Ferritin as an Inflammatory Marker in Pediatric Metabolic Syndrome: Links to Obesity and Liver Ultrasound Alterations
Abstract
1. Introduction
2. Results
2.1. Obesity Group vs. Average Weight
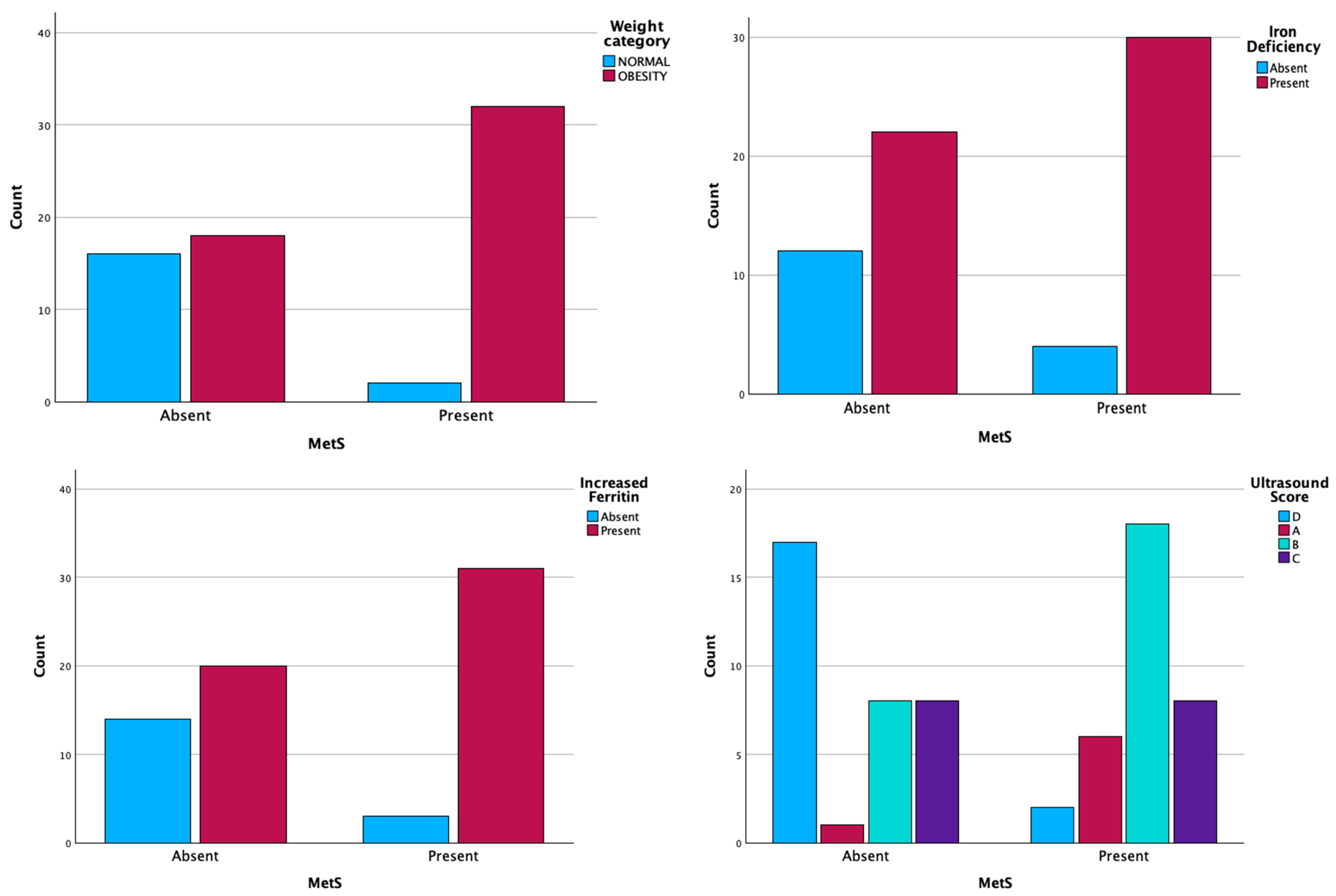
2.2. MetS+ Group vs. MetS− Group
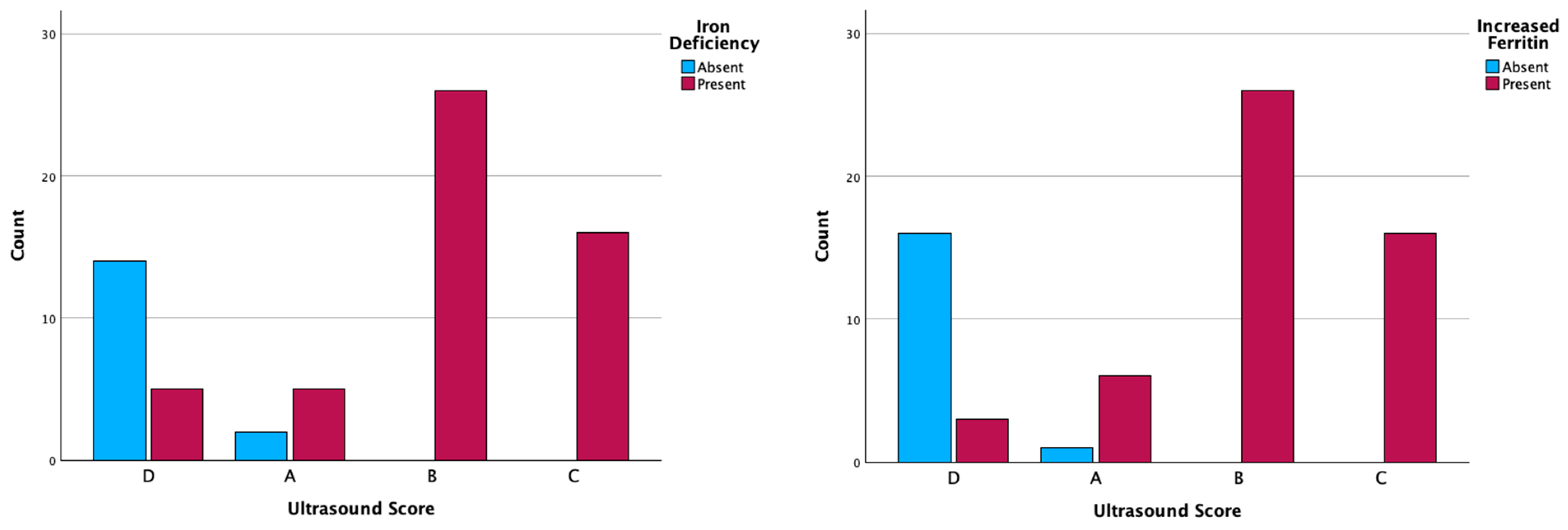
2.3. MetS and Ultrasonographic Changes
3. Discussion
4. Materials and Methods
4.1. Study Design
4.2. Study Population
4.3. Inclusion Criteria
4.4. Exclusion Criteria
4.5. Patient Assessment
4.6. Measurements
4.7. Laboratory Tests
- Children < 14 years: 29–137 μg/dL;
- Boys (14–19 years): 43–176 μg/dL;
- Girls (14–19 years): 33–170 μg/dL.
- 4–7 years: 4–67 ng/mL;
- 7–13 years:
- ○
- Males: 14–124 ng/mL;
- ○
- Females: 7–84 ng/mL;
- 13–18 years:
- ○
- Males: 14–152 ng/mL;
- ○
- Females: 13–68 ng/mL.
4.8. Definition of MetS
- Abdominal obesity—increased AP (≥90th percentile of waist circumference for patients of the same age and gender).
- Hypertriglyceridemia—TG ≥ 100 mg/dL for children aged 0–9 years and ≥130 mg/dL for those aged 10–19 years.
- Low HDL cholesterol—HDLch < 40 mg/dL.
- Hypertension—SBP or DBP ≥ 90th percentile for children of the same age, height, and gender.
4.9. Imagistic Result
- Score A: diffuse increased liver echogenicity; periportal and diaphragmatic echogenicity is still appreciable;
- Score B: diffuse increased liver echogenicity hiding periportal echogenicity but diaphragmatic echogenicity is still appreciable;
- Score C: diffuse increased liver echogenicity hiding periportal and diaphragmatic echogenicity;
- Score D: normal liver echogenicity [63].
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| MetS | metabolic syndrome |
| BMI | body mass index |
| HDLch | high-density lipoprotein cholesterol |
| SBP | systolic blood pressure |
| DBP | diastolic blood pressure |
| TG | triglycerides |
| AP | abdominal perimeter |
| CDC | Centers for Disease Control and Prevention |
| WHO | World Health Organization |
| LDLch | Low-density lipoprotein cholesterol |
| NAFLD | nonalcoholic fatty liver disease |
| MAFLD | metabolic-associated fatty liver disease |
| MRI | magnetic resonance imaging |
| IL-6 | interleukin-6 |
| CRP | C-reactive protein |
| TNF-α | tumor necrosis factor-alpha |
| FPN | ferroportin |
| ROS | reactive oxygen species |
| Fe | iron |
References
- Panuganti, K.K.; Nguyen, M.; Kshirsagar, R.K. Obesity. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- The World Health Organization (WHO) Obesity. Available online: https://www.who.int/health-topics/obesity#tab=tab_1 (accessed on 26 December 2024).
- Al-Hamad, D.; Raman, V. Metabolic Syndrome in Children and Adolescents. Transl. Pediatr. 2017, 6, 397–407. [Google Scholar] [CrossRef]
- American Academy of Child and Adolescent Psychiatry Obesity in Children and Teens. 2023. 70. Available online: https://www.aacap.org/AACAP/Families_and_Youth/Facts_for_Families/FFF-Guide/Obesity-In-Children-And-Teens-079.aspx (accessed on 26 December 2024).
- Orsini, F.; D’Ambrosio, F.; Scardigno, A.; Ricciardi, R.; Calabrò, G.E. Epidemiological Impact of Metabolic Syndrome in Overweight and Obese European Children and Adolescents: A Systematic Literature Review. Nutrients 2023, 15, 3895. [Google Scholar] [CrossRef] [PubMed]
- Graf, C.; Ferrari, N. Metabolic Syndrome in Children and Adolescents. Visc. Med. 2016, 32, 357–362. [Google Scholar] [CrossRef] [PubMed]
- Gepstein, V.; Weiss, R. Obesity as the Main Risk Factor for Metabolic Syndrome in Children. Front. Endocrinol. 2019, 10, 568. [Google Scholar] [CrossRef] [PubMed]
- Esposito, K.; Giugliano, D. The Metabolic Syndrome and Inflammation: Association or Causation? Nutr. Metab. Cardiovasc. Dis. 2004, 14, 228–232. [Google Scholar] [CrossRef]
- Minihane, A.M.; Vinoy, S.; Russell, W.R.; Baka, A.; Roche, H.M.; Tuohy, K.M.; Teeling, J.L.; Blaak, E.E.; Fenech, M.; Vauzour, D.; et al. Low-Grade Inflammation, Diet Composition and Health: Current Research Evidence and Its Translation. Br. J. Nutr. 2015, 114, 999–1012. [Google Scholar] [CrossRef]
- Codazzi, V.; Frontino, G.; Galimberti, L.; Giustina, A.; Petrelli, A. Mechanisms and Risk Factors of Metabolic Syndrome in Children and Adolescents. Endocrine 2024, 84, 16–28. [Google Scholar] [CrossRef]
- Hotamisligil, G.S. Inflammation and Metabolic Disorders. Nature 2006, 444, 860–867. [Google Scholar] [CrossRef]
- Aguirre, L.G.; Urrunaga-Pastor, D.; Moncada-Mapelli, E.; Guarnizo-Poma, M.; Lazaro-Alcantara, H.; Benites-Zapata, V.A. High Serum Ferritin Levels Are Associated with Insulin Resistance but Not with Impaired Glucose Tolerance in a Healthy People Population. Diabetes Metab. Syndr. Clin. Res. Rev. 2017, 11, S983–S988. [Google Scholar] [CrossRef]
- Moreira, A.C.; Mesquita, G.; Gomes, M.S. Ferritin: An Inflammatory Player Keeping Iron at the Core of Pathogen-Host Interactions. Microorganisms 2020, 8, 589. [Google Scholar] [CrossRef]
- Kotla, N.K.; Dutta, P.; Parimi, S.; Das, N.K. The Role of Ferritin in Health and Disease: Recent Advances and Understandings. Metabolites 2022, 12, 609. [Google Scholar] [CrossRef] [PubMed]
- Sobieska, K.; Buczyńska, A.; Krętowski, A.J.; Popławska-Kita, A. Iron Homeostasis and Insulin Sensitivity: Unraveling the Complex Interactions. Rev. Endocr. Metab. Disord. 2024, 25, 925–939. [Google Scholar] [CrossRef] [PubMed]
- Puiu, I.; Stancu, P.; Bulucea, D.; Niculescu, C.; Nicolescu, V.E.; Stoian, F. Diagnosis of Tuberculosis Lymphadenitis in Children. Pediatrics 2008, 121, S130–S131. [Google Scholar] [CrossRef]
- Pahwa, R.; Goyal, A.; Jialal, I. Chronic Inflammation. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Monteiro, R.; Azevedo, I. Chronic Inflammation in Obesity and the Metabolic Syndrome. Mediat. Inflamm. 2010, 2010, 289645. [Google Scholar] [CrossRef]
- Suárez-Ortegón, M.F.; Blanco, E.; McLachlan, S.; Fernandez-Real, J.M.; Burrows, R.; Wild, S.H.; Lozoff, B.; Gahagan, S. Ferritin Levels throughout Childhood and Metabolic Syndrome in Adolescent Stage. Nutr. Metab. Cardiovasc. Dis. 2019, 29, 268–278. [Google Scholar] [CrossRef]
- Abril-Ulloa, V.; Flores-Mateo, G.; Solà-Alberich, R.; Manuel-y-Keenoy, B.; Arija, V. Ferritin Levels and Risk of Metabolic Syndrome: Meta-Analysis of Observational Studies. BMC Public Health 2014, 14, 483. [Google Scholar] [CrossRef]
- Selvakumar, P.K.C.; Kabbany, M.N.; Nobili, V.; Alkhouri, N. Nonalcoholic Fatty Liver Disease in Children. Pediatr. Clin. N. Am. 2017, 64, 659–675. [Google Scholar] [CrossRef]
- Boccatonda, A.; Andreetto, L.; D’Ardes, D.; Cocco, G.; Rossi, I.; Vicari, S.; Schiavone, C.; Cipollone, F.; Guagnano, M.T. From NAFLD to MAFLD: Definition, Pathophysiological Basis and Cardiovascular Implications. Biomedicines 2023, 11, 883. [Google Scholar] [CrossRef]
- Basil, B.; Myke-Mbata, B.K.; Eze, O.E.; Akubue, A.U. From Adiposity to Steatosis: Metabolic Dysfunction-Associated Steatotic Liver Disease, a Hepatic Expression of Metabolic Syndrome—Current Insights and Future Directions. Clin. Diabetes Endocrinol. 2024, 10, 39. [Google Scholar] [CrossRef]
- Stepan, M.D.; Vintilescu, Ș.B.; Ionele, C.M.; Dumitra, G.G.; Podeanu, M.A.; Bigea, C.C.; Sacerdoțianu, V.M.; Anastasescu, C.M.; Florescu, D.N. Associations of Ultrasound Findings with Serum Iron and Ferritin Levels in Children with Obesity. Life 2024, 14, 484. [Google Scholar] [CrossRef]
- Zhang, J.; Cao, J.; Xu, H.; Dong, G.; Huang, K.; Wu, W.; Ye, J.; Fu, J. Ferritin as a Key Risk Factor for Nonalcoholic Fatty Liver Disease in Children with Obesity. J. Clin. Lab. Anal. 2021, 35, e23602. [Google Scholar] [CrossRef] [PubMed]
- Khanna, D.; Peltzer, C.; Kahar, P.; Parmar, M.S. Body Mass Index (BMI): A Screening Tool Analysis. Cureus 2022, 14, e22119. [Google Scholar] [CrossRef] [PubMed]
- Callahan, E.A. Translating Knowledge of Foundational Drivers of Obesity into Practice; National Academies Press: Washington, DC, USA, 2023; ISBN 978-0-309-70203-4. [Google Scholar]
- Freedman, D.S.; Wang, J.; Thornton, J.C.; Mei, Z.; Sopher, A.B.; Pierson, R.N.; Dietz, W.H.; Horlick, M. Classification of Body Fatness by Body Mass Index–for-Age Categories Among Children. Arch. Pediatr. Adolesc. Med. 2009, 163, 805. [Google Scholar] [CrossRef] [PubMed]
- U.S. Centers for Disease Control and Prevention (CDC). Available online: https://www.cdc.gov/bmi/child-teen-calculator/index.html (accessed on 8 January 2025).
- Weiss, R.; Bremer, A.A.; Lustig, R.H. What Is Metabolic Syndrome, and Why Are Children Getting It? Ann. N. Y. Acad. Sci. 2013, 1281, 123–140. [Google Scholar] [CrossRef]
- Quarta, A.; Quarta, M.T.; Mastromauro, C.; Chiarelli, F.; Giannini, C. Influence of Nutrition on Growth and Development of Metabolic Syndrome in Children. Nutrients 2024, 16, 3801. [Google Scholar] [CrossRef]
- Wentzel, A.; Mabhida, S.E.; Ndlovu, M.; Mokoena, H.; Esterhuizen, B.; Sekgala, M.D.; Dludla, P.V.; Kengne, A.P.; Mchiza, Z.J. Prevalence of Metabolic Syndrome in Children and Adolescents with Obesity: A Systematic Review and Meta-analysis. Obesity 2025, 33, 12–32. [Google Scholar] [CrossRef]
- Díaz-Ortega, J.L.; Yupari-Azabache, I.L.; Vidal, J.A.C.; Conde-Parada, N.E.; Gamboa, A.F.R. Criteria in the Diagnosis of Metabolic Syndrome in Children: A Scoping Review. Diabetes Metab. Syndr. Obes. 2023, 16, 3489–3500. [Google Scholar] [CrossRef]
- Reddy, P.; Lent-Schochet, D.; Ramakrishnan, N.; McLaughlin, M.; Jialal, I. Metabolic Syndrome Is an Inflammatory Disorder: A Conspiracy between Adipose Tissue and Phagocytes. Clin. Chim. Acta 2019, 496, 35–44. [Google Scholar] [CrossRef]
- Šebeková, K.; Staruchová, M.; Mišľanová, C.; Líšková, A.; Horváthová, M.; Tulinská, J.; Lehotská Mikušová, M.; Szabová, M.; Gurecká, R.; Koborová, I.; et al. Association of Inflammatory and Oxidative Status Markers with Metabolic Syndrome and Its Components in 40-To-45-Year-Old Females: A Cross-Sectional Study. Antioxidants 2023, 12, 1221. [Google Scholar] [CrossRef]
- Goral, J. TNF-Alpha and Carbohydrate and Lipid Parameters in Overweight and Obese Children. Ann. Acad. Medicae Stetin. 2008, 54, 14–21. [Google Scholar]
- Welty, F.K.; Alfaddagh, A.; Elajami, T.K. Targeting Inflammation in Metabolic Syndrome. Transl. Res. 2016, 167, 257–280. [Google Scholar] [CrossRef] [PubMed]
- Podeanu, M.-A.; Turcu-Stiolica, A.; Subțirelu, M.S.; Stepan, M.D.; Ionele, C.-M.; Gheonea, D.-I.; Vintilescu, B.Ș.; Sandu, R.E. C-Reactive Protein as a Marker of Inflammation in Children and Adolescents with Metabolic Syndrome: A Systematic Review and Meta-Analysis. Biomedicines 2023, 11, 2961. [Google Scholar] [CrossRef] [PubMed]
- Medise, B.E. The Role of Iron for Supporting Children’s Growth and Development. World Nutr. J. 2021, 5, 16–24. [Google Scholar] [CrossRef]
- Bermejo, F.; García-López, S. A Guide to Diagnosis of Iron Deficiency and Iron Deficiency Anemia in Digestive Diseases. World J. Gastroenterol. 2009, 15, 4638. [Google Scholar] [CrossRef]
- Mohammadi, M.; Gozashti, M.H.; Aghadavood, M.; Mehdizadeh, M.R.; Hayatbakhsh, M.M. Clinical Significance of Serum IL-6 and TNF-α Levels in Patients with Metabolic Syndrome. Rep. Biochem. Mol. Biol. 2017, 6, 74–79. [Google Scholar]
- Tarancon-Diez, L.; Iriarte-Gahete, M.; Sanchez-Mingo, P.; Muñoz-Fernandez, M.Á.; Navarro-Gomez, M.L.; Pacheco, Y.M.; Leal, M. Impact of Obesity on Iron Metabolism and the Effect of Intravenous Iron Supplementation in Obese Patients with Absolute Iron Deficiency. Sci. Rep. 2025, 15, 1343. [Google Scholar] [CrossRef]
- Berton, P.F.; Gambero, A. Hepcidin and Inflammation Associated with Iron Deficiency in Childhood Obesity—A Systematic Review. J. Pediatr. (Rio J.) 2024, 100, 124–131. [Google Scholar] [CrossRef]
- Kell, D.B.; Pretorius, E. Serum Ferritin Is an Important Inflammatory Disease Marker, as It Is Mainly a Leakage Product from Damaged Cells. Metallomics 2014, 6, 748–773. [Google Scholar] [CrossRef]
- Gensluckner, S.; Wernly, B.; Datz, C.; Aigner, E. Iron, Oxidative Stress, and Metabolic Dysfunction—Associated Steatotic Liver Disease. Antioxidants 2024, 13, 208. [Google Scholar] [CrossRef]
- Srivastav, S.K.; Mir, I.A.; Bansal, N.; Singh, P.K.; Kumari, R.; Deshmukh, A. Serum Ferritin in Metabolic Syndrome—Mechanisms and Clinical Applications. Pathophysiology 2022, 29, 319–325. [Google Scholar] [CrossRef]
- Xing, Y.; Fan, J.; Wang, H.-J.; Wang, H. Comparison of MAFLD and NAFLD Characteristics in Children. Children 2023, 10, 560. [Google Scholar] [CrossRef] [PubMed]
- Băloşeanu, C.L.; Streba, C.T.; Vere, C.C.; Comănescu, V.; Rogoveanu, I. Association between Liver Histology, Carotid Ultrasonography and Retinal Vascular Changes in Patients with Nonalcoholic Fatty Liver Disease (NAFLD). Rom. J. Morphol. Embryol. 2012, 53, 609–614. [Google Scholar] [PubMed]
- Ferraioli, G.; Soares Monteiro, L.B. Ultrasound-Based Techniques for the Diagnosis of Liver Steatosis. World J. Gastroenterol. 2019, 25, 6053–6062. [Google Scholar] [CrossRef] [PubMed]
- Ozcan, H.N.; Oguz, B.; Haliloglu, M.; Orhan, D.; Karcaaltincaba, M. Imaging Patterns of Fatty Liver in Pediatric Patients. Diagn. Interv. Radiol. 2015, 21, 355–360. [Google Scholar] [CrossRef]
- Paik, J.M.; Golabi, P.; Younossi, Y.; Mishra, A.; Younossi, Z.M. Changes in the Global Burden of Chronic Liver Diseases From 2012 to 2017: The Growing Impact of NAFLD. Hepatology 2020, 72, 1605–1616. [Google Scholar] [CrossRef]
- Ko, J.S. New Perspectives in Pediatric Nonalcoholic Fatty Liver Disease: Epidemiology, Genetics, Diagnosis, and Natural History. Pediatr. Gastroenterol. Hepatol. Nutr. 2019, 22, 501. [Google Scholar] [CrossRef]
- Shannon, A.; Alkhouri, N.; Carter-Kent, C.; Monti, L.; Devito, R.; Lopez, R.; Feldstein, A.E.; Nobili, V. Ultrasonographic Quantitative Estimation of Hepatic Steatosis in Children With NAFLD. J. Pediatr. Gastroenterol. Nutr. 2011, 53, 190–195. [Google Scholar] [CrossRef]
- Baker, J.L.; Farpour-Lambert, N.J.; Nowicka, P.; Pietrobelli, A.; Weiss, R. Evaluation of the Overweight/Obese Child—Practical Tips for the Primary Health Care Provider: Recommendations from the Childhood Obesity Task Force of the European Association for the Study of Obesity. Obes. Facts 2010, 3, 5. [Google Scholar] [CrossRef]
- MSD Manuals. Available online: https://www.msdmanuals.com/professional/multimedia/clinical-calculator/blood-pressure-percentiles-for-boys-2-17-years (accessed on 20 December 2024).
- MSD Manuals. Available online: https://www.msdmanuals.com/professional/multimedia/clinical-calculator/blood-pressure-percentiles-for-girls-2-17-years (accessed on 20 December 2024).
- MSD Manuals. Professional Version. National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents. The Fourth Report on the Diagnosis, Evaluation, and Treatment of High Blood Pressure in Chil-Dren and Adolescents. Pediatrics 2004, 114, 555–576. [Google Scholar] [CrossRef]
- Zimmet, P.; Alberti, K.G.M.; Kaufman, F.; Tajima, N.; Silink, M.; Arslanian, S.; Wong, G.; Bennett, P.; Shaw, J.; Caprio, S. The Metabolic Syndrome in Children and Adolescents ? An IDF Consensus Report. Pediatr. Diabetes 2007, 8, 299–306. [Google Scholar] [CrossRef]
- Bitew, Z.W.; Alemu, A.; Ayele, E.G.; Tenaw, Z.; Alebel, A.; Worku, T. Metabolic Syndrome among Children and Adolescents in Low and Middle Income Countries: A Systematic Review and Meta-Analysis. Diabetol. Metab. Syndr. 2020, 12, 93. [Google Scholar] [CrossRef] [PubMed]
- Expert Panel on Integrated Guidelines for Cardiovascular Health and Risk Reduction in Children and Adolescents: Summary Report. Pediatrics 2011, 128, S213–S256. [CrossRef] [PubMed]
- Zimmet, P.; Alberti, G.; Kaufman, F.; Tajima, N.; Silink, M.; Arslanian, S.; Wong, G.; Bennett, P.; Shaw, J.; Caprio, S. The Metabolic Syndrome in Children and Adolescents. Lancet 2007, 369, 2059–2061. [Google Scholar] [CrossRef]
- Saggese, G.; Vierucci, F.; Prodam, F.; Cardinale, F.; Cetin, I.; Chiappini, E.; de’ Angelis, G.L.; Massari, M.; Miraglia Del Giudice, E.; Miraglia Del Giudice, M.; et al. Vitamin D in Pediatric Age: Consensus of the Italian Pediatric Society and the Italian Society of Preventive and Social Pediatrics, Jointly with the Italian Federation of Pediatricians. Ital. J. Pediatr. 2018, 44, 51. [Google Scholar] [CrossRef]
- Zhang, Y.N.; Fowler, K.J.; Hamilton, G.; Cui, J.Y.; Sy, E.Z.; Balanay, M.; Hooker, J.C.; Szeverenyi, N.; Sirlin, C.B. Liver Fat Imaging—A Clinical Overview of Ultrasound, CT, and MR Imaging. Br. J. Radiol. 2018, 91, 20170959. [Google Scholar] [CrossRef]

| Parameters | Average Weight (n = 18) | Obesity (n = 50) |
|---|---|---|
| Age (years) | 11 (8.5–12) | 10 (8–12) |
| BMI (kg/m2) | 17.3 (16.57–18.25) | 27.8 (24.85–30.4) |
| Serum iron (μg/dL) | 73 (41.25–83.25) | 10 (7–12) |
| Serum ferritin (ng/mL) | 41 (31.5–55) | 203 (185.5–221) |
| Height (cm) | 138 (126.5–146.25) | 144.5 (134–156.25) |
| AP (cm) | 73.2 ± 6.3 | 90 ± 12.1 |
| SBP (mmHg) | 108 (105–112.5) | 119 (109.75–130.25) |
| DBP (mmHg) | 59 (57.75–61.25) | 74 (64–83.5) |
| Glycemia (mg/dL) | 70.2 ± 8.1 | 87.6 ± 9.4 |
| HDLch (mg/dL) | 59.37 (57.1–63.87) | 29.16 (22.91–42.65) |
| TG (mg/dL) | 108 (88–115.25) | 161.5 (99–223.75) |
| Parameters (No. Cases) (%) | Average Weight (n = 18) | Obesity (n = 50) | p-Value (x2 Test) | |
|---|---|---|---|---|
| Gender | Female | 2 (11.1%) | 16 (32%) | p = 0.08 |
| Male | 16 (88.9%) | 34 (68%) | ||
| Environment | Urban | 6 (33.3%) | 31 (62%) | p = 0.03 |
| Rural | 12 (66.7%) | 19 (38%) | ||
| Ultrasonography | Score A | 0 | 8 (16%) | p < 0.001 |
| Score B | 0 | 25 (50%) | ||
| Score C | 0 | 16 (32%) | ||
| Score D | 18 (100%) | 1 (2%) | ||
| Deficit serum iron | 4 (22.2%) | 48 (96%) | p < 0.001 | |
| Increased serum ferritin | 2 (11.1%) | 49 (98%) | p < 0.001 | |
| High AP | 2 (11.1%) | 46 (92%) | p < 0.001 | |
| High SBP | p90–95 | 0 | 3 (6%) | p = 0.01 |
| p95–99 | 0 | 7 (14%) | ||
| p > 99 | 0 | 11 (22%) | ||
| High DBP | p90–95 | 0 | 4 (8%) | p = 0.01 |
| p95–99 | 0 | 10 (20%) | ||
| p > 99 | 0 | 7 (14%) | ||
| High SBP/DBP | 0 | 27 (54%) | p < 0.001 | |
| High Glycemia | 0 | 4 (8%) | p > 0.05 | |
| High TG | 2 (11.1%) | 29 (58%) | p = 0.001 | |
| Low HDLch | 2 (11.1%) | 35 (70%) | p < 0.001 | |
| MetS + | 2 (11.1%) | 32 (64%) | p < 0.001 | |
| Parameters (No. Cases) (%) | MetS + (n = 34) | MetS − (n = 34) | |
|---|---|---|---|
| AP | High | 34 (100%) | 14 (41.2%) |
| cm | 92.8 ± 11.3 | 78.2 ± 10.7 | |
| High SBP | p90–95 | 3 (8.8%) | 0 (0%) |
| p95–99 | 5 (14.7%) | 2 (5.9%) | |
| p > 99 | 9 (26.5%) | 2 (5.9%) | |
| SBP | mmHg | 120 (111.75–132.25) | 109 (105.75–113.25) |
| High DBP | p90–95 | 3 (8.8%) | 1 (2.9%) |
| p95–99 | 8 (23.5%) | 2 (5.9%) | |
| p > 99 | 7 (20.6%) | 0 (0%) | |
| DBP | mmHg | 79 (66.75–95.25) | 62 (58–66) |
| SBP/DBP | High | 22 (64.7%) | 5 (14.7%) |
| Glycemia | High | 3 (8.8%) | 1 (2.9%) |
| mg/dL | 87.7 ± 9.6 | 78.2 ± 12.2 | |
| TG | High | 31 (91.2%) | 0 (50%) |
| mg/dL | 204.5 (157.75–247.5) | 99 (92.75–109) | |
| HDLch | Low | 32 (94.1%) | 5 (14.7%) |
| mg/dL | 27.29 (22.62–34.03) | 56.28 (43.17–61.82) | |
| MetS number of criteria | 0 | - | 19 (27.9%) |
| 1 | - | 6 (8.8%) | |
| 2 | - | 9 (13.2%) | |
| 3 | 16 (23.5%) | - | |
| 4 | 16 (23.5%) | - | |
| 5 | 2 (2.9%) | - | |
| Parameters (No. Cases) (%) | MetS + (n = 34) | MetS − (n = 34) | p-Value | |
|---|---|---|---|---|
| Age (years) | 11 (9–12) | 9 (7–12) | - | |
| BMI (kg/m2) | 27.9 (24.85–30.8) | 20.8 (17.52–26.35) | p < 0.001 | |
| Average weight | 2 (5.9%) | 16 (47.1%) | ||
| Obesity | 32 (94.1%) | 18 (52.9%) | ||
| Serum iron | μg/dL | 10 (7–12.5) | 17 (9–67) | p = 0.02 |
| Deficit | 30 (88.2%) | 22 (64.7%) | ||
| Serum ferritin | ng/mL | 203.5 (176.75–223) | 166 (41.75–203.25) | p = 0.002 |
| Increased | 31 (91.2%) | 20 (58.8%) | ||
| Ultrasound | Score A | 7 (20.6%) | 1 (2.9%) | p < 0.001 |
| Score B | 17 (50%) | 8 (23.5) | ||
| Score C | 8 (23.5) | 8 (23.5) | ||
| Score D | 2 (5.9%) | 17 (50%) | ||
| Parameters (No. Cases) (%) | Ultrasonographic Score | p-Value | ||||
|---|---|---|---|---|---|---|
| A (n = 8) | B (n = 25) | C (n = 16) | D (n = 19) | |||
| Serum iron | Normal level | 2 (2.9%) | 0 | 0 | 14 (20.5%) | p < 0.001 |
| Deficit | 6 (8.8%) | 25 (36.7%) | 16 (23.5%) | 5 (7.3%) | ||
| Serum ferritin | Normal level | 1 (1.4%) | 0 | 0 | 16 (23.5%) | p < 0.001 |
| Increased | 7 (10.2%) | 25 (36.7%) | 16 (23.5%) | 3 (4.4%) | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Podeanu, M.-A.; Vintilescu, Ș.B.; Sandu, R.E.; Ionele, C.M.; Niculescu, C.E.; Florescu, M.-M.; Șelaru, E.-L.; Stepan, M.D. Ferritin as an Inflammatory Marker in Pediatric Metabolic Syndrome: Links to Obesity and Liver Ultrasound Alterations. Int. J. Mol. Sci. 2025, 26, 3793. https://doi.org/10.3390/ijms26083793
Podeanu M-A, Vintilescu ȘB, Sandu RE, Ionele CM, Niculescu CE, Florescu M-M, Șelaru E-L, Stepan MD. Ferritin as an Inflammatory Marker in Pediatric Metabolic Syndrome: Links to Obesity and Liver Ultrasound Alterations. International Journal of Molecular Sciences. 2025; 26(8):3793. https://doi.org/10.3390/ijms26083793
Chicago/Turabian StylePodeanu, Mihaela-Andreea, Ștefănița Bianca Vintilescu, Raluca Elena Sandu, Claudiu Marinel Ionele, Carmen Elena Niculescu, Mirela-Marinela Florescu, Elena-Loredana Șelaru, and Mioara Desdemona Stepan. 2025. "Ferritin as an Inflammatory Marker in Pediatric Metabolic Syndrome: Links to Obesity and Liver Ultrasound Alterations" International Journal of Molecular Sciences 26, no. 8: 3793. https://doi.org/10.3390/ijms26083793
APA StylePodeanu, M.-A., Vintilescu, Ș. B., Sandu, R. E., Ionele, C. M., Niculescu, C. E., Florescu, M.-M., Șelaru, E.-L., & Stepan, M. D. (2025). Ferritin as an Inflammatory Marker in Pediatric Metabolic Syndrome: Links to Obesity and Liver Ultrasound Alterations. International Journal of Molecular Sciences, 26(8), 3793. https://doi.org/10.3390/ijms26083793



.jpg)






