Cremastra appendiculata Polysaccharides Alleviate Neurodegenerative Diseases in Caenorhabditis elegans: Targeting Amyloid-β Toxicity, Tau Toxicity and Oxidative Stress
Abstract
1. Introduction
2. Results
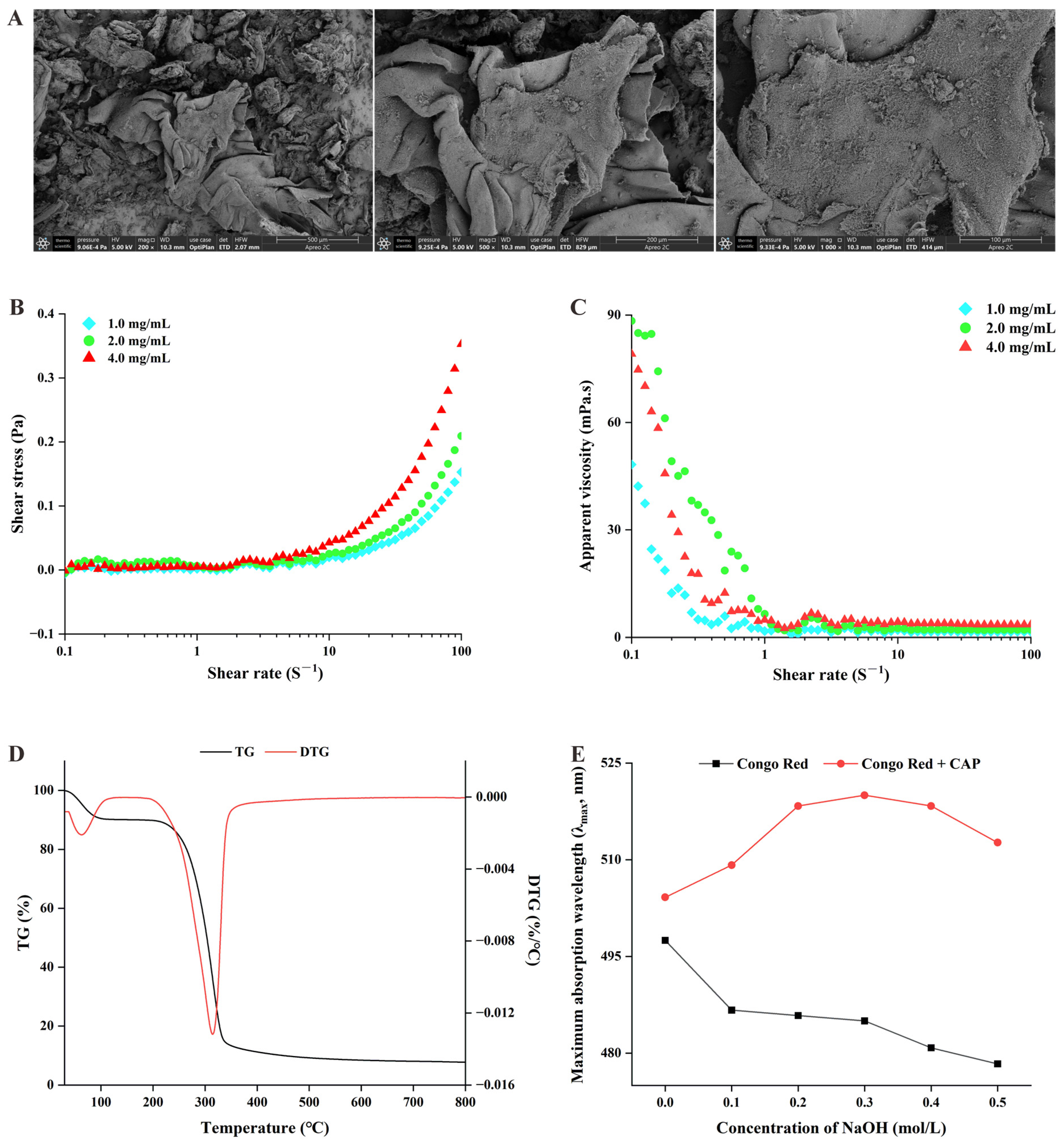
2.1. Physicochemical Characterization of Cremastra Appendiculata Polysaccharides (CAPs)
- Chemical composition of CAP
- 2.
- Microscopic morphology of CAP
- 3.
- The apparent viscosity of CAP
- 4.
- Thermal properties of CAP
- 5.
- Identification of the triple helix structure of CAP
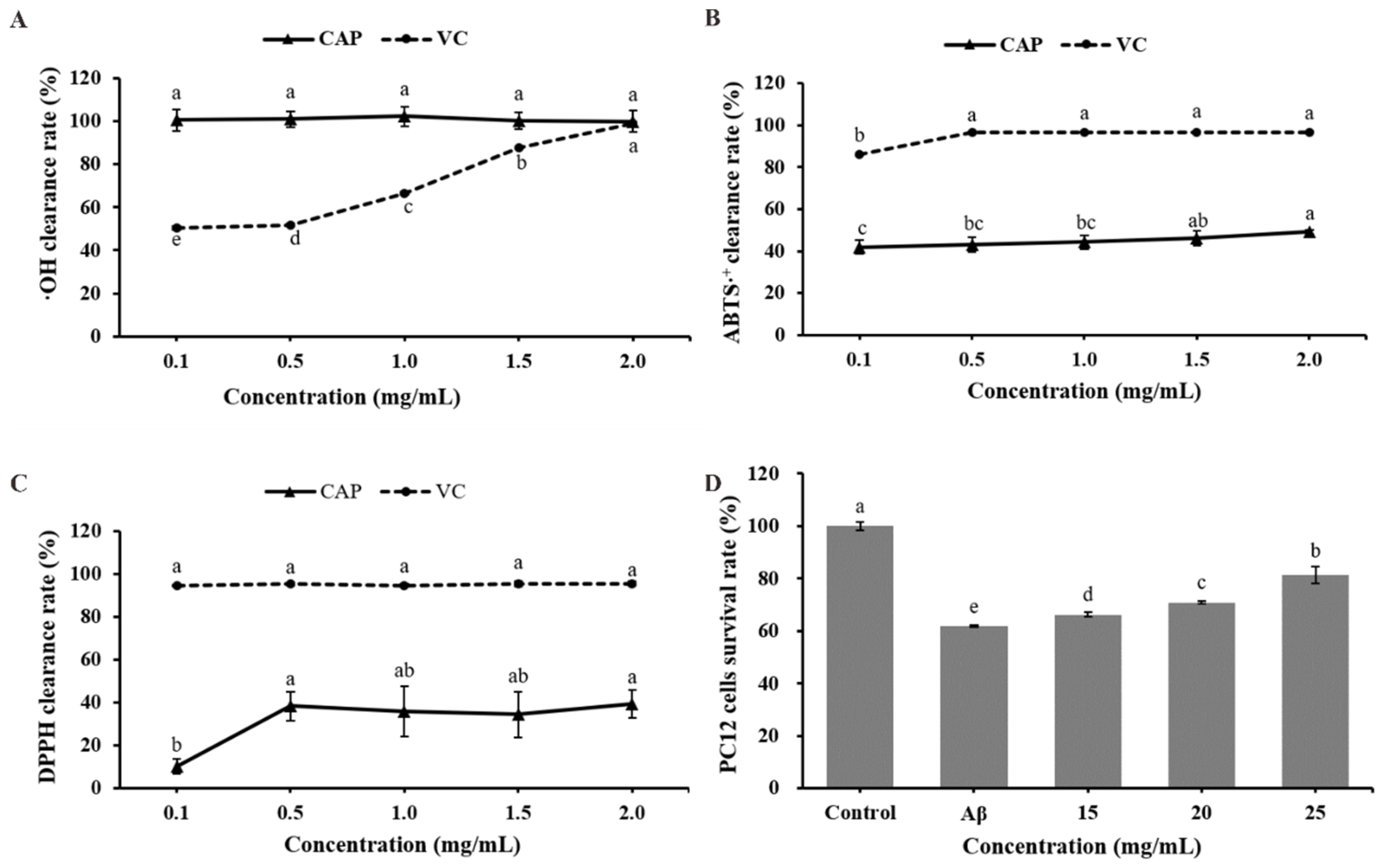
2.2. Evaluation of In Vitro Capacity of CAP
2.3. Effect of CAP on Tau-Induced Toxicity in Caenorhabditis elegans (C. elegans)
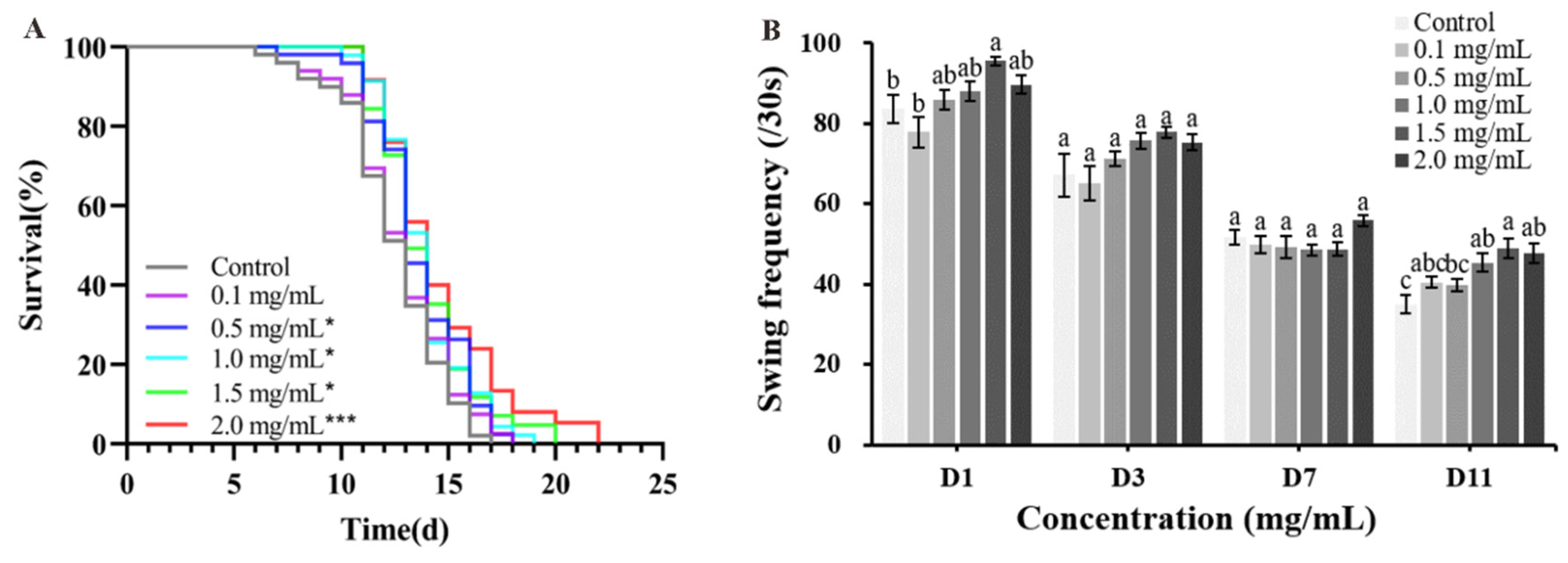
2.4. Effect of CAP on Lifespan, Growth, Development, and Motility of Alzheimer’s Disease (AD) Model C. elegans
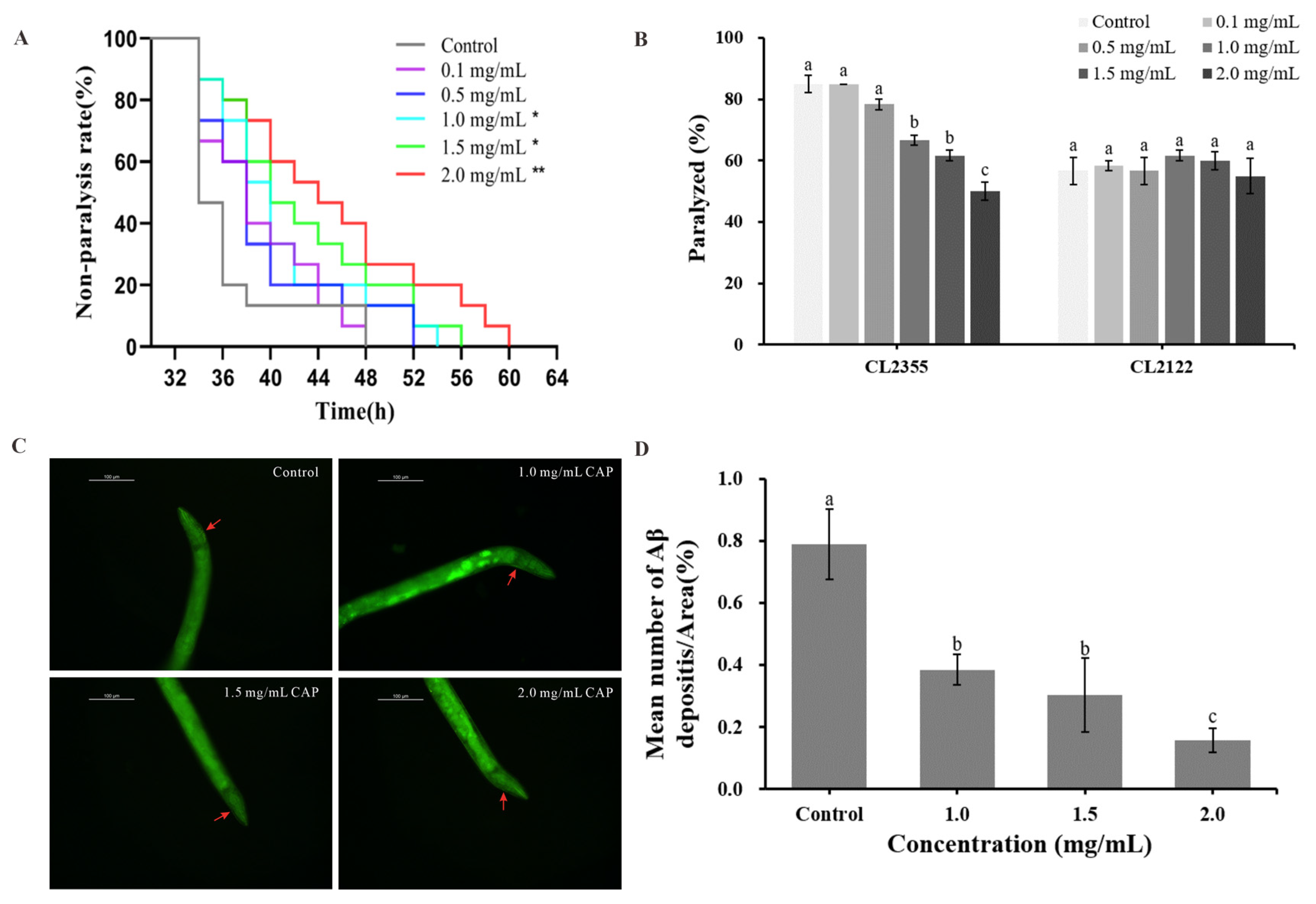
2.5. Effects of CAP on Amyloid-Beta (Aβ)-Induced Paralysis and 5-Hydroxytryptamine (5-HT) Hypersensitivity
2.6. Effect of CAP on Aβ Deposition
2.7. Resistance Analysis
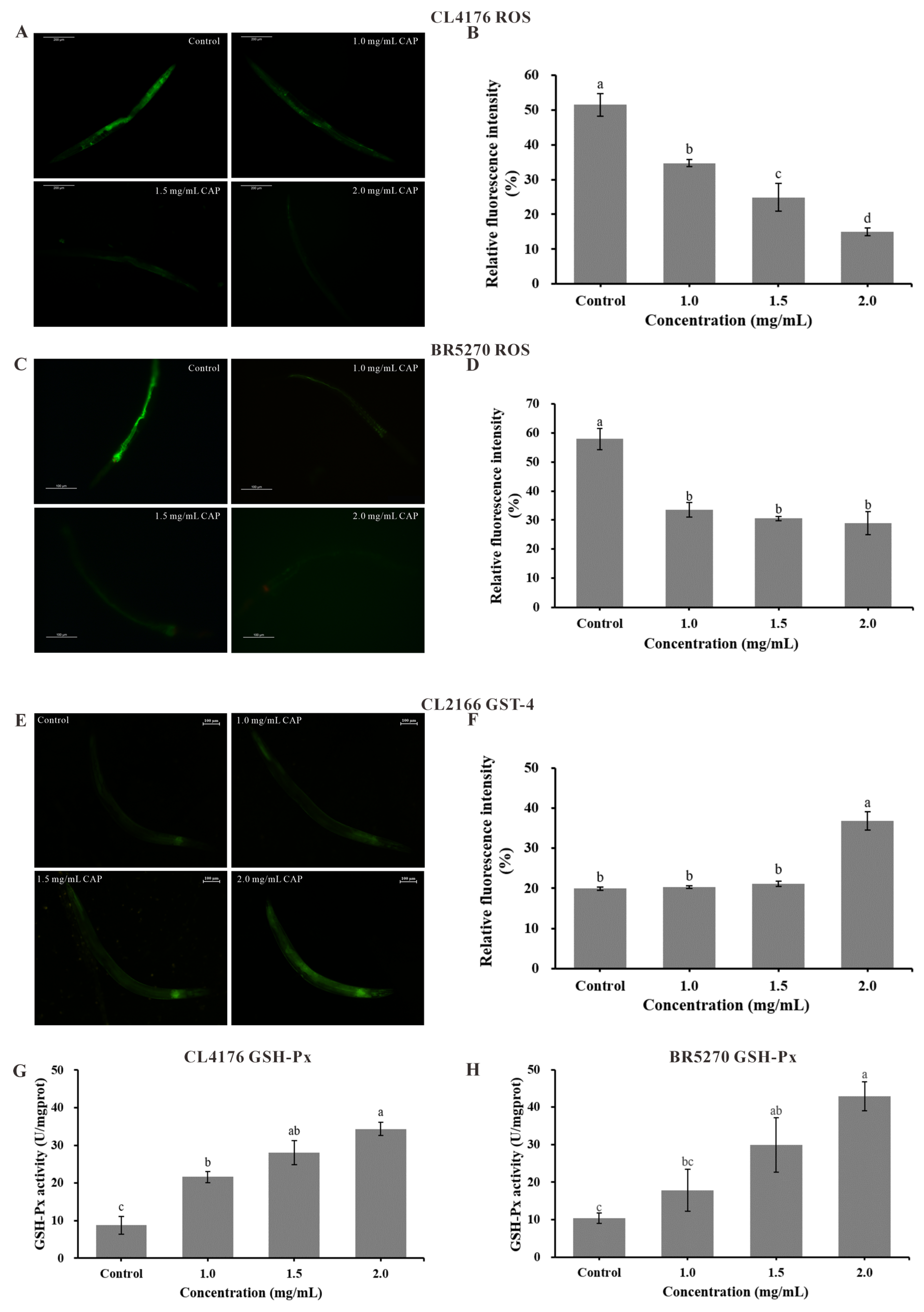
2.8. Reactive Oxygen Species (ROS) Level and Antioxidant Enzyme Activities
2.9. Effect of CAP on Gene Expression in AD Model C. elegans
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Extraction, Isolation, and Physicochemical Characterization of CAPs
4.3. Determination of In Vitro Antioxidant Capacity of CAPs
- (1)
- Hydroxyl radical (·OH) scavenging activity
- (2)
- ABTS·+ radical scavenging activity
- (3)
- DPPH radical scavenging activity
4.4. Determination of In Vitro Anti-AD Capacity of CAP
4.5. C. elegans Strain and Experimental Design
4.6. Determination of Physiological Indexes
- (1)
- Lifespan Assay:
- (2)
- Pharyngeal Pumping and Body Size:
- (3)
- Motility Assay:
4.7. Stress Resistance Assays
4.8. Paralysis, 5-Hydroxytryptamine Hypersensitivity Assays
4.9. Aβ Deposition
4.10. Determination of Intracellular ROS and Antioxidant Enzyme Activities
4.11. Real-Time Quantitative Polymerase Chain Reaction
4.12. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mao, K.; Zhang, G. The role of PARP1 in neurodegenerative diseases and aging. FEBS J. 2022, 289, 2013–2024. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Wu, J. Amyloid-β: A double agent in Alzheimer’s disease? Biomed. Pharmacother. 2021, 139, 111575. [Google Scholar] [CrossRef]
- Fares, A.; Borrmann, D. Neurochemical Aspects of Alzheimer’s Disease and Movement Disturbances: A Theory of β-Amyloid and τ-Protein. Am. J. Alzheimers Dis. Other Demen. 2018, 33, 535–540. [Google Scholar] [CrossRef]
- Bonda, D.J.; Wang, X.L.; Lee, H.G.; Smith, M.A.; Perry, G.; Zhu, X.W. Neuronal failure in Alzheimer’s disease: A view through the oxidative stress looking-glass. Neurosci. Bull. 2014, 30, 243–252. [Google Scholar] [CrossRef]
- Chen, Z.C.; Zhong, C.J. Oxidative stress in Alzheimer’s disease. Neurosci. Bull. 2014, 30, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Farooqui, T.; Farooqui, A.A. Aging: An important factor for the pathogenesis of neurodegenerative diseases. Mech. Ageing Dev. 2009, 130, 203–215. [Google Scholar] [CrossRef]
- Schweers, O.; Mandelkow, E.M.; Biernat, J.; Mandelkow, E. Oxidation of cysteine-322 in the repeat domain of microtubule-associated protein tau controls the in vitro assembly of paired helical filaments. Proc. Natl. Acad. Sci. USA 1995, 92, 8463–8467. [Google Scholar] [CrossRef]
- Tamagno, E.; Bardini, P.; Obbili, A.; Vitali, A.; Borghi, R.; Zaccheo, D.; Pronzato, M.A.; Danni, O.; Smith, M.A.; Perry, G.; et al. Oxidative stress increases expression and activity of BACE in NT2 neurons. Neurobiol. Dis. 2002, 10, 279–288. [Google Scholar] [CrossRef] [PubMed]
- Oda, A.; Tamaoka, A.; Araki, W. Oxidative stress up-regulates presenilin 1 in lipid rafts in neuronal cells. J. Neurosci. Res. 2010, 88, 1137–1145. [Google Scholar] [CrossRef]
- Breijyeh, Z.; Karaman, R. Comprehensive Review on Alzheimer’s Disease: Causes and Treatment. Molecules 2020, 25, 5789. [Google Scholar] [CrossRef]
- Gonzalez-Moragas, L.; Roig, A.; Laromaine, A. C. elegans as a tool for in vivo nanoparticle assessment. Adv. Colloid Interface Sci. 2015, 219, 10–26. [Google Scholar] [CrossRef] [PubMed]
- Markaki, M.; Tavernarakis, N. Caenorhabditis elegans as a model system for human diseases. Curr. Opin. Biotechnol. 2020, 63, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Roussos, A.; Kitopoulou, K.; Borbolis, F.; Palikaras, K. Caenorhabditis elegans as a model system to study human neurodegenerative disorders. Biomolecules 2023, 13, 478. [Google Scholar] [CrossRef] [PubMed]
- Koch, K.; Weldle, N.; Baier, S.; Büchter, C.; Wätjen, W. Hibiscus sabdariffa L. extract prolongs lifespan and protects against amyloid-β toxicity in Caenorhabditis elegans: Involvement of the FoxO and Nrf2 orthologues DAF-16 and SKN-1. Eur. J. Nutr. 2020, 59, 137–150. [Google Scholar] [CrossRef]
- Li, H.; Yu, X.X.; Li, C.X.; Ma, L.; Zhao, Z.Y.; Guan, S.W.; Wang, L.P. Caffeic acid protects against Aβ toxicity and prolongs lifespan in Caenorhabditis elegans models. Food Funct. 2021, 12, 1219–1231. [Google Scholar] [CrossRef]
- Romero-Márquez, J.M.; Navarro-Hortal, M.D.; Jiménez-Trigo, V.; Vera-Ramírez, L.; Forbes-Hernández, T.J.; Esteban-Muñoz, A.; Giampieri, F.; Bullón, P.; Battino, M.; Sánchez-González, C.; et al. An oleuropein rich-olive (Olea europaea L.) leaf extract reduces β-amyloid and tau proteotoxicity through regulation of oxidative- and heat shock-stress responses in Caenorhabditis elegans. Food Chem. Toxicol. 2022, 162, 112914. [Google Scholar] [CrossRef]
- Peng, G.; Li, M.; Meng, Z.L. Polysaccharides: Potential bioactive macromolecules for Alzheimer’s disease. Front. Nutr. 2023, 10, 1249018. [Google Scholar] [CrossRef]
- Du, Q.; Zhu, X.Y.; Si, J.R. Angelica polysaccharide ameliorates memory impairment in Alzheimer’s disease rat through activating BDNF/TrkB/CREB pathway. Exp. Biol. Med. 2020, 245, 1–10. [Google Scholar] [CrossRef]
- Zhang, Z.H.; Wang, Y.J.; Gao, T.; Tang, Z.Z.; Zhou, L.J.; Chen, T.; Feng, S.L.; Ding, C.B.; Yuan, S.; Yuan, M. Basic characterization and Alzheimer’s disease relieving property of a glucose riched polysaccharide from Cibotium barometz. Arab. J. Chem. 2023, 16, 104597. [Google Scholar] [CrossRef]
- State Pharmacopoeia Committee. Pharmacopoeia of the People’s Republic of China; China Pharmaceutical Science and Technology Press: Beijing, China, 2020; Volume I. [Google Scholar]
- Wang, Q.; Wu, J.S.; Huang, J.; Yang, L.J.; Tao, J.; Nie, J.T.; Zhao, J.Y.; Wang, Y.N. Cremastra appendiculata polysaccharides improve stress resistance and prolong the lifespan of Caenorhabditis elegans via daf-16 in the insulin signaling pathway. Int. J. Biol. Macromol. 2023, 229, 496–506. [Google Scholar] [CrossRef]
- Fang, Y.; Ning, A.; Li, S.; Zhou, S.; Liu, L.; Joseph, T.P.; Zhong, M.; Jiao, J.; Zhang, W.; Shi, Y.; et al. Polysaccharides Extracted from Rhizoma Pleionis Have Antitumor Properties In Vitro and in an H22 Mouse Hepatoma Ascites Model In Vivo. Int. J. Mol. Sci. 2018, 19, 1386. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Bi, C.L.; Shi, H.C.; Li, X.J. Structural studies of a mannoglucan from Cremastra appendiculata (Orchidaceae) by chemical and enzymatic methods. Carbohydr. Polym. 2021, 272, 118524. [Google Scholar] [CrossRef]
- Li, Q.; Tu, Y.; Zhu, C.; Luo, W.; Huang, W.; Liu, W.; Li, Y. Cholinesterase, β-amyloid aggregation inhibitory and antioxidant capacities of Chinese medicinal plants. Ind. Crops Prod. 2017, 108, 512–519. [Google Scholar] [CrossRef]
- Jiang, S.; Wang, M.Y.; Zafar, S.; Jiang, L.; Luo, J.Y.; Zhao, H.M.; Tian, S.Y.; Zhu, Y.Q.; Peng, C.Y.; Wang, W. Phytochemistry, pharmacology and clinical applications of the traditional Chinese herb Pseudobulbus Cremastrae seu Pleiones (Shancigu): A review. Arab. J. Chem. 2022, 15, 104090. [Google Scholar] [CrossRef]
- Wang, Z.C.; Zheng, Y.; Lai, Z.R.; Hu, X.L.; Wang, L.; Wang, X.Q.; Li, Z.T.; Gao, M.J.; Yang, Y.H.; Wang, Q.; et al. Effect of monosaccharide composition and proportion on the bioactivity of polysaccharides: A review. Int. J. Biol. Macromol. 2024, 254, 127955. [Google Scholar] [CrossRef]
- Yi, J.P.; Li, X.; Wang, S.; Wu, T.T.; Liu, P. Steam explosion pretreatment of Achyranthis bidentatae radix: Modified polysaccharide and its antioxidant activities. Food Chem. 2022, 375, 131746. [Google Scholar] [CrossRef]
- He, P.F.; Zhang, A.Q.; Zhang, F.M.; Linhardt, R.J.; Sun, P.L. Structure and bioactivity of a polysaccharide containing uronic acid from Polyporus umbellatus sclerotia. Carbohydr. Polym. 2016, 152, 222–230. [Google Scholar] [CrossRef] [PubMed]
- He, P.F.; Zhang, A.Q.; Wang, X.L.; Qu, L.; Li, G.L.; Li, Y.P.; Sun, P.L. Structure elucidation and antioxidant activity of a novel polysaccharide from Polyporus umbellatus sclerotia. Int. J. Biol. Macromol. 2016, 82, 411–417. [Google Scholar] [CrossRef]
- Scheltens, P.; Strooper, B.D.; Kivipelto, M.; Holstege, H.; Chételat, G.; Teunissen, C.E.; Cummings, J.; van der Flier, W.M. Alzheimer’s disease. Lancet 2021, 397, 1577–1590. [Google Scholar] [CrossRef]
- Hu, Y.R.; Xing, S.L.; Chen, C.; Shen, D.Z.; Chen, J.L. Codonopsis pilosula Polysaccharides Alleviate Aβ1-40-Induced PC12 Cells Energy Dysmetabolism via CD38/NAD+ Signaling Pathway. Curr. Alzheimer Res. 2021, 18, 208–221. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.J.; Wang, B.M.; Liu, C.; Zhu, X.T.; Zhang, P.Q.; Yu, H.K.; Li, Y.Q.; Li, Z.H.; Li, M.Q. Inhibiting c-Jun N-terminal kinase (JNK)-mediated apoptotic signaling pathway in PC12 cells by a polysaccharide (CCP) from Coptis chinensis against Amyloid-β (Aβ)-induced neurotoxicity. Int. J. Biol. Macromol. 2019, 134, 565–574. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Song, S.; Li, X.; Wang, J.; Bao, Y.; Wang, X.; Lian, L.; Liu, X.; Ma, W. Neuroprotective Effect of Codonopsis pilosula Polysaccharide on Aβ25-35-Induced Damage in PC12 Cells via the p38MAPK Signaling Pathways. Pharmaceuticals 2024, 17, 1231. [Google Scholar] [CrossRef]
- Drake, J.; Link, C.D.; Butterfield, D.A. Oxidative stress precedes fibrillar deposition of Alzheimer’s disease amyloid β-peptide (1–42) in a transgenic Caenorhabditis elegans model. Neurobiol. Aging 2003, 24, 415–420. [Google Scholar] [CrossRef]
- Fatouros, C.; Pir, G.J.; Biernat, J.; Koushika, S.P.; Mandelkow, E.; Mandelkow, E.M.; Schmidt, E.; Baumeister, R. Inhibition of tau aggregation in a novel Caenorhabditis elegans model of tauopathy mitigates proteotoxicity. Hum. Mol. Genet. 2012, 21, 3587–3603. [Google Scholar] [CrossRef]
- Li, Y.J.; Guan, S.W.; Liu, C.; Chen, X.H.; Zhu, Y.M.; Xie, Y.T.; Wang, J.B.; Ji, X.; Li, L.Q.; Li, Z.H.; et al. Neuroprotective effects of Coptis chinensis Franch polysaccharide on amyloid-beta (Aβ)-induced toxicity in a transgenic Caenorhabditis elegans model of Alzheimer’s disease (AD). Int. J. Biol. Macromol. 2018, 113, 991–995. [Google Scholar] [CrossRef]
- Xiao, C.X.; Chen, T.J.; Yuan, M.; Li, Y.; Wang, F.W. A Novel Polysaccharide DSPP-1 from Durian Seed: Structure Characterization and Its Protective Effects Against Alzheimer’s Disease in a Transgenic Caenorhabditis elegans Model. Plant Foods Hum. Nutr. 2023, 78, 329–335. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, H.; Wang, J.; Jin, W.Q.; Yan, X.C.; Chen, X.N.; Wang, D.D.; Zhao, D.Q.; Wang, Y.F.; Cong, D.Y.; et al. Ginsenoside Rf inhibits human tau proteotoxicity and causes specific LncRNA, miRNA and mRNA expression changes in Caenorhabditis elegans model of tauopathy. Eur. J. Pharmacol. 2022, 922, 174887. [Google Scholar] [CrossRef] [PubMed]
- Dosanjh, L.E.; Brown, M.K.; Rao, G.; Link, C.D.; Luo, Y. Behavioral phenotyping of a transgenic Caenorhabditis elegans expressing neuronal amyloid-β. J. Alzheimers Dis. 2010, 19, 681–690. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Chen, X.; Zhang, Y.; Liu, X.; Wang, C.; Teng, L.; Wang, D. Protective roles of Amanita caesarea polysaccharides against Alzheimer’s disease via Nrf2 pathway. Int. J. Biol. Macromol. 2019, 121, 29–37. [Google Scholar] [CrossRef]
- Shanavas, A.; Papasozomenos, S.C. τ kinases in the rat heat shock model: Possible implications for Alzheimer disease. Proc. Natl. Acad. Sci. USA 2000, 97, 14139–14144. [Google Scholar] [CrossRef]
- Kim, S.; Shin, S.J.; Nam, Y.; Park, Y.H.; Kim, B.H.; Park, H.H.; Kumar, V.; Yoo, D.H.; Lee, Y.Y.; Hoe, H.S.; et al. Korean red ginseng polysaccharide as a potential therapeutic agent targeting tau pathology in Alzheimer’s disease. Int. J. Biol. Macromol. 2024, 263, 130516. [Google Scholar] [CrossRef] [PubMed]
- Seidler, P.M.; Boyer, D.R.; Rodriguez, J.A.; Sawaya, M.R.; Cascio, D.; Murray, K.; Gonen, T.; Eisenberg, D.S. Structure-based inhibitors of tau aggregation. Nat. Chem. 2018, 10, 170–176. [Google Scholar] [CrossRef]
- Kim, S.; Nam, Y.; Shin, S.J.; Prajapati, R.; Shin, S.M.; Kim, M.J.; Kim, H.S.; Leem, S.H.; Kim, T.J.; Park, Y.H.; et al. Dual modulators of aggregation and dissociation of amyloid beta and tau: In vitro, in vivo, and in silico studies of Uncaria rhynchophylla and its bioactive components. Biomed. Pharmacother. 2022, 156, 113865. [Google Scholar] [CrossRef]
- Cutler, R.G.; Kelly, J.; Storie, K.; Pedersen, W.A.; Tammara, A.; Hatanpaa, K.; Troncoso, J.C.; Mattson, M.P. Involvement of oxidative stress-induced abnormalities in ceramide and cholesterol metabolism in brain aging and Alzheimer’s disease. Proc. Natl. Acad. Sci. USA 2004, 101, 2070–2075. [Google Scholar] [CrossRef] [PubMed]
- Arimon, M.; Takeda, S.; Post, K.L.; Svirsky, S.; Hyman, B.T.; Berezovska, O. Oxidative stress and lipid peroxidation are upstream of amyloid pathology. Neurobiol. Dis. 2015, 84, 109–119. [Google Scholar] [CrossRef]
- Tamagno, E.; Guglielmotto, M.; Vasciaveo, V.; Tabaton, M. Oxidative Stress and Beta Amyloid in Alzheimer’s Disease. Which Comes First: The Chicken or the Egg? Antioxidants 2021, 10, 1479. [Google Scholar] [CrossRef]
- Dubey, S.; Singh, E. Antioxidants: An approach for restricting oxidative stress induced neurodegeneration in Alzheimer’s disease. Inflammopharmacology 2023, 31, 717–730. [Google Scholar] [CrossRef] [PubMed]
- Kang, N.X.; Luan, Y.G.; Jiang, Y.; Cheng, W.H.; Liu, Y.J.; Su, Z.J.; Liu, Y.G.; Tan, P. Neuroprotective effects of Oligosaccharides in Rehmanniae Radix on transgenic Caenorhabditis elegans models for Alzheimer’s Disease. Front. Pharmacol. 2022, 13, 878631. [Google Scholar] [CrossRef]
- Du, F.Z.; Zhao, H.P.; Yao, M.J.; Yang, Y.Y.; Jiao, J.X.; Li, C.Y. Deer antler extracts reduce amyloid-beta toxicity in a Caenorhabditis elegans model of Alzheimer’s disease. J. Ethnopharmacol. 2022, 285, 114850. [Google Scholar] [CrossRef]
- Zhang, Z.P.; Bai, X.; Cui, W.B.; Chen, X.H.; Liu, X.; Zhi, D.J.; Zhang, Z.X.; Fei, D.Q.; Wang, D.S. Diterpenoid Caesalmin C Delays Aβ-Induced Paralysis Symptoms via the DAF-16 Pathway in Caenorhabditis elegans. Int. J. Mol. Sci. 2022, 23, 6871. [Google Scholar] [CrossRef]
- Tullet, J.M.A.; Green, J.W.; Au, C.; Benedetto, A.; Thompson, M.A.; Clark, E.; Gilliat, A.F.; Young, A.; Schmeisser, K.; Gems, D. The SKN-1/Nrf2 transcription factor can protect against oxidative stress and increase lifespan in C. elegans by distinct mechanisms. Aging Cell 2017, 16, 1191–1194. [Google Scholar] [CrossRef] [PubMed]
- Govindan, S.; Amirthalingam, M.; Duraisamy, K.; Govindhan, T.; Sundararaj, N.; Palanisamy, S. Phytochemicals-induced hormesis protects Caenorhabditis elegans against α-synuclein protein aggregation and stress through modulating HSF-1 and SKN-1/Nrf2 signaling pathways. Biomed. Pharmacother. 2018, 102, 812–822. [Google Scholar] [CrossRef] [PubMed]
- Pohl, F.; Castro, A.T.; Costa, M.D.; Lindsay, V.; Fernandes, J.F.; Goua, M.; Bermano, G.; Russell, W.; Maciel, P.; Lin, P.K.T. GST-4-Dependent Suppression of Neurodegeneration in C. elegans Models of Parkinson’s and Machado-Joseph Disease by Rapeseed Pomace Extract Supplementation. Front. Neurosci. 2019, 13, 1091. [Google Scholar] [CrossRef]
- Abubakar, A.S.; Ahmad, B.; Ahmad, N.; Liu, L.; Liu, B.; Qu, Y.; Chen, J.; Chen, P.; Zhao, H.; Chen, J.; et al. Physicochemical evaluation; structural characterization, in vitro and in vivo bioactivities of water-soluble polysaccharides from Luobuma (Apocynum L.) tea. Food Chem. 2024, 460, 140453. [Google Scholar] [CrossRef] [PubMed]
- Zeng, S.; Wang, B.; Lv, W.; Li, B.; Xiao, H.; Lin, R. Physicochemical properties, structure and biological activity of ginger polysaccharide: Effect of microwave infrared dual-field coupled drying. Int. J. Biol. Macromol. 2024, 281, 136474. [Google Scholar] [CrossRef]
- Liu, X.P.; Wang, Q.Y.; Wang, J.; Guo, L.; Chu, Y.H.; Ma, C.Y.; Kang, W.Y. Structural characterization, chain conformation and immunomodulatory activity of a heteropolysaccharide from Inonotus hispidus. Int. J. Biol. Macromol. 2024, 260, 129187. [Google Scholar] [CrossRef]
- Liu, Y.; Hu, C.F.; Feng, X.; Cheng, L.; Ibrahim, S.A.; Wang, C.T.; Huang, W. Isolation, characterization and antioxidant of polysaccharides from Stropharia rugosoannulata. Int. J. Biol. Macromol. 2020, 155, 883–889. [Google Scholar] [CrossRef]
- Hu, X.Y.; Liu, C.; Wang, K.C.; Zhao, L.X.; Qiu, Y.; Chen, H.Z.; Hu, J.M.; Xu, J.R. Multifunctional Anti-Alzheimer’s Disease Effects of Natural Xanthone Derivatives: A Primary Structure-Activity Evaluation. Front. Chem. 2022, 10, 842208. [Google Scholar] [CrossRef]
- Porta-de-la-Riva, M.; Fontrodona, L.; Villanueva, A.; Cerón, J. Basic Caenorhabditis elegans methods: Synchronization and observation. J. Vis. Exp. 2012, 64, 4019. [Google Scholar] [CrossRef]
- Liao, V.H.; Yu, C.W.; Chu, Y.J.; Li, W.H.; Hsieh, Y.C.; Wang, T.T. Curcumin-mediated lifespan extension in Caenorhabditis elegans. Mech. Ageing Dev. 2011, 132, 480–487. [Google Scholar] [CrossRef]
- Wei, C.C.; Li, S.W.; Wu, C.T.; How, C.M.; Pan, M.H. Dietary Methylglyoxal Exposure Induces Alzheimer’s Disease by Promoting Amyloid β Accumulation and Disrupting Autophagy in Caenorhabditis elegans. J. Agric. Food Chem. 2022, 70, 10011–10021. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.T.; Zhao, X.T.; Zhang, Y.Q.; Xie, J.B.; Zhou, A.M. 6‴-Feruloylspinosin alleviated beta-amyloid induced toxicity by promoting mitophagy in Caenorhabditis elegans (GMC101) and PC12 cells. Sci. Total Environ. 2020, 715, 136953. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, H.; Wang, Q.; Zhou, Y.; Chen, H.; Tao, J.; Huang, J.; Miao, Y.; Zhao, J.; Wang, Y. Cremastra appendiculata Polysaccharides Alleviate Neurodegenerative Diseases in Caenorhabditis elegans: Targeting Amyloid-β Toxicity, Tau Toxicity and Oxidative Stress. Int. J. Mol. Sci. 2025, 26, 3900. https://doi.org/10.3390/ijms26083900
Xu H, Wang Q, Zhou Y, Chen H, Tao J, Huang J, Miao Y, Zhao J, Wang Y. Cremastra appendiculata Polysaccharides Alleviate Neurodegenerative Diseases in Caenorhabditis elegans: Targeting Amyloid-β Toxicity, Tau Toxicity and Oxidative Stress. International Journal of Molecular Sciences. 2025; 26(8):3900. https://doi.org/10.3390/ijms26083900
Chicago/Turabian StyleXu, Huaying, Qian Wang, Yihan Zhou, Haiyu Chen, Jin Tao, Jing Huang, Yuzhi Miao, Jiayuan Zhao, and Yanan Wang. 2025. "Cremastra appendiculata Polysaccharides Alleviate Neurodegenerative Diseases in Caenorhabditis elegans: Targeting Amyloid-β Toxicity, Tau Toxicity and Oxidative Stress" International Journal of Molecular Sciences 26, no. 8: 3900. https://doi.org/10.3390/ijms26083900
APA StyleXu, H., Wang, Q., Zhou, Y., Chen, H., Tao, J., Huang, J., Miao, Y., Zhao, J., & Wang, Y. (2025). Cremastra appendiculata Polysaccharides Alleviate Neurodegenerative Diseases in Caenorhabditis elegans: Targeting Amyloid-β Toxicity, Tau Toxicity and Oxidative Stress. International Journal of Molecular Sciences, 26(8), 3900. https://doi.org/10.3390/ijms26083900






