sciences. 2025; 26(8):3531. https://doi.org/10.3390/ijms26083531
Chicago/Turabian StyleAderibigbe, Oluwagbemisola, Levi B. Wood, and Susan S. Margulies. 2025. "Cyclosporine A Accelerates Neurorecovery Transcriptional Trajectory in a Swine Model of Diffuse Traumatic Brain Injury" International Journal of Molecular Sciences 26, no. 8: 3531. https://doi.org/10.3390/ijms26083531
APA StyleAderibigbe, O., Wood, L. B., & Margulies, S. S. (2025). Cyclosporine A Accelerates Neurorecovery Transcriptional Trajectory in a Swine Model of Diffuse Traumatic Brain Injury. International Journal of Molecular Sciences, 26(8), 3531. https://doi.org/10.3390/ijms26083531






