Spatiotemporal Angiogenic Patterns in the Development of the Mouse Fetal Blood–Brain Barrier System During Pregnancy
Abstract
1. Introduction
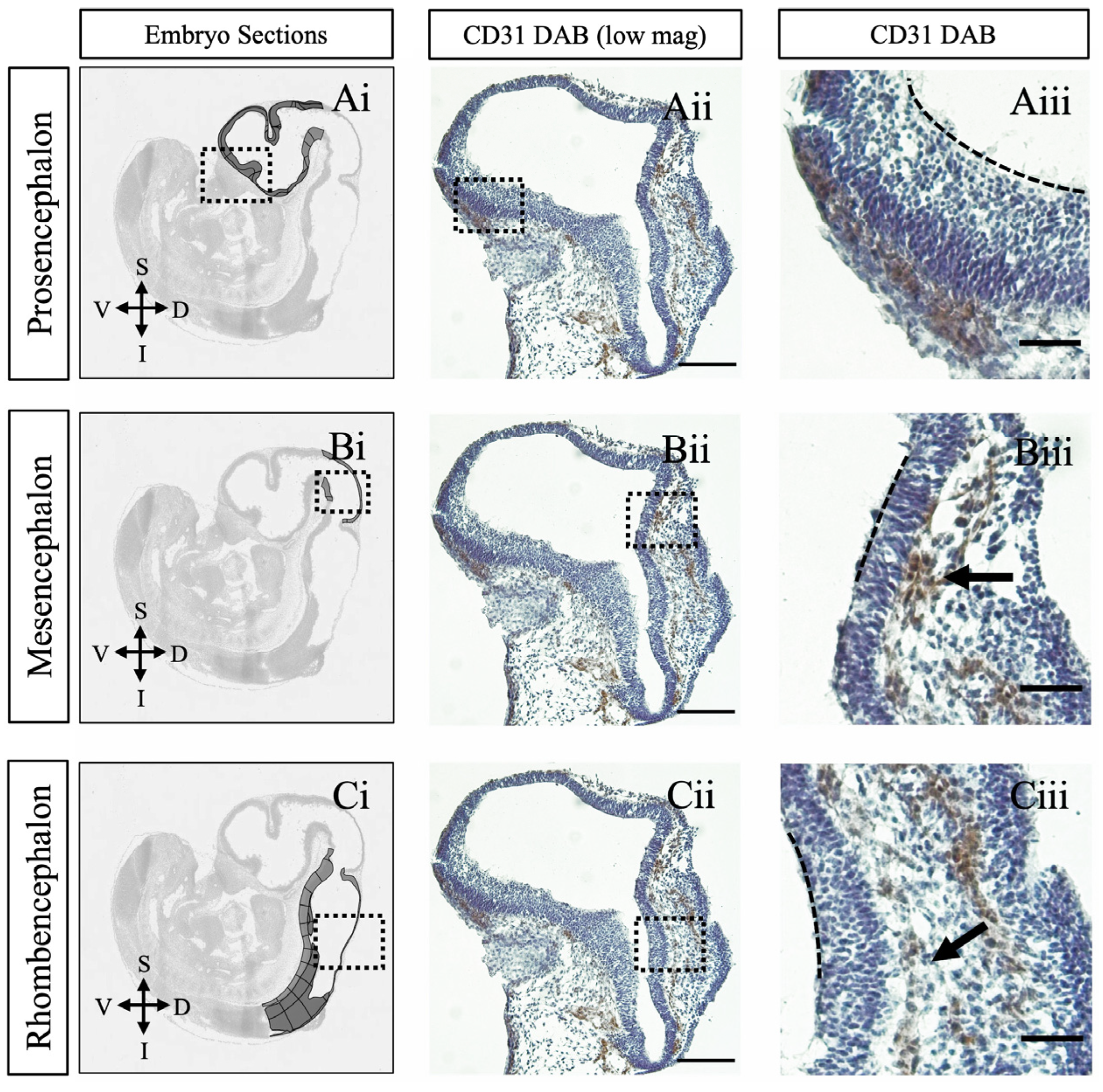
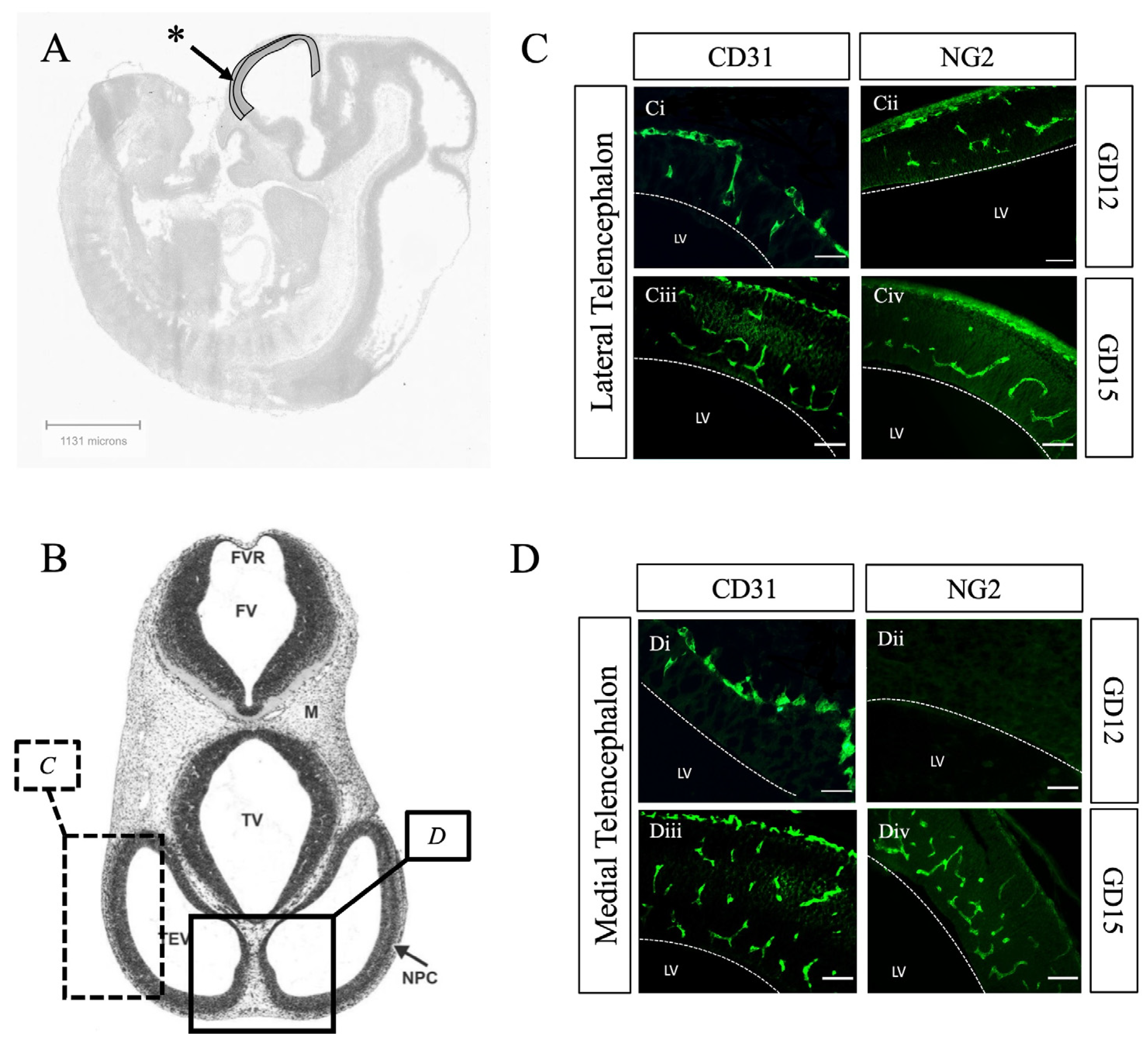
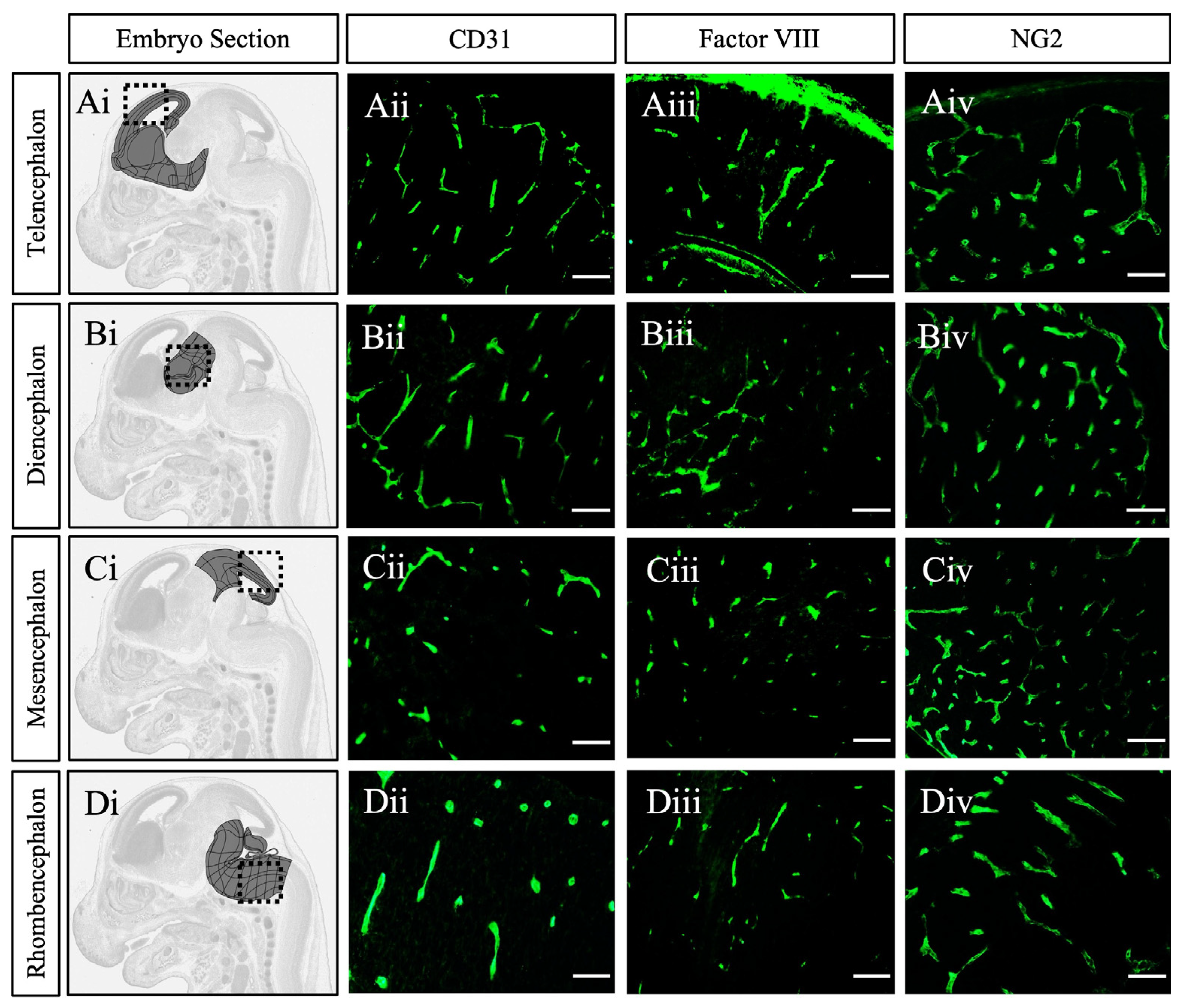
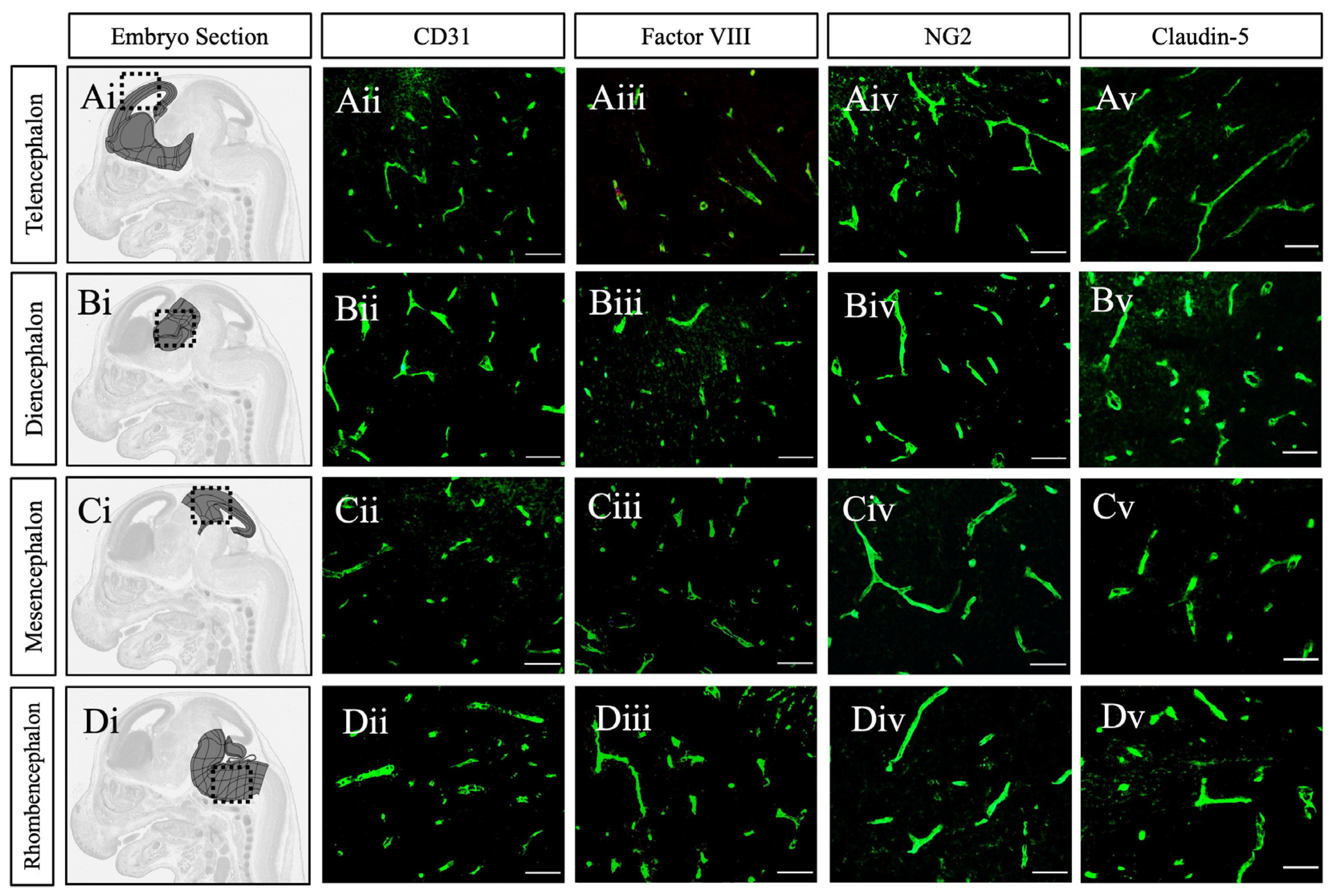
2. Results and Discussion
3. Materials and Methods
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BBB | Blood–brain barrier |
| GD | Gestational day |
| FGR | Fetal growth restriction |
| PNVP | Perineural vascular plexus |
| SVP | Subventricular vascular plexus |
References
- Goasdoué, K.; Miller, S.M.; Colditz, P.B.; Björkman, S.T. Review: The blood-brain barrier; protecting the developing fetal brain. Placenta 2017, 54, 111–116. [Google Scholar] [CrossRef]
- Sandovici, I.; Hoelle, K.; Angiolini, E.; Constância, M. Placental adaptations to the maternal-fetal environment: Implications for fetal growth and developmental programming. Reprod. Biomed. Online 2012, 25, 68–89. [Google Scholar] [CrossRef]
- Saunders, N.R.; Dreifuss, J.J.; Dziegielewska, K.M.; Johansson, P.A.; Habgood, M.D.; Møllgård, K.; Bauer, H.C. The rights and wrongs of blood-brain barrier permeability studies: A walk through 100 years of history. Front. Neurosci. 2014, 8, 404. [Google Scholar] [CrossRef] [PubMed]
- Daneman, R.; Zhou, L.; Kebede, A.A.; Barres, B.A. Pericytes are required for blood-brain barrier integrity during embryogenesis. Nature 2010, 468, 562–566. [Google Scholar] [CrossRef]
- Paredes, I.; Vieira, J.R.; Shah, B.; Ramunno, C.F.; Dyckow, J.; Adler, H.; Richter, M.; Schermann, G.; Giannakouri, E.; Schirmer, L.; et al. Oligodendrocyte precursor cell specification is regulated by bidirectional neural progenitor-endothelial cell crosstalk. Nat. Neurosci. 2021, 24, 478–488. [Google Scholar] [CrossRef] [PubMed]
- Paredes, I.; Himmels, P.; Ruiz de Almodóvar, C. Neurovascular Communication during CNS Development. Dev. Cell 2018, 45, 10–32. [Google Scholar] [CrossRef] [PubMed]
- Daneman, R.; Agalliu, D.; Zhou, L.; Kuhnert, F.; Kuo, C.J.; Barres, B.A. Wnt/beta-catenin signaling is required for CNS, but not non-CNS, angiogenesis. Proc. Natl. Acad. Sci. USA 2009, 106, 641–646. [Google Scholar] [CrossRef]
- Mamber, C.; Kamphuis, W.; Haring, N.L.; Peprah, N.; Middeldorp, J.; Hol, E.M. GFAPδ expression in glia of the developmental and adolescent mouse brain. PLoS ONE 2012, 7, e52659. [Google Scholar] [CrossRef]
- Sasson, E.; Anzi, S.; Bell, B.; Yakovian, O.; Zorsky, M.; Deutsch, U.; Engelhardt, B.; Sherman, E.; Vatine, G.; Dzikowski, R.; et al. Nano-scale architecture of blood-brain barrier tight-junctions. eLife 2021, 10, e63253. [Google Scholar] [CrossRef]
- Steinemann, A.; Galm, I.; Chip, S.; Nitsch, C.; Maly, I.P. Claudin-1, -2 and -3 Are Selectively Expressed in the Epithelia of the Choroid Plexus of the Mouse from Early Development and into Adulthood While Claudin-5 is Restricted to Endothelial Cells. Front. Neuroanat. 2016, 10, 16. [Google Scholar] [CrossRef]
- Knuesel, I.; Chicha, L.; Britschgi, M.; Schobel, S.A.; Bodmer, M.; Hellings, J.A.; Toovey, S.; Prinssen, E.P. Maternal immune activation and abnormal brain development across CNS disorders. Nat. Rev. Neurol. 2014, 10, 643–660. [Google Scholar] [CrossRef] [PubMed]
- Hagberg, H.; Mallard, C.; Ferriero, D.M.; Vannucci, S.J.; Levison, S.W.; Vexler, Z.S.; Gressens, P. The role of inflammation in perinatal brain injury. Nat. Rev. Neurol. 2015, 11, 192–208. [Google Scholar] [CrossRef]
- Wang, L.W.; Tu, Y.F.; Huang, C.C.; Ho, C.J. JNK signaling is the shared pathway linking neuroinflammation, blood-brain barrier disruption, and oligodendroglial apoptosis in the white matter injury of the immature brain. J. Neuroinflamm. 2012, 9, 175. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.Z.; Moster, D.; Harmon, Q.E.; Wilcox, A.J. Association of Preeclampsia in Term Births with Neurodevelopmental Disorders in Offspring. JAMA Psychiatry 2020, 77, 823–829. [Google Scholar] [CrossRef] [PubMed]
- Mathiesen, L.; Mørck, T.A.; Poulsen, M.S.; Nielsen, J.K.S.; Mose, T.; Long, M.; Bonefeld-Jørgensen, E.; Bossi, R.; Knudsen, L.E. Placental transfer of pesticides studied in human placental perfusion. Basic Clin. Pharmacol. Toxicol. 2020, 127, 505–515. [Google Scholar] [CrossRef]
- O’Loughlin, E.; Pakan, J.M.P.; Yilmazer-Hanke, D.; McDermott, K.W. Acute in utero exposure to lipopolysaccharide induces inflammation in the pre- and postnatal brain and alters the glial cytoarchitecture in the developing amygdala. J. Neuroinflamm. 2017, 14, 212. [Google Scholar] [CrossRef]
- Ek, C.J.; Dziegielewska, K.M.; Stolp, H.; Saunders, N.R. Functional effectiveness of the blood-brain barrier to small water-soluble molecules in developing and adult opossum (Monodelphis domestica). J. Comp. Neurol. 2006, 496, 13–26. [Google Scholar] [CrossRef] [PubMed]
- Saunders, N.R.; Dziegielewska, K.M.; Møllgård, K.; Habgood, M.D. Physiology and molecular biology of barrier mechanisms in the fetal and neonatal brain. J. Physiol. 2018, 596, 5723–5756. [Google Scholar] [CrossRef]
- Sohet, F.; Lin, C.; Munji, R.N.; Lee, S.Y.; Ruderisch, N.; Soung, A.; Arnold, T.D.; Derugin, N.; Vexler, Z.S.; Yen, F.T.; et al. LSR/angulin-1 is a tricellular tight junction protein involved in blood-brain barrier formation. J. Cell Biol. 2015, 208, 703–711. [Google Scholar] [CrossRef]
- Ben-Zvi, A.; Lacoste, B.; Kur, E.; Andreone, B.J.; Mayshar, Y.; Yan, H.; Gu, C. Mfsd2a is critical for the formation and function of the blood-brain barrier. Nature 2014, 509, 507–511. [Google Scholar] [CrossRef]
- Ben-Zvi, A.; Liebner, S. Developmental regulation of barrier- and non-barrier blood vessels in the CNS. J. Intern. Med. 2022, 292, 31–46. [Google Scholar] [CrossRef] [PubMed]
- Virgintino, D.; Errede, M.; Girolamo, F.; Capobianco, C.; Robertson, D.; Vimercati, A.; Serio, G.; Di Benedetto, A.; Yonekawa, Y.; Frei, K.; et al. Fetal blood-brain barrier P-glycoprotein contributes to brain protection during human development. J. Neuropathol. Exp. Neurol. 2008, 67, 50–61. [Google Scholar] [CrossRef]
- Tata, M.; Ruhrberg, C.; Fantin, A. Vascularisation of the central nervous system. Mech. Dev. 2015, 138, 26–36. [Google Scholar] [CrossRef]
- Ruhrberg, C.; Bautch, V.L. Neurovascular development and links to disease. Cell Mol. Life Sci. 2013, 70, 1675–1684. [Google Scholar] [CrossRef] [PubMed]
- Puelles, L.; Martínez-Marin, R.; Melgarejo-Otalora, P.; Ayad, A.; Valavanis, A.; Ferran, J.L. Patterned Vascularization of Embryonic Mouse Forebrain, and Neuromeric Topology of Major Human Subarachnoidal Arterial Branches: A Prosomeric Mapping. Front. Neuroanat. 2019, 13, 59. [Google Scholar] [CrossRef]
- Allen Reference Atlas—Mouse Brain [Brain Atlas]. Available online: https://atlas.brain-map.org/ (accessed on 13 February 2025).
- Vasudevan, A.; Bhide, P.G. Angiogenesis in the embryonic CNS: A new twist on an old tale. Cell Adhes. Migr. 2008, 2, 167–169. [Google Scholar] [CrossRef]
- Fantin, A.; Vieira, J.M.; Gestri, G.; Denti, L.; Schwarz, Q.; Prykhozhij, S.; Peri, F.; Wilson, S.W.; Ruhrberg, C. Tissue macrophages act as cellular chaperones for vascular anastomosis downstream of VEGF-mediated endothelial tip cell induction. Blood 2010, 116, 829–840. [Google Scholar] [CrossRef] [PubMed]
- Arnold, T.D.; Niaudet, C.; Pang, M.F.; Siegenthaler, J.; Gaengel, K.; Jung, B.; Ferrero, G.M.; Mukouyama, Y.S.; Fuxe, J.; Akhurst, R.; et al. Excessive vascular sprouting underlies cerebral hemorrhage in mice lacking αVβ8-TGFβ signaling in the brain. Development 2014, 141, 4489–4499. [Google Scholar] [CrossRef]
- Ruiz-Reig, N.; Studer, M. Rostro-Caudal and Caudo-Rostral Migrations in the Telencephalon: Going Forward or Backward? Front. Neurosci. 2017, 11, 692. [Google Scholar] [CrossRef]
- Armulik, A.; Genové, G.; Mäe, M.; Nisancioglu, M.H.; Wallgard, E.; Niaudet, C.; He, L.; Norlin, J.; Lindblom, P.; Strittmatter, K.; et al. Pericytes regulate the blood-brain barrier. Nature 2010, 468, 557–561. [Google Scholar] [CrossRef]
- Chen, V.S.; Morrison, J.P.; Southwell, M.F.; Foley, J.F.; Bolon, B.; Elmore, S.A. Histology Atlas of the Developing Prenatal and Postnatal Mouse Central Nervous System, with Emphasis on Prenatal Days E7.5 to E18.5. Toxicol. Pathol. 2017, 45, 705–744. [Google Scholar] [CrossRef] [PubMed]
- Baeten, K.M.; Akassoglou, K. Extracellular matrix and matrix receptors in blood-brain barrier formation and stroke. Dev. Neurobiol. 2011, 71, 1018–1039. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Shen, M.; Stockton, M.E.; Zhao, X. Hippocampal deficits in neurodevelopmental disorders. Neurobiol. Learn. Mem. 2019, 165, 106945. [Google Scholar] [CrossRef]
- Cieślik, M.; Gassowska-Dobrowolska, M.; Zawadzka, A.; Frontczak-Baniewicz, M.; Gewartowska, M.; Dominiak, A.; Czapski, G.A.; Adamczyk, A. The Synaptic Dysregulation in Adolescent Rats Exposed to Maternal Immune Activation. Front. Mol. Neurosci. 2020, 13, 555290. [Google Scholar] [CrossRef] [PubMed]
- Makinodan, M.; Tatsumi, K.; Manabe, T.; Yamauchi, T.; Makinodan, E.; Matsuyoshi, H.; Shimoda, S.; Noriyama, Y.; Kishimoto, T.; Wanaka, A. Maternal immune activation in mice delays myelination and axonal development in the hippocampus of the offspring. J. Neurosci. Res. 2008, 86, 2190–2200. [Google Scholar] [CrossRef]
- Noubade, R.; del Rio, R.; McElvany, B.; Zachary, J.F.; Millward, J.M.; Wagner, D.D.; Offner, H.; Blankenhorn, E.P.; Teuscher, C. von-Willebrand factor influences blood brain barrier permeability and brain inflammation in experimental allergic encephalomyelitis. Am. J. Pathol. 2008, 173, 892–900. [Google Scholar] [CrossRef]
- Suidan, G.L.; Brill, A.; De Meyer, S.F.; Voorhees, J.R.; Cifuni, S.M.; Cabral, J.E.; Wagner, D.D. Endothelial Von Willebrand factor promotes blood-brain barrier flexibility and provides protection from hypoxia and seizures in mice. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 2112–2120. [Google Scholar] [CrossRef]
- van Leeuwen, L.M.; Evans, R.J.; Jim, K.K.; Verboom, T.; Fang, X.; Bojarczuk, A.; Malicki, J.; Johnston, S.A.; van der Sar, A.M. A transgenic zebrafish model for the in vivo study of the blood and choroid plexus brain barriers using claudin 5. Biol. Open 2018, 7, bio030494. [Google Scholar] [CrossRef]
- Zhao, X.; Bhattacharyya, A. Human Models Are Needed for Studying Human Neurodevelopmental Disorders. Am. J. Hum. Genet. 2018, 103, 829–857. [Google Scholar] [CrossRef]
- Shi, Y.; Wu, Q.; Wang, X. Modeling brain development and diseases with human cerebral organoids. Curr. Opin. Neurobiol. 2021, 66, 103–115. [Google Scholar] [CrossRef]
- Fan, X.; Rai, A.; Kambham, N.; Sung, J.F.; Singh, N.; Petitt, M.; Dhal, S.; Agrawal, R.; Sutton, R.E.; Druzin, M.L.; et al. Endometrial VEGF induces placental sFLT1 and leads to pregnancy complications. J. Clin. Investig. 2014, 124, 4941–4952. [Google Scholar] [CrossRef] [PubMed]
- Anamthathmakula, P.; Shallie, P.D.; Nayak, N.; Dhal, S.; Vivian, J.L.; Mor, G.; Soares, M.J.; Nayak, N.R. Variable Cre Recombination Efficiency in Placentas of Cyp19-Cre ROSAmT/mG Transgenic Mice. Cells 2023, 12, 2096. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Krieg, S.; Kuo, C.J.; Wiegand, S.J.; Rabinovitch, M.; Druzin, M.L.; Brenner, R.M.; Giudice, L.C.; Nayak, N.R. VEGF blockade inhibits angiogenesis and reepithelialization of endometrium. FASEB J. 2008, 22, 3571–3580. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brown, S.N.; Shallie, P.; Sierra, C.A.; Nayak, N.; Odibo, A.O.; Monaghan-Nichols, P.; Nayak, N.R. Spatiotemporal Angiogenic Patterns in the Development of the Mouse Fetal Blood–Brain Barrier System During Pregnancy. Int. J. Mol. Sci. 2025, 26, 3862. https://doi.org/10.3390/ijms26083862
Brown SN, Shallie P, Sierra CA, Nayak N, Odibo AO, Monaghan-Nichols P, Nayak NR. Spatiotemporal Angiogenic Patterns in the Development of the Mouse Fetal Blood–Brain Barrier System During Pregnancy. International Journal of Molecular Sciences. 2025; 26(8):3862. https://doi.org/10.3390/ijms26083862
Chicago/Turabian StyleBrown, Samuel Nofsinger, Philemon Shallie, Connor A. Sierra, Neha Nayak, Anthony O. Odibo, Paula Monaghan-Nichols, and Nihar R. Nayak. 2025. "Spatiotemporal Angiogenic Patterns in the Development of the Mouse Fetal Blood–Brain Barrier System During Pregnancy" International Journal of Molecular Sciences 26, no. 8: 3862. https://doi.org/10.3390/ijms26083862
APA StyleBrown, S. N., Shallie, P., Sierra, C. A., Nayak, N., Odibo, A. O., Monaghan-Nichols, P., & Nayak, N. R. (2025). Spatiotemporal Angiogenic Patterns in the Development of the Mouse Fetal Blood–Brain Barrier System During Pregnancy. International Journal of Molecular Sciences, 26(8), 3862. https://doi.org/10.3390/ijms26083862





