Sialyllactose Attenuates Inflammation and Injury of Intestinal Epithelial Cells upon Enterotoxigenic Escherichia coli Infection
Abstract
1. Introduction
2. Results
2.1. SL Treatment Dose and Duration
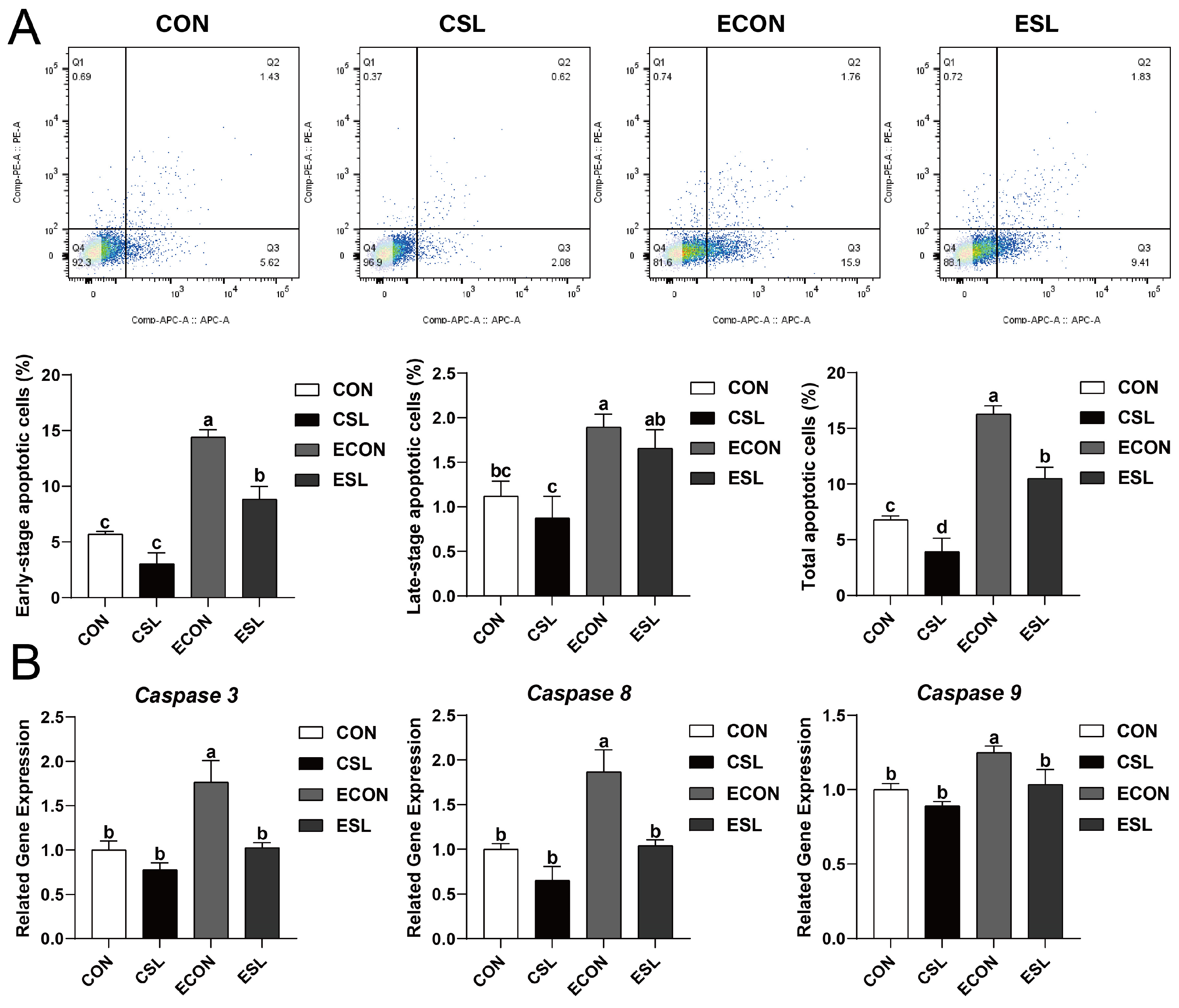
2.2. Protective Effect of SL on Intestinal Epithelial Cells (IECs) Challenged by ETEC
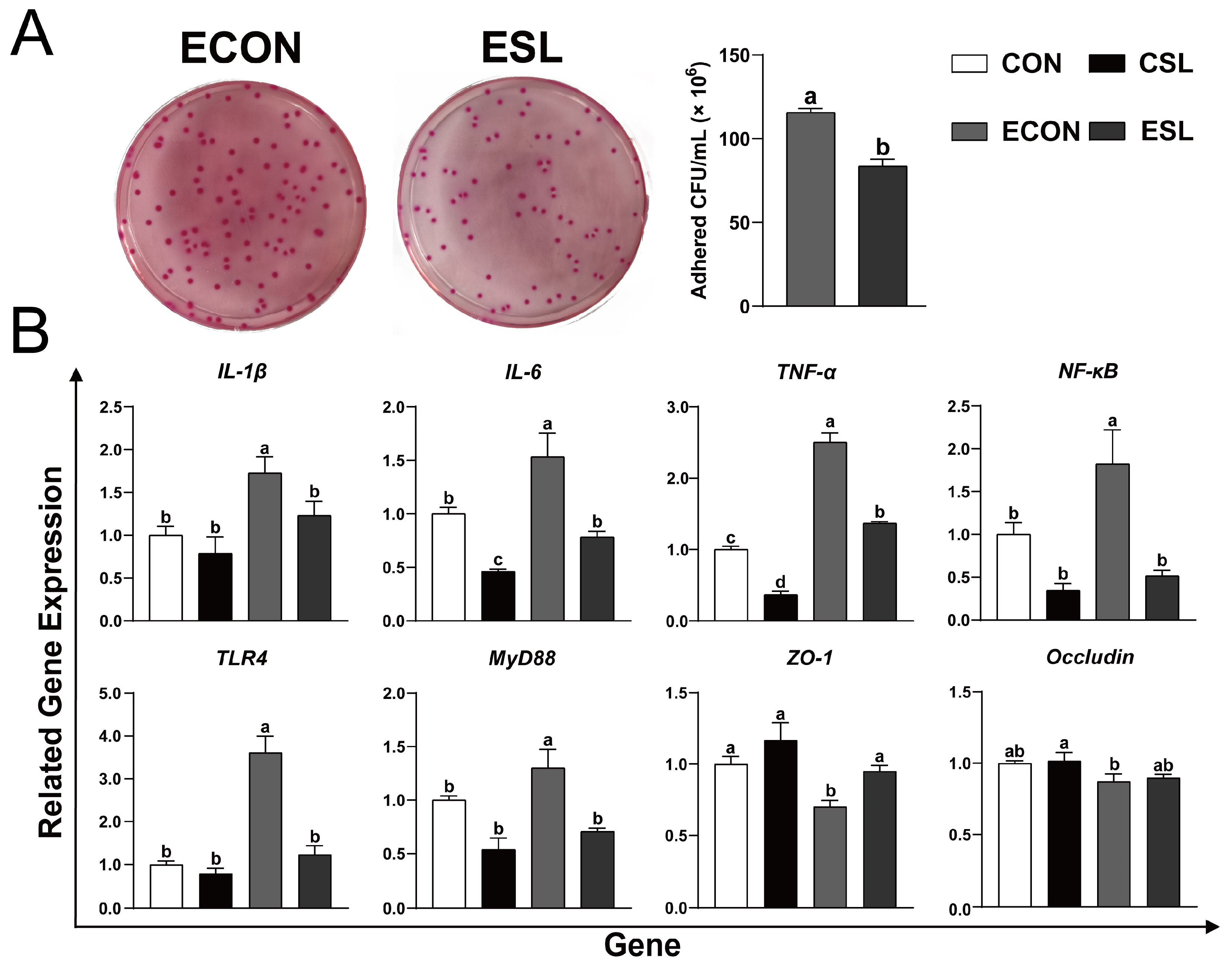
2.3. SL Alleviates Intestinal Inflammation by Preventing the Adhesion of ETEC K88 on IECs
2.4. SL Attenuated Inflammation and Injury of the IECs Challenged by LPS
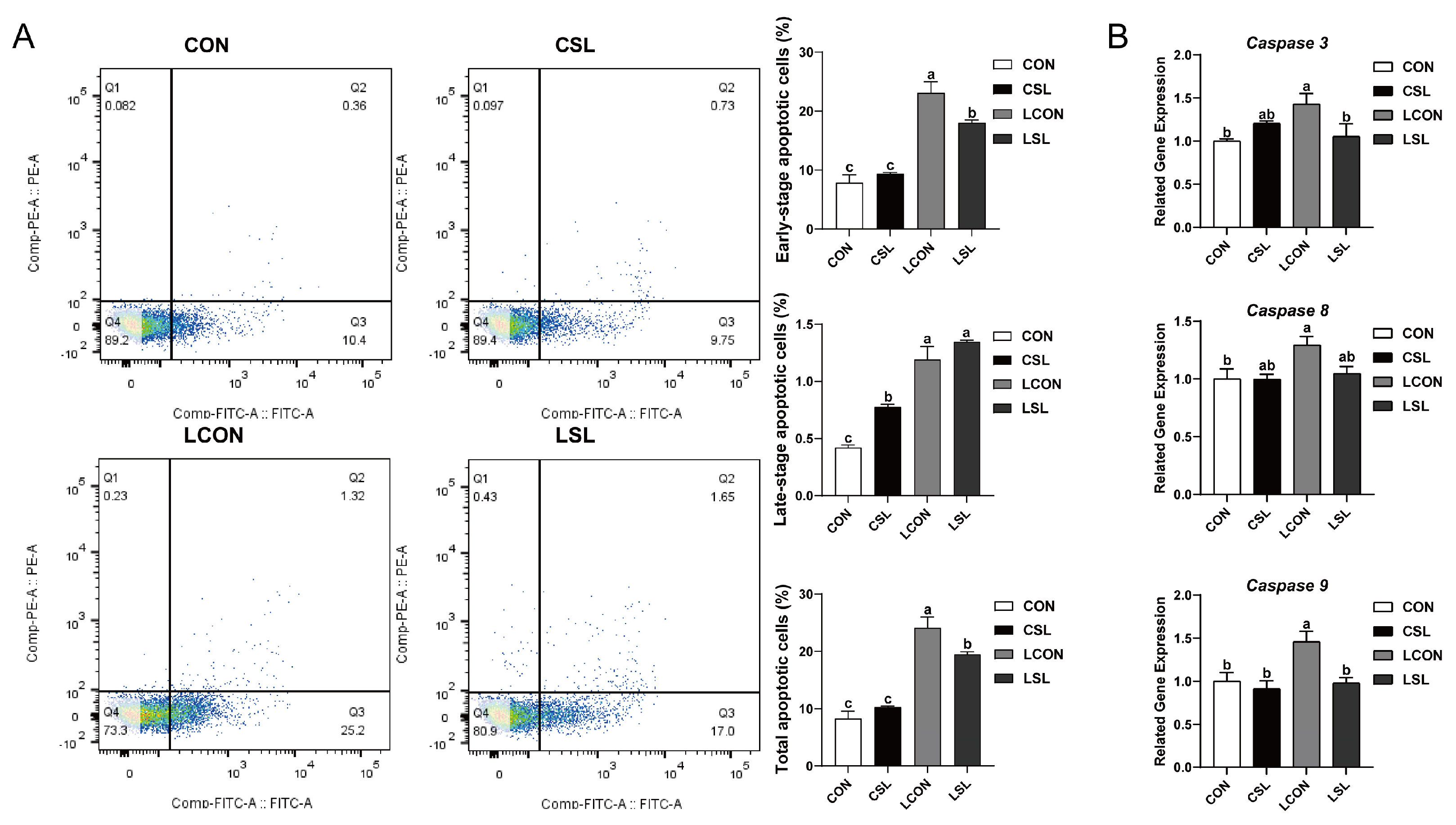
2.5. Protective Effect of SL on IECs Challenged by LPS
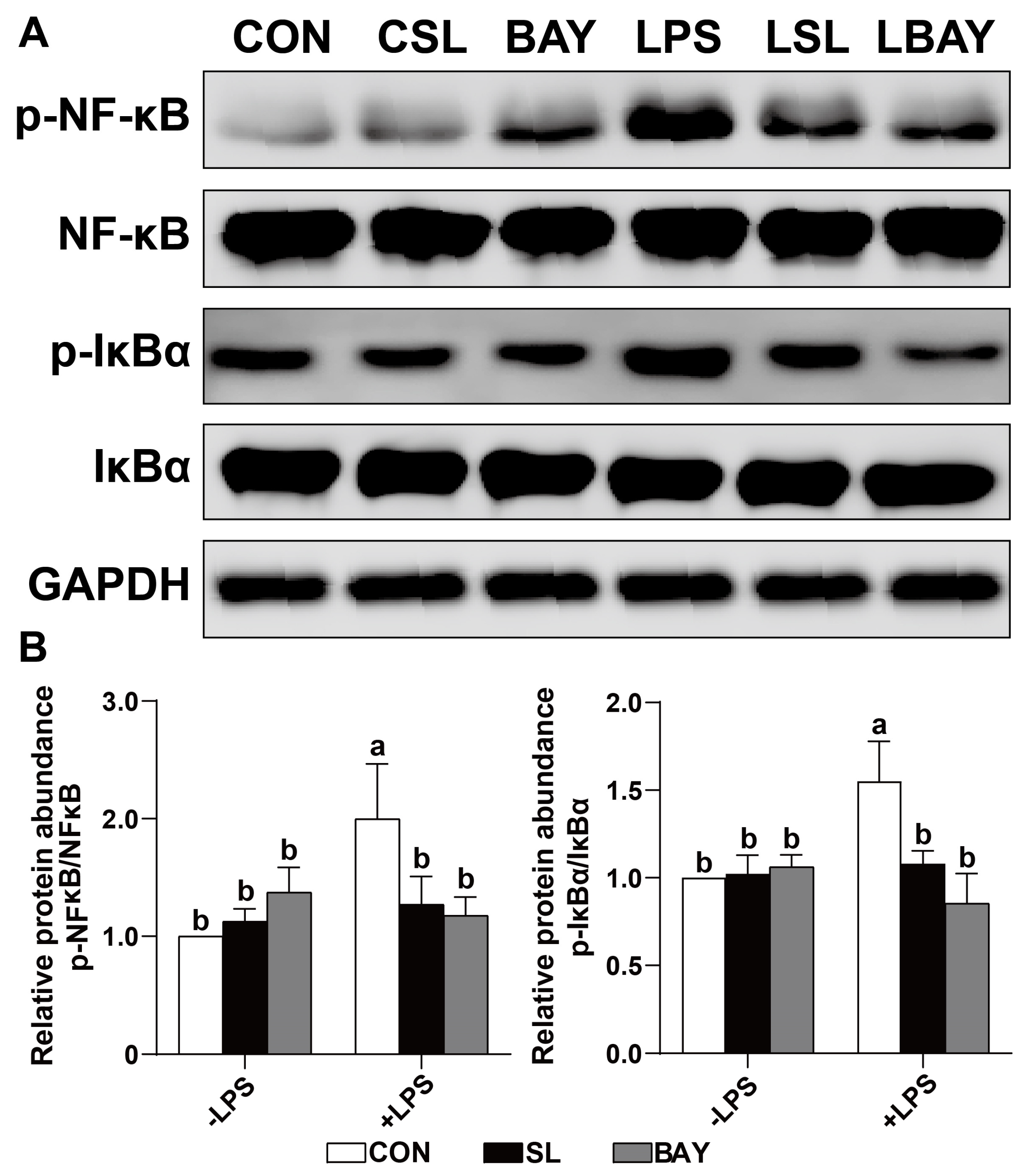
2.6. SL Inhibits the Phosphorylation of IκB-α and NF-κB by Preventing the Adhesion of LPS on IECs
2.7. SL and BAY 11-7082 Attenuated Inflammation and Injury of the IECs Challenged by LPS
2.8. SL and BAY 11-7082 Inhibit Apoptosis on IECs Challenged by LPS
2.9. SL and BAY 11-7082 Decreased the Phosphorylation of the Critical Inflammation-Associated Proteins IκB-α and NF-κB in LPS-Challenged Cells
3. Discussion
4. Materials and Methods
4.1. Bacterial Strains and Culture
4.2. Sialylactose
4.3. Cell Culture
4.4. ETEC Treatment
4.5. LPS Treatment
4.6. BAY 11-7082 Treatment
4.7. Assay of ETEC K88 Adhesion onto IPEC-J2 Cells
4.8. Immunofluorescence Assay
4.9. RNA Extraction and Quantitative Real-Time PCR (qPCR)
4.10. Detection of Cell Apoptosis
4.11. Total Protein Extraction and Western Blot Analysis
4.12. Statistics Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MOs | Milk oligosaccharides |
| SL | Sialyllactose |
| CON | Control group |
| CSL | SL group |
| ESL | ETEC + SL group |
| ETEC | Enterotoxigenic Escherichia coli |
| IκBα | Inhibitor of NF-κB Alpha |
| IL-1β | Interleukin-1 beta |
| IL-6 | Interleukin-6 |
| IPEC-J2 | Intestinal Porcine Epithelial Cell Line-J2 |
| LPS | Lipopolysaccharide |
| LBAY | BAY 11-7082 + LPS group |
| LSL | LPS + SL group |
| MyD88 | Myeloid Differentiation Primary Response 88 |
| NF-κB | Nuclear factor kappa B |
| TLR4 | Toll-like receptor 4 |
| TNF-α | Tumor Necrosis Factor Alpha |
| ZO-1 | Zonula Occludens-1 Protein |
References
- Turner, J.R. Intestinal mucosal barrier function in health and disease. Nat. Rev. Immunol. 2009, 9, 799–809. [Google Scholar] [CrossRef] [PubMed]
- Walker, R.I. An assessment of enterotoxigenic Escherichia coli and Shigella vaccine candidates for infants and children. Vaccine 2015, 33, 954–965. [Google Scholar] [CrossRef] [PubMed]
- Dubreuil, J.D.; Isaacson, R.E.; Schifferli, D.M. Animal Enterotoxigenic Escherichia coli. EcoSal Plus 2016, 7, 10–1128. [Google Scholar] [CrossRef]
- Dubreuil, J.D. Enterotoxigenic Escherichia coli targeting intestinal epithelial tight junctions: An effective way to alter the barrier integrity. Microb. Pathog. 2017, 113, 129–134. [Google Scholar] [CrossRef]
- Luppi, A.; Gibellini, M.; Gin, T.; Vangroenweghe, F.; Vandenbroucke, V.; Bauerfeind, R.; Bonilauri, P.; Labarque, G.; Hidalgo, Á. Prevalence of virulence factors in enterotoxigenic Escherichia coli isolated from pigs with post-weaning diarrhoea in Europe. Porc. Health Manag. 2016, 2, 20. [Google Scholar] [CrossRef]
- Pohl, C.S.; Medland, J.E.; Mackey, E.; Edwards, L.L.; Bagley, K.D.; DeWilde, M.P.; Williams, K.J.; Moeser, A.J. Early weaning stress induces chronic functional diarrhea, intestinal barrier defects, and increased mast cell activity in a porcine model of early life adversity. Neurogastroenterol. Motil. 2017, 29, e13118. [Google Scholar] [CrossRef]
- Dinleyici, M.; Barbieur, J.; Dinleyici, E.C.; Vandenplas, Y. Functional effects of human milk oligosaccharides (HMOs). Gut Microbes 2023, 15, 2186115. [Google Scholar] [CrossRef] [PubMed]
- Wiciński, M.; Sawicka, E.; Gębalski, J.; Kubiak, K.; Malinowski, B. Human Milk Oligosaccharides: Health Benefits, Potential Applications in Infant Formulas, and Pharmacology. Nutrients 2020, 12, 266. [Google Scholar] [CrossRef]
- Zhang, B.; Li, L.-Q.; Liu, F.; Wu, J.-Y. Human milk oligosaccharides and infant gut microbiota: Molecular structures, utilization strategies and immune function. Carbohydr. Polym. 2022, 276, 118738. [Google Scholar] [CrossRef]
- Bode, L. The functional biology of human milk oligosaccharides. Early Hum. Dev. 2015, 91, 619–622. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhang, J.; Zhang, W.; Mu, W. Recent progress on health effects and biosynthesis of two key sialylated human milk oligosaccharides, 3’-sialyllactose and 6’-sialyllactose. Biotechnol. Adv. 2023, 62, 108058. [Google Scholar] [CrossRef] [PubMed]
- Sarabia-Sainz, H.M.; Armenta-Ruiz, C.; Sarabia-Sainz, J.A.; Guzmán-Partida, A.M.; Ledesma-Osuna, A.I.; Vázquez-Moreno, L.; Ramos-Clamont Montfort, G. Adhesion of enterotoxigenic Escherichia coli strains to neoglycans synthesised with prebiotic galactooligosaccharides. Food Chem. 2013, 141, 2727–2734. [Google Scholar] [CrossRef]
- Coppa, G.V.; Facinelli, B.; Magi, G.; Marini, E.; Zampini, L.; Mantovani, V.; Galeazzi, T.; Padella, L.; Marchesiello, R.L.; Santoro, L.; et al. Human milk glycosaminoglycans inhibit in vitro the adhesion of Escherichia coli and Salmonella fyris to human intestinal cells. Pediatr. Res. 2016, 79, 603–607. [Google Scholar] [CrossRef]
- Schwegmann, C.; Zimmer, G.; Yoshino, T.; Enss, M.; Herrler, G. Comparison of the sialic acid binding activity of transmissible gastroenteritis coronavirus and E. coli K99. Virus Res. 2001, 75, 69–73. [Google Scholar] [CrossRef] [PubMed]
- Seignole, D.; Mouricout, M.; Duval-Iflah, Y.; Quintard, B.; Julien, R. Adhesion of K99 fimbriated Escherichia coli to pig intestinal epithelium: Correlation of adhesive and non-adhesive phenotypes with the sialoglycolipid content. J. Gen. Microbiol. 1991, 137, 1591–1601. [Google Scholar] [CrossRef][Green Version]
- Mouricout, M.; Petit, J.M.; Carias, J.R.; Julien, R. Glycoprotein glycans that inhibit adhesion of Escherichia coli mediated by K99 fimbriae: Treatment of experimental colibacillosis. Infect. Immun. 1990, 58, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Wan, J.; Zhang, J.; Xu, Q.; Yin, H.; Chen, D.; Yu, B.; He, J. Alginate oligosaccharide protects against enterotoxigenic Escherichia coli-induced porcine intestinal barrier injury. Carbohydr. Polym. 2021, 270, 118316. [Google Scholar] [CrossRef]
- Coppa, G.V.; Zampini, L.; Galeazzi, T.; Facinelli, B.; Ferrante, L.; Capretti, R.; Orazio, G. Human milk oligosaccharides inhibit the adhesion to Caco-2 cells of diarrheal pathogens: Escherichia coli, Vibrio cholerae, and Salmonella fyris. Pediatr. Res. 2006, 59, 377–382. [Google Scholar] [CrossRef]
- Sarabia-Sainz, A.; Ramos-Clamont, G.; Del Candia-Plata, M.M.C.; Vázquez-Moreno, L. Biorecognition of Escherichia coli K88 adhesin for glycated porcine albumin. Int. J. Biol. Macromol. 2009, 44, 175–181. [Google Scholar] [CrossRef]
- Bode, L. Human milk oligosaccharides: Every baby needs a sugar mama. Glycobiology 2012, 22, 1147–1162. [Google Scholar] [CrossRef]
- Piotrowski, M.; Wultańska, D.; Pituch, H. The prebiotic effect of human milk oligosaccharides 3’- and 6’-sialyllactose on adhesion and biofilm formation by Clostridioides difficile—Pilot study. Microbes Infect. 2022, 24, 104929. [Google Scholar] [CrossRef]
- Facinelli, B.; Marini, E.; Magi, G.; Zampini, L.; Santoro, L.; Catassi, C.; Monachesi, C.; Gabrielli, O.; Coppa, G.V. Breast milk oligosaccharides: Effects of 2′-fucosyllactose and 6′-sialyllactose on the adhesion of Escherichia coli and Salmonella fyris to Caco-2 cells. J. Matern. Fetal Neonatal Med. 2019, 32, 2950–2952. [Google Scholar] [CrossRef]
- Liu, M.; Kang, W.; Hu, Z.; Wang, C.; Zhang, Y. Targeting MyD88: Therapeutic mechanisms and potential applications of the specific inhibitor ST2825. Inflamm. Res. 2023, 72, 2023–2036. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Zhang, Y.; Li, H.; Wang, F.; Yao, S. Toll-like receptor 4: A potential therapeutic target for multiple human diseases. Biomed. Pharmacother. 2023, 166, 115338. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.M.; Balda, M.S.; Fanning, A.S. The structure and regulation of tight junctions. Curr. Opin. Cell Biol. 1993, 5, 772–778. [Google Scholar] [CrossRef] [PubMed]
- Qiao, J.; Sun, Z.; Liang, D.; Li, H. Lactobacillus salivarius alleviates inflammation via NF-κB signaling in ETEC K88-induced IPEC-J2 cells. J. Anim. Sci. Biotechnol. 2020, 11, 76. [Google Scholar] [CrossRef]
- Shi, L.; Fang, B.; Yong, Y.; Li, X.; Gong, D.; Li, J.; Yu, T.; Gooneratne, R.; Gao, Z.; Li, S.; et al. Chitosan oligosaccharide-mediated attenuation of LPS-induced inflammation in IPEC-J2 cells is related to the TLR4/NF-κB signaling pathway. Carbohydr. Polym. 2019, 219, 269–279. [Google Scholar] [CrossRef]
- Farkas, O.; Palócz, O.; Pászti-Gere, E.; Gálfi, P. Polymethoxyflavone Apigenin-Trimethylether Suppresses LPS-Induced Inflammatory Response in Nontransformed Porcine Intestinal Cell Line IPEC-J2. Oxid. Med. Cell. Longev. 2015, 2015, 673847. [Google Scholar] [CrossRef]
- Zhao, L.; Li, M.; Sun, K.; Su, S.; Geng, T.; Sun, H. Hippophae rhamnoides polysaccharides protect IPEC-J2 cells from LPS-induced inflammation, apoptosis and barrier dysfunction in vitro via inhibiting TLR4/NF-κB signaling pathway. Int. J. Biol. Macromol. 2020, 155, 1202–1215. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, O.; Akira, S. Pattern recognition receptors and inflammation. Cell 2010, 140, 805–820. [Google Scholar] [CrossRef]
- Fu, Q.; Lin, Q.; Chen, D.; Yu, B.; Luo, Y.; Zheng, P.; Mao, X.; Huang, Z.; Yu, J.; Luo, J.; et al. β-defensin 118 attenuates inflammation and injury of intestinal epithelial cells upon enterotoxigenic Escherichia coli challenge. BMC Vet. Res. 2022, 18, 142. [Google Scholar] [CrossRef] [PubMed]
- Sodhi, C.P.; Wipf, P.; Yamaguchi, Y.; Fulton, W.B.; Kovler, M.; Niño, D.F.; Zhou, Q.; Banfield, E.; Werts, A.D.; Ladd, M.R.; et al. The human milk oligosaccharides 2’-fucosyllactose and 6’-sialyllactose protect against the development of necrotizing enterocolitis by inhibiting toll-like receptor 4 signaling. Pediatr. Res. 2021, 89, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Won, S.; Sayeed, I.; Peterson, B.L.; Wali, B.; Kahn, J.S.; Stein, D.G. Vitamin D prevents hypoxia/reoxygenation-induced blood-brain barrier disruption via vitamin D receptor-mediated NF-kB signaling pathways. PLoS ONE 2015, 10, e0122821. [Google Scholar] [CrossRef]
- Lee, J.-W.; Bae, C.J.; Choi, Y.-J.; Kim, S.-I.; Kim, N.-H.; Lee, H.J.; Kim, S.-S.; Kwon, Y.-S.; Chun, W. 3,4,5-Trihydroxycinnamic Acid Inhibits LPS-Induced iNOS Expression by Suppressing NF-κB Activation in BV2 Microglial Cells. Korean J. Physiol. Pharmacol. 2012, 16, 107–112. [Google Scholar] [CrossRef]
- Croy, C.H.; Bergqvist, S.; Huxford, T.; Ghosh, G.; Komives, E.A. Biophysical characterization of the free IkappaBalpha ankyrin repeat domain in solution. Protein Sci. 2004, 13, 1767–1777. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, T. The nuclear factor NF-kappaB pathway in inflammation. Cold Spring Harb. Perspect. Biol. 2009, 1, a001651. [Google Scholar] [CrossRef]
- Hsuan, C.-F.; Hsu, H.-F.; Tseng, W.-K.; Lee, T.-L.; Wei, Y.-F.; Hsu, K.-L.; Wu, C.-C.; Houng, J.-Y. Glossogyne tenuifolia Extract Inhibits TNF-α-Induced Expression of Adhesion Molecules in Human Umbilical Vein Endothelial Cells via Blocking the NF-kB Signaling Pathway. Molecules 2015, 20, 16908–16923. [Google Scholar] [CrossRef]
- Duan, Q.; Chen, D.; Yu, B.; Huang, Z.; Luo, Y.; Zheng, P.; Mao, X.; Yu, J.; Luo, J.; Yan, H.; et al. Effect of sialyllactose on growth performance and intestinal epithelium functions in weaned pigs challenged by enterotoxigenic Escherichia coli. J. Anim. Sci. Biotechnol. 2022, 13, 30. [Google Scholar] [CrossRef]
- Letourneau, J.; Levesque, C.; Berthiaume, F.; Jacques, M.; Mourez, M. In Vitro assay of bacterial adhesion onto mammalian epithelial cells. J. Vis. Exp. 2011, 51, e2873. [Google Scholar] [CrossRef]
- Fleige, S.; Walf, V.; Huch, S.; Prgomet, C.; Sehm, J.; Pfaffl, M.W. Comparison of relative mRNA quantification models and the impact of RNA integrity in quantitative real-time RT-PCR. Biotechnol. Lett. 2006, 28, 1601–1613. [Google Scholar] [CrossRef]




| Gene * | Primer Sequence (5′–3′) | Product Size (bp) |
|---|---|---|
| β-Actin | F: TGGAACGGTGAAGGTGACAGC | 177 |
| R: GCTTTTGGGAAGGCAGGGACT | ||
| Caspase 3 | F: GGGATTGAGACGGACAGTGG | 136 |
| R: TGAACCAGGATCCGTCCTTTG | ||
| Caspase 8 | F: TCTGCGGACTGGATGTGATT | 165 |
| R: TCTGAGGTTGCTGGTCACAC | ||
| Caspase 9 | F: AATGCCGATTTGGCTTACGT | 195 |
| R: CATTTGCTTGGCAGTCAGGTT | ||
| IL-1β | F: GTGATGCCAACGTGCAGTCT | 97 |
| R: AGGTGGAGAGCCTTCAGCAT | ||
| IL-6 | F: TGGCTACTGCCTTCCCTACC | 153 |
| R: CACACATCTCCTTTCTCATTGC | ||
| MyD88 | F: CCATTCGAGATGACCCCCTG | 183 |
| R: TAGCAATGGACCAGACGCAG | ||
| NF-κB | F: GTGTGTAAAGAAGCGGGACCT | 139 |
| R: CACTGTCACCTGGAAGCAGAG | ||
| Occludin | F: CTACTCGTCCAACGGGAAAG | 158 |
| R: ACGCCTCCAAGTTACCACTG | ||
| TNF-α | F: GCATCGCCGTCTCCTACCAG | 173 |
| R: GGGCAGGTTGATCTCGGCAC | ||
| TLR4 | F: TTACAGAAGCTGGTTGCCGT | 152 |
| R: TCCAGGTTGGGCAGGTTAGA | ||
| ZO-1 | F: CAGCCCCCGTACATGGAGA | 114 |
| R: GCGCAGACGGTGTTCATAGTT |
| Name | Supplier and Catalog Number | Dilution Factor |
|---|---|---|
| Rabbit anti-ZO-1 | Abcam plc. (Cambridge, UK) | 1:150 |
| Mouse anti-E. coli LPS | Abcam plc. (Cambridge, UK) | 1:50 |
| FITC-conjugated goat anti-rabbit IgG antibody | Abcam plc. (Cambridge, UK) | 1:2500 |
| TRITC-conjugated goat anti-mouse IgG | Abcam plc. (Cambridge, UK) | 1:2500 |
| p-NF-κB p65 | Cell Signaling Technology 3033S (Danvers, MA, USA) | 1:1000 |
| NF-κB p65 | Cell Signaling Technology 6956S (Danvers, MA, USA) | 1:1000 |
| p-IκBα | Invitrogen MA5-15224 (Danvers, MA, USA) | 1:1000 |
| IκBα | Cell Signaling Technology4814S (Danvers, MA, USA) | 1:1000 |
| GAPDH | Cell Signaling Technology 2118S (Danvers, MA, USA) | 1:1000 |
| Anti-rabbit IgG | Cell Signaling Technology 7074S (Danvers, MA, USA) | 1:2500 |
| Anti-mouse IgG | Cell Signaling Technology 7076S (Danvers, MA, USA) | 1:2500 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duan, Q.; Yu, B.; Huang, Z.; Luo, Y.; Zheng, P.; Mao, X.; Yu, J.; Luo, J.; Yan, H.; He, J. Sialyllactose Attenuates Inflammation and Injury of Intestinal Epithelial Cells upon Enterotoxigenic Escherichia coli Infection. Int. J. Mol. Sci. 2025, 26, 3860. https://doi.org/10.3390/ijms26083860
Duan Q, Yu B, Huang Z, Luo Y, Zheng P, Mao X, Yu J, Luo J, Yan H, He J. Sialyllactose Attenuates Inflammation and Injury of Intestinal Epithelial Cells upon Enterotoxigenic Escherichia coli Infection. International Journal of Molecular Sciences. 2025; 26(8):3860. https://doi.org/10.3390/ijms26083860
Chicago/Turabian StyleDuan, Qiming, Bing Yu, Zhiqing Huang, Yuheng Luo, Ping Zheng, Xiangbing Mao, Jie Yu, Junqiu Luo, Hui Yan, and Jun He. 2025. "Sialyllactose Attenuates Inflammation and Injury of Intestinal Epithelial Cells upon Enterotoxigenic Escherichia coli Infection" International Journal of Molecular Sciences 26, no. 8: 3860. https://doi.org/10.3390/ijms26083860
APA StyleDuan, Q., Yu, B., Huang, Z., Luo, Y., Zheng, P., Mao, X., Yu, J., Luo, J., Yan, H., & He, J. (2025). Sialyllactose Attenuates Inflammation and Injury of Intestinal Epithelial Cells upon Enterotoxigenic Escherichia coli Infection. International Journal of Molecular Sciences, 26(8), 3860. https://doi.org/10.3390/ijms26083860









