Chalcopyrite Flotation, Molecular Design and Smart Industry: A Review
Abstract
1. Introduction
2. Materials and Methods
3. Relevant Sections
3.1. General Information and Initial Investigations (1979–2015)
3.2. Analytical Techniques and Innovative Reagents (2009–2024)
3.3. Molecular Design and Machine Learning (2018–2024)
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Schlesinger, M.; Sole, K.; Davenport, W.; Alvear, G. Extractive Metallurgy of Copper, 6th ed.; Elsevier: Amsterdam, The Netherland, 2021; ISBN 9780080967899. [Google Scholar]
- Barros, K.S.; Vielmo, V.S.; Moreno, B.G.; Riveros, G.; Cifuentes, G.; Bernardes, A.M. Chemical Composition Data of the Main Stages of Copper Production from Sulfide Minerals in Chile: A Review to Assist Circular Economy Studies. Minerals 2022, 12, 250. [Google Scholar] [CrossRef]
- U.S. Geological Survey. Mineral Commodity Summaries 2024; U.S. Geological Survey: Reston, VA, USA, 2024. [Google Scholar]
- Abbas, S.; Saqib, N.; Shahzad, U. Global export flow of Chilean copper: The role of environmental innovation and renewable energy transition. Geosci. Front. 2024, 15, 101697. [Google Scholar] [CrossRef]
- Islam, M.M.; Sohag, K.; Hammoudeh, S.; Mariev, O.; Samargandi, N. Minerals import demands and clean energy transitions: A disaggregated analysis. Energy Econ. 2022, 113, 106205. [Google Scholar] [CrossRef]
- Schipper, B.W.; Lin, H.C.; Meloni, M.A.; Wansleeben, K.; Heijungs, R.; van der Voet, E. Estimating global copper demand until with regression and stock dynamics. Resour. Conserv. Recycl. 2018, 132, 28–36. [Google Scholar] [CrossRef]
- Tanda, B.C.; Eksteen, J.J.; Oraby, E.A. An investigation into the leaching behaviour of copper oxide minerals in aqueous alkaline glycine solutions. Hydrometallurgy 2017, 167, 153–162. [Google Scholar] [CrossRef]
- Feng, Q.; Yang, W.; Wen, S.; Wang, H.; Zhao, W.; Han, G. Flotation of copper oxide minerals: A review. Int. J. Min. Sci. Technol. 2022, 32, 1351–1364. [Google Scholar] [CrossRef]
- Lee, K.; Archibald, D.; McLean, J.; Reuter, M.A. Flotation of mixed copper oxide and sulphide minerals with xanthate and hydroxamate collectors. Miner. Eng. 2009, 22, 395–401. [Google Scholar] [CrossRef]
- Castellón, C.I.; Toro, N.; Gálvez, E.; Robles, P.; Leiva, W.H.; Jeldres, R.I. Froth Flotation of Chalcopyrite/Pyrite Ore: A Critical Review. Materials 2022, 15, 6536. [Google Scholar] [CrossRef]
- Crespo, J.; Reich, M.; Barra, F.; Verdugo, J.J.; Martínez, C.; Leisen, M.; Romero, R.; Morata, D.; Marquardt, C. Occurrence and Distribution of Silver in the World–Class Río Blanco Porphyry Cu–Mo Deposit, Central Chile. Econ. Geol. 2020, 115, 1619–1644. [Google Scholar] [CrossRef]
- Garza, M.R.; Carrillo, F.R.; Picazo, N.G.; Soria, M.J.; Almaguer, I.; Chaidez, J. Effects of pretreatment and leaching medium on the extraction efficiency of Au and Ag from a chalcopyrite leaching by-product. DYNA 2021, 88, 119–126. [Google Scholar] [CrossRef]
- Hao, J.; Liu, J.; Yu, Y.; Gao, H.; Qin, X.; Bai, X. Depressants for separation of chalcopyrite and molybdenite: Review and prospects. Miner. Eng. 2023, 201, 108209. [Google Scholar] [CrossRef]
- Bogdanović, G.D.; Petrović, S.; Sokić, M.; Antonijević, M.M. Chalcopyrite leaching in acid media: A review. Metall. Mater. Eng. 2020, 26, 177–198. [Google Scholar] [CrossRef] [PubMed]
- Bulatovic, S.M. Handbook of Flotation Reagents: Chemistry, Theory and Practice Flotation of Sulfide Ores; Elsevier: Amsterdam, The Netherland, 2007; ISBN 9780444530295. [Google Scholar]
- Fuerstenau, M.C.; Jameson, G.J.; Yoon, R.H. Froth Flotation: A Century of Innovation; Society for Mining, Metallurgy, and Exploration (SME): Littleton, CO, USA, 2007; ISBN 0873352521. [Google Scholar]
- Wang, C.; Liu, R.; Wu, M.; Zhai, Q.; Luo, Y.; Jing, N.; Xie, F.; Sun, W. Selective separation of chalcopyrite from sphalerite with a novel depressant fenugreek gum: Flotation and adsorption mechanism. Miner. Eng. 2022, 184, 107653. [Google Scholar] [CrossRef]
- Shen, Z.; Feng, Q.; Wen, S.; Wang, H.; Cao, J.; Song, Z. Insights into flotation separation mechanism of chalcopyrite from galena using mercaptosuccinic acid as a novel depressant: Experimental and MDS studies. Powder Technol. 2024, 445, 120103. [Google Scholar] [CrossRef]
- Pan, G.; Shi, Q.; Zhang, G.; Huang, G. Selective depression of talc in chalcopyrite flotation by xanthan gum: Flotation response and adsorption mechanism. Colloids Surf. A Physicochem. Eng. Asp. 2020, 600, 124902. [Google Scholar] [CrossRef]
- Owusu, C.; Brito, S.; Skinner, W.; Addai, J.; Zanin, M. The influence of pyrite content on the flotation of chalcopyrite/pyrite mixtures. Miner. Eng. 2014, 55, 87–95. [Google Scholar] [CrossRef]
- Kalegowda, Y.; Chan, Y.L.; Wei, D.H.; Harmer, S.L. X-PEEM, XPS and ToF-SIMS characterisation of xanthate induced chalcopyrite flotation: Effect of pulp potential. Surf. Sci. 2015, 635, 70–77. [Google Scholar] [CrossRef]
- Zhang, W.; Sun, W.; Zheng, M.; Xu, S.; Zheng, R.; Cao, J.; Jin, X.; Gao, Z.; Feng, Z. Prediction of collector flotation performance based on machine learning and quantum chemistry: A case of sulfide minerals. Sep. Purif. Technol. 2024, 342, 126954. [Google Scholar] [CrossRef]
- Ackerman, P.K.; Harris, G.H.; Klimpel, R.R.; Aplan, F.F. Evaluation of Flotation Collectors for Copper Sulfides and Pyrite, I. Common Sulfhydryl Collectors. Int. J. Miner. Process. 1987, 21, 105–127. [Google Scholar] [CrossRef]
- Gardner, J.; Woods, R. An Electrochemical Investigation of the Natural Flotability of Chalcopyrite. Int. J. Miner. Process. 1979, 6, 1–16. [Google Scholar] [CrossRef]
- Chander, S. Electrochemistry of sulfide flotation: Growth characteristics of surface coatings and their properties, with special reference to chalcopyrite and pyrite. Int. J. Miner. Process. 1991, 33, 121–134. [Google Scholar] [CrossRef]
- Senior, G.D.; Trahar, W.J. The influence of metal hydroxides and collector on the flotation of chalcopyrite. Int. J. Miner. Process. 1991, 33, 321–341. [Google Scholar] [CrossRef]
- Kant, C.; Rao, S.R.; Finch, J.A. Distribution of surface metal ions among the products of chalcopyrite flotation. Miner. Eng. 1994, 7, 905–916. [Google Scholar] [CrossRef]
- Peng, Y.; Grano, S.; Fornasiero, D.; Ralston, J. Control of grinding conditions in the flotation of chalcopyrite and its separation from pyrite. Int. J. Miner. Process. 2003, 69, 87–100. [Google Scholar] [CrossRef]
- Peng, Y.; Grano, S. Effect of iron contamination from grinding media on the flotation of sulphide minerals of different particle size. Int. J. Miner. Process. 2010, 97, 1–6. [Google Scholar] [CrossRef]
- He, S.; Skinner, W.; Fornasiero, D. Effect of oxidation potential and zinc sulphate on the separation of chalcopyrite from pyrite. Int. J. Miner. Process. 2006, 80, 169–176. [Google Scholar] [CrossRef]
- Owusu, C.; Addai, J.; Fornasiero, D.; Zanin, M. Estimating the electrochemical reactivity of pyrite ores—Their impact on pulp chemistry and chalcopyrite flotation behaviour. Adv. Powder Technol. 2013, 24, 801–809. [Google Scholar] [CrossRef]
- Owusu, C.; Fornasiero, D.; Addai, J.; Zanin, M. Influence of pulp aeration on the flotation of chalcopyrite with xanthate in chalcopyrite/pyrite mixtures. Int. J. Miner. Process. 2015, 134, 50–57. [Google Scholar] [CrossRef]
- Bai, L.M.; Han, Y.X.; Yuan, Z.T.; Li, G.Z. Study on Different Depressants to Influence on Chalcopyrite Flotation. Adv. Mater. Res. 2012, 454, 337–341. [Google Scholar] [CrossRef]
- Wills, B.A.; Finch, J.A. Wills’ Mineral Processing Technology: An Introduction to the Practical Aspects of Ore Treatment and Mineral Recovery, 8th ed.; Butterworth–Heinemann: Oxford, UK, 2015; ISBN 9780080970530. [Google Scholar]
- Leja, J. Surface Chemistry of Froth Flotation, 1st ed.; Springer: New York, NY, USA, 1982; ISBN 9781461579779. [Google Scholar]
- Liu, R.; Sun, W.; Hu, Y.; Wang, D. Effect of Organic Depressant Lignosulfonate Calcium on Separation of Chalcopyrite from Pyrite. J. Cent. South Univ. Technol. 2009, 16, 753–757. [Google Scholar] [CrossRef]
- Mierczynska, A.; Beattie, D.A. Adsorption of Tailored Carboxymethyl Cellulose Polymers on Talc and Chalcopyrite: Correlation Between Coverage, Wettability, and Flotation. Miner. Eng. 2010, 23, 985–993. [Google Scholar] [CrossRef]
- Wang, Z.; Yunlou, Q.; Long, X.; Bo, D.; Jun, X.; Kaibin, F. Selective chalcopyrite flotation from pyrite with glycerine–xanthate as depressant. Miner. Eng. 2015, 74, 86–90. [Google Scholar] [CrossRef]
- He, Z.; Liu, G.; Yang, X.; Liu, W. A novel surfactant, N,N–diethyl–N′–cyclohexylthiourea: Synthesis, flotation and adsorption on chalcopyrite. J. Ind. Eng. Chem. 2016, 37, 107–114. [Google Scholar] [CrossRef]
- Qu, X.; Xiao, J.; Liu, G.; Liu, S.; Zhang, Z. Investigation on the flotation behavior and adsorption mechanism of 3–hexyl–4–amino–1,2,4–triazole–5–thione to chalcopyrite. Miner. Eng. 2016, 89, 10–17. [Google Scholar] [CrossRef]
- Peng, H.; Wu, D.; Abdelmonem, M. Flotation performances and surface properties of chalcopyrite with xanthate collector added before and after grinding. Results Phys. 2017, 7, 3567–3573. [Google Scholar] [CrossRef]
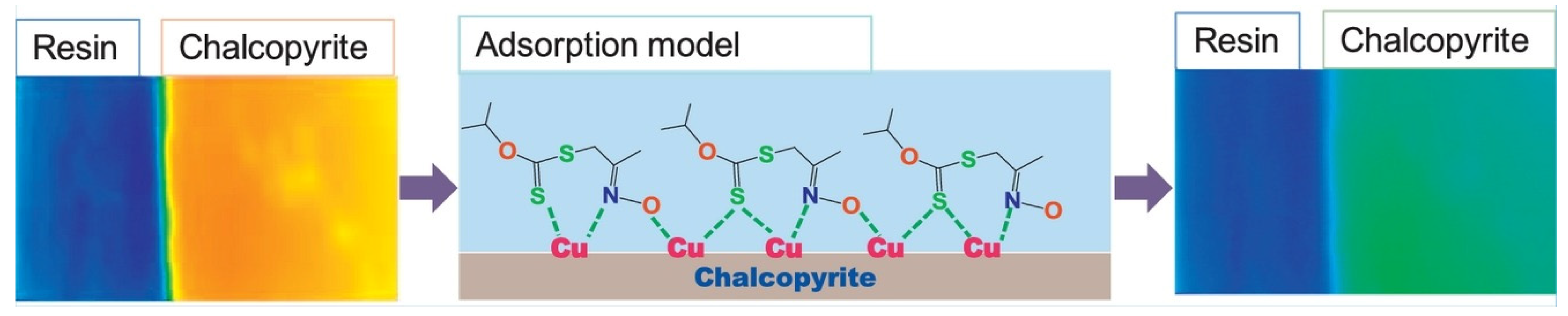
- Xiao, J.; Liu, G.; Zhong, H.; Huang, Y.; Cao, Z. The flotation behavior and adsorption mechanism of O–isopropyl–S– [2–(hydroxyimino) propyl] dithiocarbonate ester to chalcopyrite. J. Taiwan Inst. Chem. Eng. 2017, 71, 38–46. [Google Scholar] [CrossRef]
- He, G.C.; Ding, J.; Huang, C.H.; Kang, Q. Synthesis of nanoparticle emulsion collector HNP and its application in microfine chal-copyrite flotation. IOP Conf. Ser. Mater. Sci. Eng. 2018, 292, 012029. [Google Scholar] [CrossRef]
- Liu, J.; Wang, Y.; Luo, D.; Zeng, Y. Use of ZnSO4 and SDD mixture as sphalerite depressant in copper flotation. Miner. Eng. 2018, 121, 31–38. [Google Scholar] [CrossRef]
- Chen, W.; Chen, T.; Bu, X.; Chen, F.; Ding, Y.; Zhang, C.; Deng, S.; Song, Y. The selective flotation of chalcopyrite against galena using alginate as a depressant. Miner. Eng. 2019, 141, 105848. [Google Scholar] [CrossRef]
- Chen, X.; Gu, G.H.; Chen, Z.X. Seaweed glue as a novel polymer depressant for the selective separation of chalcopyrite and galena. Int. J. Miner. Metall. Mater. 2019, 26, 1495–1503. [Google Scholar] [CrossRef]
- Huang, X.; Huang, K.; Jia, Y.; Wang, S.; Cao, Z.; Zhong, H. Investigating the selectivity of a xanthate derivative for the flotation separation of chalcopyrite from pyrite. Chem. Eng. Sci. 2019, 205, 220–229. [Google Scholar] [CrossRef]
- Jia, Y.; Huang, K.; Wang, S.; Cao, Z.; Zhong, H. The selective flotation behavior and adsorption mechanism of thiohexanamide to chalcopyrite. Miner. Eng. 2019, 137, 187–199. [Google Scholar] [CrossRef]
- Pan, G.; Zhang, G.; Shi, Q.; Chen, W. The Effect of Sodium Alginate on Chlorite and Serpentine in Chalcopyrite Flotation. Minerals 2019, 9, 196. [Google Scholar] [CrossRef]
- Yuan, D.; Cadien, K.; Liu, Q.; Zeng, H. Flotation separation of Cu-Mo sulfides by O-Carboxymethyl chitosan. Miner. Eng. 2019, 134, 202–205. [Google Scholar] [CrossRef]
- Chimonyo, W.; Fletcher, B.; Peng, Y. The differential depression of an oxidized starch on the flotation of chalcopyrite and graphite. Miner. Eng. 2020, 146, 106114. [Google Scholar] [CrossRef]
- Gutierrez, L.; Uribe, L.; Hernandez, V.; Vidal, C.; Texeira, R. Assessment of the use of lignosulfonates to separate chalcopyrite and molybdenite by flotation. Powder Technol. 2020, 359, 216–225. [Google Scholar] [CrossRef]
- Jia, Y.; Wang, S.; Ma, X.; Yang, J.; Zhong, H. Synthesis of thioxopropanamide surfactants for studying the flotation performance and adsorption mechanism on chalcopyrite. Appl. Surf. Sci. 2020, 505, 144539. [Google Scholar] [CrossRef]
- Liu, J.; Liu, G.; Yang, X.; Dong, Y.; Zhang, Z. 6–Hexyl–1,2,4,5–tetrazinane–3–thione: Flotation selectivity and mechanism to copper sulfide mineral. Miner. Eng. 2020, 152, 106345. [Google Scholar] [CrossRef]
- Duan, H.; Huang, X.; Cao, X.; Cao, Z.; Zhong, H.; Zeng, J.; Zhou, H.; Xue, J.; Liu, Y. Investigating the flotation performance and interfacial adsorption mechanism of N–benzoyl–N’,N’–diethyl thiourea on chalcopyrite and pyrite. Miner. Eng. 2021, 172, 107178. [Google Scholar] [CrossRef]
- He, S.; Huang, Y.; Wang, M.; Zhang, Y.; Chen, L.; Jia, Y.; Liu, H. An efficient solid-liquid interface adsorption mode in chalcopyrite flotation with a novel di–minerophilic group surfactant 5–methyl isobutylxanthate–1,3,4–oxadiazole–2–thione. J. Mol. Liq. 2022, 345, 118254. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, X.G.; Wei, X.X.; Cheng, S.Y.; Hu, X.Q.; Luo, Y.C.; Xu, P.F. Selective depression of galena by sodium polyaspartate in chalcopyrite flotation. Miner. Eng. 2022, 180, 107464. [Google Scholar] [CrossRef]
- Zou, S.; Wang, S.; Ma, X.; Zhong, H. Underlying synergistic collection mechanism of an emerging mixed reagent scheme in chalcopyrite flotation. J. Mol. Liq. 2022, 364, 119948. [Google Scholar] [CrossRef]
- He, H.; Fang, J.; Qiu, Z.; Liu, D.; Xie, H.; Shen, P.; Peng, R.; Peng, L.; Qin, S.; Dong, S. Selective galena depression mechanism of tea polyphenol in chalcopyrite flotation. Miner. Eng. 2023, 202, 108259. [Google Scholar] [CrossRef]
- Jiang, X.; Zhang, W.; Fan, R.; Zhang, Z.; Chen, S.; Pooley, S.; Yang, L.; Gao, Z. Improved flotation of chalcopyrite from galena and pyrite by employing Cu–affinity phosphate collector. Miner. Eng. 2023, 197, 108064. [Google Scholar] [CrossRef]
- Wang, J.; Jing, G.; Zheng, R.; Huang, Z.; Sun, W.; Gao, Z. Ethylenediamine tetramethylenephosphonic acid as a selective collector for the improved separation of chalcopyrite against pyrite at low alkalinity. Int. J. Min. Sci. Technol. 2023, 33, 873–882. [Google Scholar] [CrossRef]
- Perera, T.D.; Hsia, T.; Ritchie, C.; Thang, S.H. Flotation efficiency and surface adsorption mechanism on chalcopyrite and pyrite by a novel cardanol derivative 3–pentadecylphenyl 4–(3,3–diethylthiouredo–4–oxobutanoate). Miner. Eng. 2024, 207, 108566. [Google Scholar] [CrossRef]
- He, S.; Li, X.; Huang, Y.; Wang, C.; Si, Z.; Chen, L.; Zhang, Y. A non-inhibitor flotation process for selective enrichment of chalcopyrite from molybdenite by using 5–methyl diethyl dithiocarbamate–1,3,4–oxadiazole–2–thione. Colloids Surf. A Physicochem. Eng. Asp. 2024, 694, 134141. [Google Scholar] [CrossRef]
- Liang, G.; Zhang, S.; Xian, Y.; Chen, L. Depressing molybdenite using calcium lignosulfonate in Cu-Mo flotation separation: Interaction and desorption insights. Adv. Powder Technol. 2024, 35, 104665. [Google Scholar] [CrossRef]
- Perera, T.D.; Fan, B.; Hsia, T.; Ritchie, C.; Thang, S.H. Polymers with Biobased Hydrophobic Cardanyl Acrylate and O–Ethyl Acetylcarbamothioate Functionality for Chalcopyrite Selective Flotation. ACS Appl. Polym. Mater. 2024, 6, 12719–12733. [Google Scholar] [CrossRef]
- Sun, X.; Yu, J.; Zhang, W.; Li, P.; Li, Y.; Han, Y. Flotation separation of chalcopyrite from pyrite and sphalerite using a novel collector: An experimental and mechanism investigation. Appl. Surf. Sci. 2024, 661, 160059. [Google Scholar] [CrossRef]
- Sun, X.; Yu, J.; Li, Y.; Han, Y.; Gao, P. A novel selective collector MIBATC and its performance on flotation separation of chalcopyrite from sphalerite and pyrite. Miner. Eng. 2024, 216, 108875. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, R.; Li, X.; Huang, Y.; Chen, L. A Novel Chelating Collector with Di-minerophilic Group 5–Methyl Butylxanthate–1,2,4–Triazole–3–Thione: Its Synthesis and Selective Flotation Mechanism of Chalcopyrite from Pyrite. JOM 2024, 76, 2478–2490. [Google Scholar] [CrossRef]
- Bu, Y.; Hu, Y.; Sun, W.; Gao, Z.; Liu, R. Fundamental Flotation Behaviors of Chalcopyrite and Galena using O–Isopropyl–N–Ethyl Thionocarbamate as a Collector. Minerals 2018, 8, 115. [Google Scholar] [CrossRef]
- Dai, P.; Chen, H.; Chen, L.; Liu, Y.; Wei, Z. Depression mechanism of peracetic acid for flotation separation of chalcopyrite from arsenopyrite based on coordination chemistry. Miner. Eng. 2022, 186, 107757. [Google Scholar] [CrossRef]
- Yang, B.; Liu, J.; Wang, L.; Ai, G.; Ren, S. Enhanced collection of chalcopyrite by styrene-butyl acrylate polymer nanospheres in the presence of serpentine. Colloids Surf. A Physicochem. Eng. Asp. 2022, 640, 128408. [Google Scholar] [CrossRef]
- Dong, W.; Liu, R.; Wang, C.; Zhu, X.; Xie, Z.; Sun, W. Insight into selective depression of sodium thioglycollate on arsenopyrite flotation: Adsorption mechanism and constructure. J. Mol. Liq. 2023, 377, 121480. [Google Scholar] [CrossRef]
- Gao, H.; Liu, J.; Hao, J.; Bai, X.; Liao, R. Novel synergistic mechanism of oxalic acid –CMC at the solid–liquid interface: For selective depression of talc from chalcopyrite. Surf. Interfaces 2024, 55, 105345. [Google Scholar] [CrossRef]
- Shen, Z.; Feng, Q.; Wen, S.; Wang, H. Flotation separation of chalcopyrite from galena based on selective adsorption of pyrogallic acid: Experiments and MD simulations. Appl. Surf. Sci. 2024, 667, 160398. [Google Scholar] [CrossRef]
- Shen, Z.; Feng, Q.; Wen, S.; Wang, H.; Lai, H. Mechanistic insights into flotation separation of galena and chalcopyrite with polymaleic acid as Pb-affinity depressant: Experiments and MD simulations. Sep. Purif. Technol. 2024, 347, 127607. [Google Scholar] [CrossRef]
- Yuan, J.; Li, Y.; Ding, Z.; Yu, A.; Zhang, Y.; Wen, S.; Bai, S. Influence and mechanism of new environmentally friendly depressant carboxymethyl–β–cyclodextrin on the flotation separation of chalcopyrite and pyrite. Colloids Surf. A Physicochem. 2024, 699, 134576. [Google Scholar] [CrossRef]
- Zeng, Y.; Zhang, X.; Dong, Y.; Hua, Z.; Wu, X.; Sun, W.; Wang, L.; Tang, H.; Guan, Q. Flotation behavior and mechanism of tannic acid as a depressant on Cu–Mo flotation separation. J. Ind. Eng. Chem. 2024, 131, 623–634. [Google Scholar] [CrossRef]
- Zhang, S.; Feng, Q.; Wen, S.; Xian, Y.; Liu, J.; Liang, G. Flotation separation of chalcopyrite from molybdenite with sodium thioglycolate: Mechanistic insights from experiments and MD simulations. Sep. Purif. Technol. 2024, 342, 126958. [Google Scholar] [CrossRef]
- Lin, Q.; Wang, S.; Ma, X.; Yang, J.; Zhong, H. O–Isobutyl–N–hydroxyethyl Thionocarbamate: Molecular Behavior and Flotation Mechanism to Chalcopyrite. Ind. Eng. Chem. Res. 2023, 62, 10090–10100. [Google Scholar] [CrossRef]
- He, J.; Wang, L.; Zhang, C.; Sun, W.; Yin, Z.; Zhang, H.; Chen, D.; Pei, Y. A high throughput screening model of solidophilic flotation reagents for chalcopyrite based on quantum chemistry calculations and machine learning. Miner. Eng. 2022, 177, 107375. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, J.; Li, Y.; Ding, M.; Hu, H. Adsorption behaviors of dibutyl dithiophosphate and sodium–diisobutyl dithiophosphinate (3418A) on chalcopyrite: A combined experimental and theoretical study. Appl. Surf. Sci. 2023, 636, 157810. [Google Scholar] [CrossRef]
- Cook, R.; Monyake, K.C.; Hayat, M.B.; Kumar, A.; Alagha, L. Prediction of flotation efficiency of metal sulfides using an original hybrid machine learning model. Eng. Rep. 2020, 2, e12167. [Google Scholar] [CrossRef]
- Koh, E.J.; Amini, E.; Spier, C.A.; McLachlan, G.J.; Xie, W.; Beaton, N. A mineralogy characterisation technique for copper ore in flotation pulp using deep learning machine vision with optical microscopy. Miner. Eng. 2024, 205, 108481. [Google Scholar] [CrossRef]
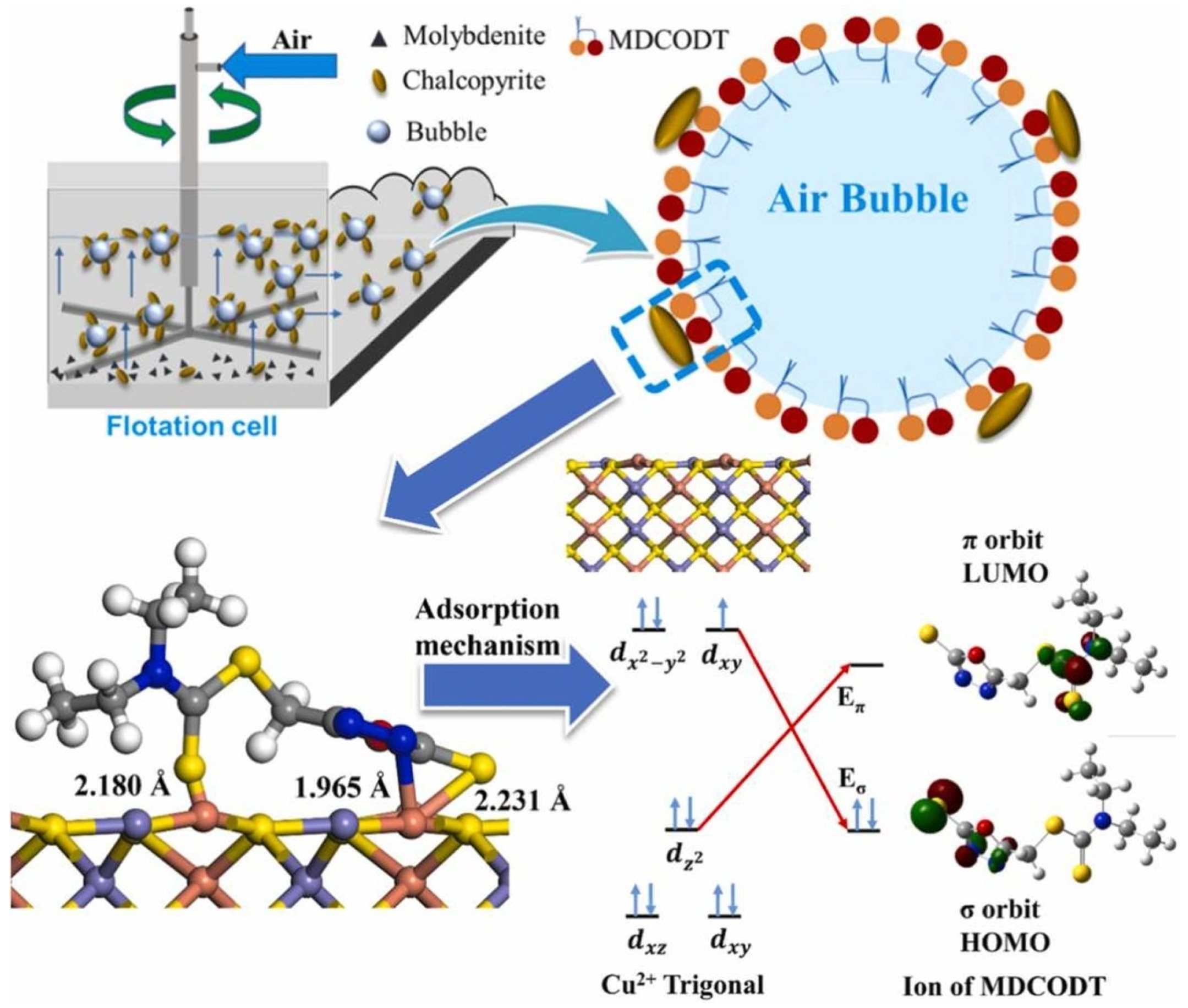
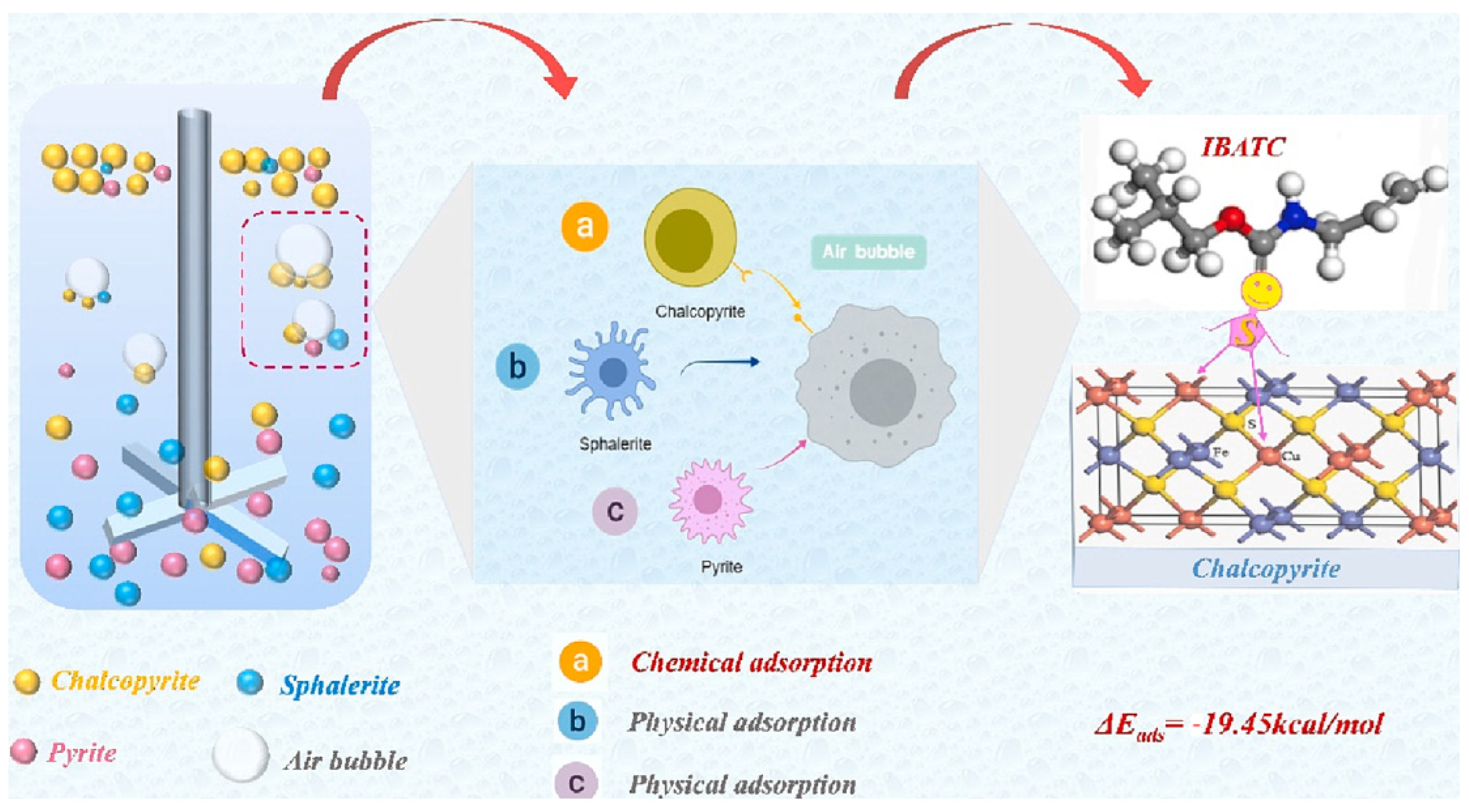
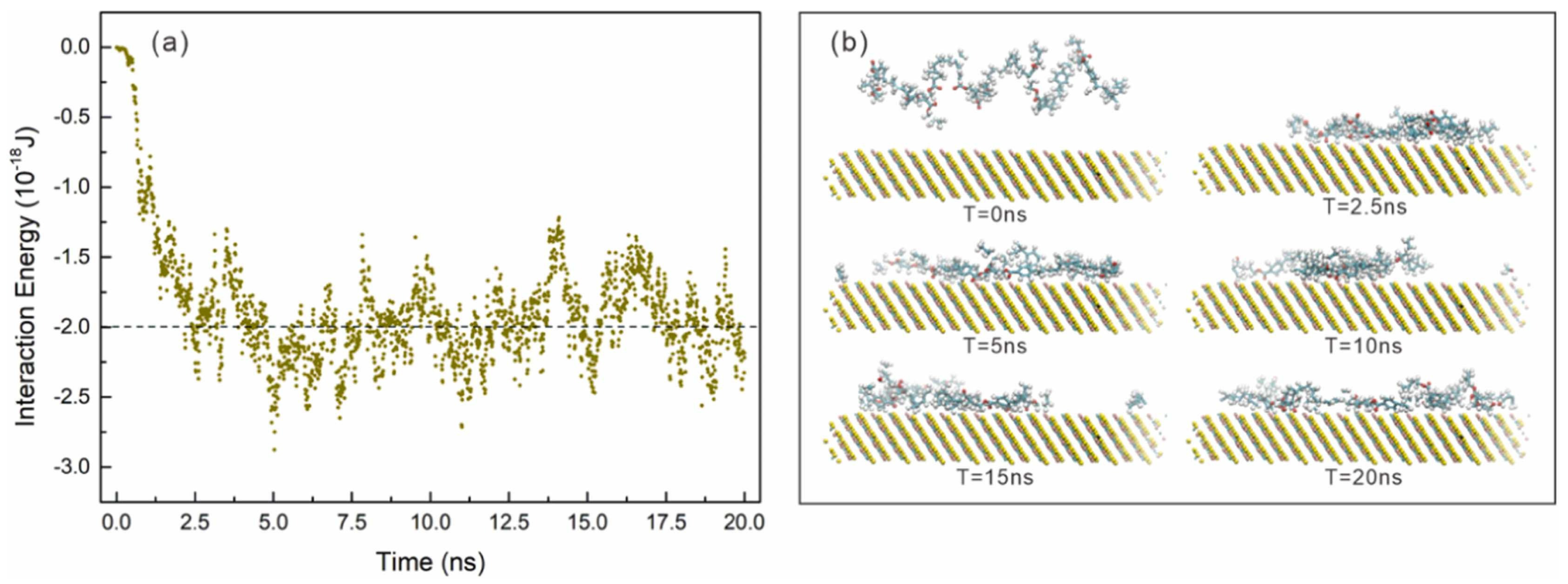
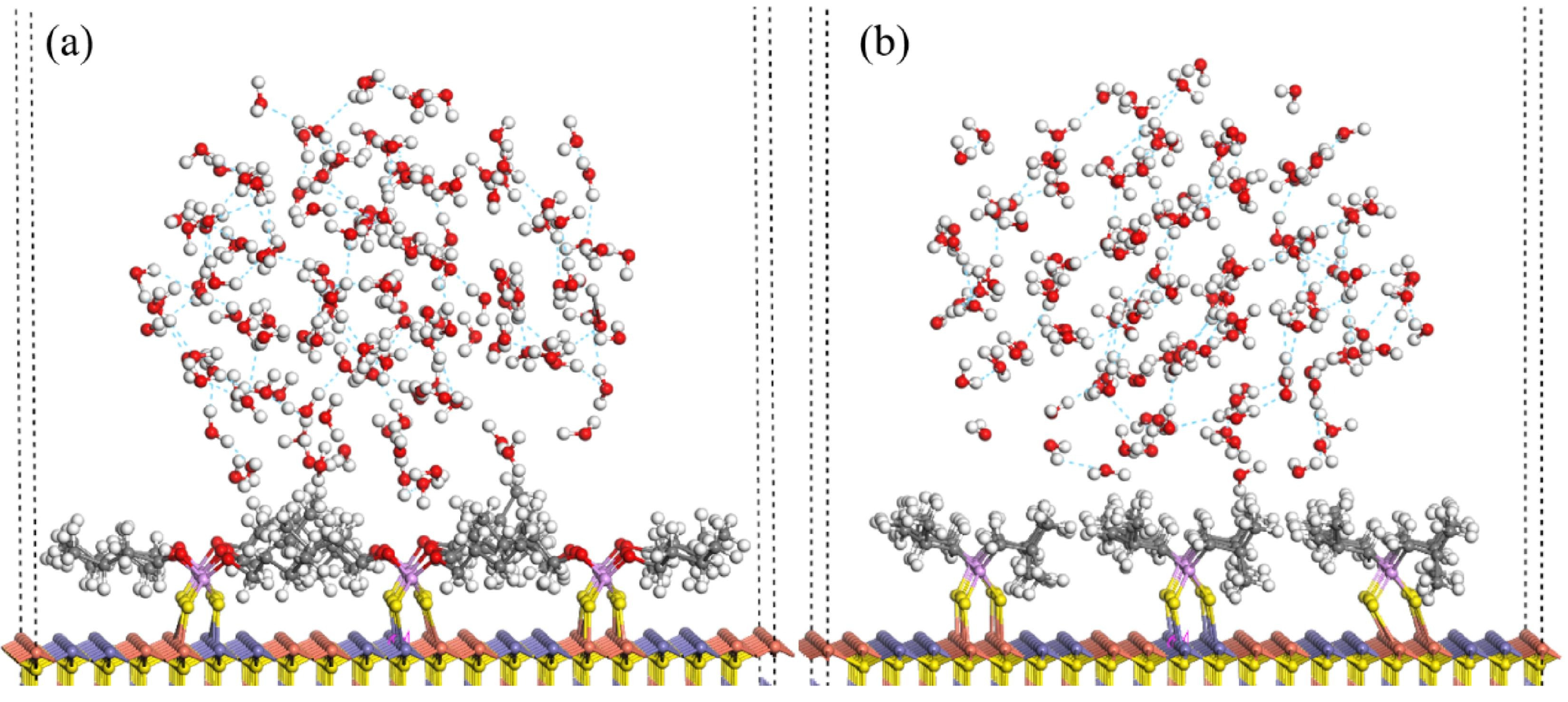

| Search Query | Number of Results |
|---|---|
| Chalcopyrite flotation and copper recovery | 81 |
| Chalcopyrite flotation and reagent effect | 104 |
| Chalcopyrite flotation and surface characteristics | 29 |
| Chalcopyrite flotation and analytical techniques | 37 |
| Chalcopyrite flotation and molecular dynamics | 19 |
| Chalcopyrite flotation machine learning | 8 |
| Total results | 278 |
| Section | Years | Number of Publications |
|---|---|---|
| First section | 1979–2015 | 15 |
| Second section | 2009–2024 | 33 |
| Third section | 2018–2024 | 17 |
| Technique Name | Abbreviation |
|---|---|
| X-ray photoelectron spectroscopy | XPS |
| Fourier transform infrared spectroscopy | FTIR |
| Ultraviolet–visible spectroscopy | UV–Vis |
| Nuclear magnetic resonance spectroscopy | NMR |
| Local electrochemical impedance spectroscopy | LEIS |
| Atomic absorption spectroscopy | AAS |
| Raman spectroscopy | - |
| Secondary ion mass spectrometry | SIMS |
| Inductively coupled plasma mass spectrometry | ICP-MS |
| Atomic force microscopy | AFM |
| Scanning electron microscopy | SEM |
| Transmission electron microscopy | TEM |
| Scanning electrochemical microscopy | SECM |
| X-ray diffractometry | XRD |
| Reagent | Methods | Results | Reference |
|---|---|---|---|
| Collector: Butyl xanthate (BX). Depressants: Lignosulfonate calcium (LSC). Frother: 2# oil. | Separation of chalcopyrite and pyrite. Particle size < 106 µm. pH 9–10 fixed with NaOH. XFG-1600 flotation machine. FTIR to analyze LSC adsorption on mineral surfaces. | LSC selectively depressed pyrite without affecting chalcopyrite flotation. Optimal separation at pH 9–10 using 150 mg/L of LSC, 0.1 mmol/L of BX, and 15 mg/L of 2# oil. The copper concentrate grade reached 24.73% with a recovery of 80.36%. FTIR analysis confirmed that LSC adsorbed on pyrite, making it hydrophilic, whereas chalcopyrite was unaffected due to prior xanthate adsorption. | [36] |
| Collector: N/A. Depressant: Carboxymethyl cellulose (CMC) polymers with varying degrees of substitution. Frother: Methyl isobutyl carbinol (MIBC). | Separation of chalcopyrite and talc. Particle size < 850 µm. pH 9 fixed with HCl/KOH. Denver flotation cell. AFM to analyze adsorption. Contact angle to assess wettability. XPS and electron microprobe to confirm purity. | LSLB polymer reduced talc recovery (54%), while chalcopyrite recovery remained high (~91%). AFM and contact angle showed that CMCs with lower substitution and random distribution exhibited greater surface adsorption, reducing talc hydrophobicity and enhancing depressant performance. | [37] |
| Collector: Sodium butyl xanthate (SBX). Depressant: Sodium glycerine–xanthate (SGX). Frother: Pine oil. | Separation of chalcopyrite and pyrite. Particle size +38–74 µm. pH 7–10. microflotation cell. Zeta potential to assess SGX adsorption. UV–Vis to quantify SBX and SGX in solution. FTIR to identify adsorbed functional groups. | SGX effectively depressed pyrite (<10% recovery at 45 mg/L) while maintaining high chalcopyrite recovery (~80–90%). Zeta potential and UV–Vis confirmed stronger SGX adsorption on pyrite. FTIR showed SGX blocked active sites, preventing SBX adsorption and enabling efficient separation. | [38] |
| Collector: N,N–diethyl–N′–cyclohexylthiourea (DECHTU). Depressant: N/A. Frother: N/A. | Separation of chalcopyrite. Particle size +38−76 µm. pH 4–8. Hallimond cell. UV–Vis to quantify DECHTU in solution and calculate adsorption. Zeta potential to detect surface charge changes. FTIR and XPS to confirm chemisorption and surface complex formation. | DECHTU enabled effective chalcopyrite flotation (up to ~90% recovery) at pH 4–8. Adsorption followed Langmuir isotherm and pseudo–second–order kinetics. Thermodynamic parameters indicated spontaneous, exothermic chemisorption. Zeta potential and FTIR confirmed anionic adsorption. XPS verified Cu(I)–DECHTU complex formation. | [39] |
| Collector: 3–hexyl–4–amino–1,2,4–triazole–5–thione (HATT). Depressant: N/A. Frother: Methyl isobutyl carbinol (MIBC). | Chalcopyrite flotation. Particle size +37−74 µm. pH 3–10. Hallimond cell. UV–Vis to quantify HATT in solution. Zeta potential to assess surface adsorption. FTIR and XPS to confirm chemisorption and surface complex formation. | HATT enabled high chalcopyrite recovery (up to 96.07% at 2 × 10−5 mol/L) across pH 3–10, outperforming SIBX (88.77%). Adsorption followed Langmuir isotherm and pseudo–second–order kinetics. Thermodynamic data indicated spontaneous, endothermic chemisorption. Zeta and FTIR confirmed HATT–Cu complex formation. | [40] |
| Collector: Sodium butyl xanthate (SBX). Depressant: N/A. Frother: Terpenic oil. | Chalcopyrite flotation. Particle size +74–500 µm. pH 10. XFG flotation cell. Pulp potential measurement to assess Eh. XPS to analyze surface composition (<20 µm) and collector adsorption. | Adding SBX before grinding yielded higher recovery (93.62% vs. 90.03% at 2 × 10⁻³ mol/L) and higher pulp potential. XPS revealed higher collector adsorption (C: 20.11%), more free oxygen (O: 51.04%), and less oxidized Fe and Cu species in fine particles, improving flotation of <20 µm fraction. | [41] |
| Collector: O–isopropyl–S–[2–(hydroxyimino)propyl] dithiocarbonate ester (IPXPO). Depressant: N/A. Frother: Methyl isobutyl carbinol (MIBC). | Separation of chalcopyrite and pyrite. Particle size +38–76 µm. pH 4–9. Hallimond cell. UV–Vis to calculate adsorption. Zeta potential to assess surface interactions. In situ SECM to visualize surface coverage. FTIR to identify functional groups adsorbed. | IPXPO enabled high chalcopyrite recovery (~95%) and low pyrite recovery (~20%) at pH 4–9. Adsorption followed Langmuir isotherm and pseudo–second–order kinetics. Zeta, SECM and FTIR confirmed chemisorption of IPXPO, forming Cu–S, Cu–N and Cu–O bonds on the surface. | [42] |
| Collector: Nanoparticle emulsion. Depressant: N/A. Frother: N/A. | Separation of chalcopyrite, pyrite and quartz. Particle size < 23 µm. pH 2–10. Microflotation cell. FTIR to confirm chemical adsorption. Zeta potential to evaluate surface charge changes. SEM to observe morphology and adsorption. | HNP enabled high chalcopyrite recovery (up to 96.32% with 1 mL) at pH 6, selectively over pyrite (66.44%) and quartz (25.88%). FTIR confirmed chemical bonding between HNP and chalcopyrite. Zeta potential indicated anionic collector behavior. SEM showed strong adsorption on chalcopyrite and limited interaction with other minerals. | [43] |
| Collector: Butyl xanthate (BX). Depressant: Zinc sulfate (ZnSO4) and sodium dimethyl dithiocarbamate (SDD). Frother: N/A | Separation of chalcopyrite and sphalerite. Particle size +40–75 µm. pH 4–12. XFG microflotation cell. Zeta potential to evaluate adsorption. LEIS to map local electrochemical impedance and confirm surface coverage. | Optimal separation at pH 10 using 10−4 mol/L ZnSO4+SDD (3:1) and 10−5 mol/L BX. A concentrate containing 30.21% Cu with 86.79% recovery was obtained. Zn content was 4.20%, with a recovery of 5.48%. Zeta potential and LEIS confirmed that ZnSO4+SDD significantly prevented BX adsorption on sphalerite while allowing it on chalcopyrite. | [44] |
| Collector: Ammonium dibutyl dithiophosphate (ADD). Depressant: Sodium alginate (NaAl). Frother: Not specified. | Separation of chalcopyrite and galena. Particle size +74–106 µm. pH 7–12. XFG microflotation cell. Zeta potential to assess surface interactions. FTIR to identify adsorbed functional groups. XPS for surface analysis and adsorption confirmation. | NaAl reduced galena recovery (<10% at 15 mg/L), while maintaining chalcopyrite recovery (>80% across pH 7–12). In 1:1 mixed feed, NaAl+ADD yielded a concentrate with 65.69% chalcopyrite and 82.34% recovery. Zeta and FTIR confirmed strong NaAl chemisorption on galena, but not on chalcopyrite. XPS verified COO– groups and Pb signal changes, indicating selective surface coverage. | [45] |
| Collector: Butyl xanthate (BX). Depressant: Seaweed glue (SEG). Frother: Methyl isobutyl carbinol (MIBC). | Separation of chalcopyrite and galena. Particle size +38–74 µm. pH 8. Microflotation cell. Contact angle to assess wettability. UV–Vis to quantify adsorption. Zeta potential for surface interaction. FTIR to identify adsorbed functional groups. | SEG reduced galena recovery from 94.89% to 7.55% at 15 mg/L, while chalcopyrite recovery remained above 75%. In mixed ore (8.29% Cu), a concentrate with 23.68% Cu and 81.52% recovery was obtained. Analytical results confirmed strong chemisorption of SEG on galena and weak physisorption on chalcopyrite, enabling selective depression. | [46] |
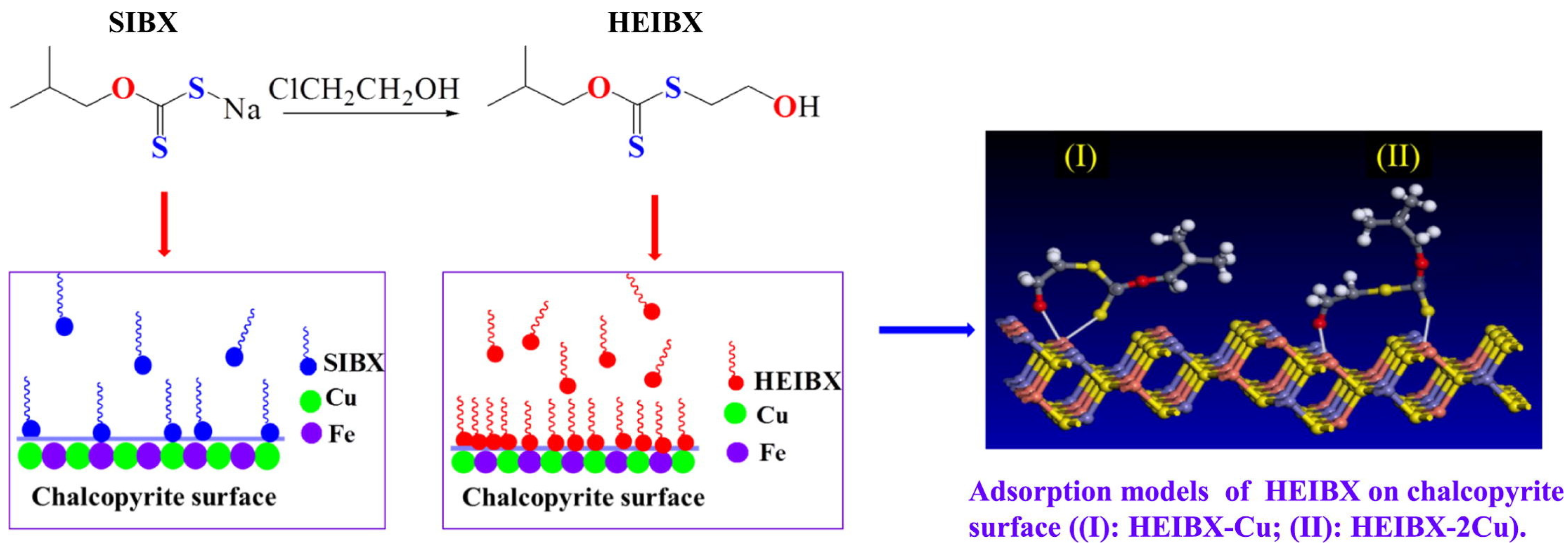
| Collector: S–hydroxyethyl–O–isobutyl xanthate (HEIBX) compared with Sodium isobutyl xanthate (SIBX). Depressant: N/A. Frother: Methyl isobutyl carbinol (MIBC). | Separation of chalcopyrite and pyrite. Particle size +38−76 µm. pH 8. Microflotation cell. DFT to analyze molecular reactivity. UV–Vis to assess metal–ion interaction. Contact angle for wettability. Zeta potential, FTIR and XPS to confirm adsorption mechanisms. | HEIBX achieved 93.94% chalcopyrite recovery and 24.39% pyrite recovery (at 4 × 10−5 mol/L). DFT and UV–Vis confirmed selective interaction of C=S and OH groups with Cu⁺/Cu2+ forming Cu–O and Cu–S bonds. Zeta, FTIR and XPS showed strong chemisorption on chalcopyrite and weak interaction with pyrite, enabling efficient separation. | [47] |
| Collector: Thiohexanamide (THA) compared with O–isopropyl–N–ethyl thionocarbamate (IPETC) and sodium isobutyl xanthate (SIBX). Depressants: N/A. Frother: Methyl isobutyl carbinol (MIBC). | Separation of chalcopyrite, pyrite and galena. Particle size +38–76 µm. pH 8. Microflotation cell and bench-scale tests. UV–Vis to assess metal ion interaction. Zeta potential, FTIR and XPS to confirm adsorption mechanisms. DFT to explore electronic interactions with Cu. | THA achieved 97.1% chalcopyrite recovery (at 5 × 10−5 mol/L), while pyrite and galena were 34.8% and 3.5%, respectively. In mixed ore, the concentrate had 13.05% Cu and 94.22% recovery. UV–Vis confirmed selective THA–Cu⁺ interaction. Zeta, FTIR and XPS showed strong chemisorption on chalcopyrite and weak interaction with pyrite and galena. DFT supported Cu–THA complex formation via Cu–S and Cu–N bonding. | [48] |
| Collector: Sodium amyl xanthate (SAX). Depressant: Sodium alginate (NaAl). Frother: Methyl isobutyl carbinol (MIBC). | Separation of chalcopyrite, chlorite and serpentine. Particle size +38–75 µm. pH 9. Microflotation and batch cell. TOC to measure NaAl adsorption density. Zeta potential to track surface charge shifts. FTIR to confirm chemical adsorption. | NaAl reduced chlorite (60% to <5%) and serpentine (36% to <5%) recovery while maintaining chalcopyrite (~90%). In ternary mix, concentrate reached 31% Cu and 90% recovery. TOC showed selective adsorption on gangues (0.14–0.24 mg/m2), negligible on chalcopyrite (<0.02 mg/m2). FTIR and zeta confirmed selective chemisorption. | [49] |
| Collector: Potassium isobutyl xanthate (KIBX). Depressant: O–carboxymethyl chitosan (O–CMC). Frother: Methyl isobutyl carbinol (MIBC). | Separation of chalcopyrite and molybdenite. Particle size +74–150 µm. pH 3–11. Hallimond cell. AFM to observe surface adsorption of O–CMC on treated minerals. | O–CMC (150 ppm) strongly depressed molybdenite (<12% recovery) across pH 3–11, while chalcopyrite flotation remained largely unaffected (>93% recovery). AFM confirmed irreversible adsorption on molybdenite and negligible adsorption on chalcopyrite, enabling selective separation. | [50] |
| Collector: Sodium ethyl xanthate (SEX). Depressant: Native wheat starch (NWS) and oxidized starch (Ox 5/120). Frother: polyglycol ether mix (FZS180). | Separation of chalcopyrite and graphite. Particle size P80 = 212 µm. pH 7.5. Flotation cell. Adsorption to measure surface density. AFM to visualize polymer layer morphology on graphite. | Ox 5/120 (8 mg/L) depressed graphite (<42%) with limited effect on chalcopyrite (~79%). At higher dosage (20 mg/L), graphite recovery dropped more than chalcopyrite, indicating strong selectivity. AFM showed Ox 5/120 formed a well-structured network on graphite, increasing wettability. Lower adsorption on chalcopyrite (1.70 mg/m2) vs NWS (5.54 mg/m2) explained reduced depression. | [51] |
| Collector: Sodium isopropyl xanthate (SIPX), potassium amyl xanthate (PAX), thionocarbamate (TC). Depressant: Modified and commercial lignosulfonates (KLS, CLS). Frother: Methyl isobutyl carbinol (MIBC). | Separation of chalcopyrite and molybdenite. Particle size +75–150 and +38–75 µm, respectively. pH 6–9. Partridge–Smith cell. FTIR to identify functional groups. ICP–OES to measure Ca, Na. Electrophoretic mobility to assess surface interaction. | Lignosulfonates strongly depressed molybdenite, while PAX preserved chalcopyrite flotation (>90% recovery). SIPX led to strong depression of chalcopyrite. FTIR and mobility data confirmed interaction with surface Ca2+ sites on molybdenite. PAX hindered lignosulfonate adsorption on chalcopyrite. KLS were as effective as CLS. | [52] |
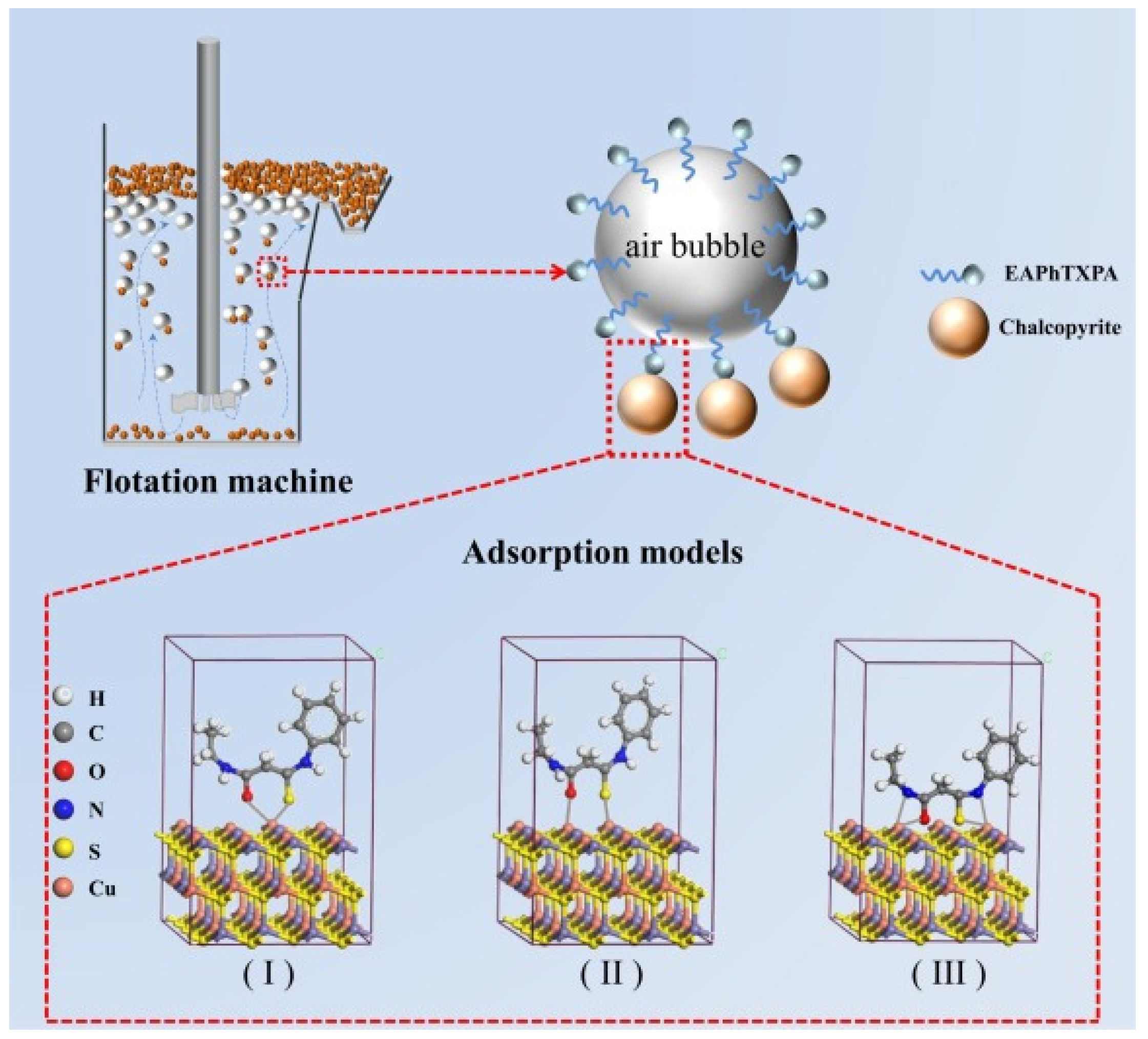
| Collector: 3–ethylamino–N–phenyl–3–thioxopropanamide (EAPhTXPA). Depressant: N/A. Frother: N/A. | Chalcopyrite flotation. Particle size +38–76 µm. pH 3–10. XFGCII flotation cell. Contact angle to assess hydrophobicity. UV–Vis to quantify residual collector. DFT for electronic reactivity. XPS to confirm Cu–S, Cu–O and Cu–N bonding. | EAPhTXPA achieved chalcopyrite recovery up to 97.5% (pH 8, 4 × 10−5 mol/L). Highest contact angle (97°), showing stronger collecting ability than EAMTXPA and PhATXPA. Adsorption was spontaneous, endothermic and chemisorptive. DFT identified C=S, C=O and NH as active sites. XPS confirmed Cu–EAPhTXPA complex formation. | [53] |
| Collector: 6–hexyl–1,2,4,5–tetrazinane–3–thione) (HTT) vs sodium hexyl xanthate (SHX). Depressant: N/A. Frother: Methyl isobutyl carbinol (MIBC). | Separation of chalcopyrite and pyrite. Particle size +38–76 µm. pH 6–11.5. Hallimond cell. AFM to observe surface coverage. Contact angle for hydrophobicity. UV–Vis for interaction with metal ions. DFT for electronic reactivity. Zeta potential for surface charge. XPS to confirm bonding. | HTT achieved selective flotation, with chalcopyrite (97.2%) and pyrite (22.4%) at pH 10.5 (2 × 10−5 mol/L). Contact angle rose to ~95° (vs. ~88.5° for SHX). AFM showed dense HTT adsorption. UV–Vis confirmed HTT prefers Cu(I/II) over Fe(II/III). DFT indicated higher electron-accepting ability. XPS confirmed Cu–S and Cu–N bonding on surface. | [54] |
| Collector: N–benzoyl–N’,N’–diethyl thiourea (BDETU) compared with O–isopropyl–N–etil tionocarbamato (IPETC). Depressant: N/A. Frother: Methyl isobutyl carbinol (MIBC). | Separation of chalcopyrite and pyrite. Particle size +38–76 µm. pH 2–11. Hallimond cell. Contact angle for hydrophobicity. UV–Vis for interaction with metal ions. FTIR to identify bonding groups. DFT to analyze active sites and binding energy. | BDETU achieved 98.0% chalcopyrite recovery (at pH 8, 2 × 10−5 mol/L), while pyrite recovery remained low (20.65%). Contact angle increased to 90.5° on chalcopyrite with minimal change on pyrite. UV–Vis and FTIR confirmed selective interaction via C=O and C=S groups, forming C–O–metal and C–S–metal bonds. DFT indicated six–membered chelate ring formation, enhancing adsorption. | [55] |
| Collector: 5–methyl isobutylxanthate–1,3,4–oxadiazole–2–thione (MIXODT) vs. Sodium Isobutyl Xanthate (SIBX). Depressant: N/A. Frother: Methyl isobutyl carbinol (MIBC). | Flotation of chalcopyrite. Particle size: +38–76 µm. pH 3–11. Microflotation cell. Contact angle for hydrophobicity. Zeta potential to assess surface adsorption. UV–Vis to detect interaction with metal ions. FTIR and XPS to confirm bonding. DFT to predict adsorption structures and binding energy. | MIXODT achieved 95% chalcopyrite recovery (1 × 10−5 mol/L, pH 3–9), outperforming 77% SIBX. Contact angle >80°, indicating enhanced hydrophobicity. Zeta potential showed anionic adsorption. UV–Vis, FTIR and XPS confirmed surface complex formation with Cu(I) via Cu–S and Cu–N bonds. DFT supported stable chemisorption models. | [56] |
| Collector: Ammonium dibutyl dithiophosphate (ADD). Depressant: Sodium polyaspartate (PASP). Frother: Methyl isobutyl carbinol (MIBC). | Separation of chalcopyrite and galena. Particle size +38–74 µm. pH 10. XFGCII cell. TOC to quantify PASP adsorption. UV–Vis to measure ADD adsorption. FTIR and XPS to analyze functional groups and surface bonding. | PASP effectively depressed galena (1.1% recovery), maintaining high chalcopyrite recovery (91.9%) with 10 mg/L PASP. TOC showed higher PASP adsorption on galena. UV–Vis confirmed that PASP reduced ADD adsorption on galena. FTIR and XPS verified chemical adsorption of PASP on both minerals, with stronger interaction on galena. | [57] |
| Collector: O–isopropyl–N–ethyl thionocarbamate (IPETC) + BEAT (O,O′–bis(2–butoxyethyl) ammonium dithiophosphate). Depressant: N/A. Frother: Methyl isobutyl carbinol (MIBC). | Separation of chalcopyrite and pyrite. Particle size +38–74 µm. pH 7.0–7.5. XFG cell. Foaming tests to assess stability. Contact angle for wettability. Surface tension to evaluate solubility. FTIR to analyze adsorption on chalcopyrite. Intermolecular interaction parameter (β) calculation. | The IPETC/BEAT mixture (60% molar BEAT) achieved highest chalcopyrite recovery (92.43%) and low pyrite recovery (~14.2%) at 4 × 10−5 mol/L. Contact angle on chalcopyrite rose to 117.7°, indicating improved hydrophobicity. Lower surface tension and CMC values suggested enhanced solubility. FTIR confirmed co–adsorption on chalcopyrite. Negative β (−3.05) confirmed synergistic effect. | [58] |
| Collector: Sodium isoamyl xanthate (SIX). Depressant: Tea polyphenols (TP). Frother: Methyl isobutyl carbinol (MIBC). | Separation of chalcopyrite and galena. Particle size: 45–75 µm. pH 8. XFGCII cell. Contact angle for hydrophobicity. Zeta potential to study surface interactions. UV–Vis to quantify unadsorbed collector. ToF–SIMS to detect adsorbed ionic species. XPS to analyze surface chemical changes. | TP reduced galena recovery to 6.01%, while chalcopyrite recovery remained at 85.63% (TP 4 × 10−4 mol/L, SIX 2 × 10−4 mol/L). UV–Vis showed that TP inhibited SIX adsorption on galena. Contact angle and zeta results confirmed stronger hydrophobicity on chalcopyrite and preferential TP adsorption on galena. XPS and ToF-SIMS revealed Pb–TP complex formation on galena, blocking active sites. | [59] |
| Collector: Dibutyl phosphonate (HDBP). Depressant: N/A. Frother: Terpineol. | Separation of chalcopyrite, galena and pyrite. Particle size +37–74 µm. pH 6–8. XFG flotation cell. XPS to analyze elemental composition and surface bonding. CV and Tafel for electrochemical reactivity. DFT to simulate adsorption energy and bonding on mineral surfaces. | HDBP enabled selective flotation of chalcopyrite (>85% recovery at 5 × 10−5 mol/L) over galena (≤19%) and pyrite (≤8%). XPS showed P–O and P=O bonding only on chalcopyrite. Electrochemical tests confirmed strong reactivity with chalcopyrite but minimal changes for galena and pyrite. DFT showed highest adsorption energy on chalcopyrite, forming stable Cu–O–P and Cu–O=P chelation bonds. | [60] |
| Collector: Ethylenediamine tetramethylenephosphonic acid (EDTMPA). Depressant: N/A. Frother: Terpineol. | Separation of chalcopyrite and pyrite. Particle size +35−74 µm. pH 6–11. XFG cell. XPS to analyze surface bonding. FTIR to identify adsorbed functional groups. CV and Tafel for electrochemical reactivity. DFT and crystal chemistry to evaluate adsorption energy and surface affinity. | EDTMPA achieved high chalcopyrite recovery (~88.36%) and low pyrite recovery (~13.93%) at pH 9.0 (1 × 10⁻⁴ mol/L). XPS and FTIR showed strong binding of P=O and P–O groups to Cu/Fe sites on chalcopyrite. DFT revealed higher adsorption energy on chalcopyrite. Crystal chemistry confirmed greater metal atom density and valence. | [61] |
| Collector: 3–pentadecylphenyl 4–(3,3–diethylthiouredo–4–oxobutanoate) (cardanol derivative DP089). Depressant: N/A. Frother: Methyl isobutyl carbinol (MIBC). | Separation of chalcopyrite and pyrite. Particle size +53–104 µm. pH 8. Denver cell. UV–Vis to assess metal ions affinity. FTIR to identify bonding. Contact angle for hydrophobicity. Adsorption isotherms for kinetic modeling. | DP089 showed strong selectivity for chalcopyrite (92.1% recovery) and low recovery of pyrite (11.2%) at pH 8. In mixed mineral flotation, 87.3% chalcopyrite recovery and 12.7% pyrite recovery were achieved. UV–Vis confirmed selective interaction with Cu⁺. FTIR revealed formation of C–O–Cu and C–S–Cu bonds. Contact angle increased to 97.3° in chalcopyrite, confirming enhanced hydrophobicity. | [62] |
| Collector: 5–methyl diethyl dithiocarbamate–1,3,4–oxadiazole–2–thione (MDCODT). Depressant: N/A. Frother: Not specified. | Separation of chalcopyrite and molybdenite. pH 9. XFG–II flotation cell. Zeta potential to assess surface charge. UV–Vis to analyze adsorption behavior. Contact angle for hydrophobicity. AFM to examine surface morphology. FTIR and XPS to confirm bonding. Electrochemistry and DFT to evaluate interaction and adsorption mechanism. | MDCODT achieved selective flotation of chalcopyrite (~95% recovery) and low molybdenite recovery (<20%) at 1 × 10⁻⁵ mol/L and pH 9. Zeta potential and contact angle (76°) confirmed selective adsorption. UV–Vis, FTIR and XPS verified Cu–S and Cu–N bonding. AFM and electrochemical tests showed dense surface adsorption. DFT supported stable chemisorption via Cu complexation. | [63] |
| Collector: Potassium ethyl xanthate (KEX). Depressant: Calcium lignosulfonate (CLS). Frother: Methyl isobutyl carbinol (MIBC). | Separation of chalcopyrite and molybdenite. Particle size <38 µm. pH 8. XFG cell. FTIR for chemical adsorption. XPS to identify surface bonding. UV–Vis to quantify CLS adsorption/desorption. | CLS depressed molybdenite flotation (~18% recovery at 50 mg/L) with minimal effect on chalcopyrite (~80%). In mixed minerals, Cu recovery was 84% and Mo 35%, with grades of 29.56% Cu and 5.17% Mo. FTIR and XPS confirmed chemisorption on chalcopyrite and hydrophobic interaction on molybdenite. UV–Vis showed higher CLS desorption from chalcopyrite than from molybdenite. | [64] |
| Collector: Poly(CA4–co–ACOEA14 (RAFT) with O–ethyl acetylcarbamothioate functionality. Depressant: N/A. Frother: N/A. | Separation and flocculation of chalcopyrite and pyrite. Particle size: 53–104 µm (flotation). pH 8. Contact angle to assess hydrophobicity. UV–Vis for adsorption quantification. FTIR and XPS to confirm chemical interaction. | In mixed mineral, poly(CA4–co–ACOEA14) achieved 82.3% chalcopyrite and 17.6% pyrite recovery (1 × 10−5 mol/L). FTIR and XPS confirmed selective chemisorption on chalcopyrite. Higher contact angle indicated greater hydrophobicity. | [65] |
| Collector: O–isobutyl–N–allyl thionocarbamate (IBATC). Depressant: N/A. Frother: Not specified. | Separation of chalcopyrite from pyrite and sphalerite. Particle size −38 µm. pH 9.5–10. XFG cell. FTIR and XPS to confirm chemisorption. UV–Vis to quantify adsorption. Zeta potential for surface interactions. DFT to calculate adsorption energy. | IBATC achieved high chalcopyrite recovery (>80%) at pH 9.5–10, with low sphalerite (<40%) and pyrite (~20%) recovery. Adsorption was 81–128% higher on chalcopyrite. FTIR and XPS confirmed chemisorption of sulfur atom onto Cu. | [66] |
| Collector: O–methylisobutyl-N–allyl thionocarbamate (MIBATC). Depressant: N/A. Frother: Terpineol. | Separation of chalcopyrite, sphalerite and pyrite. Particle size: −38 μm. pH 2–12. XFG flotation cell. FTIR and XPS to analyze adsorption and active sites. Zeta potential for surface change. UV–Vis to determine adsorbed amount. DFT to calculate adsorption energy and orbital interaction. | Chalcopyrite recovery > 80% at pH 9.5–10 with 30–40 mg/L MIBATC. Adsorption on chalcopyrite was 167% and 125% higher than on sphalerite and pyrite. Zeta potential showed positive shift. FTIR and XPS confirmed chemisorption via Cu–S bond. DFT indicated spontaneous, exothermic reaction. | [67] |
| Collector: 5–methyl butylxanthate–1,2,4–triazole–3–thione (MBuXTT). Depressant: N/A.Frother: N/A. | Separation of chalcopyrite and pyrite. Particle size +38–74 µm. XFG-II flotation cell. Contact angle to assess hydrophobicity. UV–Vis to study interaction with metal ions. Zeta potential to assess surface affinity. FTIR and XPS to identify adsorbed groups. DFT to confirm bonding behavior. | MBuXTT achieved 96% chalcopyrite recovery at pH 7 with 3 × 10−5 mol/L, with 23% pyrite recovery. Contact angle reached 89.5° (vs 83.5° for SBX). Zeta potential indicated higher chalcopyrite affinity. FTIR and XPS confirmed Cu–S and Cu–N bonding. DFT supported stable surface complex formation. | [68] |
| Software Name | Reference |
|---|---|
| Materials Studio 2020 | [69,70,71,72,73,74,75,76,77,78] |
| LAMMPS 2020 | [71] |
| Gaussian 16 | [22,79,80] |
| DBTF+ | [81] |
| CP2K 2020 | [78] |
| Reagent | Methods | Results | Reference |
|---|---|---|---|
| Collector: O-isopropyl-N-ethyl thionocarbamate (IPETC). Depressant: N/A. Frother: N/A. | Separation of chalcopyrite and galena. MDS in Materials Studio. PCFF force field. Chalcopyrite (112) and galena (110) surface. Geometry optimized with CASTEP and DMol3. Adsorption energy via Discover. NVT at 298.15 K, 2 ns, Nose thermostat. Ewald summation for interactions. Flotation −74 µm, pH 4–13, XFD cell. FTIR, UV–Vis to evaluate adsorption. | MDS showed stronger interaction of IPETC with galena (−47.39 kJ/mol) than with chalcopyrite (−29.73 kJ/mol). However, IPETC displaced H2O and OH⁻ on chalcopyrite, allowing competitive adsorption. At pH 9.5 and 7 × 10−4 mol/L IPETC, chalcopyrite recovery reached 83.6%, while galena was 61.8%, achieving effective separation. FTIR confirmed adsorption of –C=N and –CH groups on both minerals at pH 9.5. | [69] |
| Collector: Butyl xanthate (BX). Depressant: Peracetic acid (PAA). Frother: Not specified. | Separation of chalcopyrite and arsenopyrite. MDS in Materials Studio (Forcite). Universal force field. NVT at 298 K for 100 ps with Nose thermostat. Chalcopyrite (112) and arsenopyrite (001) surfaces modeled as 4 × 3 × 1 supercells with 15 Å vacuum. Adsorption energy and electronic interaction via DFT using CASTEP, GGA/PW91 functional, 400 eV cutoff, k–points grids 2 × 2 × 1 and 3 × 4 × 1. Verified with XPS. | PAA adsorbed more strongly on arsenopyrite (−550.5 kJ/mol) than chalcopyrite (−60.3 and −36.2 kJ/mol for Cu and Fe). MDS showed diffusion on arsenopyrite and aggregation on chalcopyrite, confirming preference. DOS and Mulliken analysis revealed O–Fe hybridization and spin polarization. XPS confirmed Fe(II) oxidation to Fe(III), preventing BX adsorption on arsenopyrite. | [70] |
| Collector: Styrene–butyl acrylate (St-Ba). Depressant: N/A. Frother: Methyl isobutyl carbinol (MIBC). | Separation of chalcopyrite and serpentine. MDS in LAMMPS. CVFF force field. Chalcopyrite (112) surface and St–Ba polymer model (5 monomers). Periodic box, NVT ensemble at 300 K, 0.1 MPa, Lennard–Jones potential, 1 fs timestep, 1000 outputs every 20 ps. Microflotation at −38 µm, pH 7, XFGC II cell. Contact angle, FTIR, SEM to evaluate adsorption. | St–Ba–chalcopyrite interaction energy was −1.99 × 10−18 J, indicating spontaneous adsorption. St–Ba adhered and spread on chalcopyrite at 2.5 ns. Chalcopyrite recovery increased from 89% (SBX) to 95% (St–Ba) for single mineral, and from 42% to 84% in mixture. No St–Ba adsorption on serpentine. Contact angle > 70° for chalcopyrite with St–Ba. FTIR and SEM confirmed effective adsorption. | [71] |
| Collector: Butyl xanthate (BX). Depressant: Sodium thioglycollate (STG). Frother: Methyl isobutyl carbinol (MIBC). | Separation of chalcopyrite and arsenopyrite. MDS with Materials Studio (Forcite). Universal force field. NVT at 298 K, 500 ps, 1 fs timestep. DFT using CASTEP, GGA/PW91 functional, 400 eV cutoff, k-points 2 × 2 × 1. Arsenopyrite (001) surface. Flotation at −74 + 38 µm, pH 8, 1 × 10−5 mol/L BX, 20 mg/L MIBC. LEIS, contact angle, FTIR, UV–Vis for adsorption analysis. | STG formed S–Fe and S–As bonds (up to −223.34 kJ/mol) and hydrogen bonds with water via –COO⁻. MDS confirmed stable adsorption (−212.55 kJ/mol). STG sharply reduced arsenopyrite recovery to ~5% while chalcopyrite remained ~90% at optimal dosage (1.25 × 10−4 mol/L). LEIS and FTIR validated selective adsorption. Contact angle difference (15.12°) confirmed hydrophilic film formation on arsenopyrite. | [72] |
| Collector: O–isobutil–N–hidroxietil tionocarbamato (IBHETC). Depressant: N/A. Frother: Methyl isobutyl carbinol (MIBC). | Separation of chalcopyrite and pyrite. IBHETC was synthesized and confirmed by GC, NMR, MS, FTIR. MDS were performed to study its interaction with Cu⁺. Initial structures were generated with GFN0–xTB (xtb 6.4.1), selected using Molclus, optimized by DFT in Gaussian 16, B3LYP-D3(BJ) functional, SDD basis for Cu, 6–311+G(d,p) for other, with IEF–PCM solvation. Flotation at −74 + 38 µm, pH 8, XFG–II cell. Adsorption analysis by FTIR, XPS. | MDS revealed that IBHETC forms a Cu⁺ complex via N–Cu (2.18 Å), O–Cu (2.13 Å), and S–Cu (2.31 Å) bonds, stabilized by four and five membered rings. The molecule undergoes deprotonation and isomerization, enhancing selectivity. Chalcopyrite recovery was 94.41% (vs. 81.79% with IBETC), while pyrite recovery was 12.63%. FTIR and XPS confirmed selective chemisorption on chalcopyrite. | [79] |
| Collector: Dibutyl dithiophosphate (DTP) and sodium diisobutyl dithiophosphinate (3418A). Depressant: Not specified. Frother: Not specified. | Flotation of chalcopyrite. Microflotation at −74 + 38 µm, pH 3–11, XFG5–35 cell. Microcalorimetry at 26 °C, 1×10⁻⁴ mol/L. UV–Vis for adsorption. CV for redox behavior. DFT using CASTEP, GGA–PW91 functional, 400 eV cutoff, 3 × 4 × 1 k–points. MDS with DFTB+. NVT at 298 K, 0.6 ps, 1 fs timestep. | 3418A showed higher chalcopyrite recovery (>80%) and greater adsorption than DTP. Adsorption heats of 3418A were higher. MD showed stronger hydrophobicity (RDF peak 8.2 Å vs. 6.5 Å). DFT revealed higher adsorption energy (−311.4 vs. −170.9 kJ/mol), shorter Cu–S bonds, and stronger Mulliken populations. HOMO of 3418A (−4.098 eV) closer to chalcopyrite LUMO (−3.8 eV). | [81] |
| Collector: Sodium butyl xanthate (SBX). Depressants: Oxalic acid (OA) and Carboxymethyl cellulose (CMC). Frother: Methyl isobutyl carbinol (MIBC). | Separation of chalcopyrite and talc. Microflotation at −74 + 38 µm, pH 9, XFG–II cell. Bench–scale flotation (Cu 0.60%). Contact angle, Zeta potential, AFM, SEM–EDS, FTIR, TOC used to analyze surface properties and surface interactions. MDS in Materials Studio (CASTEP, Amorphous Cell, Forcite). Compass II force field. NVT at 298 K, 200 ps. Talc (001) surface. | OA+CMC (60 + 60 mg/L) achieved 86.54% chalcopyrite and 10.16% talc recovery (pH 9). Contact angle and zeta potential showed enhanced surface hydrophobicity difference. MDS revealed OA enables multilayer CMC adsorption on talc, introducing hydrophilic groups. In Bench–scale test, OA+CMC (30 + 30 g/t) gave Cu recovery of 85.85% with MgO recovery of 10.23%. | [73] |
| Collector: Sodium butyl xanthate (SBX). Depressant: Pyrogallic acid (PA). Frother: Terpineol. | Separation of chalcopyrite and galena. Microflotation at −200 + 75 µm, pH 9, synthetic resin cell. Contact angle, FTIR, TOC, UV–Vis, ToF–SIMS and XPS to assess adsorption and surface properties. MDS in Materials Studio (Forcite). Universal force field. NVT at 298 K, 1 fs, 1000 ps. Galena (100) surface. | PA (2 × 10−4 mol/L) enabled 83.56% chalcopyrite and only 11.22% galena recovery. MDS showed PA’s affinity to galena, promoting H2O accumulation and reducing hydrophobicity. Contact angle, FTIR, TOC, XPS and ToF–SIMS confirmed strong PA adsorption on galena, suppressing SBX adsorption. | [74] |
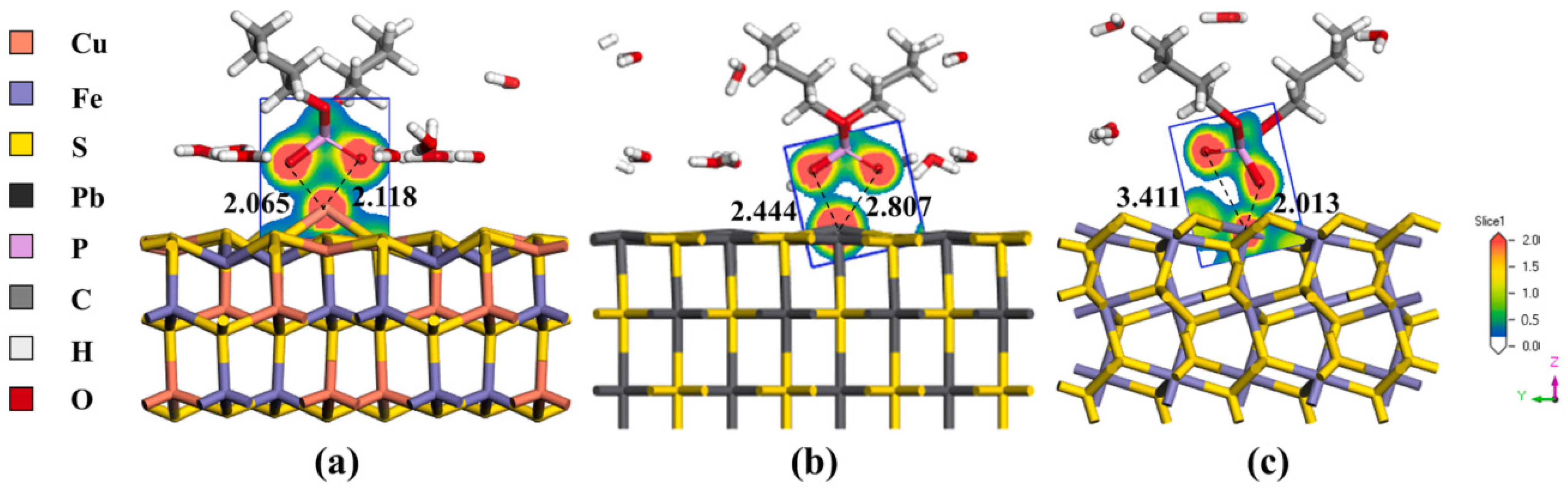
| Collector: Sodium butyl xanthate (SBX). Depressant: Mercaptosuccinic acid (MSA). Frother: Terpineol. | Separation of chalcopyrite and galena. Microflotation at pH 9. Contact angle to assess wettability. FTIR, UV–Vis, TOC, XPS, AFM to analyze adsorption and surface properties. MDS in Materials Studio (CASTEP, Amorphous Cell, Forcite). Universal force field. NVT at 298 K, 1000 ps. Galena (100) surface. | MSA reduced galena recovery to 16.12% at 8 × 10−4 M, while chalcopyrite remained > 80%. Contact angle, TOC, FTIR and XPS confirmed higher MSA and lower SBX adsorption on galena. MDS showed MSA increased H2O accumulation on galena, enhancing hydrophilicity and depression. | [18] |
| Collector: Sodium butyl xanthate (SBX). Depressant: Polymaleic acid (PMA). Frother: Terpineol. | Separation of chalcopyrite and galena. Microflotation at −74 + 38 µm, pH 9. Contact angle, FTIR, XPS, ToF–SIMS, UV–Vis, TOC to assess adsorption and surface properties. MDS in Materials Studio (Forcite). Universal force field. NVT 298 K, 1 fs, 1000 ps, 2000 H2O ± 4 PMA. | PMA (2 × 10−4 mol/L) depressed galena (−74.91%) but preserved chalcopyrite recovery (83.27%). In binary flotation, PMA improved Cu grade/recovery (28.11%/86.01%) and reduced Pb floatability. XPS, ToF–SIMS and MDS showed PMA increased hydrophilicity and water/PMA accumulation on galena. | [75] |
| Collector: Sodium butyl xanthate (SBX). Depressant: Carboxymethyl–β–cyclodextrin (CMCD). Frother: Pine oil. | Separation of chalcopyrite and pyrite. Microflotation at −74 + 38 µm, pH 6.5 ± 0.2, XFG cell. Contact angle, UV–Vis, FTIR, XPS, and AFM to assess surface interactions. MDS using Materials Studio (CASTEP, Amorphous Cell, Forcite). Universal force field. NVT at 98 K, 500 ps, 1 fs. | CMCD showed stronger affinity for pyrite (−430.10 kcal/mol) than for chalcopyrite (−179.40 kcal/mol). At 250 mg/L CMCD and 40 mg/L SBX, Cu recovery reached 73.15% with a grade of 28.06%. FTIR, XPS and AFM confirmed selective adsorption on pyrite via hydrogen bonding, preventing SBX adsorption. | [76] |
| Collector: PBX (Potassium n-butyl xanthate). Depressant: Tannic acid (TA). Frother: Methyl isobutyl carbinol (MIBC). | Separation of chalcopyrite and molybdenite. Microflotation at −76 + 38 µm, pH 10. UV, FTIR and XPS to analyze adsorption. MDS in Materials Studio (DMol3, CASTEP, Forcite). Universal force field. NVT (1 ps), NVE (50 ps), final NVT (1 ns, 1 fs timestep). Chalcopyrite (012) and molybdenite (001) surfaces. | TA enabled selective depression of molybdenite. Chalcopyrite recovery reached 95.01% and molybdenite 3.34% at 900 mg/L TA. XPS showed chemisorption on chalcopyrite via Fe, not Cu, and no chemisorption on molybdenite. MDS confirmed hydrophobic interaction as the main adsorption mechanism on molybdenite (−76.49 kcal/mol vs. −6.84 on chalcopyrite). | [77] |
| Collector: Sodium isobutyl xanthate (SIBX) and Kerosene. Depressant: Sodium thioglycolate (STG). Frother: Pine oil. | Separation of chalcopyrite and molybdenite. Microflotation at −74 + 38 μm, pH 10, XFGCII cell. Contact angle, UV, FTIR, XPS, ToF–SIMS, OCP, EIS to evaluate surface adsorption and hydrophobicity. MDS and AIMD with Materials Studio (CASTEP, Forcite, CP2K). Chalcopyrite (012) surface. COMPASS II force field. 4000 H2O, 40 STG−, 40 Na⁺. PBE–D3BJ functional. 298 K, 1 fs, 500 ps (MD), 5000 fs (AIMD). | STG enabled effective separation with 6.69% chalcopyrite and 55.26% molybdenite recovery, achieving 83.79% separation efficiency. XPS confirmed chemisorption via Cu(I)–S and Fe(III)–S bonds. Adsorption on chalcopyrite was ~4× higher than on molybdenite. MDS and AIMD showed multilayer STG adsorption, stronger water affinity, and adsorption energy of −57.48 kcal/mol. STG also removed 81% of SIBX from the chalcopyrite surface. | [78] |
| Reagent | Methods | Results | Reference |
|---|---|---|---|
| Collector: Sodium isopropyl xanthate (SIPX). Depressant: Sodium cyanide (NaCN) and zinc sulfate (ZnSO4). Frother: Methyl isobutyl carbinol (MIBC). | Flotation of chalcopyrite and galena. Box–Behnken Design (BBD) with 8 variables (reagent dosages, pH, airflow, impeller speed, time). A hybrid ML model (RF–FFA) is applied to predict Cu/Pb grades and recoveries, and to compare its performance with other models (ANN, SVM, M5P, RF). The model is trained and validated using the BBD dataset. CPI analysis is conducted to identify the most influential parameters. | The RF–FFA hybrid model outperformed all standalone models. It achieved R2 values up to 0.9864 (Pb grade) and 0.9817 (Cu recovery), with minimal RMSE, MAE, and MAPE. CPI identifies SIPX and NaCN as the most influential reagents. Accurate predictions of flotation performance using process parameters enable efficient process optimization, reducing experimental cost and time. | [82] |
| 47 ethyl–linked molecules with donor atoms (O, N, S). | QC+ML model to screen flotation reagents for chalcopyrite. DFT using B3LYP/def2–TZVP, SMD solvation and D3 dispersion was used to calculate ΔG and descriptors (15 total). Gaussian was used for QC. ML models included RR, RF, GBR and MLP. SBI to assess selectivity. | GBR was the most accurate model (R2 0.90, RMSE 4.04 for Cu⁺). QC showed higher affinity for Cu(II), moderate for Fe(II) and lower for Cu(I). ML accurately predicted ΔG and SBI, identifying 8 reagents with highest selectivity. HOMO, LUMO and molecular charge were key descriptors. ML reduced screening time from ~38.5 h (QC) to ms. Cu–selective reagents mainly contained sulfur. | [80] |
| Collector: Isopropyl ethyl thionocarbamate (IET) and Sodium diisobutyl dithiophosphate (SDD). Depressant: Sodium metabisulfite (SMBS), zinc sulfate. Frother: Methyl isobutyl carbinol (MIBC). | DL model using SOLOv2 for instance segmentation and SLIC for unsupervised clustering to classify chalcopyrite, pyrite, sphalerite, galena and gangue in flotation pulp images. SOLOv2 trained in PyTorch for coarse (+38 µm), SLIC superpixels applied to fine (−38 µm). Validation via ICP–MS and XRD. | Vision model predicted mineral grades and P50 with high accuracy (±5.4% and ±2.3 µm). Repeated analysis reduced error to ±1.3%. ICP–MS and XRPD confirmed precise classification. Enabled sulfide identification in pulp in under 5 min, improving real–time flotation monitoring. | [83] |
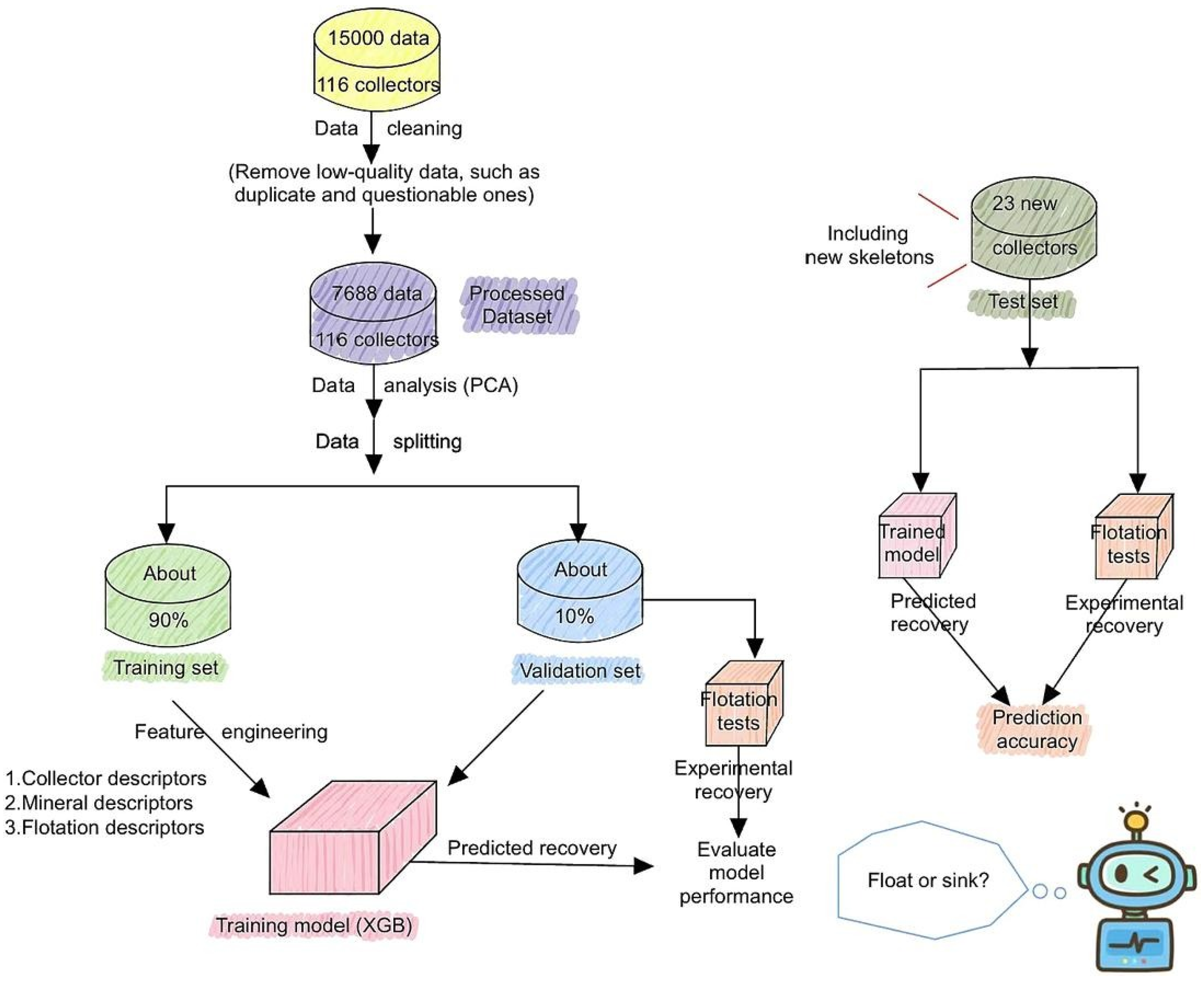
| Collector: 139 flotation collectors with diverse molecular structures. Depressant: Not specified. Frother: Not specified. | QC+ML model to predict flotation recovery of chalcopyrite, galena, pyrite and sphalerite. Descriptors from DFT (Gaussian, MN15/def2–TZVP, Multiwfn) and mineral features from CP2K (PBE–D3, 400 Ry). ML model (XGBoost) was trained on 7688 tests using 69 input features (collector, mineral, flotation conditions). | The model accurately predicted flotation performance (MAE = 10% in validation, 5.2% in test). Enabled structure–performance insights. Effective for chalcopyrite, pyrite, galena and sphalerite. New selective reagents identified. Demonstrated potential for high-throughput screening. | [22] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rios, L.A.; Barraza, M.J.; Robles, P.A.; Quezada, G.R. Chalcopyrite Flotation, Molecular Design and Smart Industry: A Review. Int. J. Mol. Sci. 2025, 26, 3613. https://doi.org/10.3390/ijms26083613
Rios LA, Barraza MJ, Robles PA, Quezada GR. Chalcopyrite Flotation, Molecular Design and Smart Industry: A Review. International Journal of Molecular Sciences. 2025; 26(8):3613. https://doi.org/10.3390/ijms26083613
Chicago/Turabian StyleRios, Luis A., Melanny J. Barraza, Pedro A. Robles, and Gonzalo R. Quezada. 2025. "Chalcopyrite Flotation, Molecular Design and Smart Industry: A Review" International Journal of Molecular Sciences 26, no. 8: 3613. https://doi.org/10.3390/ijms26083613
APA StyleRios, L. A., Barraza, M. J., Robles, P. A., & Quezada, G. R. (2025). Chalcopyrite Flotation, Molecular Design and Smart Industry: A Review. International Journal of Molecular Sciences, 26(8), 3613. https://doi.org/10.3390/ijms26083613








