NID2 Affects Prognosis of Glioma via Activating the Akt Signaling Pathway
Abstract
:1. Introduction
2. Results
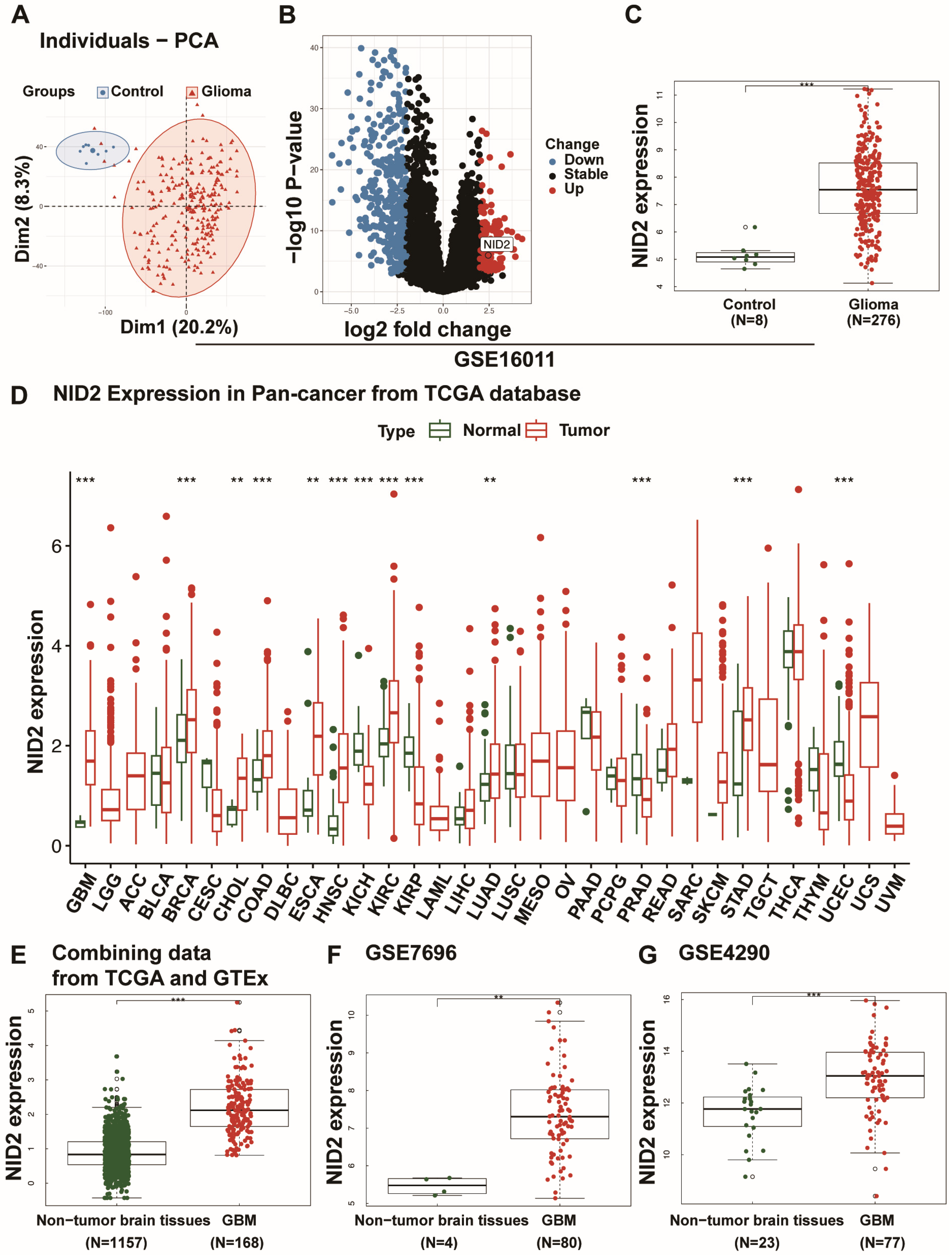
2.1. NID2 Upregulation in Glioma
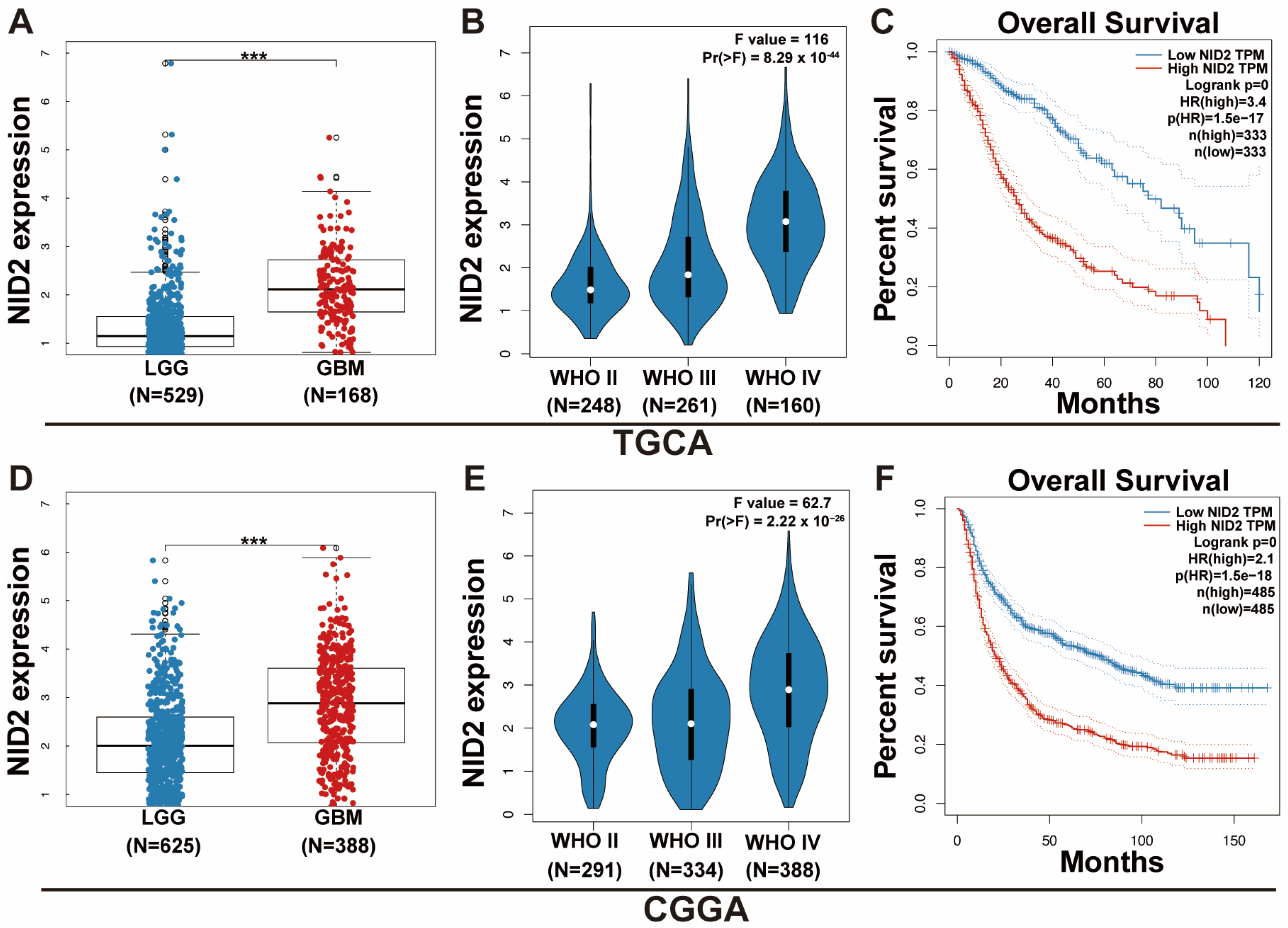
2.2. NID2 Upregulation Predicts Worse Patient Survival and Higher Tumor Grade
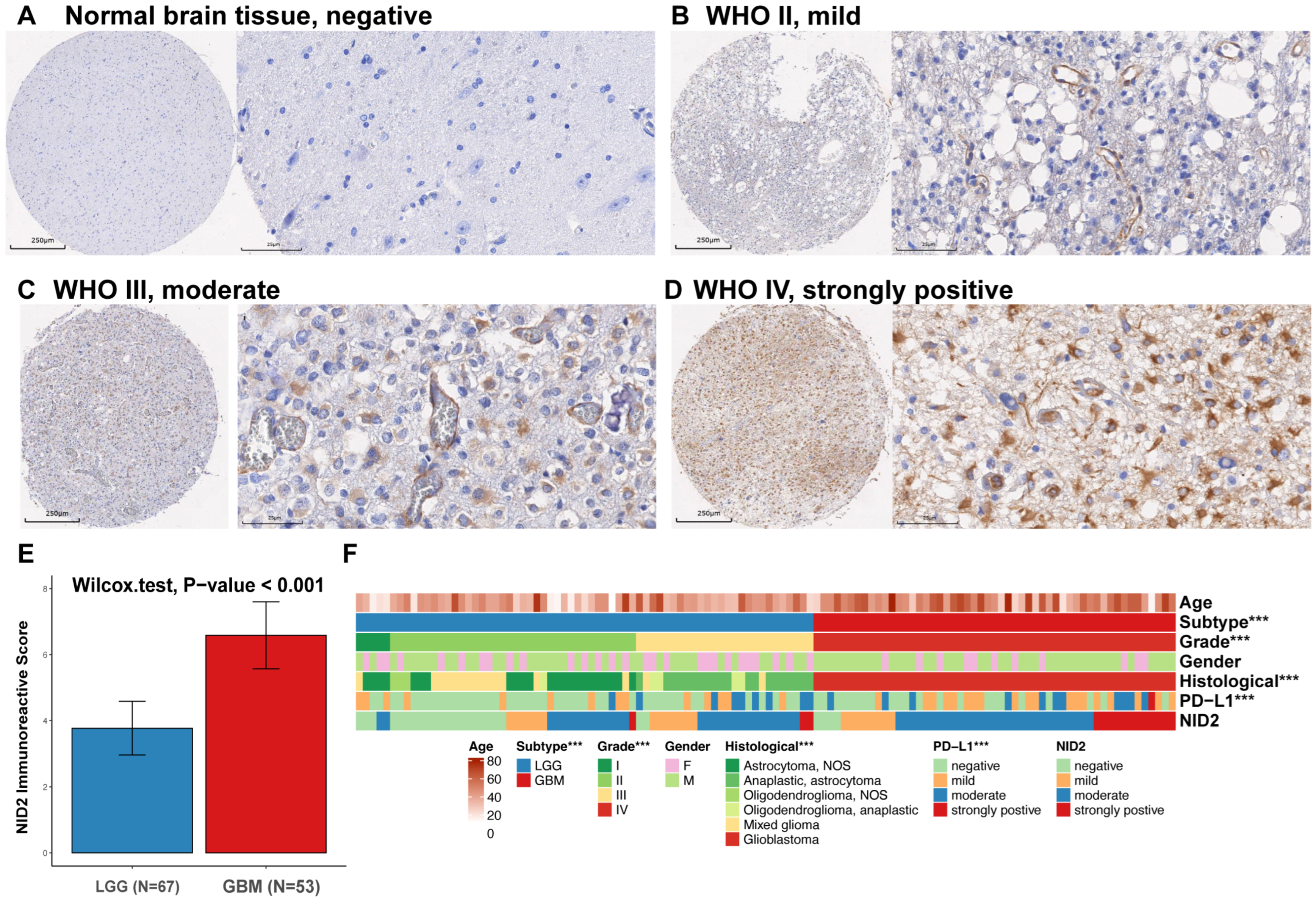
2.3. NID2 Expression Study in Glioma TMA
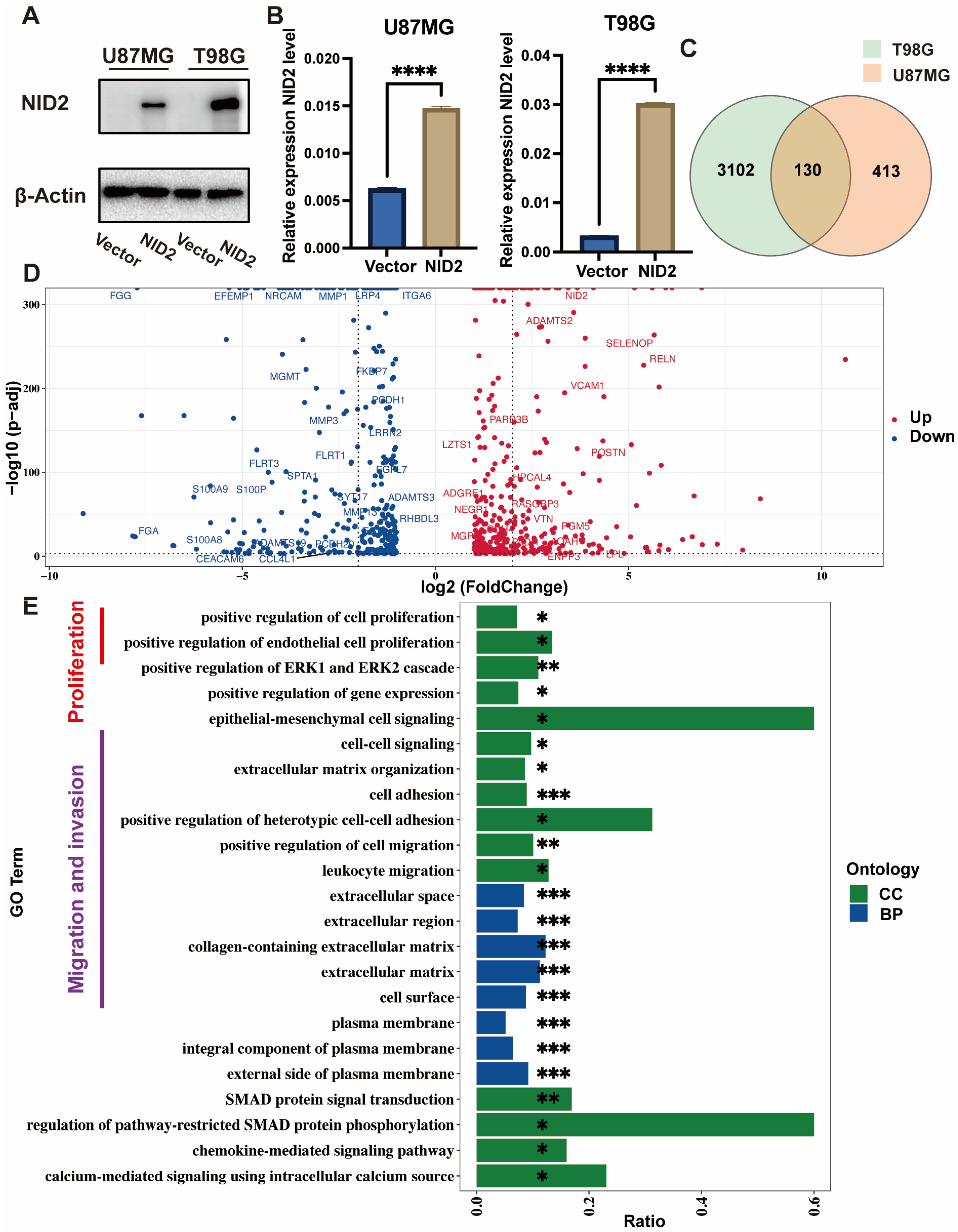
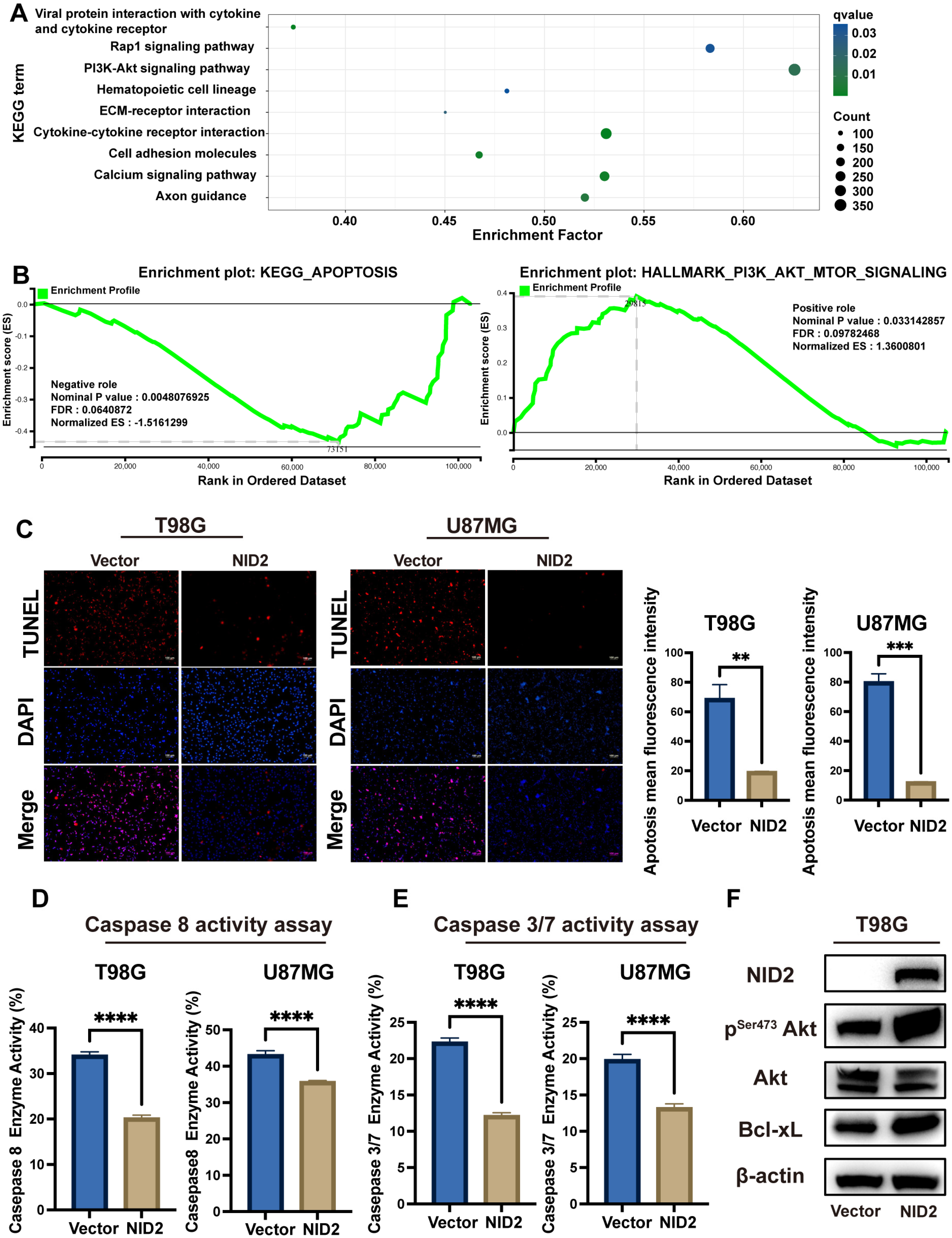
2.4. NID2-Associated DEGs Enrichment Analysis Revealed Key Tumorigenic Pathway Activation
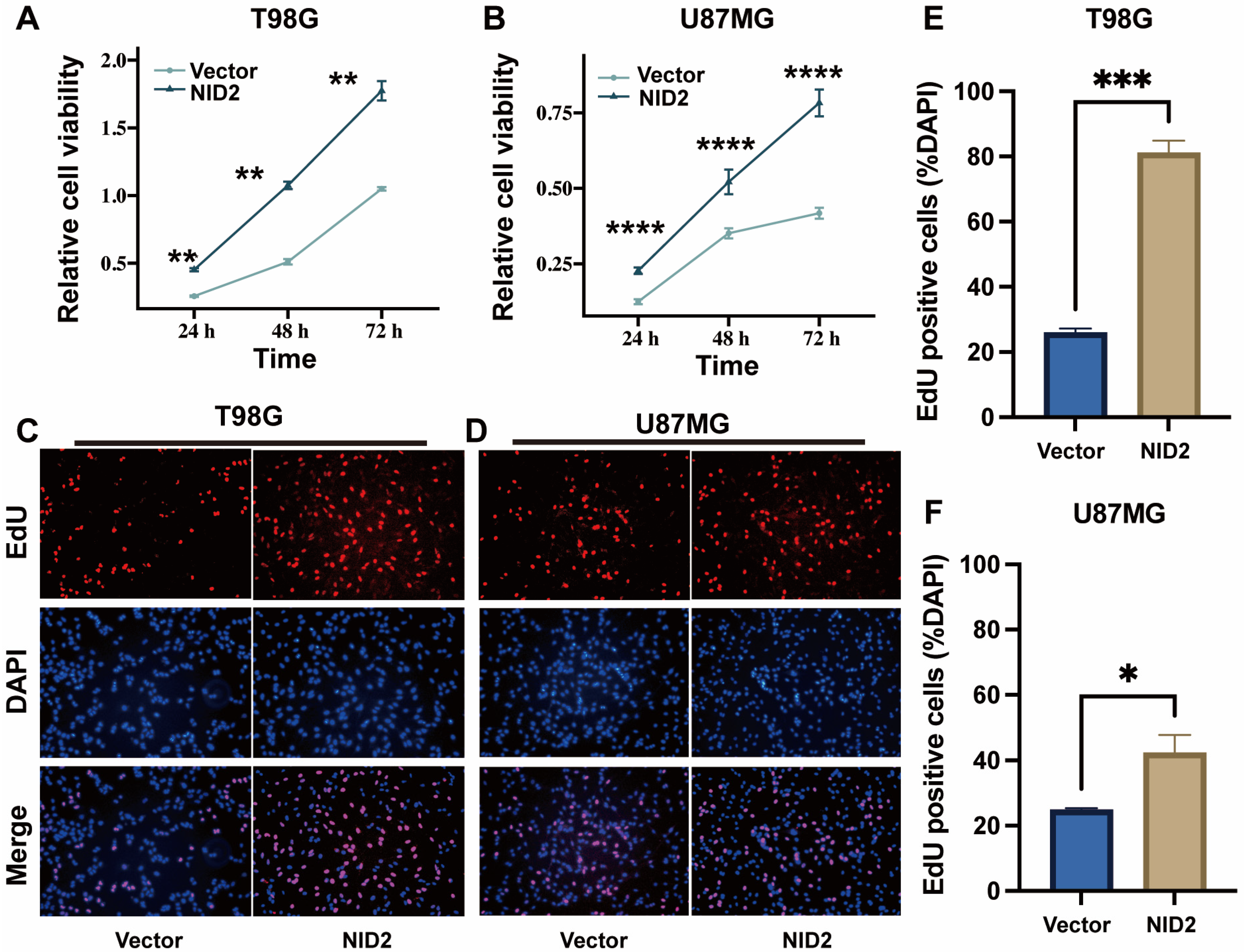
2.5. NID2 Overexpression Promoted Proliferation of Glioma Cells
2.6. NID2 Overexpression Promoted Migration and Invasion of Glioma Cells
2.7. Akt Was Activated in NID2-Overexpressing Cells
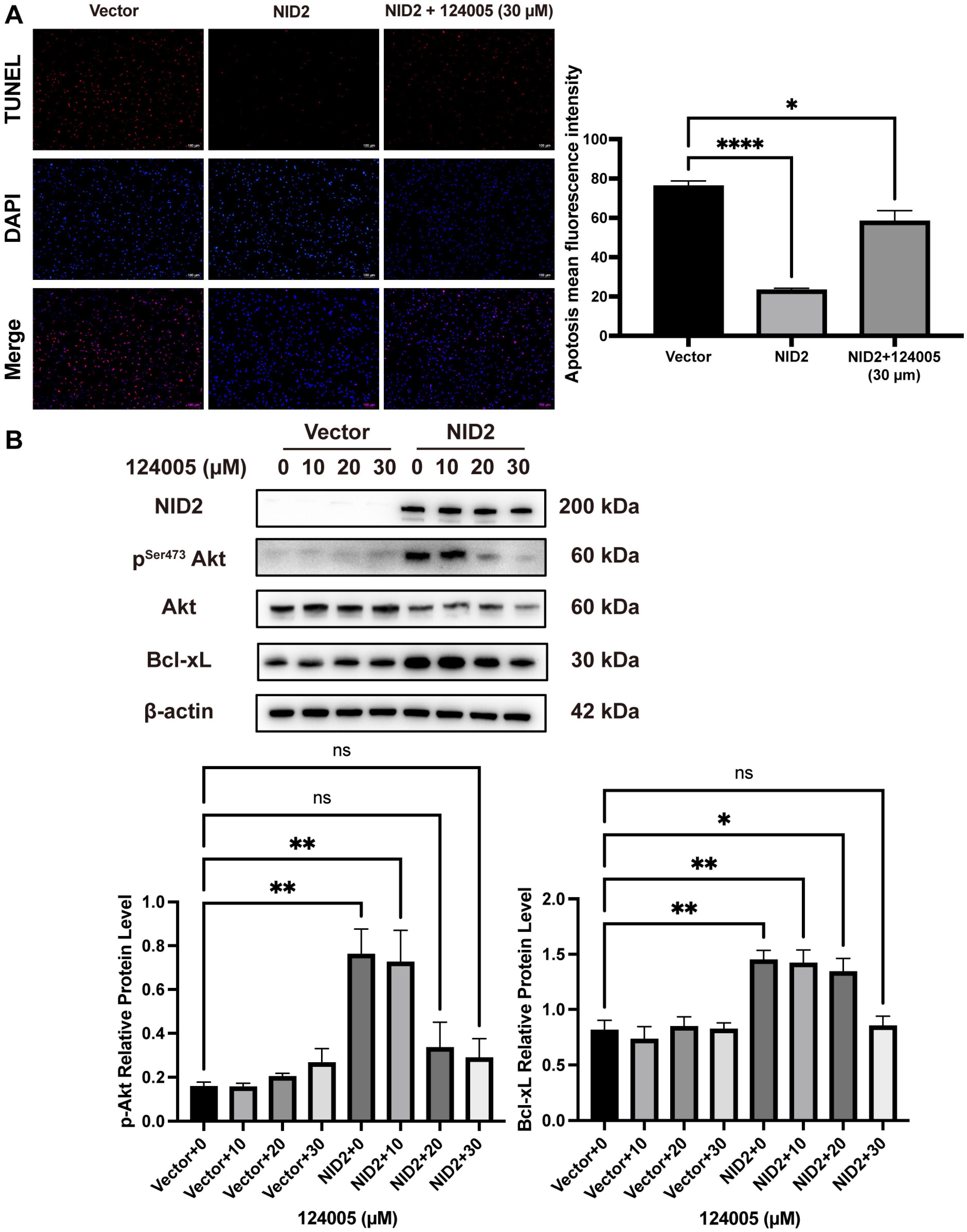
2.8. Akt Inhibitor 124005 Reversed Anti-Apoptotic Effect of NID2 Overexpression
3. Discussion
4. Materials and Methods
4.1. Cell Lines, Cell Culture, and Transfection
4.2. Datasets and Bioinformatic Processing
4.3. Glioma Tissue Microarray
4.4. Survival Analysis
4.5. Western Blotting
4.6. Reverse Transcription and RT-qPCR
4.7. Cell Counting Kit-8 and EdU Assays
4.8. Wound Healing and Transwell Assays
4.9. Apoptosis Assay
4.10. Transcriptome Sequencing (RNA-Seq) Analysis
4.11. Immunohistochemistry
4.12. Immunofluorescence
4.13. Statistics
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| NID2 | Nidogen-2 |
| ECM | Extracellular matrix |
| TCGA | Cancer Genome Atlas |
| CGGA | Chinese Glioma Genome Atlas |
| GBM | Glioblastoma multiforme |
| LGG | Lower-grade gliomas |
| WGCNA | Weighted gene co-expression network analysis |
| TMA | Tissue microarray |
References
- Lapointe, S.; Perry, A.; Butowski, N.A. Primary brain tumours in adults. Lancet 2018, 392, 432–446. [Google Scholar] [CrossRef] [PubMed]
- Antonelli, M.; Poliani, P.L. Adult type diffuse gliomas in the new 2021 WHO Classification. Pathologica 2022, 114, 397–409. [Google Scholar] [CrossRef] [PubMed]
- Weller, M.; van den Bent, M.; Preusser, M.; Le Rhun, E.; Tonn, J.C.; Minniti, G.; Bendszus, M.; Balana, C.; Chinot, O.; Dirven, L.; et al. EANO guidelines on the diagnosis and treatment of diffuse gliomas of adulthood. Nat. Rev. Clin. Oncol. 2021, 18, 170–186. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Feng, L.; Lu, Y. Glioblastoma multiforme: Diagnosis, treatment, and invasion. J. Biomed. Res. 2022, 37, 47–58. [Google Scholar] [CrossRef]
- Nishikawa, T.; Watanabe, R.; Kitano, Y.; Yamamichi, A.; Motomura, K.; Ohka, F.; Aoki, K.; Hirano, M.; Kato, A.; Yamaguchi, J.; et al. Reliability of IDH1-R132H and ATRX and/or p53 immunohistochemistry for molecular subclassification of Grade 2/3 gliomas. Brain Tumor Pathol. 2022, 39, 14–24. [Google Scholar] [CrossRef]
- Cancer Genome Atlas Research, N.; Brat, D.J.; Verhaak, R.G.; Aldape, K.D.; Yung, W.K.; Salama, S.R.; Cooper, L.A.; Rheinbay, E.; Miller, C.R.; Vitucci, M.; et al. Comprehensive, Integrative Genomic Analysis of Diffuse Lower-Grade Gliomas. N. Engl. J. Med. 2015, 372, 2481–2498. [Google Scholar] [CrossRef]
- Tom, M.C.; Cahill, D.P.; Buckner, J.C.; Dietrich, J.; Parsons, M.W.; Yu, J.S. Management for Different Glioma Subtypes: Are All Low-Grade Gliomas Created Equal? Am. Soc. Clin. Oncol. Educ. Book 2019, 39, 133–145. [Google Scholar] [CrossRef]
- Dziadek, M. Role of laminin-nidogen complexes in basement membrane formation during embryonic development. Experientia 1995, 51, 901–913. [Google Scholar] [CrossRef]
- Bader, B.L.; Smyth, N.; Nedbal, S.; Miosge, N.; Baranowsky, A.; Mokkapati, S.; Murshed, M.; Nischt, R. Compound genetic ablation of nidogen 1 and 2 causes basement membrane defects and perinatal lethality in mice. Mol. Cell Biol. 2005, 25, 6846–6856. [Google Scholar] [CrossRef]
- Kohfeldt, E.; Sasaki, T.; Gohring, W.; Timpl, R. Nidogen-2: A new basement membrane protein with diverse binding properties. J. Mol. Biol. 1998, 282, 99–109. [Google Scholar] [CrossRef]
- Bose, K.; Nischt, R.; Page, A.; Bader, B.L.; Paulsson, M.; Smyth, N. Loss of nidogen-1 and -2 results in syndactyly and changes in limb development. J. Biol. Chem. 2006, 281, 39620–39629. [Google Scholar] [CrossRef] [PubMed]
- Muffler, S.; Stark, H.J.; Amoros, M.; Falkowska-Hansen, B.; Boehnke, K.; Buhring, H.J.; Marme, A.; Bickenbach, J.R.; Boukamp, P. A stable niche supports long-term maintenance of human epidermal stem cells in organotypic cultures. Stem Cells 2008, 26, 2506–2515. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Chen, S.; Pei, Y.A.; Pei, M. Nidogen: A matrix protein with potential roles in musculoskeletal tissue regeneration. Genes Dis. 2022, 9, 598–609. [Google Scholar] [CrossRef] [PubMed]
- Prakash, J.; Shaked, Y. The Interplay between Extracellular Matrix Remodeling and Cancer Therapeutics. Cancer Discov. 2024, 14, 1375–1388. [Google Scholar] [CrossRef]
- Kefalides, N.A.; Borel, J.P. Structural Macromolecules: Laminins, Entactin/Nidogen, and Proteoglycans (Perlecan, Agrin). In Basement Membranes: Cell and Molecular Biology; Springer: Berlin/Heidelberg, Germany, 2005; pp. 147–197. [Google Scholar]
- Torky, H.A.; Sherif, A.; Abo-Louz, A.; Ali, M.; Ahmed, A.; Ali, A. Evaluation of Serum Nidogen-2 as a Screening and Diagnostic Tool for Ovarian Cancer. Gynecol. Obstet. Investig. 2018, 83, 461–465. [Google Scholar] [CrossRef]
- Kuk, C.; Gunawardana, C.G.; Soosaipillai, A.; Kobayashi, H.; Li, L.; Zheng, Y.; Diamandis, E.P. Nidogen-2: A new serum biomarker for ovarian cancer. Clin. Biochem. 2010, 43, 355–361. [Google Scholar] [CrossRef]
- Buckanovich, R.J.; Sasaroli, D.; O’Brien-Jenkins, A.; Botbyl, J.; Hammond, R.; Katsaros, D.; Sandaltzopoulos, R.; Liotta, L.A.; Gimotty, P.A.; Coukos, G. Tumor Vascular Proteins As Biomarkers in Ovarian Cancer. J. Clin. Oncol. 2007, 25, 852–861. [Google Scholar] [CrossRef]
- Rokavec, M.; Jaeckel, S.; Hermeking, H. Nidogen-1/NID1 Function and Regulation during Progression and Metastasis of Colorectal Cancer. Cancers 2023, 15, 5316. [Google Scholar] [CrossRef]
- Rokavec, M.; Bouznad, N.; Hermeking, H. Paracrine Induction of Epithelial-Mesenchymal Transition Between Colorectal Cancer Cells and its Suppression by a p53/miR-192/215/NID1 Axis. Cell Mol. Gastroenterol. Hepatol. 2019, 7, 783–802. [Google Scholar] [CrossRef]
- Yu, Z.H.; Wang, Y.M.; Jiang, Y.Z.; Ma, S.J.; Zhong, Q.; Wan, Y.Y.; Wang, X.W. NID2 can serve as a potential prognosis prediction biomarker and promotes the invasion and migration of gastric cancer. Pathol. Res. Pract. 2019, 215, 152553. [Google Scholar] [CrossRef]
- Aleckovic, M.; Wei, Y.; LeRoy, G.; Sidoli, S.; Liu, D.D.; Garcia, B.A.; Kang, Y. Identification of Nidogen 1 as a lung metastasis protein through secretome analysis. Genes Dev. 2017, 31, 1439–1455. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Xu, C.; Liu, J.; Yang, J.; Gao, Q.; Ye, F. Nidogen-1 expression is associated with overall survival and temozolomide sensitivity in low-grade glioma patients. Aging 2021, 13, 9085–9107. [Google Scholar] [CrossRef] [PubMed]
- Tan, A.C.; Ashley, D.M.; Lopez, G.Y.; Malinzak, M.; Friedman, H.S.; Khasraw, M. Management of glioblastoma: State of the art and future directions. CA Cancer J. Clin. 2020, 70, 299–312. [Google Scholar] [CrossRef] [PubMed]
- Omuro, A.; DeAngelis, L.M. Glioblastoma and other malignant gliomas: A clinical review. JAMA 2013, 310, 1842–1850. [Google Scholar] [CrossRef]
- Schiff, D.; Brown, P.D.; Giannini, C. Outcome in adult low-grade glioma: The impact of prognostic factors and treatment. Neurology 2007, 69, 1366–1373. [Google Scholar] [CrossRef]
- Xiao, Y.; Yu, D. Tumor microenvironment as a therapeutic target in cancer. Pharmacol. Ther. 2021, 221, 107753. [Google Scholar] [CrossRef]
- Cruz-Acuna, R.; Vunjak-Novakovic, G.; Burdick, J.A.; Rustgi, A.K. Emerging technologies provide insights on cancer extracellular matrix biology and therapeutics. iScience 2021, 24, 102475. [Google Scholar] [CrossRef]
- Sleeboom, J.J.F.; van Tienderen, G.S.; Schenke-Layland, K.; van der Laan, L.J.W.; Khalil, A.A.; Verstegen, M.M.A. The extracellular matrix as hallmark of cancer and metastasis: From biomechanics to therapeutic targets. Sci. Transl. Med. 2024, 16, eadg3840. [Google Scholar] [CrossRef]
- Tsai, P.J.; Lai, Y.H.; Manne, R.K.; Tsai, Y.S.; Sarbassov, D.; Lin, H.K. Akt: A key transducer in cancer. J. Biomed. Sci. 2022, 29, 76. [Google Scholar] [CrossRef]
- Liu, X.; He, H.; Zhang, F.; Hu, X.; Bi, F.; Li, K.; Yu, H.; Zhao, Y.; Teng, X.; Li, J.; et al. m6A methylated EphA2 and VEGFA through IGF2BP2/3 regulation promotes vasculogenic mimicry in colorectal cancer via PI3K/AKT and ERK1/2 signaling. Cell Death Dis. 2022, 13, 483. [Google Scholar] [CrossRef]
- Verdugo, E.; Puerto, I.; Medina, M.A. An update on the molecular biology of glioblastoma, with clinical implications and progress in its treatment. Cancer Commun. 2022, 42, 1083–1111. [Google Scholar] [CrossRef] [PubMed]
- Schaefer, T.; Lengerke, C. SOX2 protein biochemistry in stemness, reprogramming, and cancer: The PI3K/AKT/SOX2 axis and beyond. Oncogene 2020, 39, 278–292. [Google Scholar] [CrossRef] [PubMed]
- Bhutani, J.; Sheikh, A.; Niazi, A.K. Akt inhibitors: Mechanism of action and implications for anticancer therapeutics. Infect. Agent. Cancer 2013, 8, 49. [Google Scholar] [CrossRef] [PubMed]
- Kwiatkowska, A.; Krawczyk, D.; Kułak, K. Can Nidogen-1 and Nidogen-2 improve our preoperative cancer detection rate? J. Pre-Clin. Clin. Res. 2021, 15, 80–86. [Google Scholar] [CrossRef]
- Cancer Genome Atlas Research, N. Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature 2008, 455, 1061–1068. [Google Scholar] [CrossRef]
- Ritchie, M.E.; Phipson, B.; Wu, D.; Hu, Y.; Law, C.W.; Shi, W.; Smyth, G.K. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015, 43, e47. [Google Scholar] [CrossRef]
- Zhao, Z.; Zhang, K.-N.; Wang, Q.; Li, G.; Zeng, F.; Zhang, Y.; Wu, F.; Chai, R.; Wang, Z.; Zhang, C.; et al. Chinese Glioma Genome Atlas (CGGA): A Comprehensive Resource with Functional Genomic Data from Chinese Glioma Patients. Genom. Proteom. Bioinform. 2021, 19, 1–12. [Google Scholar] [CrossRef]
- Gravendeel, L.A.; Kouwenhoven, M.C.; Gevaert, O.; de Rooi, J.J.; Stubbs, A.P.; Duijm, J.E.; Daemen, A.; Bleeker, F.E.; Bralten, L.B.; Kloosterhof, N.K.; et al. Intrinsic gene expression profiles of gliomas are a better predictor of survival than histology. Cancer Res. 2009, 69, 9065–9072. [Google Scholar] [CrossRef]
- Murat, A.; Migliavacca, E.; Gorlia, T.; Lambiv, W.L.; Shay, T.; Hamou, M.-F.; De Tribolet, N.; Regli, L.; Wick, W.; Kouwenhoven, M.C.M.; et al. Stem Cell–Related “Self-Renewal” Signature and High Epidermal Growth Factor Receptor Expression Associated With Resistance to Concomitant Chemoradiotherapy in Glioblastoma. J. Clin. Oncol. 2008, 26, 3015–3024. [Google Scholar] [CrossRef]
- Sun, L.; Hui, A.-M.; Su, Q.; Vortmeyer, A.; Kotliarov, Y.; Pastorino, S.; Passaniti, A.; Menon, J.; Walling, J.; Bailey, R.; et al. Neuronal and glioma-derived stem cell factor induces angiogenesis within the brain. Cancer Cell 2006, 9, 287–300. [Google Scholar] [CrossRef]
- Davis, S.; Meltzer, P.S. GEOquery: A bridge between the Gene Expression Omnibus (GEO) and BioConductor. Bioinformatics 2007, 23, 1846–1847. [Google Scholar] [CrossRef] [PubMed]
- Langfelder, P.; Horvath, S. WGCNA: An R package for weighted correlation network analysis. BMC Bioinform. 2008, 9, 559. [Google Scholar] [CrossRef] [PubMed]
- Lonsdale, J.; Thomas, J.; Salvatore, M.; Phillips, R.; Lo, E.; Shad, S.; Hasz, R.; Walters, G.; Garcia, F.; Young, N.; et al. The Genotype-Tissue Expression (GTEx) project. Nat. Genet. 2013, 45, 580–585. [Google Scholar] [CrossRef] [PubMed]
- Fedchenko, N.; Reifenrath, J. Different approaches for interpretation and reporting of immunohistochemistry analysis results in the bone tissue—A review. Diagn. Pathol. 2014, 9, 221. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lan, Z.; Xiao, Y.; Liao, Y.; Li, X.; Zhang, Y.; Wang, H.; Zhang, W. NID2 Affects Prognosis of Glioma via Activating the Akt Signaling Pathway. Int. J. Mol. Sci. 2025, 26, 3859. https://doi.org/10.3390/ijms26083859
Lan Z, Xiao Y, Liao Y, Li X, Zhang Y, Wang H, Zhang W. NID2 Affects Prognosis of Glioma via Activating the Akt Signaling Pathway. International Journal of Molecular Sciences. 2025; 26(8):3859. https://doi.org/10.3390/ijms26083859
Chicago/Turabian StyleLan, Zhangzhang, Yanlin Xiao, Youyou Liao, Xuan Li, Yi Zhang, Huajie Wang, and Wenyong Zhang. 2025. "NID2 Affects Prognosis of Glioma via Activating the Akt Signaling Pathway" International Journal of Molecular Sciences 26, no. 8: 3859. https://doi.org/10.3390/ijms26083859
APA StyleLan, Z., Xiao, Y., Liao, Y., Li, X., Zhang, Y., Wang, H., & Zhang, W. (2025). NID2 Affects Prognosis of Glioma via Activating the Akt Signaling Pathway. International Journal of Molecular Sciences, 26(8), 3859. https://doi.org/10.3390/ijms26083859




