Cucurbitacin B Exhibits Antitumor Effects on Chordoma Cells via Disruption of Brachyury
Abstract
1. Introduction
2. Results
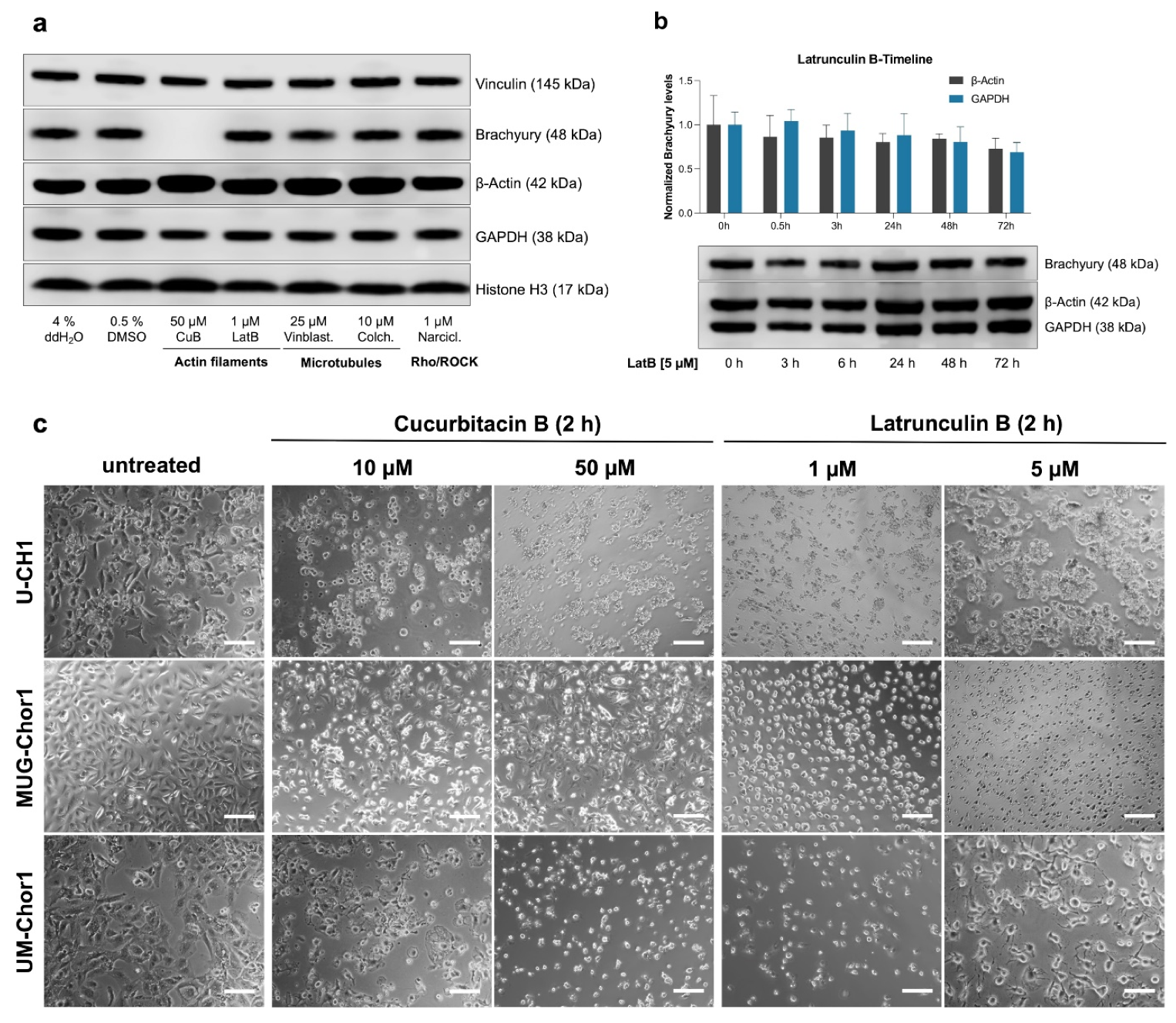
2.1. Evaluation of Cytoskeletal Inhibitors on Brachyury Levels in Chordoma Cells
2.2. Cucurbitacin B Has Antiproliferative Effects in Chordoma Cell Lines
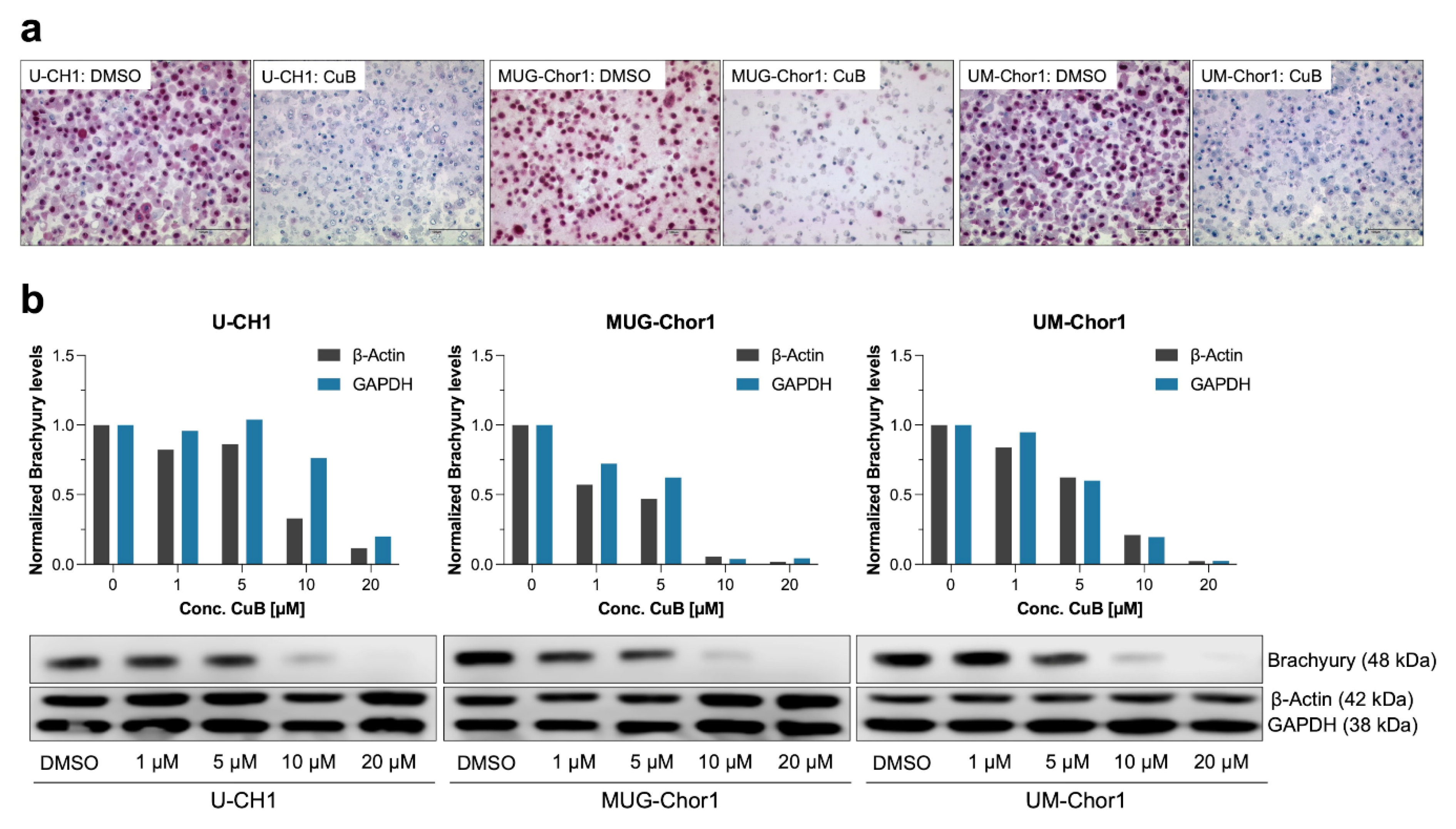
2.3. Dose-Dependent Reduction in Brachyury Protein in Chordoma Cell Lines
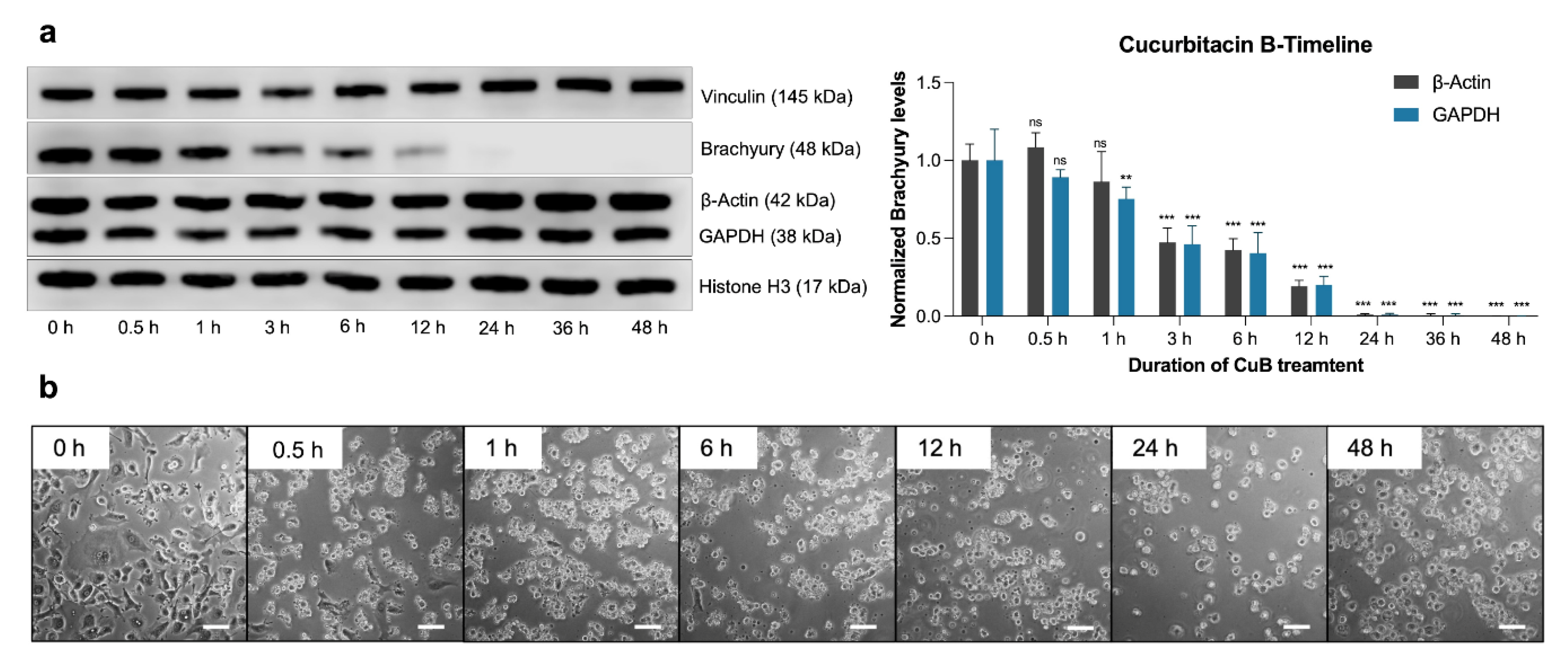
2.4. Time-Dependent Effects of Cucurbitacin B on Brachyury Levels
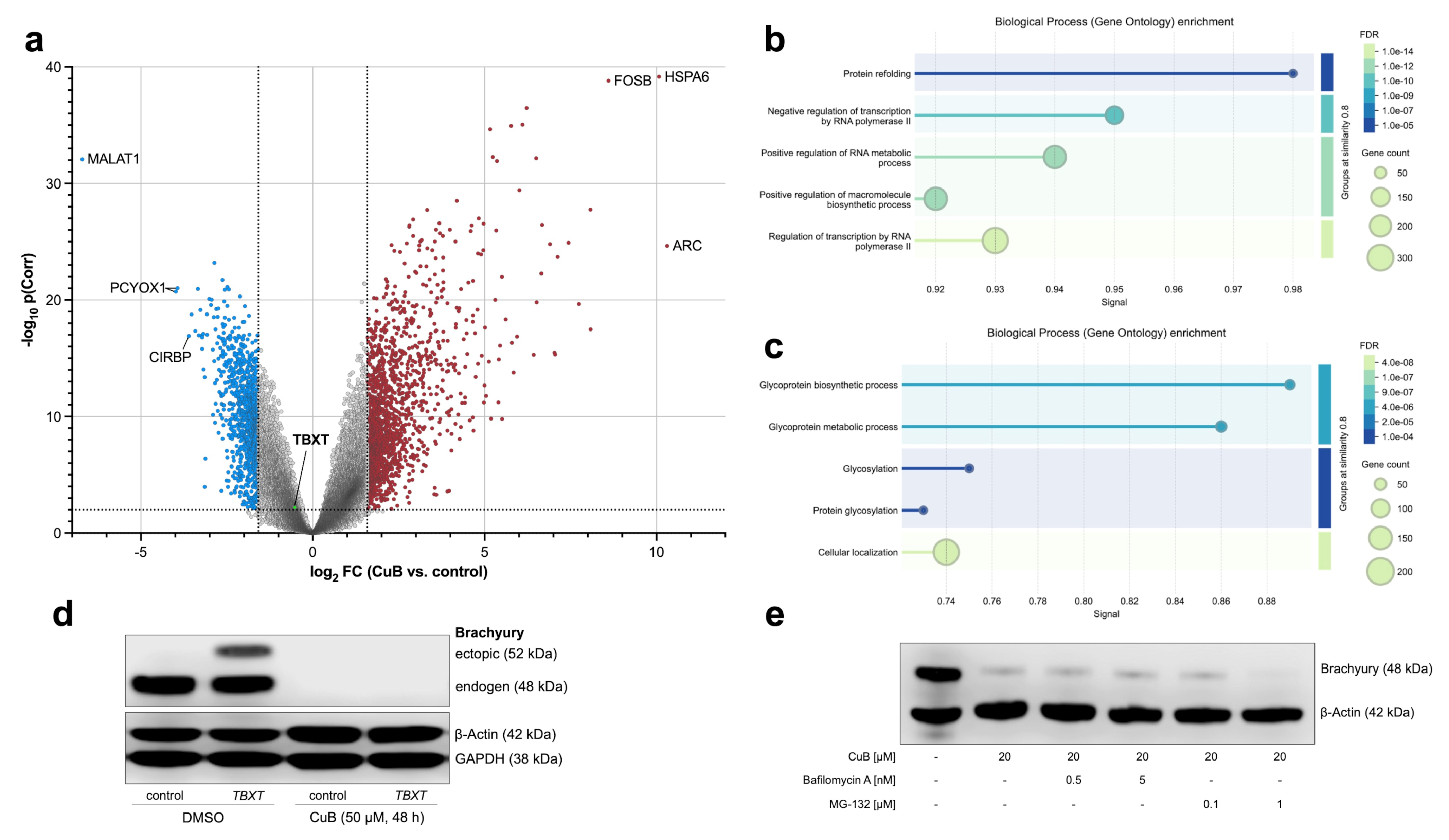
2.5. Transcriptional and Post-Transcriptional Regulation of Brachyury by Cucurbitacin B
3. Discussion
4. Material and Methods
4.1. Cell Culture
4.2. Reagents
4.3. Cell Viability Assay
4.4. Assessment of Cytotoxic Effects of Cucurbitacin B
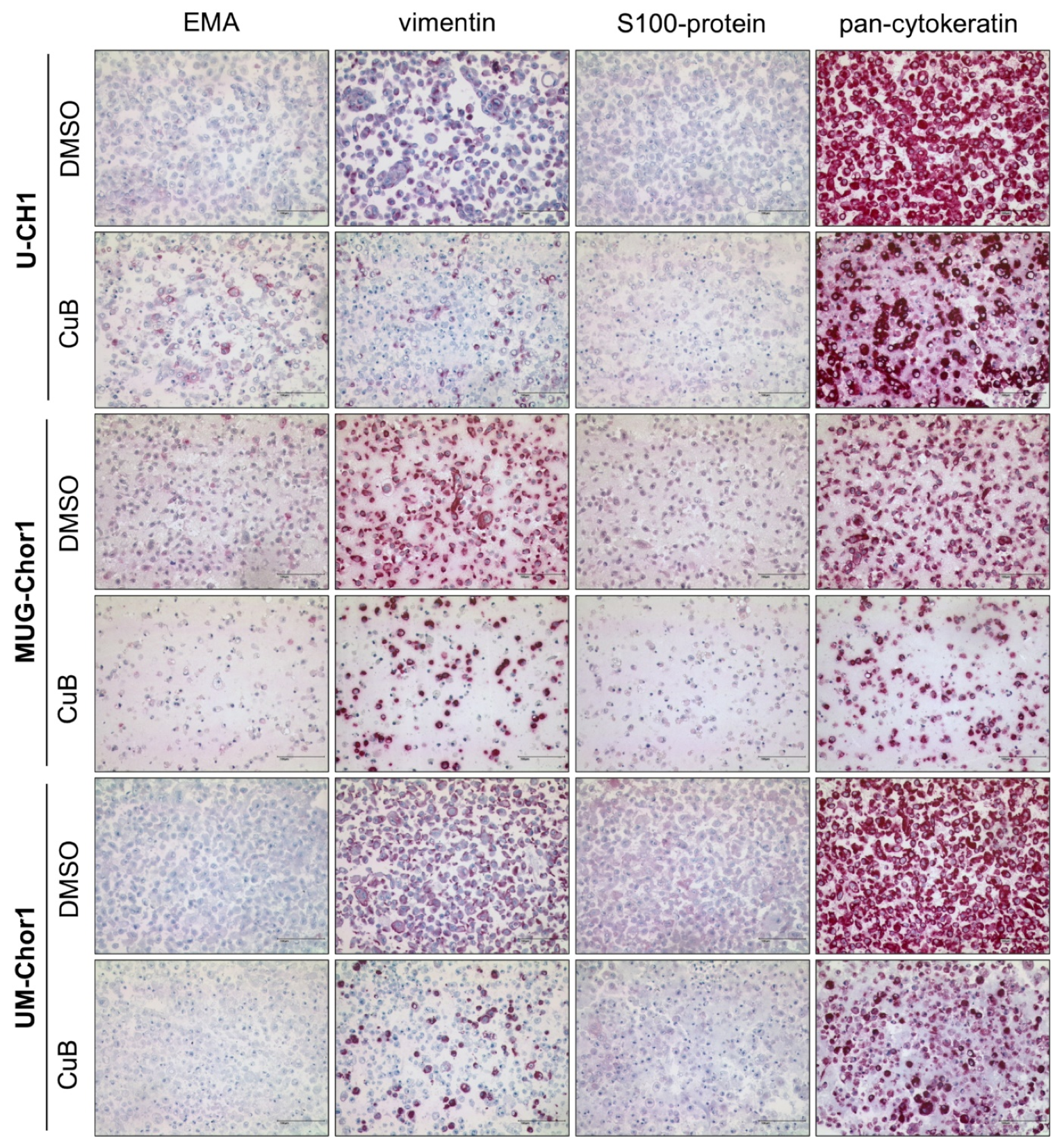
4.5. Immunostaining of Formalin-Fixed and Paraffin-Embedded Cell Blocks
4.6. Western Blot Analysis
4.7. Assessment of Time- and Dose-Dependency of Cucurbitacin B-Induced Brachyury Depletion
4.8. TBXT Open Reading Frame (ORF) Rescue Experiment
4.9. Inhibition of Autophagy and Proteasome System
4.10. RNA Isolation and cDNA Synthesis from Compound-Treated Cells
4.11. Reverse Transcription Quantitative Polymerase Chain Reaction (qRT-PCR)
4.12. Microarray Gene Expression Analysis
4.13. GO-Term Analysis
4.14. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ARC | Activity-Regulated Cytoskeleton-Associated Protein |
| cDNA | Complementary DNA |
| Colch. | Colchicine |
| CuB | Cucurbitacin B |
| ddH2O | Double-distilled water |
| DMSO | Dimethyl Sulfoxide |
| EMA | Epithelial Membrane Antigen |
| ER | Endoplasmic Reticulum |
| FDR | False Discovery Rate |
| GAPDH | Glyceraldehyde-3-Phosphate Dehydrogenase |
| GO | Gene Ontology |
| HSPA6 | Heat Shock Protein Family A Member 6 |
| IC50 | Half Maximal Inhibitory Concentration |
| JAK2 | Janus Kinase 2 |
| LatB | Latrunculin B |
| LDH | Lactate Dehydrogenase |
| MALAT1 | Metastasis Associated Lung Adenocarcinoma Transcript 1 |
| MAPK | Mitogen-Activated Protein Kinase |
| MTS | 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4- sulfophenyl)-2H-tetrazolium |
| Narcil. | Narciclasine |
| ORF | Open Reading Frame |
| qRT-PCR | Quantitative Reverse Transcription Polymerase Chain Reaction |
| Rho/ROCK | Rho-associated coiled-coil kinase pathway |
| STAT3 | Signal Transducer and Activator of Transcription 3 |
| TBXT | T-Box Transcription Factor |
| UPR | Unfolded Protein Response |
| Vinblast. | Vinblastine |
References
- Flanagan, A.M.; Yamaguchi, T. WHO Classification of Tumors and Soft Tissue and Bones, 4th ed.; Fletcher, C.D.M., Bridge, J.A., Hogendoorn, P.C.W., Mertens, F., Eds.; Lyon IARC Press: Lyon, France, 2013; pp. 328–329. [Google Scholar]
- Jäger, D.; Barth, T.F.; Brüderlein, S.; Scheuerle, A.; Rinner, B.; von Witzleben, A.; Lechel, A.; Meyer, P.; Mayer-Steinacker, R.; Baer, A.V.; et al. HOXA7, HOXA9, and HOXA10 are differentially expressed in clival and sacral chordomas. Sci. Rep. 2017, 7, 2032. [Google Scholar] [CrossRef] [PubMed]
- Hoch, B.L.; Nielsen, G.P.; Liebsch, N.J.; Rosenberg, A.E. Base of skull chordomas in children and adolescents: A clinicopathologic study of 73 cases. Am. J. Surg. Pathol. 2006, 30, 811–818. [Google Scholar] [CrossRef] [PubMed]
- Young, V.A.; Curtis, K.M.; Temple, H.T.; Eismont, F.J.; DeLaney, T.F.; Hornicek, F.J. Characteristics and Patterns of Metastatic Disease from Chordoma. Sarcoma 2015, 2015, 517657. [Google Scholar] [CrossRef]
- D’Amore, T.; Boyce, B.; Mesfin, A. Chordoma of the mobile spine and sacrum: Clinical management and prognosis. J. Spine Surg. 2018, 4, 546–552. [Google Scholar] [CrossRef]
- Stacchiotti, S.; Sommer, J.; Chordoma Global Consensus, G. Building a global consensus approach to chordoma: A position paper from the medical and patient community. Lancet Oncol. 2015, 16, e71–e83. [Google Scholar] [CrossRef]
- Stacchiotti, S.; Longhi, A.; Ferraresi, V.; Grignani, G.; Comandone, A.; Stupp, R.; Bertuzzi, A.; Tamborini, E.; Pilotti, S.; Messina, A.; et al. Phase II study of imatinib in advanced chordoma. J. Clin. Oncol. 2012, 30, 914–920. [Google Scholar] [CrossRef]
- Schuetze, S.M.; Bolejack, V.; Choy, E.; Ganjoo, K.N.; Staddon, A.P.; Chow, W.A.; Tawbi, H.A.; Samuels, B.L.; Patel, S.R.; von Mehren, M.; et al. Phase 2 study of dasatinib in patients with alveolar soft part sarcoma, chondrosarcoma, chordoma, epithelioid sarcoma, or solitary fibrous tumor. Cancer 2017, 123, 90–97. [Google Scholar] [CrossRef]
- Asklund, T.; Sandstrom, M.; Shahidi, S.; Riklund, K.; Henriksson, R. Durable stabilization of three chordoma cases by bevacizumab and erlotinib. Acta Oncol. 2014, 53, 980–984. [Google Scholar] [CrossRef]
- Magnaghi, P.; Salom, B.; Cozzi, L.; Amboldi, N.; Ballinari, D.; Tamborini, E.; Gasparri, F.; Montagnoli, A.; Raddrizzani, L.; Somaschini, A.; et al. Afatinib Is a New Therapeutic Approach in Chordoma with a Unique Ability to Target EGFR and Brachyury. Mol. Cancer Ther. 2018, 17, 603–613. [Google Scholar] [CrossRef]
- Fujii, R.; Schlom, J.; Hodge, J.W. A potential therapy for chordoma via antibody-dependent cell-mediated cytotoxicity employing NK or high-affinity NK cells in combination with cetuximab. J. Neurosurg. 2018, 128, 1419–1427. [Google Scholar] [CrossRef]
- George, S.; Merriam, P.; Maki, R.G.; Van den Abbeele, A.D.; Yap, J.T.; Akhurst, T.; Harmon, D.C.; Bhuchar, G.; O’Mara, M.M.; D’Adamo, D.R.; et al. Multicenter phase II trial of sunitinib in the treatment of nongastrointestinal stromal tumor sarcomas. J. Clin. Oncol. 2009, 27, 3154–3160. [Google Scholar] [CrossRef] [PubMed]
- Bompas, E.; Le Cesne, A.; Tresch-Bruneel, E.; Lebellec, L.; Laurence, V.; Collard, O.; Saada-Bouzid, E.; Isambert, N.; Blay, J.Y.; Amela, E.Y.; et al. Sorafenib in patients with locally advanced and metastatic chordomas: A phase II trial of the French Sarcoma Group (GSF/GETO). Ann. Oncol. 2015, 26, 2168–2173. [Google Scholar] [CrossRef] [PubMed]
- Tarpey, P.S.; Behjati, S.; Young, M.D.; Martincorena, I.; Alexandrov, L.B.; Farndon, S.J.; Guzzo, C.; Hardy, C.; Latimer, C.; Butler, A.P.; et al. The driver landscape of sporadic chordoma. Nat. Commun. 2017, 8, 890. [Google Scholar] [CrossRef]
- Scheil, S.; Bruderlein, S.; Liehr, T.; Starke, H.; Herms, J.; Schulte, M.; Moller, P. Genome-wide analysis of sixteen chordomas by comparative genomic hybridization and cytogenetics of the first human chordoma cell line, U-CH1. Genes. Chromosomes Cancer 2001, 32, 203–211. [Google Scholar] [CrossRef]
- Von Witzleben, A.; Goerttler, L.T.; Marienfeld, R.; Barth, H.; Lechel, A.; Mellert, K.; Bohm, M.; Kornmann, M.; Mayer-Steinacker, R.; von Baer, A.; et al. Preclinical Characterization of Novel Chordoma Cell Systems and Their Targeting by Pharmocological Inhibitors of the CDK4/6 Cell-Cycle Pathway. Cancer Res. 2015, 75, 3823–3831. [Google Scholar] [CrossRef]
- Stacchiotti, S.; Marrari, A.; Tamborini, E.; Palassini, E.; Virdis, E.; Messina, A.; Crippa, F.; Morosi, C.; Gronchi, A.; Pilotti, S.; et al. Response to imatinib plus sirolimus in advanced chordoma. Ann. Oncol. 2009, 20, 1886–1894. [Google Scholar] [CrossRef]
- Seeling, C.; Mosca, E.; Mantel, E.; Moller, P.; Barth, T.F.E.; Mellert, K. Prognostic Relevance and In Vitro Targeting of Concomitant PTEN and p16 Deficiency in Chordomas. Cancers 2023, 15, 1977. [Google Scholar] [CrossRef]
- Vujovic, S.; Henderson, S.; Presneau, N.; Odell, E.; Jacques, T.S.; Tirabosco, R.; Boshoff, C.; Flanagan, A.M. Brachyury, a crucial regulator of notochordal development, is a novel biomarker for chordomas. J. Pathol. 2006, 209, 157–165. [Google Scholar] [CrossRef]
- Chen, M.; Wu, Y.; Zhang, H.; Li, S.; Zhou, J.; Shen, J. The Roles of Embryonic Transcription Factor BRACHYURY in Tumorigenesis and Progression. Front. Oncol. 2020, 10, 961. [Google Scholar] [CrossRef]
- Sheppard, H.E.; Dall’Agnese, A.; Park, W.D.; Shamim, M.H.; Dubrulle, J.; Johnson, H.L.; Stossi, F.; Cogswell, P.; Sommer, J.; Levy, J.; et al. Targeted brachyury degradation disrupts a highly specific autoregulatory program controlling chordoma cell identity. Cell Rep. Med. 2021, 2, 100188. [Google Scholar] [CrossRef]
- Hsu, W.; Mohyeldin, A.; Shah, S.R.; Ap Rhys, C.M.; Johnson, L.F.; Sedora-Roman, N.I.; Kosztowski, T.A.; Awad, O.A.; McCarthy, E.F.; Loeb, D.M.; et al. Generation of chordoma cell line JHC7 and the identification of Brachyury as a novel molecular target. J. Neurosurg. 2011, 115, 760–769. [Google Scholar] [CrossRef] [PubMed]
- Fernando, R.I.; Litzinger, M.; Trono, P.; Hamilton, D.H.; Schlom, J.; Palena, C. The T-box transcription factor Brachyury promotes epithelial-mesenchymal transition in human tumor cells. J. Clin. Invest. 2010, 120, 533–544. [Google Scholar] [CrossRef]
- Jung, H.I.; Shin, I.; Park, Y.M.; Kang, K.W.; Ha, K.S. Colchicine activates actin polymerization by microtubule depolymerization. Mol. Cells 1997, 7, 431–437. [Google Scholar] [CrossRef]
- Weichsel, J.; Herold, N.; Lehmann, M.J.; Krausslich, H.G.; Schwarz, U.S. A quantitative measure for alterations in the actin cytoskeleton investigated with automated high-throughput microscopy. Cytometry A 2010, 77, 52–63. [Google Scholar] [CrossRef] [PubMed]
- Brautigam, J.; Bischoff, I.; Schurmann, C.; Buchmann, G.; Epah, J.; Fuchs, S.; Heiss, E.; Brandes, R.P.; Furst, R. Narciclasine inhibits angiogenic processes by activation of Rho kinase and by downregulation of the VEGF receptor 2. J. Mol. Cell Cardiol. 2019, 135, 97–108. [Google Scholar] [CrossRef]
- Wang, X.; Tanaka, M.; Peixoto, H.S.; Wink, M. Cucurbitacins: Elucidation of their interactions with the cytoskeleton. PeerJ 2017, 5, e3357. [Google Scholar] [CrossRef]
- Chen, X.; Xu, Z.; Tang, K.; Hu, G.; Du, P.; Wang, J.; Zhang, C.; Xin, Y.; Li, K.; Zhang, Q.; et al. The Mechanics of Tumor Cells Dictate Malignancy via Cytoskeleton-Mediated APC/Wnt/beta-Catenin Signaling. Research 2023, 6, 0224. [Google Scholar] [CrossRef]
- Olson, E.N.; Nordheim, A. Linking actin dynamics and gene transcription to drive cellular motile functions. Nat. Rev. Mol. Cell Biol. 2010, 11, 353–365. [Google Scholar] [CrossRef]
- Hotta, K.; Takahashi, H.; Asakura, T.; Saitoh, B.; Takatori, N.; Satou, Y.; Satoh, N. Characterization of Brachyury-downstream notochord genes in the Ciona intestinalis embryo. Dev. Biol. 2000, 224, 69–80. [Google Scholar] [CrossRef]
- Pinto, F.; Costa, A.M.; Andrade, R.P.; Reis, R.M. Brachyury Is Associated with Glioma Differentiation and Response to Temozolomide. Neurotherapeutics 2020, 17, 2015–2027. [Google Scholar] [CrossRef]
- Dai, S.; Wang, C.; Zhao, X.; Ma, C.; Fu, K.; Liu, Y.; Peng, C.; Li, Y. Cucurbitacin B: A review of its pharmacology, toxicity, and pharmacokinetics. Pharmacol. Res. 2023, 187, 106587. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Islam, M.S.; Tian, J.; Lui, V.W.; Xiao, D. Inactivation of ATP citrate lyase by Cucurbitacin B: A bioactive compound from cucumber, inhibits prostate cancer growth. Cancer Lett. 2014, 349, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Wakimoto, N.; Yin, D.; O’Kelly, J.; Haritunians, T.; Karlan, B.; Said, J.; Xing, H.; Koeffler, H.P. Cucurbitacin B has a potent antiproliferative effect on breast cancer cells in vitro and in vivo. Cancer Sci. 2008, 99, 1793–1797. [Google Scholar] [CrossRef]
- Yin, D.; Wakimoto, N.; Xing, H.; Lu, D.; Huynh, T.; Wang, X.; Black, K.L.; Koeffler, H.P. Cucurbitacin B markedly inhibits growth and rapidly affects the cytoskeleton in glioblastoma multiforme. Int. J. Cancer 2008, 123, 1364–1375. [Google Scholar] [CrossRef]
- Nobusue, H.; Onishi, N.; Shimizu, T.; Sugihara, E.; Oki, Y.; Sumikawa, Y.; Chiyoda, T.; Akashi, K.; Saya, H.; Kano, K. Regulation of MKL1 via actin cytoskeleton dynamics drives adipocyte differentiation. Nat. Commun. 2014, 5, 3368. [Google Scholar] [CrossRef]
- Sankaran, J.; Uzer, G.; van Wijnen, A.J.; Rubin, J. Gene regulation through dynamic actin control of nuclear structure. Exp. Biol. Med. 2019, 244, 1345–1353. [Google Scholar] [CrossRef]
- Aranda-Anzaldo, A.; Dent, M.A.R.; Segura-Anaya, E.; Martinez-Gomez, A. Protein folding, cellular stress and cancer. Prog. Biophys. Mol. Biol. 2024, 191, 40–57. [Google Scholar] [CrossRef]
- Huang, J.L.; Liang, L.; Xie, P.E.; Sun, W.L.; Wang, L.; Cai, Z.W. Cucurbitacin B induces apoptosis in colorectal cells through reactive oxygen species generation and endoplasmic reticulum stress pathways. Exp. Ther. Med. 2023, 26, 484. [Google Scholar] [CrossRef]
- Zheng, X.; Tang, P.; Li, H.; Ye, T.; Zhu, X.; He, W.; Cheng, L.; Cheng, H. Cucurbitacin E elicits apoptosis in laryngeal squamous cell carcinoma by enhancing reactive oxygen species-regulated mitochondrial dysfunction and endoplasmic reticulum stress. Am. J. Cancer Res. 2024, 14, 3905–3921. [Google Scholar] [CrossRef]
- Li, H.; Chen, H.; Li, R.; Xin, J.; Wu, S.; Lan, J.; Xue, K.; Li, X.; Zuo, C.; Jiang, W.; et al. Cucurbitacin I induces cancer cell death through the endoplasmic reticulum stress pathway. J. Cell Biochem. 2019, 120, 2391–2403. [Google Scholar] [CrossRef]
- Park, A.Y.; Park, Y.S.; So, D.; Song, I.K.; Choi, J.E.; Kim, H.J.; Lee, K.J. Activity-Regulated Cytoskeleton-Associated Protein (Arc/Arg3.1) is Transiently Expressed after Heat Shock Stress and Suppresses Heat Shock Factor 1. Sci. Rep. 2019, 9, 2592. [Google Scholar] [CrossRef] [PubMed]
- Cherepanova, N.; Shrimal, S.; Gilmore, R. N-linked glycosylation and homeostasis of the endoplasmic reticulum. Curr. Opin. Cell Biol. 2016, 41, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Hetz, C. The unfolded protein response: Controlling cell fate decisions under ER stress and beyond. Nat. Rev. Mol. Cell Biol. 2012, 13, 89–102. [Google Scholar] [CrossRef] [PubMed]
- Shukla, S.; Sinha, S.; Khan, S.; Kumar, S.; Singh, K.; Mitra, K.; Maurya, R.; Meeran, S.M. Cucurbitacin B inhibits the stemness and metastatic abilities of NSCLC via downregulation of canonical Wnt/beta-catenin signaling axis. Sci. Rep. 2016, 6, 21860. [Google Scholar] [CrossRef]
- Zheng, Q.; Liu, Y.; Liu, W.; Ma, F.; Zhou, Y.; Chen, M.; Chang, J.; Wang, Y.; Yang, G.; He, G. Cucurbitacin B inhibits growth and induces apoptosis through the JAK2/STAT3 and MAPK pathways in SH-SY5Y human neuroblastoma cells. Mol. Med. Rep. 2014, 10, 89–94. [Google Scholar] [CrossRef]
- Zhang, Z.R.; Gao, M.X.; Yang, K. Cucurbitacin B inhibits cell proliferation and induces apoptosis in human osteosarcoma cells via modulation of the JAK2/STAT3 and MAPK pathways. Exp. Ther. Med. 2017, 14, 805–812. [Google Scholar] [CrossRef]
- Kavka, A.I.; Green, J.B. Tales of tails: Brachyury and the T-box genes. Biochim. Biophys. Acta 1997, 1333, F73–F84. [Google Scholar] [CrossRef]
- Sharifnia, T.; Wawer, M.J.; Chen, T.; Huang, Q.Y.; Weir, B.A.; Sizemore, A.; Lawlor, M.A.; Goodale, A.; Cowley, G.S.; Vazquez, F.; et al. Small-molecule targeting of brachyury transcription factor addiction in chordoma. Nat. Med. 2019, 25, 292–300. [Google Scholar] [CrossRef]
- Seeling, C.; Lechel, A.; Svinarenko, M.; Moller, P.; Barth, T.F.E.; Mellert, K. Molecular features and vulnerabilities of recurrent chordomas. J. Exp. Clin. Cancer Res. 2021, 40, 244. [Google Scholar] [CrossRef]
- Gellner, V.; Tomazic, P.V.; Lohberger, B.; Meditz, K.; Heitzer, E.; Mokry, M.; Koele, W.; Leithner, A.; Liegl-Atzwanger, B.; Rinner, B. Establishment of clival chordoma cell line MUG-CC1 and lymphoblastoid cells as a model for potential new treatment strategies. Sci. Rep. 2016, 6, 24195. [Google Scholar] [CrossRef]
- Szklarczyk, D.; Kirsch, R.; Koutrouli, M.; Nastou, K.; Mehryary, F.; Hachilif, R.; Gable, A.L.; Fang, T.; Doncheva, N.T.; Pyysalo, S.; et al. The STRING database in 2023: Protein-protein association networks and functional enrichment analyses for any sequenced genome of interest. Nucleic Acids Res. 2023, 51, D638–D646. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seeling, C.; Neumahr, J.; Häberle, F.; Lechel, A.; Möller, P.; Gaisa, N.T.; Barth, T.F.E.; Mellert, K. Cucurbitacin B Exhibits Antitumor Effects on Chordoma Cells via Disruption of Brachyury. Int. J. Mol. Sci. 2025, 26, 3864. https://doi.org/10.3390/ijms26083864
Seeling C, Neumahr J, Häberle F, Lechel A, Möller P, Gaisa NT, Barth TFE, Mellert K. Cucurbitacin B Exhibits Antitumor Effects on Chordoma Cells via Disruption of Brachyury. International Journal of Molecular Sciences. 2025; 26(8):3864. https://doi.org/10.3390/ijms26083864
Chicago/Turabian StyleSeeling, Carolin, Johannes Neumahr, Fabian Häberle, André Lechel, Peter Möller, Nadine T. Gaisa, Thomas F. E. Barth, and Kevin Mellert. 2025. "Cucurbitacin B Exhibits Antitumor Effects on Chordoma Cells via Disruption of Brachyury" International Journal of Molecular Sciences 26, no. 8: 3864. https://doi.org/10.3390/ijms26083864
APA StyleSeeling, C., Neumahr, J., Häberle, F., Lechel, A., Möller, P., Gaisa, N. T., Barth, T. F. E., & Mellert, K. (2025). Cucurbitacin B Exhibits Antitumor Effects on Chordoma Cells via Disruption of Brachyury. International Journal of Molecular Sciences, 26(8), 3864. https://doi.org/10.3390/ijms26083864




