Testosterone Modulates Oxidative Stress in a Sexually Dimorphic Manner in CBA/Ca Mice Infected with Plasmodium berghei ANKA
Abstract
1. Introduction
2. Results
2.1. The Combination of Letrozole and Testosterone Increased Testosterone Levels and Decreased 17β-Estradiol Levels in P. berghei ANKA-Infected Mice
2.2. Increasing Testosterone Concentrations Increased Parasitemia in Both Sexes but Affected Males to a Greater Extent
2.3. Effects of Increasing Testosterone Concentration on Oxidative Stress in the Blood of P. berghei ANKA-Infected Mice
2.4. Effects of Increasing Testosterone Concentrations on Oxidative Stress in the Spleens of P. berghei ANKA-Infected Mice
2.5. Effects of Increasing Testosterone Concentration on Oxidative Stress in the Brains of P. berghei ANKA-Infected Mice
3. Discussion
4. Materials and Methods
4.1. Mice
4.2. Parasites and Infection
4.3. Parasitemia
4.4. Testosterone and Letrozole Administration
4.5. Quantification of Testosterone, 17β-Estradiol, and DHEA
4.6. Quantification of SOD, GPx, and CAT-Specific Activities
4.7. Quantification of the MDA Concentration
4.8. Nitrite and Nitrate Quantification as an Indirect Measure of NO Levels
4.9. Experimental Design
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| SOD | Superoxide dismutase |
| GPx | Glutathione peroxidase |
| CAT | Catalase |
| MDA | Malondialdehyde |
| NO | Nitric oxide |
| ROS | Reactive oxygen species |
| ip | Intraperitoneal |
| iv | Intravenous |
| DHEA | Dehydroepiandrosterone |
| NADPH | Reduced nicotinamide adenine dinucleotide phosphate |
| eNOS | Endothelial nitric oxide synthase |
| PBS | Phosphate Buffer Solution |
References
- World Health Organization. World Malaria Report 2023; World Health Organization: Geneva, Switzerland, 2023. [Google Scholar]
- Dkhil, M.A.; Al-Shaebi, E.M.; Lubbad, M.Y.; Al-Quraishy, S. Impact of sex differences in brain response to infection with Plasmodium berghei. Parasitol. Res. 2016, 115, 415–422. [Google Scholar] [CrossRef]
- Benten, W.; Wunderlich, F.; Herrmann, R.; Kühn-Velten, W. Testrosterone-induced compared with oestradiol-induced immunosuppression against Plasmodium chabaudi malaria. J. Endocrinol. 1993, 139, 487–494. [Google Scholar] [CrossRef]
- Pathak, S.; Rege, M.; Gogtay, N.J.; Aigal, U.; Sharma, S.K.; Valecha, N.; Bhanot, G.; Kshirsagar, N.A.; Sharma, S. Age-dependent sex bias in clinical malarial disease in hypoendemic regions. PLoS ONE 2012, 7, e35592. [Google Scholar] [CrossRef]
- Singh, G.; Urhekar, R.A.D. Morbidity and Mortality associated with Plasmodium vivax and Plasmodium falciparum infection in a tertiary care hospital in Navi Mumbai, India. Era J. Med. Res. 2019, 6, 4. [Google Scholar]
- Aguilar-Castro, J.; Cervantes-Candelas, L.A.; Buendía-González, F.O.; de Jesús Nolasco-Pérez, T.; López-Padilla, M.S.; Fernández-Rivera, O.; Cervantes-Sandoval, A.; Legorreta-Herrera, M. Dimorphic effect of 17β-oestradiol on pathology and oxidative stress in experimental malaria. Immunobiology 2020, 225, 151873. [Google Scholar] [CrossRef]
- Mosqueda-Romo, N.A.; Rodriguez-Morales, A.L.; Buendia-Gonzalez, F.O.; Aguilar-Sanchez, M.; Morales-Montor, J.; Legorreta-Herrera, M. Gonadal steroids negatively modulate oxidative stress in CBA/Ca female mice infected with P. berghei ANKA. Biomed. Res. Int. 2014, 2014, 805495. [Google Scholar] [CrossRef]
- Ockenhouse, C.F.; Shear, H.L. Oxidative killing of the intraerythrocytic malaria parasite Plasmodium yoelii by activated macrophages. J. Immunol. 1984, 132, 424–431. [Google Scholar] [CrossRef]
- Strehlow, K.; Rotter, S.; Wassmann, S.; Adam, O.; Grohé, C.; Laufs, K.; Böhm, M.; Nickenig, G. Modulation of antioxidant enzyme expression and function by estrogen. Circ. Res. 2003, 93, 170–177. [Google Scholar] [CrossRef]
- Choobineh, H.; Sadighi, G.M.A.; Pasalar, P.; Jahanzad, I.; Ghorbani, R.; Hassanzadeh, G. The effects of testosterone on oxidative stress markers in mice with spinal cord injuries. Int. J. Fertil. Steril. 2016, 10, 87–93. [Google Scholar]
- Ekhoye, E.; Aloamaka, C.; Nwangwa, E. Alterations in gonadal oxidative stress markers and reproductive function of BALB/c mice infected with Plasmodium berghei. Niger. J. Physiol. Sci. 2019, 34, 131–139. [Google Scholar]
- Chainy, G.; Samantaray, S.; Samanta, L. Testosterone-induced changes in testicular antioxidant system. Andrologia 1997, 29, 343–349. [Google Scholar] [CrossRef]
- Ahlbom, E.; Prins, G.S.; Ceccatelli, S. Testosterone protects cerebellar granule cells from oxidative stress-induced cell death through a receptor mediated mechanism. Brain Res. 2001, 892, 255–262. [Google Scholar] [CrossRef]
- Chisu, V.; Manca, P.; Lepore, G.; Gadau, S.; Zedda, M.; Farina, V. Testosterone induces neuroprotection from oxidative stress. Effects on catalase activity and 3-nitro-L-tyrosine incorporation into alpha-tubulin in a mouse neuroblastoma cell line. Arch. Ital. De Biol. 2006, 144, 63–73. [Google Scholar]
- Dias, J.; Melvin, D.; Simonsick, E.; Carlson, O.; Shardell, M.; Ferrucci, L.; Chia, C.; Basaria, S.; Egan, J. Effects of aromatase inhibition vs. testosterone in older men with low testosterone: Randomized-controlled trial. Andrology 2016, 4, 33–40. [Google Scholar] [CrossRef]
- Gubbels Bupp, M.R.; Jorgensen, T.N. Androgen-induced immunosuppression. Front. Immunol. 2018, 9, 370132. [Google Scholar] [CrossRef]
- Ayi, K.; Patel, S.N.; Serghides, L.; Smith, T.G.; Kain, K.C. Nonopsonic phagocytosis of erythrocytes infected with ring-stage Plasmodium falciparum. Infect. Immun. 2005, 73, 2559–2563. [Google Scholar] [CrossRef]
- Chua, C.L.L.; Ng, I.M.J.; Yap, B.J.M.; Teo, A. Factors influencing phagocytosis of malaria parasites: The story so far. Malar. J. 2021, 20, 319. [Google Scholar] [CrossRef]
- Shank, J.; Silliker, J.; Harper, R. The effect of nitric oxide on bacteria. Appl. Microbiol. 1962, 10, 185–189. [Google Scholar] [CrossRef]
- Rockett, K.; Awburn, M.; Cowden, W.; Clark, I. Killing of Plasmodium falciparum in vitro by nitric oxide derivatives. Infect. Immun. 1991, 59, 3280–3283. [Google Scholar] [CrossRef]
- Legorreta-Herrera, M.; Rivas-Contreras, S.; Ventura-Gallegos, J.; Zentella-Dehesa, A. Nitric oxide is involved in the upregulation of IFN-γ and IL-10 mRNA expression by CD8+ T cells during the blood stages of P. chabaudi AS infection in CBA/Ca mice. Int. J. Biol. Sci. 2011, 7, 1401. [Google Scholar] [CrossRef]
- Kharazmi, A.; Jepsen, S.; Andersen, B. Generation of reactive oxygen radicals by human phagocytic cells activated by Plasmodium falciparum. Scand. J. Immunol. 1987, 25, 335–341. [Google Scholar] [CrossRef]
- Scaccabarozzi, D.; Deroost, K.; Corbett, Y.; Lays, N.; Corsetto, P.; Salè, F.O.; Van den Steen, P.E.; Taramelli, D. Differential induction of malaria liver pathology in mice infected with Plasmodium chabaudi AS or Plasmodium berghei NK65. Malar. J. 2018, 17, 18. [Google Scholar] [CrossRef]
- Atamna, H.; Ginsburg, H. Origin of reactive oxygen species in erythrocytes infected with Plasmodium falciparum. Mol. Biochem. Parasitol. 1993, 61, 231–241. [Google Scholar] [CrossRef]
- Dey, S.; Guha, M.; Alam, A.; Goyal, M.; Bindu, S.; Pal, C.; Maity, P.; Mitra, K.; Bandyopadhyay, U. Malarial infection develops mitochondrial pathology and mitochondrial oxidative stress to promote hepatocyte apoptosis. Free Radic. Biol. Med. 2009, 46, 271–281. [Google Scholar] [CrossRef]
- Huh, K.; Shin, U.-S.; Choi, J.-W.; Lee, S.-I. Effect of sex hormones on lipid peroxidation in rat liver. Arch. Pharmacal Res. 1994, 17, 109–114. [Google Scholar] [CrossRef]
- Arazi, H.; Mohammadjafari, H.; Asadi, A. Use of anabolic androgenic steroids produces greater oxidative stress responses to resistance exercise in strength-trained men. Toxicol. Rep. 2017, 4, 282–286. [Google Scholar] [CrossRef]
- Raza, A.; Varshney, S.K.; Khan, H.M.; Malik, M.A.; Mehdi, A.A.; Shukla, I. Superoxide dismutase activity in patients of cerebral malaria. Asian Pac. J. Trop. Dis. 2015, 5, S51–S53. [Google Scholar] [CrossRef]
- Long, B.J.; Jelovac, D.; Handratta, V.; Thiantanawat, A.; MacPherson, N.; Ragaz, J.; Goloubeva, O.G.; Brodie, A.M. Therapeutic strategies using the aromatase inhibitor letrozole and tamoxifen in a breast cancer model. J. Natl. Cancer Inst. 2004, 96, 456–465. [Google Scholar] [CrossRef]
- Omodeo-Sale, F.; Motti, A.; Basilico, N.; Parapini, S.; Olliaro, P.; Taramelli, D. Accelerated senescence of human erythrocytes cultured with Plasmodium falciparum. Blood 2003, 102, 705–711. [Google Scholar] [CrossRef]
- Chotivanich, K.; Udomsangpetch, R.; McGready, R.; Proux, S.; Newton, P.; Pukrittayakamee, S.; Looareesuwan, S.; White, N.J. Central role of the spleen in malaria parasite clearance. J. Infect. Dis. 2002, 185, 1538–1541. [Google Scholar] [CrossRef]
- Raza, A.; Varshney, S.K.; Shahid, M.; Khan, H.M.; Malik, M.A.; Mahdi, A.A.; Shujatullah, F. Lipid peroxidation in cerebral malaria and role of antioxidants. IOSR J. Pharm. 2013, 3, 15–18. [Google Scholar] [CrossRef]
- Mamounas, E.P.; Bandos, H.; Lembersky, B.C.; Jeong, J.-H.; Geyer, C.E.; Rastogi, P.; Fehrenbacher, L.; Graham, M.L.; Chia, S.K.; Brufsky, A.M. Use of letrozole after aromatase inhibitor-based therapy in postmenopausal breast cancer (NRG Oncology/NSABP B-42): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2019, 20, 88–99. [Google Scholar] [CrossRef]
- Cernetich, A.; Garver, L.S.; Jedlicka, A.E.; Klein, P.W.; Kumar, N.; Scott, A.L.; Klein, S.L. Involvement of gonadal steroids and gamma interferon in sex differences in response to blood-stage malaria infection. Infect. Immun. 2006, 74, 3190–3203. [Google Scholar] [CrossRef]
- Clark, I.; Hunt, N. Evidence for reactive oxygen intermediates causing hemolysis and parasite death in malaria. Infect. Immun. 1983, 39, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Krucken, J.; Dkhil, M.A.; Braun, J.V.; Schroetel, R.M.; El-Khadragy, M.; Carmeliet, P.; Mossmann, H.; Wunderlich, F. Testosterone suppresses protective responses of the liver to blood-stage malaria. Infect. Immun. 2005, 73, 436–443. [Google Scholar] [CrossRef] [PubMed]
- Mann, V.; Huber, C.; Kogianni, G.; Collins, F.; Noble, B. The antioxidant effect of estrogen and Selective Estrogen Receptor Modulators in the inhibition of osteocyte apoptosis in vitro. Bone 2007, 40, 674–684. [Google Scholar] [CrossRef]
- Borrás, C.; Gambini, J.; Gómez-Cabrera, M.C.; Sastre, J.; Pallardó, F.V.; Mann, G.E.; Viña, J. 17β-oestradiol up-regulates longevity-related, antioxidant enzyme expression via the ERK1 and ERK2 [MAPK]/NFκB cascade. Aging Cell 2005, 4, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Hobe, G.; Hillesheim, H.; Schön, R.; Reddersen, G.; Knappe, R.; Bannasch, P.; Mayer, D. Sex differences in dehydroepiandrosterone metabolism in the rat: Different plasma levels following ingestion of DHEA-supplemented diet and different metabolite patterns in plasma, bile and urine. Horm. Metab. Res. 1994, 26, 326–329. [Google Scholar] [CrossRef]
- Thomas, J.L.; Rajapaksha, M.; Mack, V.L.; DeMars, G.A.; Majzoub, J.A.; Bose, H.S. Regulation of human 3β-hydroxysteroid dehydrogenase type 2 by adrenal corticosteroids and product-feedback by androstenedione in human adrenarche. J. Pharmacol. Exp. Ther. 2015, 352, 67–76. [Google Scholar] [CrossRef]
- Nolasco-Pérez, T.d.J.; Cervantes-Candelas, L.A.; Buendía-González, F.O.; Aguilar-Castro, J.; Fernandez-Rivera, O.; Salazar-Castañón, V.H.; Legorreta-Herrera, M. Immunomodulatory effects of testosterone and letrozole during Plasmodium berghei ANKA infection. Front. Cell. Infect. Microbiol. 2023, 13, 1146356. [Google Scholar] [CrossRef]
- Benten, W.; Ulrich, P.; Kühn-Velten, W.; Vohr, H.; Wunderlich, F. Testosterone-induced susceptibility to Plasmodium chabaudi malaria: Persistence after withdrawal of testosterone. J. Endocrinol. 1997, 153, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Yadava, A.; Kumar, S.; Dvorak, J.A.; Milon, G.; Miller, L.H. Trafficking of Plasmodium chabaudi adami-infected erythrocytes within the mouse spleen. Proc. Natl. Acad. Sci. USA 1996, 93, 4595–4599. [Google Scholar] [CrossRef]
- Imai, T.; Iwawaki, T.; Akai, R.; Suzue, K.; Hirai, M.; Taniguchi, T.; Okada, H.; Hisaeda, H. Evaluating experimental cerebral malaria using oxidative stress indicator OKD48 mice. Int. J. Parasitol. 2014, 44, 681–685. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, D.; Das, U.; Ghosh, S.; Mallick, M.; Debnath, J. Testicular gametogenic and steroidogenic activities in cyclophosphamide treated rat: A correlative study with testicular oxidative stress. Drug Chem. Toxicol. 2002, 25, 281–292. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.S.; Xiong, C.; Lucero, J.; Behrens, M.M.; Dugan, L.L.; Quick, K.L. Gender differences in free radical homeostasis during aging: Shorter-lived female C57BL6 mice have increased oxidative stress. Aging Cell 2006, 5, 565–574. [Google Scholar] [CrossRef]
- Narsaria, N.; Mohanty, C.; Das, B.; Mishra, S.; Prasad, R. Oxidative stress in children with severe malaria. J. Trop. Pediatr. 2012, 58, 147–150. [Google Scholar] [CrossRef]
- Kotepui, M.; Mahittikorn, A.; Anabire, N.G.; Kotepui, K.U. Impact of malaria on glutathione peroxidase levels: A systematic review and meta-analysis. Sci. Rep. 2023, 13, 13928. [Google Scholar] [CrossRef]
- Kander, M.C.; Cui, Y.; Liu, Z. Gender difference in oxidative stress: A new look at the mechanisms for cardiovascular diseases. J. Cell. Mol. Med. 2017, 21, 1024–1032. [Google Scholar] [CrossRef]
- Park, J.; Kim, J.-R.; Shin, D.-G.; Cho, K.-H. Females with angina pectoris have altered lipoprotein metabolism with elevated cholesteryl ester transfer protein activity and impaired high-density lipoproteins-associated antioxidant enzymes. Int. J. Mol. Med. 2012, 29, 683–689. [Google Scholar] [CrossRef]
- Laughlin, M.H.; Welshons, W.V.; Sturek, M.; Rush, J.W.; Turk, J.R.; Taylor, J.A.; Judy, B.M.; Henderson, K.K.; Ganjam, V. Gender, exercise training, and eNOS expression in porcine skeletal muscle arteries. J. Appl. Physiol. 2003, 95, 250–264. [Google Scholar] [CrossRef]
- Cunnington, A.J.; Njie, M.; Correa, S.; Takem, E.N.; Riley, E.M.; Walther, M. Prolonged neutrophil dysfunction after Plasmodium falciparum malaria is related to hemolysis and heme oxygenase-1 induction. J. Immunol. 2012, 189, 5336–5346. [Google Scholar] [CrossRef] [PubMed]
- Usynin, I.; Klotz, C.; Frevert, U. Malaria circumsporozoite protein inhibits the respiratory burst in Kupffer cells. Cell. Microbiol. 2007, 9, 2610–2628. [Google Scholar] [CrossRef] [PubMed]
- Lavoie, J.-C.; Tremblay, A. Sex-specificity of oxidative stress in newborns leading to a personalized antioxidant nutritive strategy. Antioxidants 2018, 7, 49. [Google Scholar] [CrossRef]
- McCrohon, J.A.; Death, A.K.; Nakhla, S.; Jessup, W.; Handelsman, D.J.; Stanley, K.K.; Celermajer, D.S. Androgen receptor expression is greater in macrophages from male than from female donors: A sex difference with implications for atherogenesis. Circulation 2000, 101, 224–226. [Google Scholar] [CrossRef] [PubMed]
- Koukoulis, G.N.; Filiponi, M.; Gougoura, S.; Befani, C.; Liakos, P.; Bargiota, A. Testosterone and dihydrotestosterone modulate the redox homeostasis of endothelium. Cell Biol. Int. 2022, 46, 660–670. [Google Scholar] [CrossRef]
- Dockrell, H.M.; Alavi, A.; Playfair, J. Changes in oxidative burst capacity during murine malaria and the effect of vaccination. Clin. Exp. Immunol. 1986, 66, 37. [Google Scholar]
- Schneider, C.P.; Schwacha, M.G.; Samy, T.A.; Bland, K.I.; Chaudry, I.H. Androgen-mediated modulation of macrophage function after trauma-hemorrhage: Central role of 5α-dihydrotestosterone. J. Appl. Physiol. 2003, 95, 104–112. [Google Scholar] [CrossRef]
- Chignalia, A.Z.; Oliveira, M.A.; Debbas, V.; Dull, R.O.; Laurindo, F.R.; Touyz, R.M.; Carvalho, M.H.C.; Fortes, Z.B.; Tostes, R.C. Testosterone induces leucocyte migration by NADPH oxidase-driven ROS-and COX2-dependent mechanisms. Clin. Sci. 2015, 129, 39–48. [Google Scholar] [CrossRef]
- Lu, J.; Monardo, L.; Bryskin, I.; Hou, Z.; Trachtenberg, J.; Wilson, B.; Pinthus, J. Androgens induce oxidative stress and radiation resistance in prostate cancer cells though NADPH oxidase. Prostate Cancer Prostatic Dis. 2010, 13, 39–46. [Google Scholar] [CrossRef]
- Wunderlich, F.; Benten, W.P.M.; Lieberherr, M.; Guo, Z.; Stamm, O.; Wrehlke, C.; Sekeris, C.E.; Mossmann, H. Testosterone signaling in T cells and macrophages. Steroids 2002, 67, 535–538. [Google Scholar] [CrossRef]
- Pitteloud, N.; Mootha, V.K.; Dwyer, A.A.; Hardin, M.; Lee, H.; Eriksson, K.-F.; Tripathy, D.; Yialamas, M.; Groop, L.; Elahi, D. Relationship between testosterone levels, insulin sensitivity, and mitochondrial function in men. Diabetes Care 2005, 28, 1636–1642. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, I.; Newell-Fugate, A.E. Role of androgens and androgen receptor in control of mitochondrial function. Am. J. Physiol. -Cell Physiol. 2022, 323, C835–C846. [Google Scholar] [CrossRef] [PubMed]
- Dart, D.A.; Waxman, J.; Aboagye, E.O.; Bevan, C.L. Visualising androgen receptor activity in male and female mice. PLoS ONE 2013, 8, e71694. [Google Scholar] [CrossRef] [PubMed]
- Borrás, C.; Gambini, J.; López-Grueso, R.; Pallardó, F.V.; Viña, J. Direct antioxidant and protective effect of estradiol on isolated mitochondria. Biochim. Et Biophys. Acta (BBA)-Mol. Basis Dis. 2010, 1802, 205–211. [Google Scholar] [CrossRef]
- Ayres, S.; Tang, M.; Subbiah, M.R. Estradiol-17β as an antioxidant: Some distinct features when compared with common fat-soluble antioxidants. J. Lab. Clin. Med. 1996, 128, 367–375. [Google Scholar] [CrossRef]
- Xiao, H.; Deng, M.; Yang, B.; Hu, Z.; Tang, J. Pretreatment with 17β-estradiol attenuates cerebral ischemia-induced blood-brain barrier disruption in aged rats: Involvement of antioxidant signaling. Neuroendocrinology 2017, 106, 20–29. [Google Scholar] [CrossRef]
- Wang, L.; Pei, J.-H.; Jia, J.-X.; Wang, J.; Song, W.; Fang, X.; Cai, Z.-P.; Huo, D.-S.; Wang, H.; Yang, Z.-J. Inhibition of oxidative stress by testosterone improves synaptic plasticity in senescence accelerated mice. J. Toxicol. Environ. Health Part A 2019, 82, 1061–1068. [Google Scholar] [CrossRef] [PubMed]
- Meydan, S.; Kus, I.; Tas, U.; Ogeturk, M.; Sancakdar, E.; Dabak, D.O.; Zararsız, I.; Sarsılmaz, M. Effects of testosterone on orchiectomy-induced oxidative damage in the rat hippocampus. J. Chem. Neuroanat. 2010, 40, 281–285. [Google Scholar] [CrossRef]
- Francischetti, I.M.; Gordon, E.; Bizzarro, B.; Gera, N.; Andrade, B.B.; Oliveira, F.; Ma, D.; Assumpção, T.C.; Ribeiro, J.M.; Pena, M. Tempol, an intracellular antioxidant, inhibits tissue factor expression, attenuates dendritic cell function, and is partially protective in a murine model of cerebral malaria. PLoS ONE 2014, 9, e87140. [Google Scholar] [CrossRef]
- Vasquez, M.; Zuniga, M.; Rodriguez, A. Oxidative stress and pathogenesis in malaria. Front. Cell. Infect. Microbiol. 2021, 11, 768182. [Google Scholar] [CrossRef]
- Akanbi, O.; Badaki, J.; Adeniran, O.; Olotu, O. Effect of blood group and demographic characteristics on malaria infection, oxidative stress and haemoglobin levels in South Western Nigeria. Afr. J. Microbiol. Res. 2010, 4, 877–880. [Google Scholar]
- Zhang, G.; Cui, R.; Kang, Y.; Qi, C.; Ji, X.; Zhang, T.; Guo, Q.; Cui, H.; Shi, G. Testosterone propionate activated the Nrf2-ARE pathway in ageing rats and ameliorated the age-related changes in liver. Sci. Rep. 2019, 9, 18619. [Google Scholar] [CrossRef]
- Rhodes, M.E.; Frye, C.A. Androgens in the hippocampus can alter, and be altered by, ictal activity. Pharmacol. Biochem. Behav. 2004, 78, 483–493. [Google Scholar] [CrossRef] [PubMed]
- Naziroglu, M. Molecular role of catalase on oxidative stress-induced Ca(2+) signaling and TRP cation channel activation in nervous system. J. Recept. Signal Transduct. Res. 2012, 32, 134–141. [Google Scholar] [CrossRef] [PubMed]
- Akide Ndunge, O.B.; Kilian, N.; Salman, M.M. Cerebral Malaria and Neuronal Implications of Plasmodium falciparum Infection: From Mechanisms to Advanced Models. Adv. Sci. 2022, 9, e2202944. [Google Scholar] [CrossRef]
- Atallah, A.; Mhaouty-Kodja, S.; Grange-Messent, V. Chronic depletion of gonadal testosterone leads to blood-brain barrier dysfunction and inflammation in male mice. J. Cereb. Blood Flow. Metab. 2017, 37, 3161–3175. [Google Scholar] [CrossRef]
- Cioni, B.; Zaalberg, A.; van Beijnum, J.R.; Melis, M.H.M.; van Burgsteden, J.; Muraro, M.J.; Hooijberg, E.; Peters, D.; Hofland, I.; Lubeck, Y.; et al. Androgen receptor signalling in macrophages promotes TREM-1-mediated prostate cancer cell line migration and invasion. Nat. Commun. 2020, 11, 4498. [Google Scholar] [CrossRef] [PubMed]
- Fanaei, H.; Karimian, S.M.; Sadeghipour, H.R.; Hassanzade, G.; Kasaeian, A.; Attari, F.; Khayat, S.; Ramezani, V.; Javadimehr, M. Testosterone enhances functional recovery after stroke through promotion of antioxidant defenses, BDNF levels and neurogenesis in male rats. Brain Res. 2014, 1558, 74–83. [Google Scholar] [CrossRef]
- Skogastierna, C.; Hotzen, M.; Rane, A.; Ekström, L. A supraphysiological dose of testosterone induces nitric oxide production and oxidative stress. Eur. J. Prev. Cardiol. 2014, 21, 1049–1054. [Google Scholar] [CrossRef]
- Marin, D.P.; Bolin, A.P.; de Cassia Macedo dos Santos, R.; Curi, R.; Otton, R. Testosterone suppresses oxidative stress in human neutrophils. Cell Biochem. Funct. 2010, 28, 394–402. [Google Scholar] [CrossRef]
- Falanga, P.B.; Butcher, E.C. Late treatment with anti-LFA-1 (CD11a) antibody prevents cerebral malaria in a mouse model. Eur. J. Immunol. 1991, 21, 2259–2263. [Google Scholar] [CrossRef] [PubMed]
- Dogruman-Al, F.; Engin, A.B.; Bukan, N.; Evirgen-Bostanci, S.; Çeber, K. Late-stage systemic immune effectors in Plasmodium berghei ANKA infection: Biopterin and oxidative stress. Pteridines 2015, 26, 105–112. [Google Scholar] [CrossRef]
- Benten, W.; Bettenhaeuser, U.; Wunderlich, F.; Van Vliet, E.; Mossmann, H. Testosterone-induced abrogation of self-healing of Plasmodium chabaudi malaria in B10 mice: Mediation by spleen cells. Infect. Immun. 1991, 59, 4486–4490. [Google Scholar] [CrossRef] [PubMed]
- Legorreta-Herrera, M.; Retana-Ugalde, R.; Ventura-Gallegos, J.L.; Narváez, V. Pyrimethamine induces oxidative stress in Plasmodium yoelii 17XL-infected mice: A novel immunomodulatory mechanism of action for an old antimalarial drug? Exp. Parasitol. 2010, 126, 381–388. [Google Scholar] [CrossRef]
- Aebi, H. Catalase in Vitro; Methods in enzymology; Elsevier: Amsterdam, The Netherlands, 1984; Volume 105, pp. 121–126. [Google Scholar]
- Ayala, A.; Muñoz, M.F.; Argüelles, S. Lipid peroxidation: Production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxidative Med. Cell. Longev. 2014, 2014, 360438. [Google Scholar] [CrossRef]


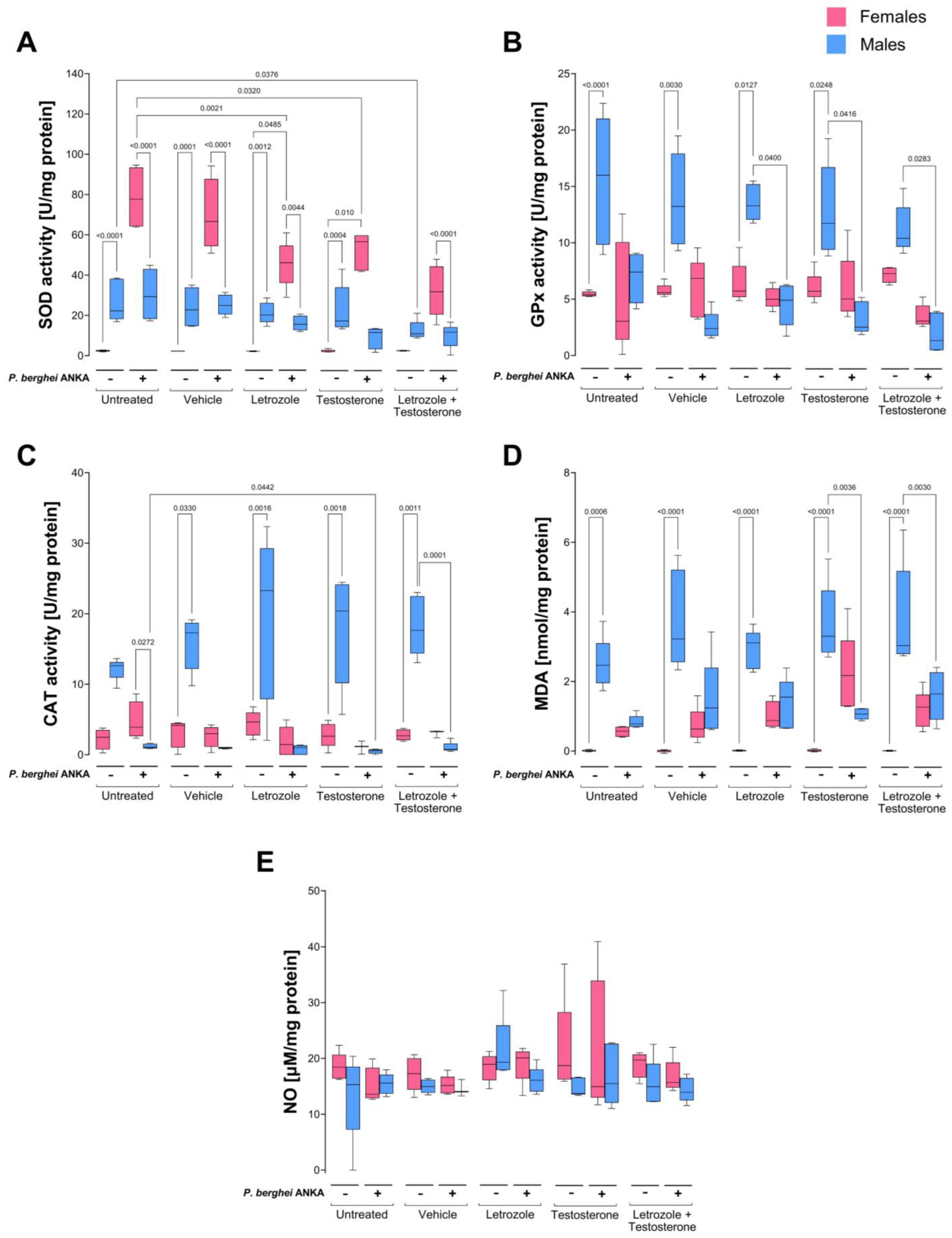
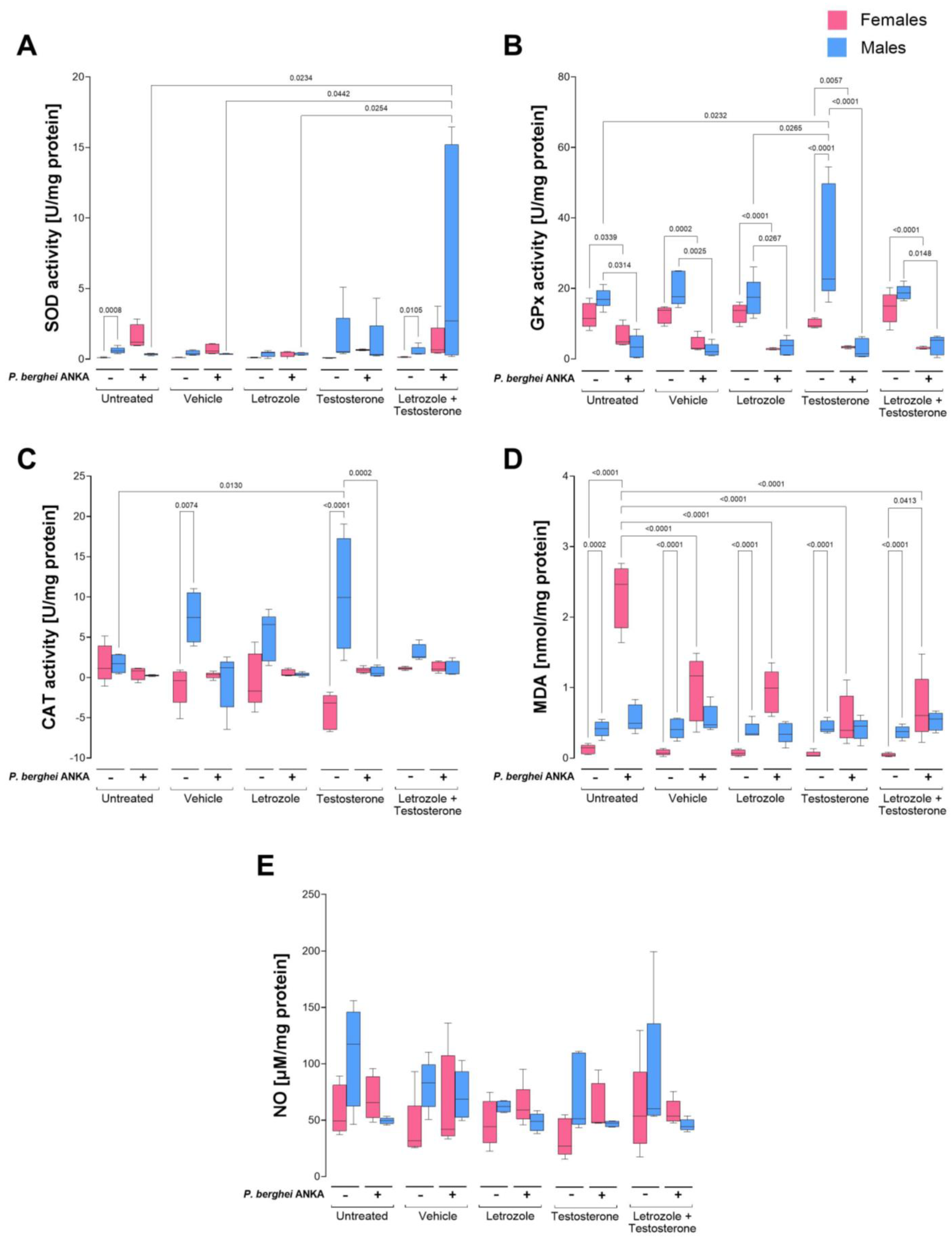


| Blood | Spleen | Brain | ||||
|---|---|---|---|---|---|---|
| P. berghei ANKA | − | + | − | + | − | + |
| SOD activity | ♀ < ♂ | ↑♀ > ♂ | ♀ > ♂ | ♀ < ↑♂ | ♀ < ♂ | ♀ < ↑♂ |
| GPx activity | ♀ < ♂ | ♀ = ↓♂ | ♀ = ♂ | ↓♀ = ↓♂ | ♀ < ♂ | ↓♀ = ↓♂ |
| CAT activity | ♀ < ♂ | ♀ > ↓♂ | ♀ < ♂ | ♀ = ↓♂ | ♀ < ♂ | ♀ = ↓♂ |
| MDA levels | ♀ < ♂ | ↑♀ < ↓♂ | ♀ < ♂ | ↑♀ = ♂ | ♀ < ♂ | ♀ = ↓♂ |
| NO levels | ♀ = ♂ | ♀ = ♂ | ♀ = ♂ | ♀ = ♂ | ♀ < ♂ | ↑♀ = ♂ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nolasco-Pérez, T.d.J.; Salazar-Castañón, V.H.; Cervantes-Candelas, L.A.; Buendía-González, F.O.; Aguilar-Castro, J.; Legorreta-Herrera, M. Testosterone Modulates Oxidative Stress in a Sexually Dimorphic Manner in CBA/Ca Mice Infected with Plasmodium berghei ANKA. Int. J. Mol. Sci. 2025, 26, 3898. https://doi.org/10.3390/ijms26083898
Nolasco-Pérez TdJ, Salazar-Castañón VH, Cervantes-Candelas LA, Buendía-González FO, Aguilar-Castro J, Legorreta-Herrera M. Testosterone Modulates Oxidative Stress in a Sexually Dimorphic Manner in CBA/Ca Mice Infected with Plasmodium berghei ANKA. International Journal of Molecular Sciences. 2025; 26(8):3898. https://doi.org/10.3390/ijms26083898
Chicago/Turabian StyleNolasco-Pérez, Teresita de Jesús, Víctor Hugo Salazar-Castañón, Luis Antonio Cervantes-Candelas, Fidel Orlando Buendía-González, Jesús Aguilar-Castro, and Martha Legorreta-Herrera. 2025. "Testosterone Modulates Oxidative Stress in a Sexually Dimorphic Manner in CBA/Ca Mice Infected with Plasmodium berghei ANKA" International Journal of Molecular Sciences 26, no. 8: 3898. https://doi.org/10.3390/ijms26083898
APA StyleNolasco-Pérez, T. d. J., Salazar-Castañón, V. H., Cervantes-Candelas, L. A., Buendía-González, F. O., Aguilar-Castro, J., & Legorreta-Herrera, M. (2025). Testosterone Modulates Oxidative Stress in a Sexually Dimorphic Manner in CBA/Ca Mice Infected with Plasmodium berghei ANKA. International Journal of Molecular Sciences, 26(8), 3898. https://doi.org/10.3390/ijms26083898






