On the Pleiotropic Actions of Glucagon-like Peptide-1 in Its Regulation of Homeostatic and Hedonic Feeding
Abstract
:1. Introduction
2. Results
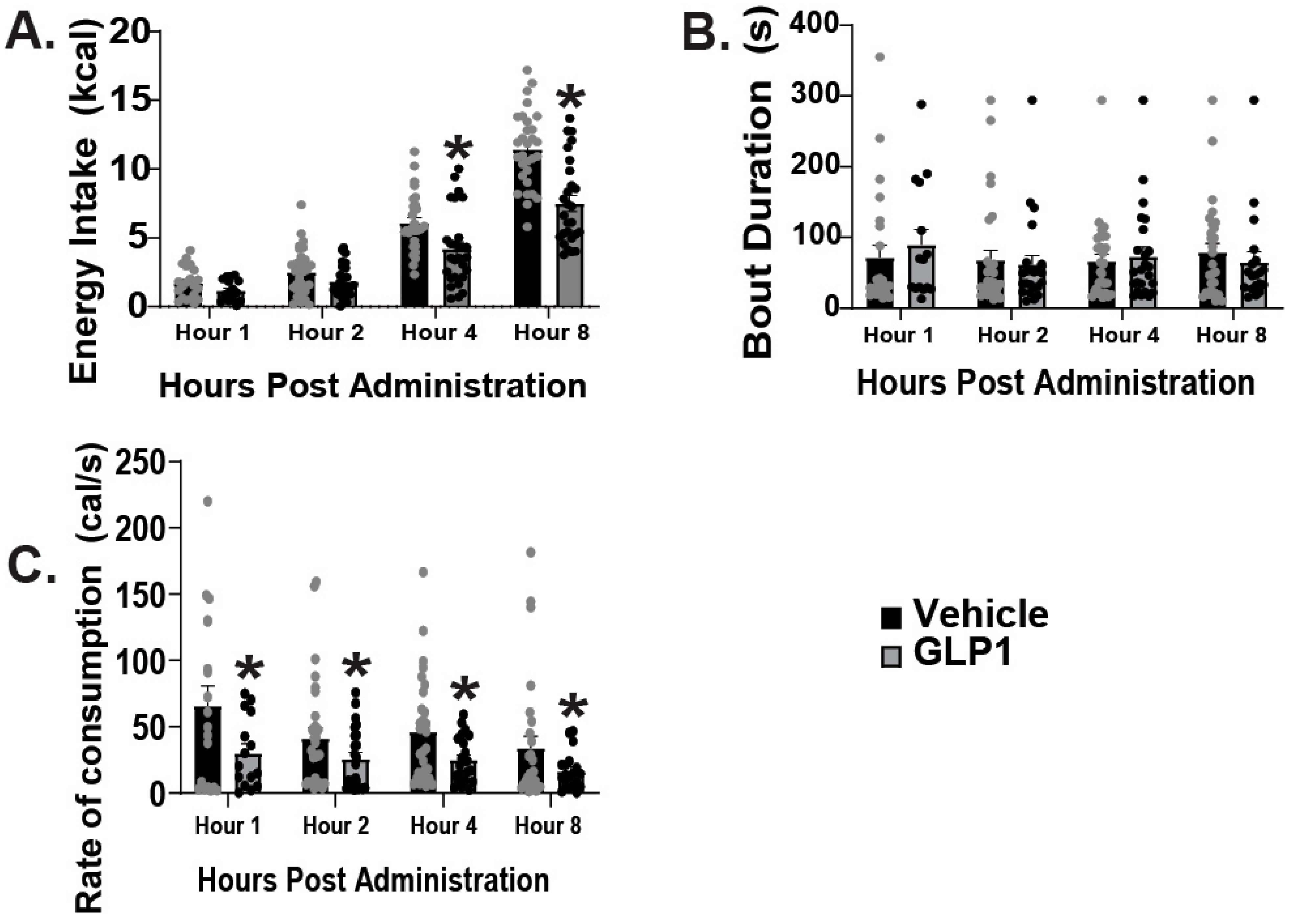
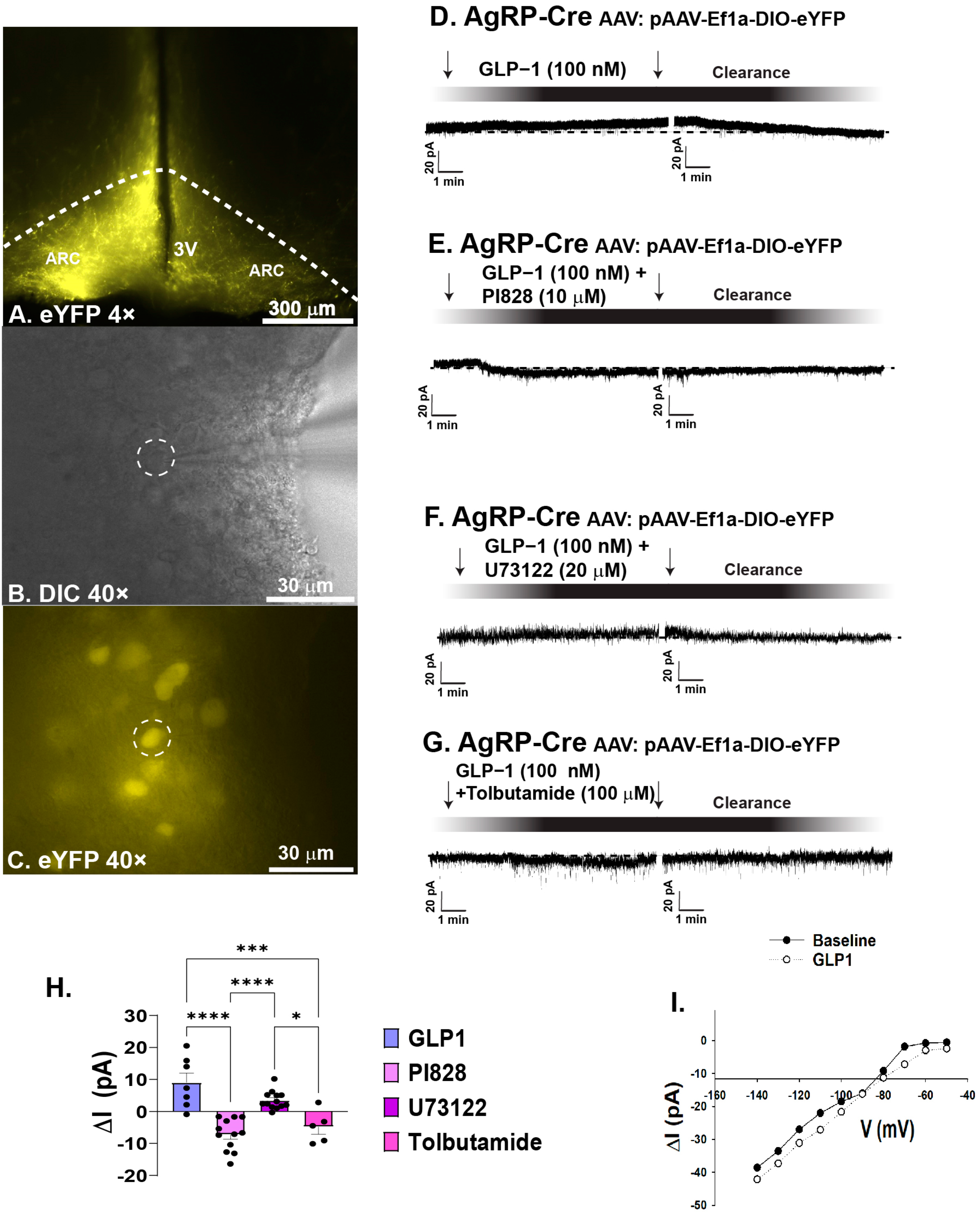
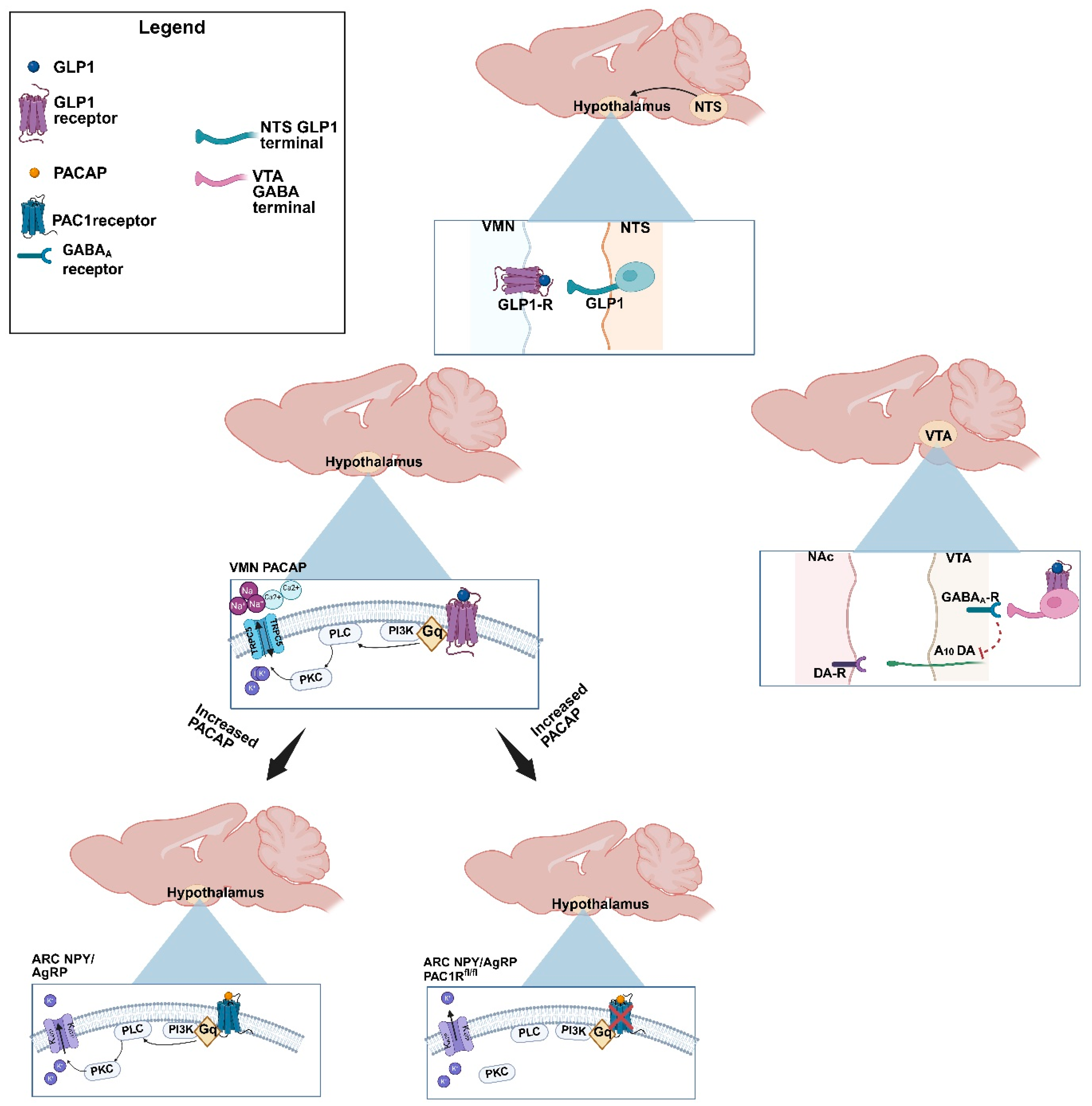
2.1. GLP1 Attenuates Feeding in the ARC, Likely Due to GLP1-Induced Inhibition of ARC NPY/AgRP Neurons
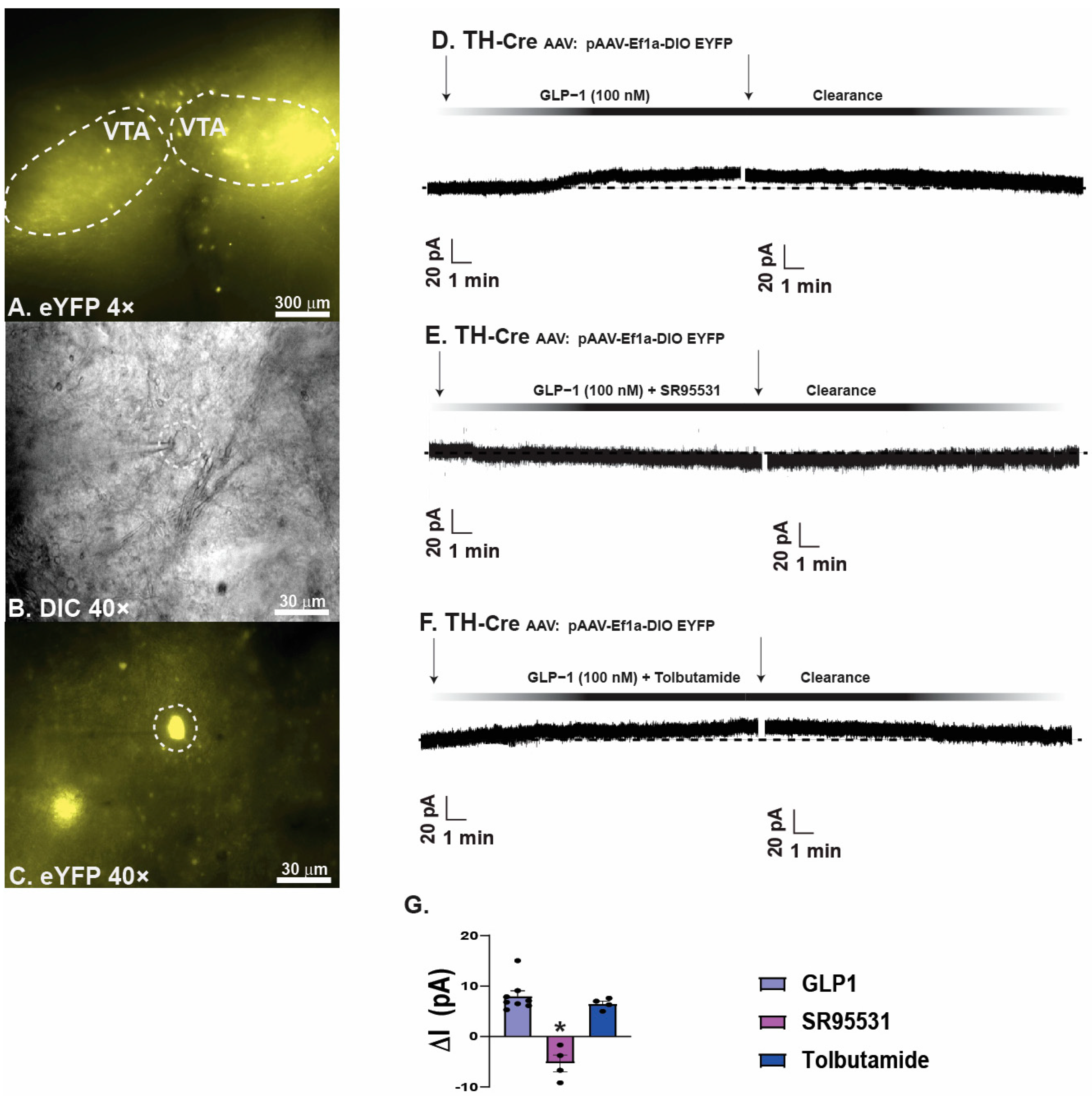
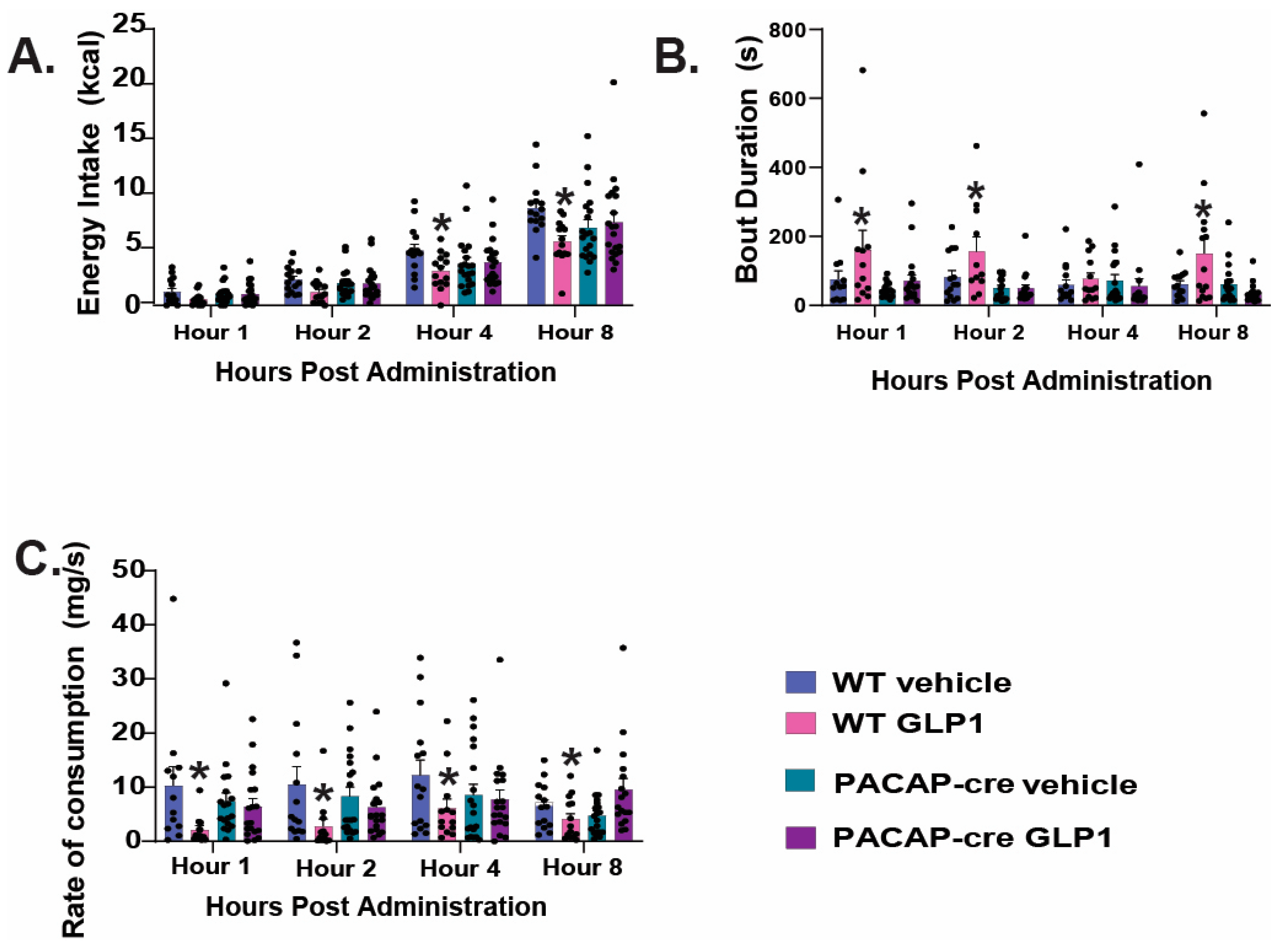
2.2. GLP1 Inhibits Hedonic Feeding via Inhibition of VTA A10 Dopamine Neurons
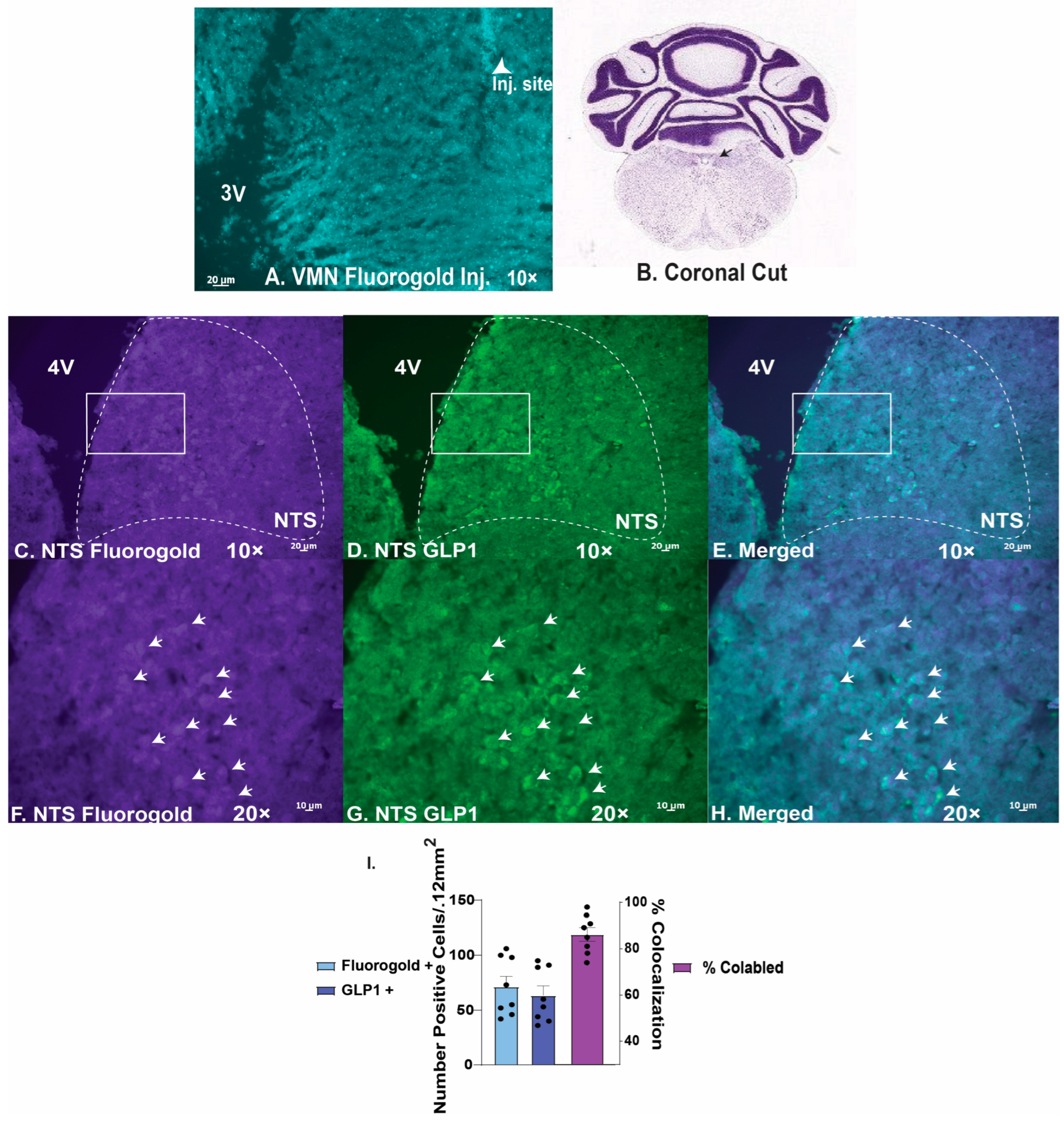
2.3. GLP1 Excites VMN PACAP Neurons via Activation of TRPC5 Channels
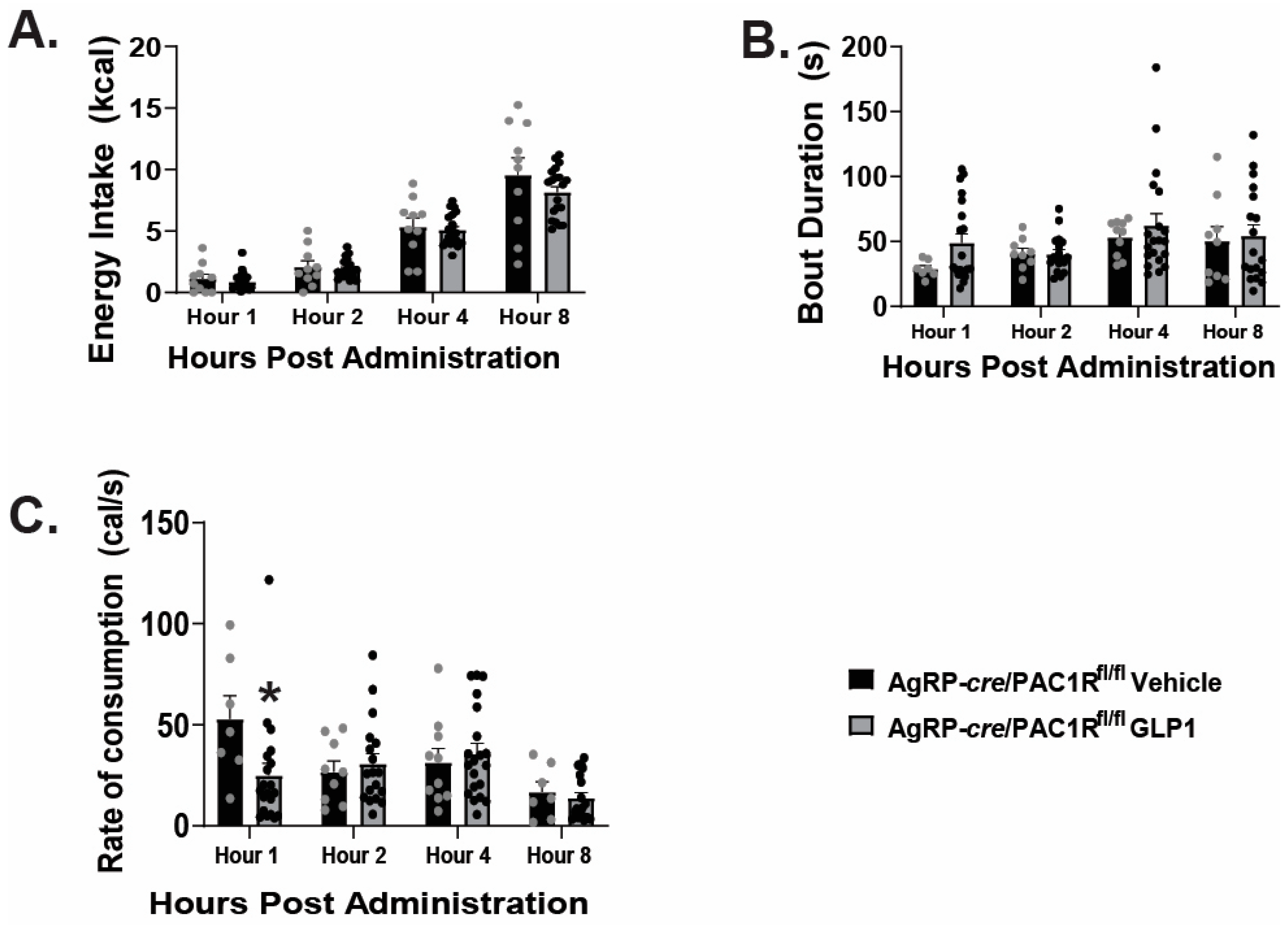
2.4. VMN PACAP Neurons and PAC1Rs Expressed in A10 Dopamine Neurons Play a Relatively Insignificant Role in GLP1-Induced Inhibition of Hedonic Feeding
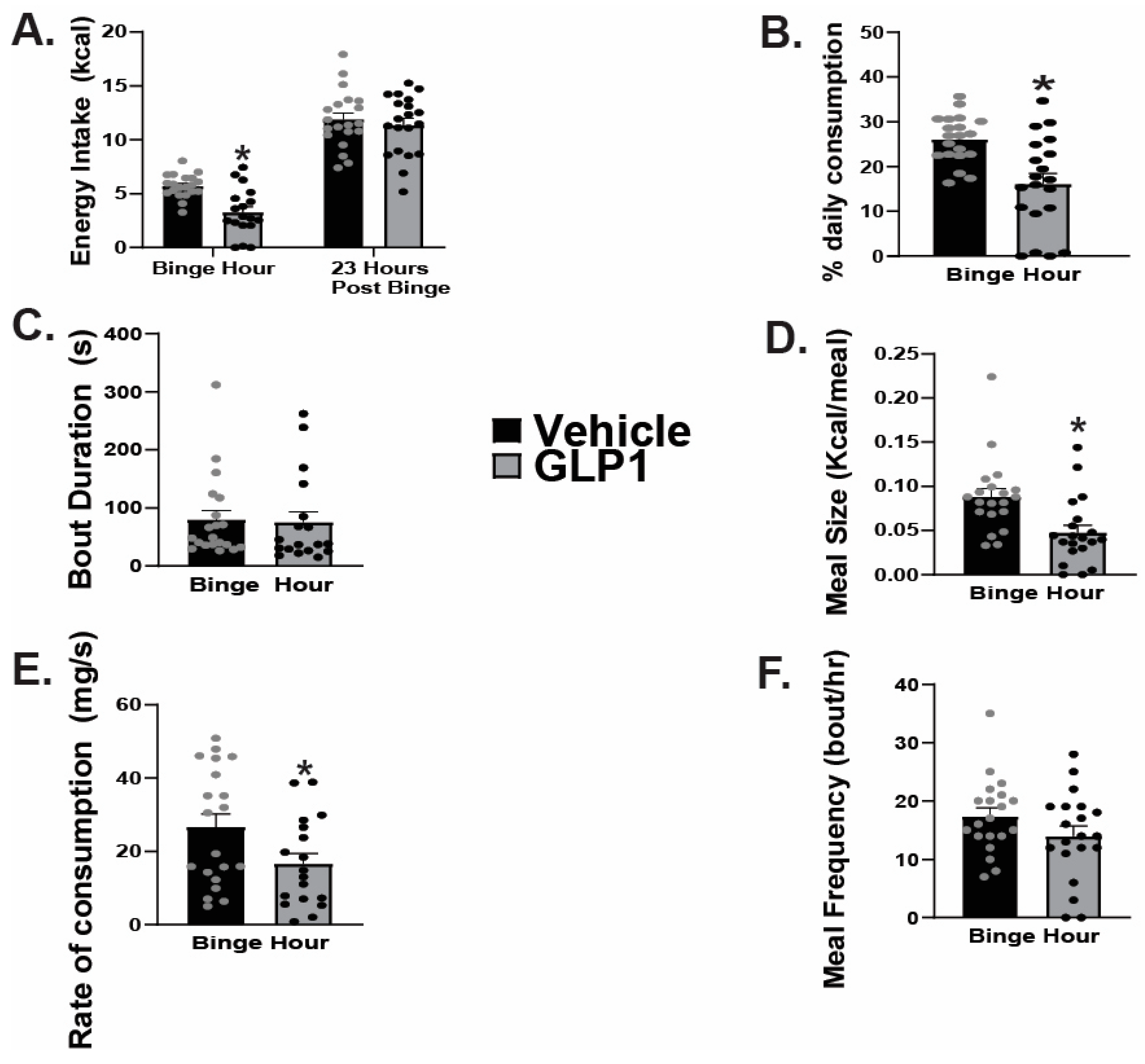
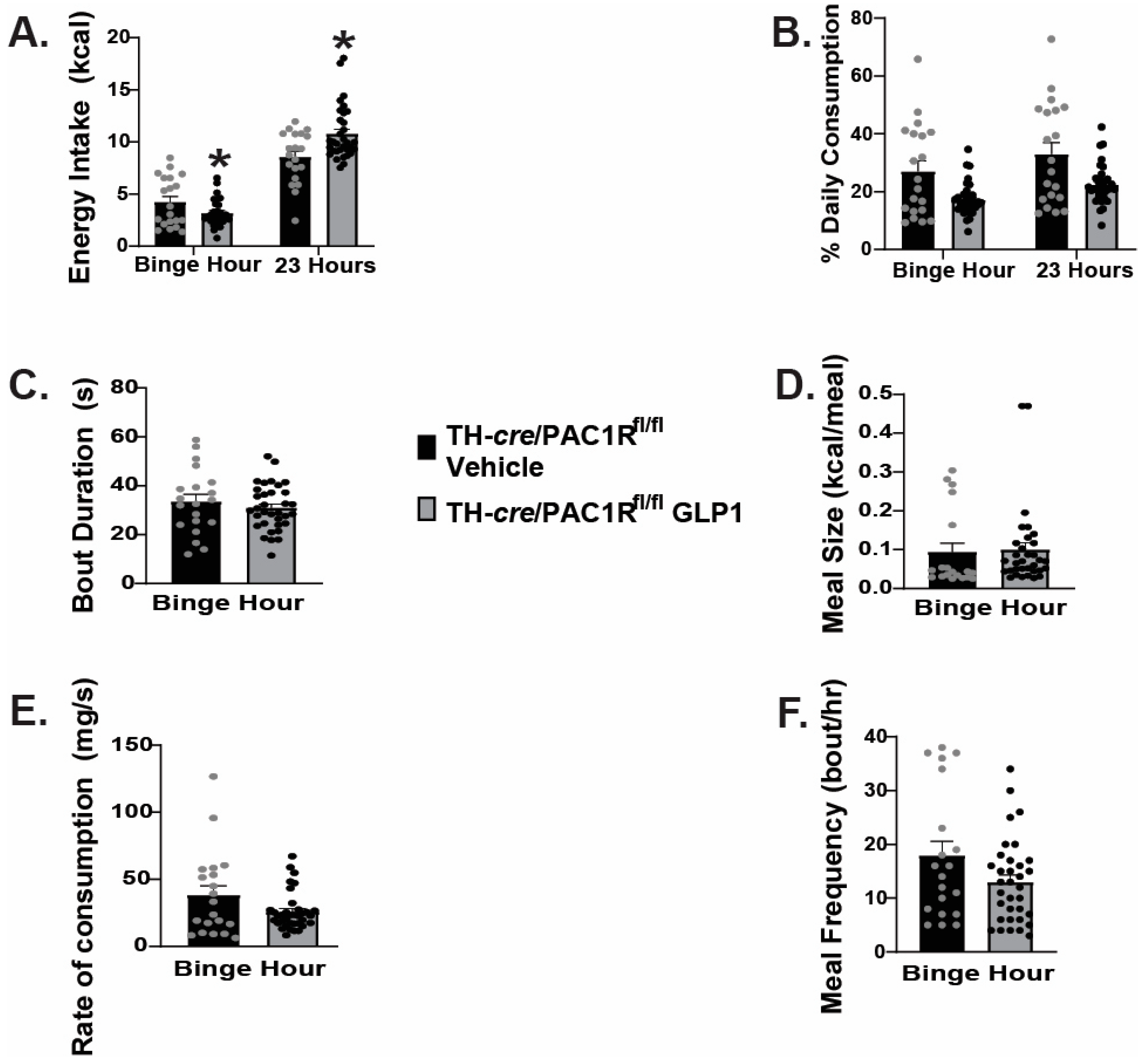
2.5. Caspase Ablation of VMN PACAP Neurons in PACAP-Cre Animals Reduces the GLP1 Induced Reduction in Homeostatic Feeding Behavior
3. Discussion
3.1. GLP1 Promotes Decreased Feeding Behavior Under Homeostatic Conditions via Excitation of VMN PACAP Neurons While Inhibiting ARC NPY/AgRP Neurons
3.2. GLP1 Influences Decreased Hedonic Feeding Behavior Through Inducing GABAA Mediated Inhibition of VTA A10 Dopamine Synaptic Output
4. Materials and Methods
4.1. Animal Models
4.2. Surgical Procedures
4.3. Drugs
4.4. Hypothalamic and Midbrain Slice Preparation
4.5. Electrophysiology
4.6. Behavioral Studies
4.7. Immunohistochemistry
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AgRP | Agouti-related peptide |
| KATP | ATP-gated K+ |
| TRPC5 | Transient receptor potential 5 channels |
| TH | Tyrosine hydroxylase |
| GLP | Glucagon-like peptide 1 |
| PVN | Paraventricular nucleus |
| VMN | Ventromedial nucleus |
| VTA | Ventral tegmental area |
| NTS | Nucleus tractus solitarius |
| POMC | Proopiomelanocortin |
| NPY | Neuropeptide Y |
| GABA | γ-aminobutyric acid |
| ARC | Arcuate |
| HFD | High fat diet |
| AAV | Adeno |
References
- Holst, J.J.; McGill, M.A. Potential new approaches to modifying intestinal GLP-1 secretion in patients with type 2 diabetes. Clin. Drug Investig. 2012, 32, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Astrup, A.; Rossner, S.; Van Gaal, L.; Rissanen, A.; Niskanen, L.; Al Hakim, M.; Madsen, J.; Rasmussen, M.F.; Lean, M.E.J. Effects of liraglutide in the treatment of obesity: A randomised, double-blind, placebo-controlled study. Lancet 2009, 374, 1606–1616. [Google Scholar] [CrossRef] [PubMed]
- Washington, M.C.; Raboin, S.J.; Thompson, W.; Larsen, C.J.; Sayegh, A.I. Exenatide reduces food intake and activates the enteric nervous system of the gastrointestinal tract and the dorsal vagal complex of the hindbrain in the rat by a GLP-1 receptor. Brain Res. 2010, 1344, 124–133. [Google Scholar] [CrossRef] [PubMed]
- Dickson, S.L.; Shirazi, R.H.; Hansson, C.; Bergquist, F.; Nissbrandt, H.; Skibicka, K.P. The glucagon-like peptide 1 (GLP-1) analogue, exendin-4, decreases the rewarding value of food: A new role for mesolimbic GLP-1 receptors. J. Neurosci. 2012, 32, 4812–4820. [Google Scholar] [CrossRef]
- Liu, J.; Conde, K.; Zhang, P.; Lilascharoen, V.; Xu, Z.; Lim, B.K.; Seeley, R.J.; Zhu, J.J.; Scott, M.M.; Pang, Z.P. Enhanced AMPA receptor trafficking mediates the anorexigenic effect of endogenous glucagon- like peptide-1 in the paraventricular hypothalamus. Neuron 2017, 96, 1–13. [Google Scholar] [CrossRef]
- Christophe, J. Is there appetite after GLP-1 and PACAP? Ann. N. Y. Acad. Sci. 1998, 865, 323–335. [Google Scholar] [CrossRef]
- Viveros, M.-P.; Bermúdez-Silva, F.-J.; Lopez-Rodriguez, A.-B.; Wagner, E.J. The endocannabinoid system as a pharmacological target derived from its CNS role in energy homeostasis and reward: Applications in eating disorders and addiction. Pharmaceuticals 2011, 4, 1101–1136. [Google Scholar] [CrossRef]
- Williams, D.L. Neural integration of satiation and food reward: Role of GLP-1 and orexin pathways. Physiol. Behav. 2014, 136, 194–199. [Google Scholar] [CrossRef]
- Biglari, N.; Gaziano, I.; Schumacher, J.; Radermacher, J.; Paeger, L.; Klemm, P.; Chen, W.; Corneliussen, S.; Wunderlich, C.M.; Sue, M.; et al. Functionally distinct POMC-expressing neuron subpopulations in hypothalamus revealed by intersectional targeting. Nat. Neurosci. 2021, 24, 913–929. [Google Scholar] [CrossRef]
- He, Z.; Gao, Y.; Lieu, L.; Afrin, S.; Cao, J.; Michael, N.J.; Dong, Y.; Sun, J.; Guo, H.; Williams, K.W. Direct and indirect effects of liraglutide on hypothalamic POMC and NPY/AgRP neurons—Implications for energy balance and glucose control. Mol. Metab. 2019, 28, 120–134. [Google Scholar] [CrossRef]
- Burmeister, M.A.; Ayala, J.E.; Smouse, H.; Landivar-Rocha, A.; Brown, J.D.; Drucker, D.J.; Stoffers, D.A.; Sandoval, D.A.; Seeley, R.J.; Ayala, J.E. The Hypothalamic Glucagon-Like Peptide 1 Receptor Is Sufficient but Not Necessary for the Regulation of Energy Balance and Glucose Homeostasis in Mice. Diabetes 2017, 66, 372–384. [Google Scholar] [CrossRef] [PubMed]
- Segal, J.P.; Stallings, N.R.; Lee, C.E.; Zhao, L.; Socci, N.; Viale, A.; Harris, T.M.; Soares, M.B.; Childs, G.; Elmquist, J.K.; et al. Use of laser-capture microdissection for the identification of marker genes for the ventromedial hypothalamic nucleus. J. Neurosci. 2005, 25, 4181–4188. [Google Scholar] [CrossRef]
- Hawke, Z.; Ivanov, T.R.; Bechtold, D.A.; Dhillon, H.; Lowell, B.B.; Luckman, S.M. PACAP neurons in the hypothalamic ventromedial nucleus are targets of central leptin signaling. J. Neurosci. 2009, 29, 14828–14835. [Google Scholar] [CrossRef] [PubMed]
- Cardinal, P.; André, C.; Quarta, C.; Bellocchio, L.; Clark, S.; Elie, M.; Leste-Lasserre, T.; Maitre, M.; Gonzales, D.; Cannich, A.; et al. CB1 cannabinoid receptor in SF1-expressing neurons of the ventromedial hypothalamus determines metabolic responses to diet and leptin. Mol. Metab. 2014, 3, 705–716. [Google Scholar] [CrossRef]
- Chang, R.; Hernandez, J.; Gastelum, C.; Guadagno, K.; Perez, L.; Wagner, E.J. Pituitary adenylate cyclase-activating polypeptide excites proopiomelanocortin neurons: Implications for the regulation of energy homeostasis. Neuroendocrinology 2021, 111, 45–69. [Google Scholar] [CrossRef] [PubMed]
- Le, N.; Hernandez, J.; Gastelum, C.; Perez, L.; Vahrson, I.; Sayers, S.; Wagner, E.J. Pituitary Adenylate Cyclase Activating Polypeptide Inhibits A(10) Dopamine Neurons and Suppresses the Binge-like Consumption of Palatable Food. Neuroscience 2021, 478, 49–64. [Google Scholar] [CrossRef]
- Lindberg, D.; Chen, P.; Li, C. Conditional viral tracing reveals that steroidogenic factor 1-positive neurons of the dorsomedial subdivision of the ventromedial hypothalamus project to the autonomic centers of the hypothalamus and hindbrain. J. Comp. Neurol. 2013, 521, 3167–3190. [Google Scholar] [CrossRef]
- Mata-Pacheco, V.; Hernandez, J.; Varma, N.; Xu, J.; Sayers, S.; Le, N.; Wagner, E.J. Dynamic, Sex- and Diet-Specific Pleiotropism in the PAC1 Receptor-Mediated Regulation of Arcuate POMC and NPY/AgRP Neuronal Excitability by Anorexigenic Ventromedial Nucleus PACAP Neurons. J. Neuroendocrinol. 2024, 36. [Google Scholar] [CrossRef]
- Sayers, S.; Le, N.; Wagner, E.J. The role of pituitary adenylate cyclase-activating polypeptide neurons in the hypothalamic ventromedial nucleus and the cognate PAC1 receptor in the regulation of hedonic feeding. Front. Nutr. 2024, 11. [Google Scholar] [CrossRef]
- Secher, A.; Jelsing, J.; Baquero, A.F.; Hecksher-Sørensen, J.; Cowley, M.A.; Dalbøge, L.S.; Hansen, G.; Grove, K.L.; Pyke, C.; Raun, K.; et al. The arcuate nucleus mediates GLP-1 receptor agonist liraglutide-dependent weight loss. J. Clin. Investig. 2014, 124, 4473–4488. [Google Scholar] [CrossRef]
- Singh, I.; Wang, L.; Xia, B.; Liu, J.; Tahiri, A.; El Ouaamari, A.; Wheeler, M.B.; Pang, Z.P. Activation of arcuate nucleus glucagon-like peptide-1 receptor-expressing neurons suppresses food intake. Cell Biosci. 2022, 12, 178. [Google Scholar] [CrossRef] [PubMed]
- Dhillon, H.; Zigman, J.M.; Ye, C.; Lee, C.E.; McGovern, R.A.; Tang, V.; Kenny, C.D.; Christiansen, L.M.; White, R.D.; Edelstein, E.A.; et al. Leptin directly activates SF1 neurons in the VMH, and this action by leptin is required for normal body-weight homeostasis. Neuron 2006, 49, 191–203. [Google Scholar] [CrossRef]
- Hurley, M.M.; Maunze, B.; Block, M.E.; Frenkel, M.M.; Reily, M.J.; Kim, E.; Chen, Y.; Li, Y.; Baker, D.A.; Liu, Q.-S.; et al. Pituitary adenylate-cyclase activating polypeptide regulates hunger- and palatability-induced binge eating. Front. Neurosci. 2016, 10, 383. [Google Scholar] [CrossRef] [PubMed]
- Iigaya, K.; Minoura, Y.; Onimaru, H.; Kotani, S.; Izumizaki, M. Effects of Feeding-Related Peptides on Neuronal Oscillation in the Ventromedial Hypothalamus. J. Clin. Med. 2019, 8, 292. [Google Scholar] [CrossRef]
- Hurley, M.M.; Anderson, E.M.; Chen, C.; Maunze, B.; Hess, E.M.; Block, M.E.; Patel, N.; Cooper, Z.; McCoy, R.; Dabra, T.; et al. Acute Blockade of PACAP-Dependent Activity in the Ventromedial Nucleus of the Hypothalamus Disrupts Leptin-Induced Behavioral and Molecular Changes in Rats. Neuroendocrinology 2020, 110, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Varela, L.; Horvath, T.L. Leptin and insulin pathways in POMC and AgRP neurons that modulate energy balance and glucose homeostasis. EMBO Rep. 2012, 13, 1079–1086. [Google Scholar] [CrossRef]
- Huang, Y.; He, Z.; Gao, Y.; Lieu, L.; Yao, T.; Sun, J.; Liu, T.; Javadi, C.; Box, M.; Afrin, S.; et al. Phosphoinositide 3-Kinase Is Integral for the Acute Activity of Leptin and Insulin in Male Arcuate NPY/AgRP Neurons. J. Endocr. Soc. 2018, 2, 518–532. [Google Scholar] [CrossRef]
- Mounien, L.; Bizet, P.; Boutelet, I.; Gourcerol, G.; Basille, M.; Gonzalez, B.; Vaudry, H.; Jegou, S. Expression of PACAP receptor mRNAs by neuropeptide Y neurons in the rat arcuate nucleus. Ann. N. Y. Acad. Sci. 2006, 1070, 457–461. [Google Scholar] [CrossRef]
- Wang, X.F.; Liu, J.J.; Xia, J.; Liu, J.; Mirabella, V.; Pang, Z.P. Endogenous Glucagon-like Peptide-1 Suppresses High-Fat Food Intake by Reducing Synaptic Drive onto Mesolimbic Dopamine Neurons. Cell Rep. 2015, 12, 726–733. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, E.; Yasoshima, Y.; Shimura, T. Systemic administration of anorexic gut peptide hormones impairs hedonic-driven sucrose consumption in mice. Physiol. Behav. 2017, 171, 158–164. [Google Scholar] [CrossRef]
- Conde, K.; Meza, C.; Kelly, M.J.; Sinchak, K.; Wagner, E.J. Estradiol rapidly attenuates ORL-1 receptor-mediated inhibition of proopiomelanocortin neurons via Gq-coupled, membrane-initiated signaling. Neuroendocrinology 2016, 103, 787–805. [Google Scholar] [CrossRef] [PubMed]
- Farhang, B.; Pietruszewski, L.; Lutfy, K.; Wagner, E.J. The role of the NOP receptor in regulating food intake, meal pattern, and the excitability of proopiomelanocortin neurons. Neuropharmacology 2010, 59, 190–200. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, J.; Perez, L.; Soto, R.; Le, N.; Gastelum, C.; Wagner, E.J. Nociceptin/orphanin FQ neurons in the Arcuate Nucleus and Ventral Tegmental Area Act via Nociceptin Opioid Peptide Receptor Signaling to Inhibit Proopiomelanocortin and A 10 Dopamine Neurons and Thereby Modulate Ingestion of Palatable Food. Physiol. Behav. 2021, 228. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sayers, S.; Wagner, E. On the Pleiotropic Actions of Glucagon-like Peptide-1 in Its Regulation of Homeostatic and Hedonic Feeding. Int. J. Mol. Sci. 2025, 26, 3897. https://doi.org/10.3390/ijms26083897
Sayers S, Wagner E. On the Pleiotropic Actions of Glucagon-like Peptide-1 in Its Regulation of Homeostatic and Hedonic Feeding. International Journal of Molecular Sciences. 2025; 26(8):3897. https://doi.org/10.3390/ijms26083897
Chicago/Turabian StyleSayers, Sarah, and Ed Wagner. 2025. "On the Pleiotropic Actions of Glucagon-like Peptide-1 in Its Regulation of Homeostatic and Hedonic Feeding" International Journal of Molecular Sciences 26, no. 8: 3897. https://doi.org/10.3390/ijms26083897
APA StyleSayers, S., & Wagner, E. (2025). On the Pleiotropic Actions of Glucagon-like Peptide-1 in Its Regulation of Homeostatic and Hedonic Feeding. International Journal of Molecular Sciences, 26(8), 3897. https://doi.org/10.3390/ijms26083897




