Freeze-Dependent Physiological and Transcriptional Changes in Olea europaea L. Cultivars with Different Cold Resistances
Abstract
1. Introduction
2. Results
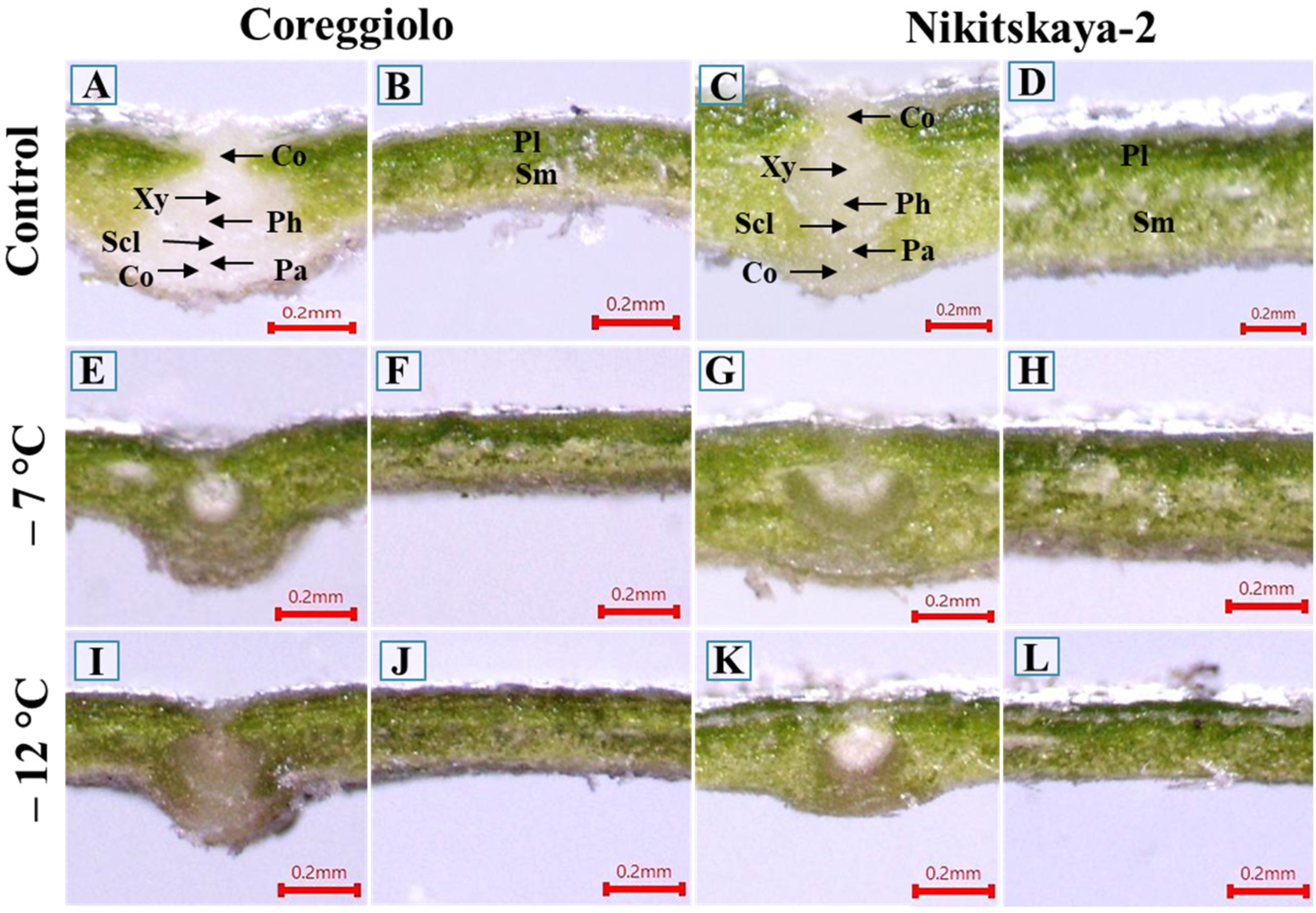
2.1. Phenotypic Changes
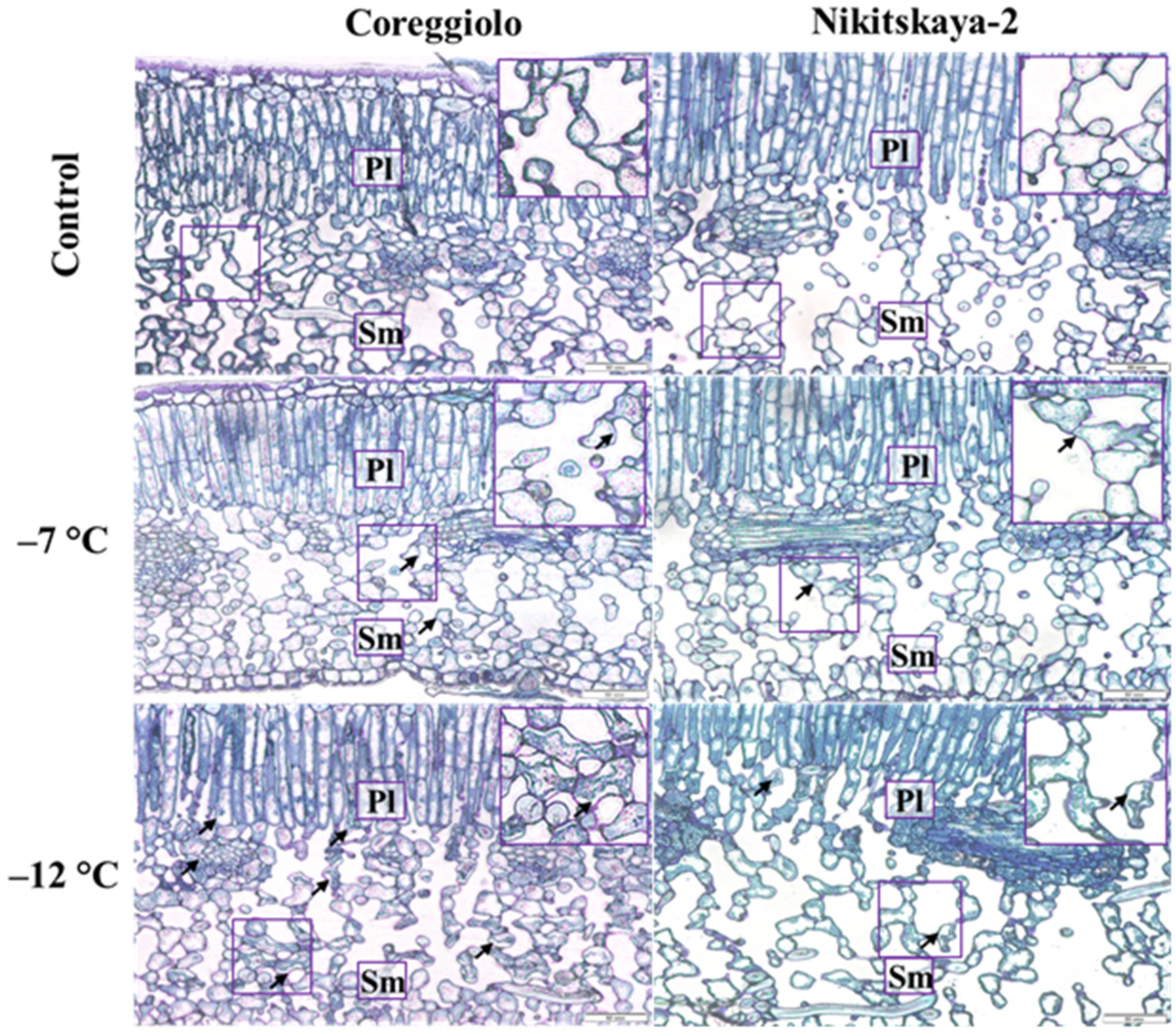
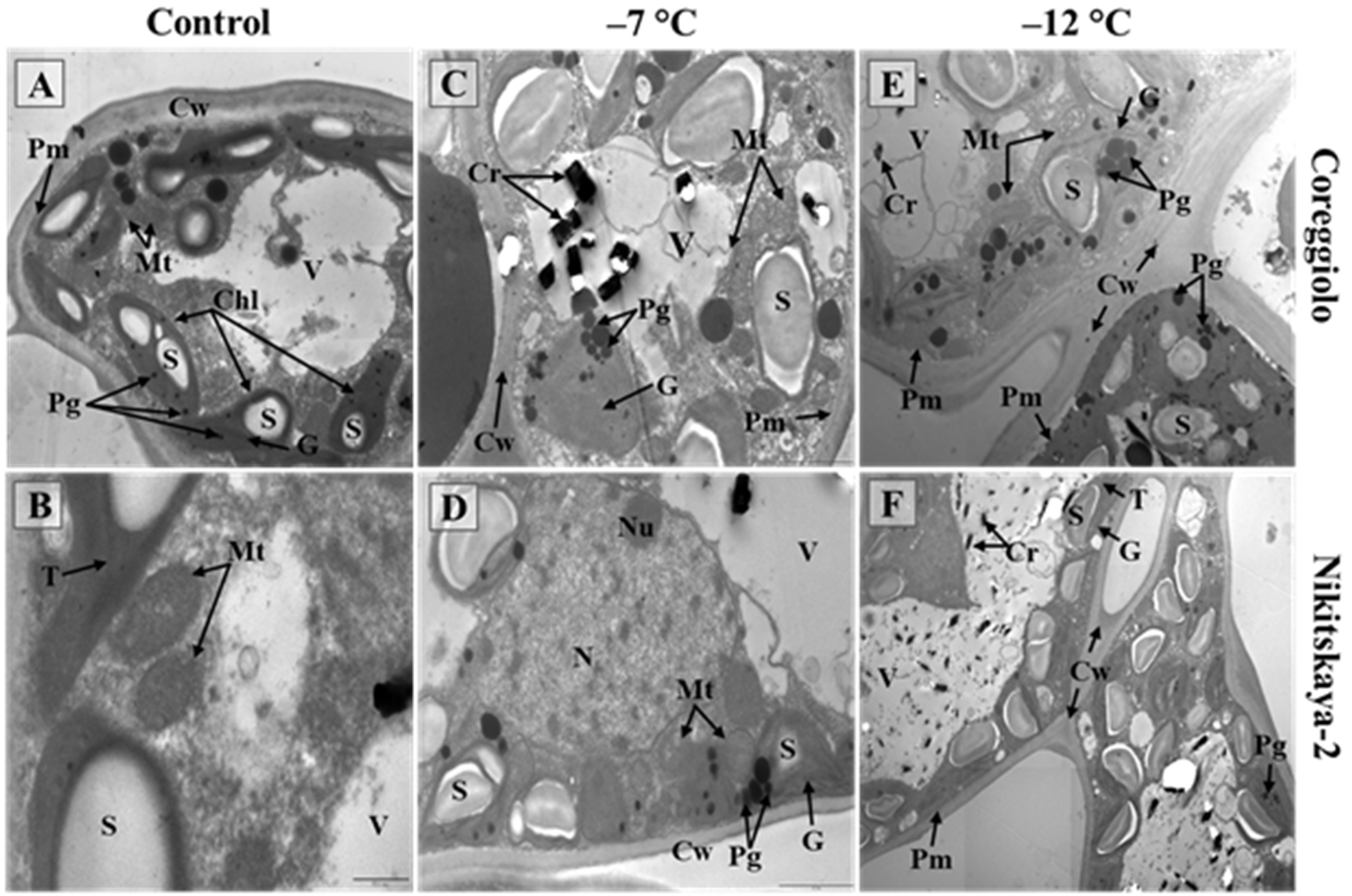
2.2. Structural Analysis of Cold-Susceptible and Cold-Tolerant Cultivars
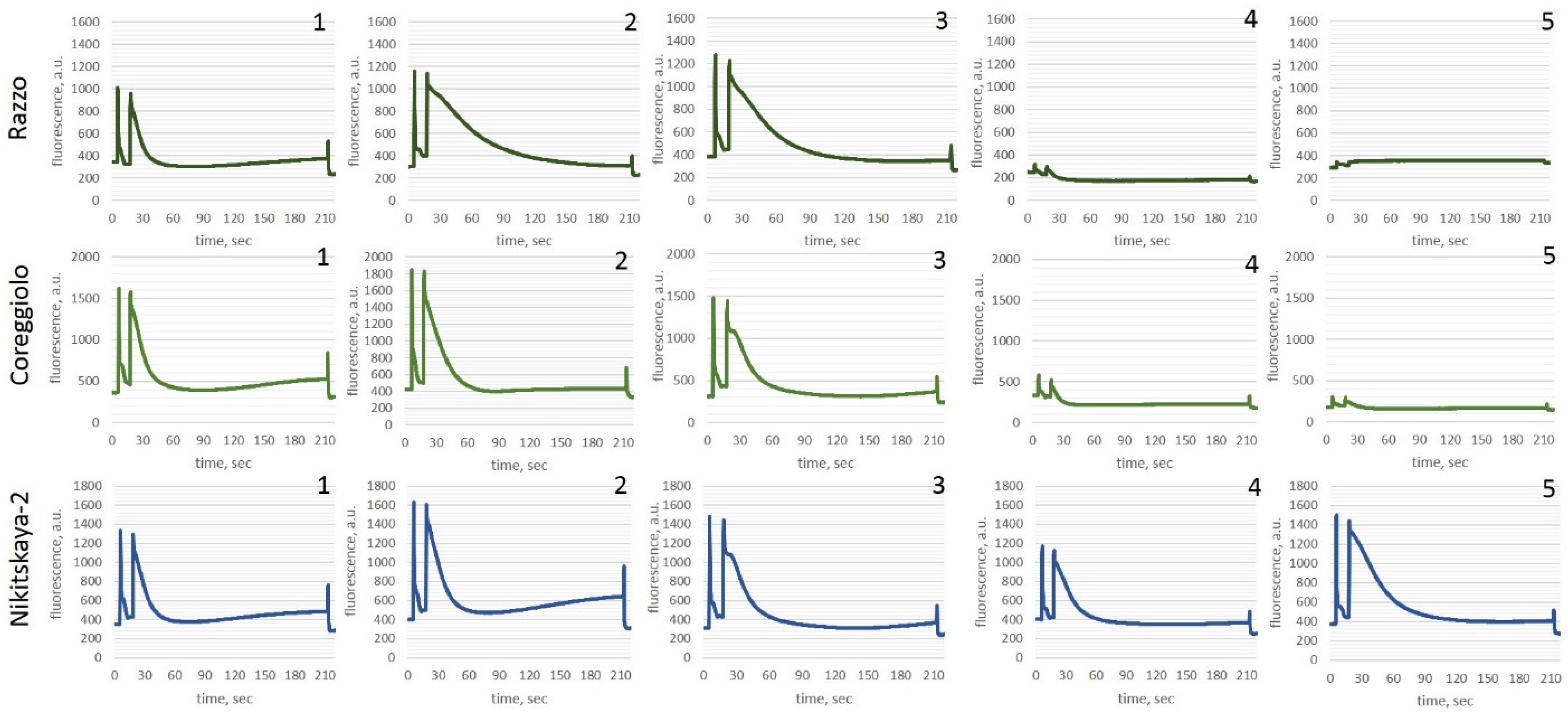
2.3. Photosynthetic Apparatus
2.3.1. Moderate Stress (−7 °C)
2.3.2. Severe Stress (−12 °C)
2.4. Enzyme Activity
2.4.1. Moderate Stress (−7 °C)
2.4.2. Severe Stress (−12 °C)
2.5. RNA-Seq and Data Processing
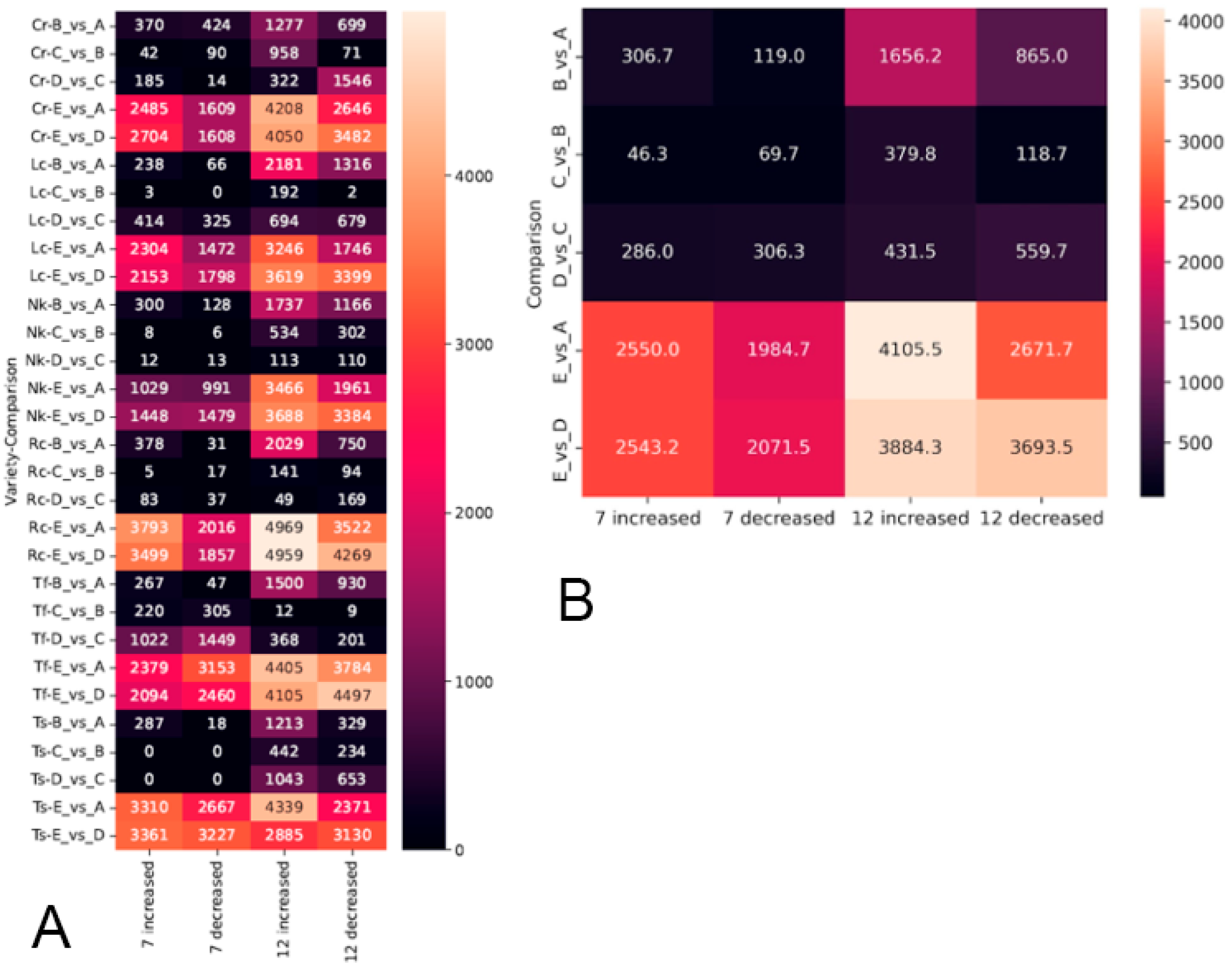
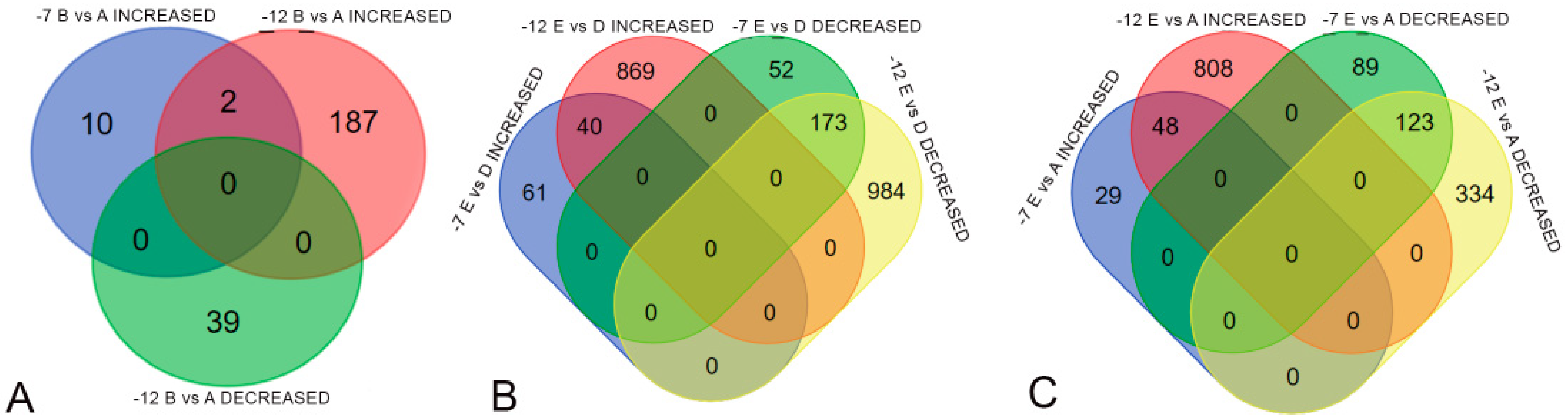
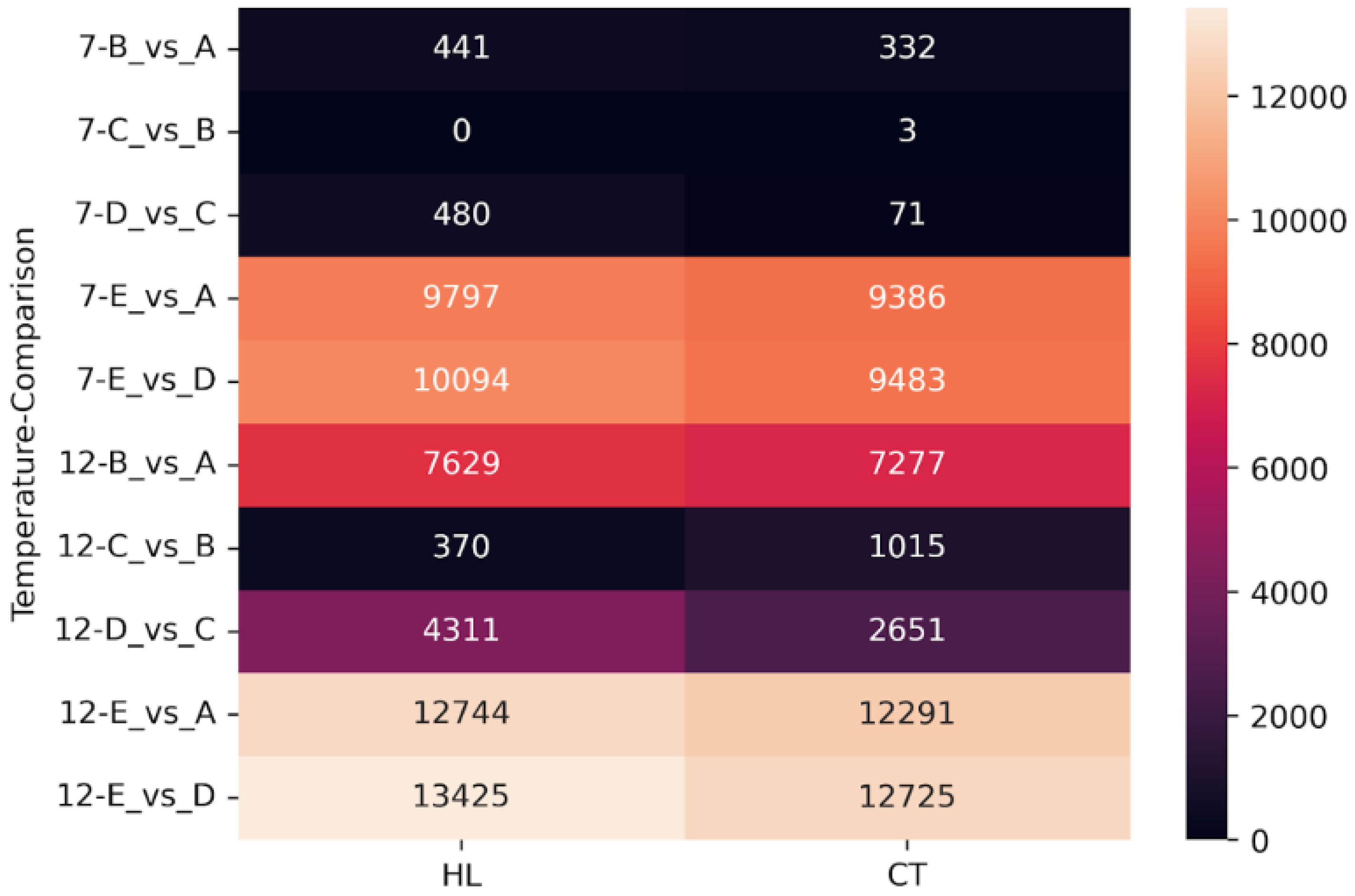
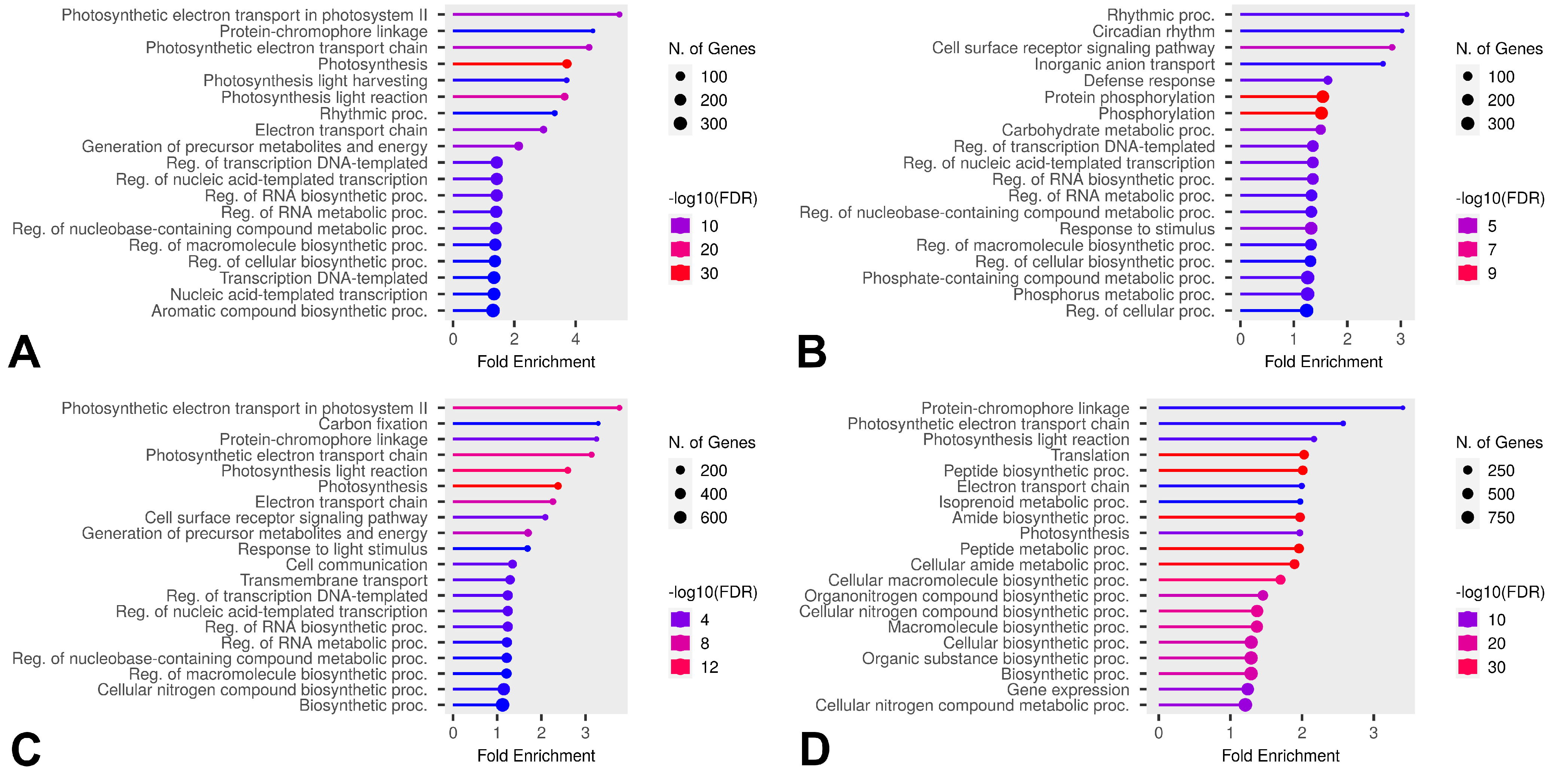
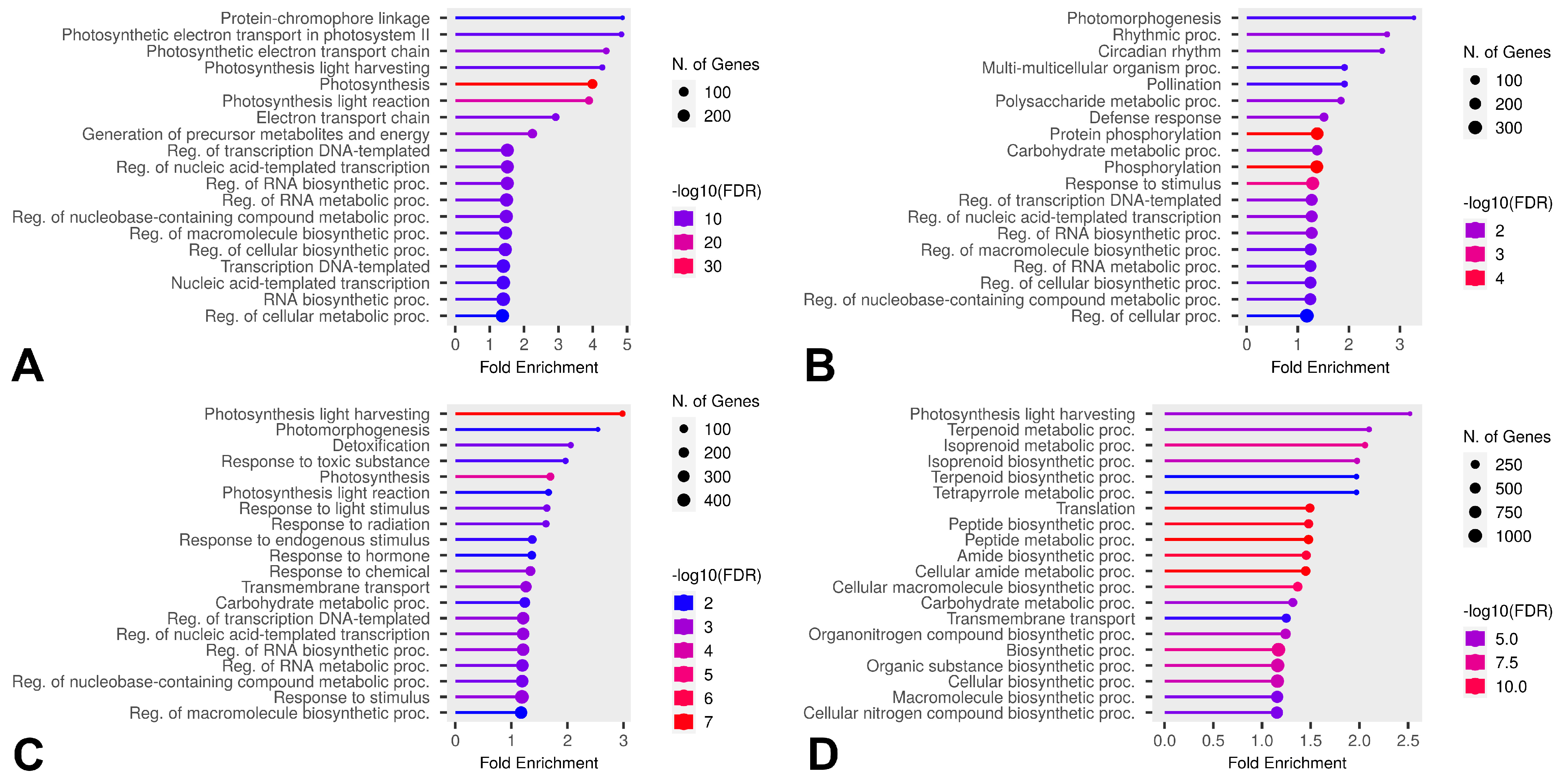
2.6. Differential Gene Expression Under Freezing Stress
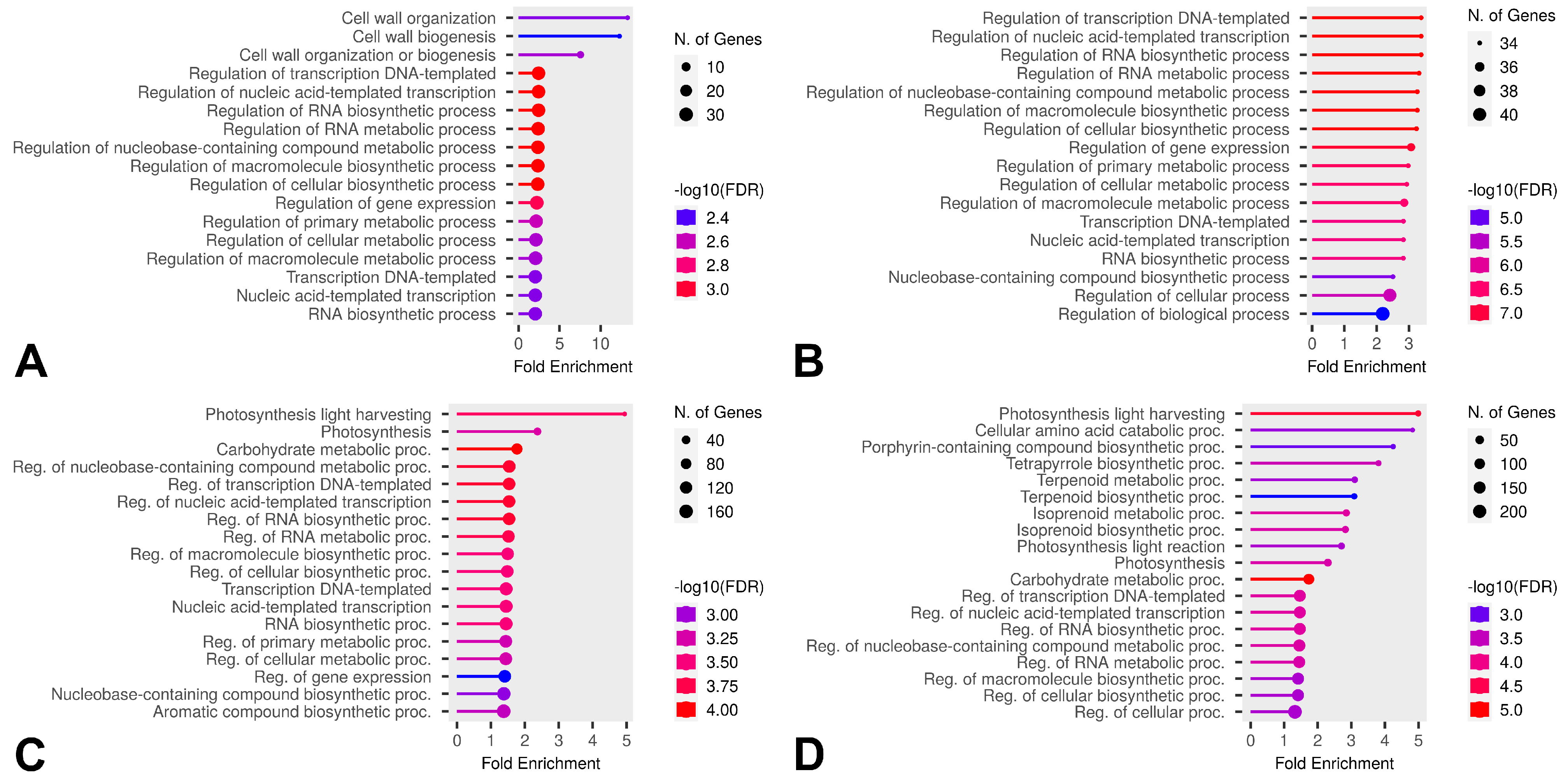
2.7. Differential Expression in Cold-Susceptible and Cold-Tolerant Cultivars
2.8. Verification by Quantitative Real-Time PCR
3. Discussion
3.1. Biochemical and Morphological Processes in Olive Leaves After Freezing
3.2. Differential Expression During Freezing and Recovery of Leaves
3.3. DEGs in Cold-Tolerant and Cold-Susceptible Cultivars
3.4. Epigenetic Modifications in Native Cold-Tolerant Cultivars
4. Materials and Methods
4.1. Plant Material
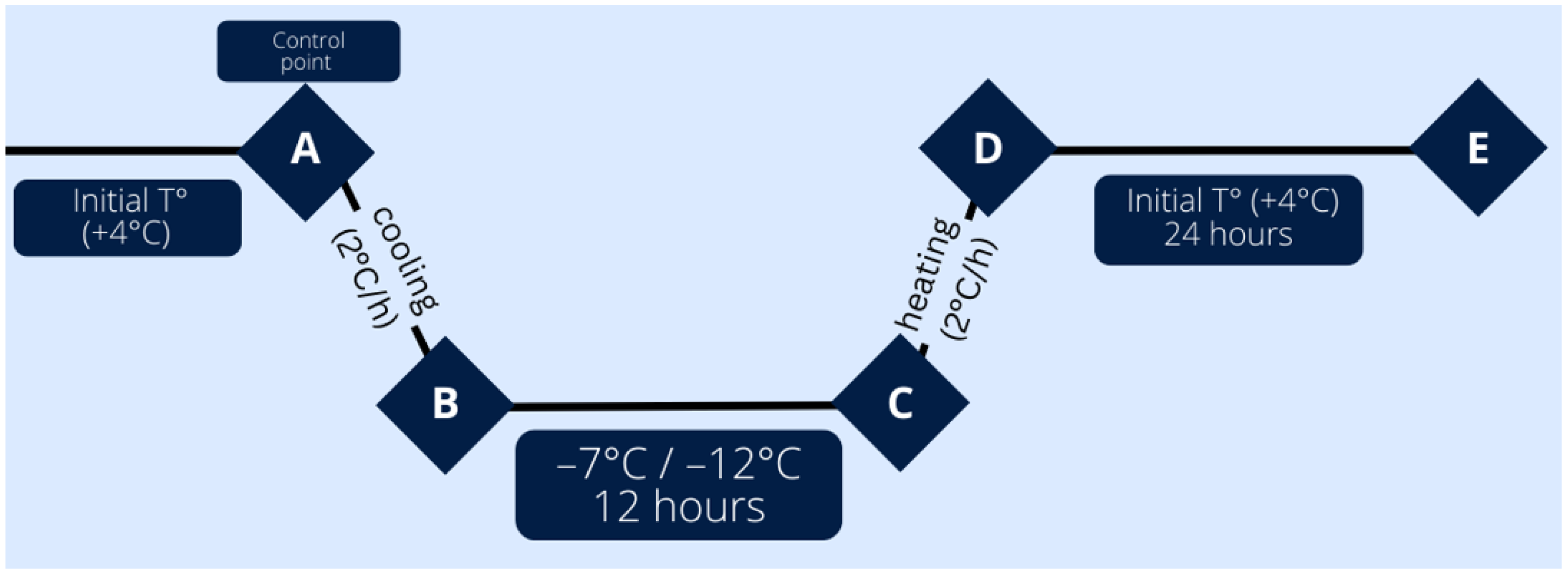
4.2. Leaf Freezing Procedure
4.3. Photosynthetic Apparatus Analysis
- Maximum photochemical quantum yield of PSII: Fv/Fm;
- Photosynthetic activity: PA = (Fm − Fs)/Fm;
- Fluorescence decrease ratio: Rfd = (Fs − Fm)/Fs;
- Effective photochemical quantum yield of PSII: Y(II) = (Fm’ − Fs)/Fm’;
- Quantum yield of regulated non-photochemical energy dissipation in PSII: Y(NPQ) = Fs/Fm’ − Fs/Fm;
- Quantum yield of non-regulated non-photochemical energy dissipation in PSII: Y(NO) = Fs/Fm.
4.4. Structural Analysis
4.5. Enzyme Activity
4.5.1. Catalase Activity
4.5.2. Peroxidase Activity
4.5.3. Polyphenol Oxidase (PPO) Activity
4.6. RNA Extraction, Library Preparation, and Sequencing
4.7. Bioinformatic Analysis
4.8. Verification by Quantitative Real-Time PCR
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fraga, H.; Moriondo, M.; Leolini, L.; Santos, J.A. Mediterranean Olive Orchards under Climate Change: A Review of Future Impacts and Adaptation Strategies. Agronomy 2020, 11, 56. [Google Scholar] [CrossRef]
- Gucci, R.; Caruso, G. Environmental Stresses and Sustainable Olive Growing. Acta Hortic. 2011, 924, 19–30. [Google Scholar] [CrossRef]
- Mougiou, N.; Baalbaki, B.; Doupis, G.; Kavroulakis, N.; Poulios, S.; Vlachonasios, K.E.; Koubouris, G.C. The Effect of Low Temperature on Physiological, Biochemical and Flowering Functions of Olive Tree in Relation to Genotype. Sustainability 2020, 12, 10065. [Google Scholar] [CrossRef]
- Petruccelli, R.; Bartolini, G.; Ganino, T.; Zelasco, S.; Lombardo, L.; Perri, E.; Durante, M.; Bernardi, R. Cold Stress, Freezing Adaptation, Varietal Susceptibility of Olea europaea L.: A Review. Plants 2022, 11, 1367. [Google Scholar] [CrossRef]
- Cansev, A.; Gulen, H.; Eris, A. Cold-Hardiness of Olive (Olea europaea L.) Cultivars in Cold-Acclimated and Non-Acclimated Stages: Seasonal Alteration of Antioxidative Enzymes and Dehydrin-like Proteins. J. Agric. Sci. 2009, 147, 51–61. [Google Scholar] [CrossRef]
- Borodin, I.F.; Budagovskii, A.V.; Budagovskaya, O.N.; Budagovskii, I.A.; Sudnik, Y.A. Using the effect of photoinduced variability of optical properties of chlorophyll-containing tissues for diagnosing the functional state of plants. Russ. Agric. Sci. 2008, 34, 357. [Google Scholar] [CrossRef]
- Gubanova, T.B.; Paliy, A.E. Physiological and Biochemical Aspects of Frost Resistance in Olea europaea L. Russ. J. Plant Physiol. 2020, 67, 671–679. [Google Scholar] [CrossRef]
- Lin, S.Z.; Zhang, Z.Y.; Liu, W.F.; Lin, Y.Z.; Zhang, Q.; Zhu, B.Q. Role of glucose-6-phosphate dehydrogenase in freezing-induced freezing resistance of Populus suaveolens. J. Plant Physiol. Mol. Biol. 2005, 31, 34. [Google Scholar]
- Mittler, R. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 2002, 7, 405–410. [Google Scholar] [CrossRef]
- Ortega-García, F.; Peragón, J. The response of phenylalanine ammonia-lyase, flavonoid oxidase and phenols to cold stress in the olive tree (Olea europaea L. Cv. Picual). J. Sci. Food Agric. 2009, 89, 1565–1573. [Google Scholar] [CrossRef]
- Francini, A.; Giro, A.; Ferrante, A. Biochemical and Molecular Regulation of Phenylpropanoids Pathway Under Abiotic Stresses. In Plant Signaling Molecules: Role and Regulation Under Stressful Environments; Khan, M., Iqbal, R., Reddy, P.S., Ferrante, A., Khan, N., Eds.; Woodhead Publishing: Chennai, India, 2019; Volume 11, pp. 183–192. ISBN 978-0-12-816452-5. [Google Scholar]
- Elhrech, H.; Aguerd, O.; El Kourchi, C.; Gallo, M.; Naviglio, D.; Chamkhi, I.; Bouyahya, A. Comprehensive Review of Olea europaea: A Holistic Exploration into Its Botanical Marvels, Phytochemical Riches, Therapeutic Potentials, and Safety Profile. Biomolecules 2024, 14, 722. [Google Scholar] [CrossRef] [PubMed]
- Guerra, D.; Lamontanara, A.; Bagnaresi, P.; Orrù, L.; Rizza, F.; Zelasco, S.; Beghè, D.; Ganino, T.; Pagani, D.; Cattivelli, L.; et al. Transcriptome Changes Associated with Cold Acclimation in Leaves of Olive Tree (Olea europaea L.). Tree Genet. Genomes 2015, 11, 113. [Google Scholar] [CrossRef]
- Azzarello, E.; Mugnai, S.; Pandolfi, C.; Masi, E.; Marone, E.; Mancuso, S. Comparing Image (Fractal Analysis) and Electrochemical (Impedance Spectroscopy and Electrolyte Leakage) Techniques for the Assessment of the Freezing Tolerance in Olive. Trees 2009, 23, 159–167. [Google Scholar] [CrossRef]
- Alfei, B.; Cavezza, G.; Santinelli, A.; Panelli, G. Osservazioni sul comportamento agronomico di alcune varietà autoctone ed alloctone di olivo nelle Marche (Agronomic behaviour of native and non-native olive varieties of the Marche Region-Italy). In Proceedings of the Atti V Convegno Nazionale Biodiversità: Biodiversità e Sistemi Ecocompatibili, Caserta, Italy, 9–10 September 1999. [Google Scholar]
- Alekseev, V.P. Olive. Olive tree. Bull. All-Union Sci. Res. Inst. Tea Subtrop. Crops 1954, 4, 86–110. [Google Scholar]
- Sokolova, N.F. Winter hardiness of olives in the conditions of the Southern coast of Crimea in connection with water balance (winter drought). Proc. State Nikitsk. Bot. Gard. 1935, 21, 3–37. [Google Scholar]
- Zhigarevich, I.A. Olive Culture; State publishing house of agricultural literature: Moscow, Russia, 1955; p. 248. [Google Scholar]
- Gubanova, T.; Brailko, V.; Myazina, L. Winter Hardiness of Some Oleaceae Species in the Collection of the Nikitskiy Botanic Gardens. Hortus Bot. 2018, 13, 250–259. [Google Scholar] [CrossRef]
- Tsiupka, V.A.; Tsiupka, S.Y.; Bulavin, I.V. Frost Resistance of Varieties and Hybrid Forms of European Olive (Olea europaea L.) from the Nikitsky Botanical Garden Collection. In Proceedings of the 7th International Conference dedicated to the 95th Anniversary of Academician of the Russian Academy of Sciences P.L. Goncharov, Novosibirsk, Russia, 10–12 April 2024; Federal Research Center Institute of Cytology and Genetics of the Siberian Branch of the Russian Academy of Sciences: Novosibirsk, Russia, 2024; pp. 388–392. [Google Scholar]
- Slobodova, N.; Sharko, F.; Gladysheva-Azgari, M.; Petrova, K.; Tsiupka, S.; Tsiupka, V.; Boulygina, E.; Rastorguev, S.; Tsygankova, S. Genetic Diversity of Common Olive (Olea europaea L.) Cultivars from Nikita Botanical Gardens Collection Revealed Using RAD-Seq Method. Genes 2023, 14, 1323. [Google Scholar] [CrossRef] [PubMed]
- Korsakova, S.P.; Korsakov, P.B. Climate References of the 2020 Seasons in the Southern Coast of the Crimea. Sci. Notes Cape Martyan Nat. Reserve 2021, 12, 6–27. [Google Scholar] [CrossRef]
- Larcher, W. Temperature Stress and Survival Ability of Mediterranean Sclerophyllous Plants. Plant Biosyst. 2000, 134, 279–295. [Google Scholar] [CrossRef]
- Lodolini, E.M.; Alfei, B.; Santinelli, A.; Cioccolanti, T.; Polverigiani, S.; Neri, D. Frost tolerance of 24 olive cultivars and subsequent vegetativere-sprouting as indication of recovery ability. Sci. Hortic. 2016, 211, 152–157. [Google Scholar] [CrossRef]
- Honorio, F.; Aguirado, C.; Paniagua, L.L.; García-Martín, A.; Rebollo, L.; Rebollo, F.J. Exploring the Climate and Topography of Olive Orchards in Extremadura, Southwestern Spain. Land 2024, 13, 495. [Google Scholar] [CrossRef]
- Çetin, Ö.; Mete, N.; Ölmez, H.; Gülen, H. Mapping of QTLs Associated with Frost Tolerance in Olive (Olea europaea L.). Turkish J. Agric. For. 2025, 49, 182–193. [Google Scholar] [CrossRef]
- Levitt, J. Responses of Plants to Environmental Stress. In Chilling, Freezing, and High Temperature Stresses; Elsevier: Amsterdam, The Netherlands, 1980; pp. 347–393. [Google Scholar]
- D’Angeli, S.; Malhó, R.; Altamura, M.M. Low-Temperature Sensing in Olive Tree: Calcium Signalling and Cold Acclimation. Plant Sci. 2003, 165, 1303–1313. [Google Scholar] [CrossRef]
- Arias, N.S.; Scholz, F.G.; Goldstein, G.; Bucci, S.J. Low-Temperature Acclimation and Legacy Effects of Summer Water Deficits in Olive Freezing Resistance. Tree Physiol. 2021, 41, 1836–1847. [Google Scholar] [CrossRef]
- de la Leyva-Pérez, M.O.; Valverde-Corredor, A.; Valderrama, R.; Jiménez-Ruiz, J.; Muñoz-Merida, A.; Trelles, O.; Barroso, J.B.; Mercado-Blanco, J.; Luque, F. Early and Delayed Long-Term Transcriptional Changes and Short-Term Transient Responses during Cold Acclimation in Olive Leaves. DNA Res. 2015, 22, 1–11. [Google Scholar] [CrossRef]
- Nievola, C.C.; Carvalho, C.P.; Carvalho, V.; Rodrigues, E. Rapid Responses of Plants to Temperature Changes. Temperature 2017, 4, 371–405. [Google Scholar] [CrossRef] [PubMed]
- Hashempour, A.; Ghasemnezhad, M.; Fotouhi Ghazvini, R.; Sohani, M.M. Effect of Freezing Stress on Lipid Peroxidation and Antioxidant Enzyme Activities of Olive cvs. ‘Fishomi’ and ‘Roughani’. Agric. Conspec. Sci. 2014, 79, 245–252. [Google Scholar]
- Pfeiffer, T.Ž.; Štolfa, I.; Žanić, M.; Pavičić, N.; Cesar, V.; Lepeduš, H. Oxidative stress in leaves of two olive cultivars under freezing conditions. Acta Biol. Hung. 2013, 64, 341–351. [Google Scholar] [CrossRef]
- Paliy, A.E.; Paliy, I.N. Some biochemical features of Olea europaea L. frost resistance. Acta Hortic. 2021, 1324, 395–400. [Google Scholar] [CrossRef]
- Arora, R. Mechanism of freeze-thaw injury and recovery: A cool retrospective and warming up to new ideas. Plant Sci. 2018, 270, 301–312. [Google Scholar] [CrossRef]
- Takahashi, D.; Willick, I.R.; Kasuga, J.; Livingston, D.P., III. Responses of the Plant Cell Wall to Sub-Zero Temperatures: A Brief Update. Plant Cell Physiol. 2021, 62, 1858–1866. [Google Scholar] [CrossRef] [PubMed]
- Sebastiani, L.; Gucci, R.; Kerem, Z.; Fernández, J.E. Physiological Responses to Abiotic Stresses. In The Olive Tree Genome. Compendium of Plant Genomes; Springer: Cham, Germany, 2016; pp. 99–122. [Google Scholar] [CrossRef]
- Saadati, S.; Baninasab, B.; Mobli, M.; Gholami, M. Cold tolerance in olive leaves of three cultivars related to some physiological parameters during cold acclimation and de-acclimation stages. J. Agric. Sci. Technol. 2020, 22, 1313–1326. [Google Scholar]
- Mayer, A.M. Polyphenol Oxidases in Plants and Fungi: Going Places? A Review. Phytochemistry 2006, 67, 2318–2331. [Google Scholar] [CrossRef]
- Bartolozzi, F.; Cerquaglia, F.; Coppari, L.; Fontanazza, G. Frost tolerance induced by cold acclimation in olive (Olea europaea L.). Acta Hortic. 2002, 586, 473–477. [Google Scholar] [CrossRef]
- Saadati, S.; Baninasab, B.; Mobli, M.; Gholami, M. Measurements of freezing tolerance and their relationship with some biochemical and physiological parameters in seven olive cultivars. Acta Physiol. Plant. 2019, 41, 51. [Google Scholar] [CrossRef]
- Jiang, C.; Hu, W.; Lu, H.; Chen, L.; Niu, E.; Zhu, S.; Shen, G. Alterations of Phenotype, Physiology, and Functional Substances Reveal the Chilling-Tolerant Mechanism in Two Common Olea europaea Cultivars. Front. Plant Sci. 2023, 14, 1046719. [Google Scholar] [CrossRef]
- Vafadar, M.; Rezaei, M.; Khadivi, A. Frost Hardiness of 10 Olive Cultivars after Natural and Controlled Freezing. Sci. Hortic. 2024, 325, 112687. [Google Scholar] [CrossRef]
- Theocharis, A.; Clément, C.; Barka, E.A. Physiological and molecular changes in plants grown at low temperatures. Planta 2012, 235, 1091–1105. [Google Scholar] [CrossRef]
- Adam, S.; Murthy, S.D.S. Effect of cold stress on photosynthesis of plants and possible protection mechanisms. In Approaches to Plant Stress and Their Management; Gaur, R.K., Sharma, P., Eds.; Springer: New Delhi, India, 2014; pp. 207–218. [Google Scholar]
- Banerjee, A.; Roychoudhury, A. Chapter 2: Cold stress and photosynthesis. In Photosynthesis, Productivity and Environmental Stress; Ahmad, P., Ahanger, M.A., Alyemeni, M.N., Alam, P., Eds.; John Wiley & Sons, Ltd.: Chennai, India, 2020; pp. 27–37. [Google Scholar]
- Gubanova, T.B. The Influence of Negative Temperatures on Photosynthetic Activity in Some Evergreen Species of Oleaceae Family. Subtrop. Ornam. Hortic. 2019, 70, 158–167. [Google Scholar] [CrossRef]
- Gubanova, T.B. Resistance of Olea europaea L. varieties and forms to unfavorable winter conditions on the southern coast of the Crimea. Pomic. Small Fruits Cult. Russ. 2019, 57, 32–41. [Google Scholar] [CrossRef]
- Wu, W.; Jiang, C.; Wei, Q.; He, L.; Jin, G.; Zheng, Y.; Qi, J.; Zhang, R.; Yao, Y.; Wan, D.; et al. Comparative Transcriptome Provides New Insights into the Molecular Regulation of Olive Trees to Chilling Stress. Tree Physiol. 2024, 44, tpae075. [Google Scholar] [CrossRef]
- Simkeshzadeh, N.; Mobli, M.; Etemadi, N.; Baninasab, B. Assessment of the Frost Resistance in Some Olive Cultivars Using Visual Injuries and Chlorophyll Fluorescence. J. Hortic. Sci. 2011, 2, 163–169. [Google Scholar]
- Afshar, M.M.; Rezaei, S.; Malekroudi, R.M. The Impact of cold stress on two olive cultivars. J. Plant Process Funct. 2012, 1, 1–11. Available online: https://www.sid.ir/en/journal/ViewPaper.aspx?id=305233 (accessed on 11 November 2021).
- Leonardo, B.; Picardi, E.; Pacenza, M.; Chiappetta, A.; Muto, A.; Gagliardi, O.; Muzzalupo, I.; Pesole, G.; Bitonti, M.B. Changes in gene expression and metabolic profile of drupes of Olea europaea L. cv Carolea in relation to maturation stage and cultivation area. BMC Plant Biol. 2019, 19, 428. [Google Scholar]
- Gilmore, A.M.; Matsubara, S.; Ball, M.C. Excitation energy flow in the photosynthetic apparatus over winter in evergreens. Plant Cell Environ. 2003, 26, 1021–1034. [Google Scholar] [CrossRef]
- Corcuera, L.; Morales, F.; Abadía, A.; Gil-Pelegrín, E. Seasonal changes in photosynthesis and photoprotection in a Quercus ilex subsp. ballota woodland located in its upper altitudinal extreme in the Iberian Peninsula. Tree Physiol. 2005, 25, 599–608. [Google Scholar] [CrossRef]
- Adams, W.W.; Demmig-Adams, B.; Rosenstiel, T.N.; Ebbert, V. Dependence of photosynthesis and energy dissipation activity upon growth form and light environment during the winter. Photosynth. Res. 2001, 67, 51–62. [Google Scholar] [CrossRef]
- Verhoeven, A.S. Recovery kinetics of photochemical efficiency in winter stressed conifers: The effects of growth light environment, extent of the season and species. Physiol. Plant 2013, 147, 147–158. [Google Scholar] [CrossRef]
- Sobieszczuk-Nowicka, E.; Wrzesiński, T.; Bagniewska-Zadworna, A.; Kubala, S.; Rucińska-Sobkowiak, R.; Polcyn, W.; Misztal, L.; Mattoo, A.K. Physio-Genetic Dissection of Dark-Induced Leaf Senescence and Timing Its Reversal in Barley. Plant Physiol. 2018, 2, 654–671. [Google Scholar] [CrossRef]
- Lichtenthaler, H.; Buschmann, C.; Knapp, M. Measurement of chlorophyll fluorescence kinetics (Kautsky effect) and the chlorophyll fluorescence decrease ratio (RFd-values) with the PAM-fluorometer. In Analytical Methods in Plant Stress Biology; Filek, M., Biesaga-Kocielniak, J., Marciska, I., Eds.; The Franciszek Gorski Inst. Plant Physiol Polish Academy of Sciences: Krakow, Poland, 2004; pp. 93–111. [Google Scholar]
- Lichtenthaler, H.K.; Babani, K. Light Adaptation and Senescence of the Photosynthetic Apparatus. Changes in Pigment Composition, Chlorophyll Fluorescence Parameters and Photosynthetic Activity. In Chlorophyll Fluorescence: A Signature of Photosynthesis; Papageorgiou, G.C., Govindjee, Eds.; Springer: Dordrecht, The Netherlands, 2004; pp. 713–736. [Google Scholar]
- Rinderle, U.; Lichtenthaler, H.K. The chlorophyll fluorescence ratio F690/F735 as a possible stress indicator. In Applications of Chlorophyll Fluorescence; Lichtenthaler, H.K., Ed.; Kluwer Acad. Publ.: Dordrecht, The Netherlands, 1988; pp. 189–196. [Google Scholar]
- Lichtenthaler, H.K.; Buschmann, C.; Knapp, M. How to correctly determine the different chlorophyll fluorescence parameters and the chlorophyll fluorescence decrease ratio RFd of leaves with the PAM fluorometer. Photosynthetica 2005, 43, 379–393. [Google Scholar] [CrossRef]
- Yuan, P.; Yang, T.; Poovaiah, B.W. Calcium Signaling-Mediated Plant Response to Cold Stress. Int. J. Mol. Sci. 2018, 19, 3896. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Bullock, D.A.; Alonso, J.M.; Stepanova, A.N. To Fight or to Grow: The Balancing Role of Ethylene in Plant Abiotic Stress Responses. Plants 2021, 11, 33. [Google Scholar] [CrossRef] [PubMed]
- Ensminger, I.; Busch, F.; Huner, N.P.A. Photostasis and Cold Acclimation: Sensing Low Temperature through Photosynthesis. Physiol. Plant. 2006, 126, 28–44. [Google Scholar] [CrossRef]
- Kidokoro, S.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Transcriptional Regulatory Network of Plant Cold-Stress Responses. Trends Plant Sci. 2022, 27, 922–935. [Google Scholar] [CrossRef]
- Medina, J.; Catalá, R.; Salinas, J. The CBFs: Three Arabidopsis Transcription Factors to Cold Acclimate. Plant Sci. 2011, 180, 3–11. [Google Scholar] [CrossRef]
- Du, X.; Lu, Y.; Sun, H.; Duan, W.; Hu, Y.; Yan, Y. Genome-Wide Analysis of Wheat GATA Transcription Factor Genes Reveals Their Molecular Evolutionary Characteristics and Involvement in Salt and Drought Tolerance. Int. J. Mol. Sci. 2022, 24, 27. [Google Scholar] [CrossRef]
- Abdulla, M.F.; Mostafa, K.; Aydin, A.; Kavas, M.; Aksoy, E. GATA Transcription Factor in Common Bean: A Comprehensive Genome-Wide Functional Characterization, Identification, and Abiotic Stress Response Evaluation. Plant Mol. Biol. 2024, 114, 43. [Google Scholar] [CrossRef]
- Zhang, X.; Ma, J.; Yang, S.; Yao, W.; Zhang, N.; Hao, X.; Xu, W. Analysis of GATA Transcription Factors and Their Expression Patterns under Abiotic Stress in Grapevine (Vitis vinifera L.). BMC Plant Biol. 2023, 23, 611. [Google Scholar] [CrossRef]
- Zhang, H.; Wu, T.; Li, Z.; Huang, K.; Kim, N.-E.; Ma, Z.; Kwon, S.-W.; Jiang, W.; Du, X. OsGATA16, a GATA Transcription Factor, Confers Cold Tolerance by Repressing OsWRKY45–1 at the Seedling Stage in Rice. Rice 2021, 14, 42. [Google Scholar] [CrossRef]
- Marchese, A.; Balan, B.; Trippa, D.A.; Bonanno, F.; Caruso, T.; Imperiale, V.; Marra, F.P.; Giovino, A. NGS Transcriptomic Analysis Uncovers the Possible Resistance Mechanisms of Olive to Spilocea Oleagina Leaf Spot Infection. Front. Plant Sci. 2023, 14, 1219580. [Google Scholar] [CrossRef]
- Tang, X.; Wang, Q.; Yuan, H.; Huang, X. Chilling-Induced DNA Demethylation Is Associated with the Cold Tolerance of Hevea Brasiliensis. BMC Plant Biol. 2018, 18, 70. [Google Scholar] [CrossRef]
- Viejo, M.; Tengs, T.; Yakovlev, I.; Cross, H.; Krokene, P.; Olsen, J.E.; Fossdal, C.G. Epitype-Inducing Temperatures Drive DNA Methylation Changes during Somatic Embryogenesis in the Long-Lived Gymnosperm Norway Spruce. Front. Plant Sci. 2023, 14, 1196806. [Google Scholar] [CrossRef] [PubMed]
- Bartolozzi, F.; Rocchi, P.; Camerini, F.; Fontanazza, G. Paramtri biochimici in foglie di olvo (Olea europaea L.) e loro potenziale relazione con la resistenza al cogelamento. Olivo Olio 1999, 6, 18–26. [Google Scholar]
- Bartolozzi, F.; Mencuccini, M.; Fontanazza, G. Enhancement of frost tolerance in olive shoots in vitro by cold acclimation and sucrose increase in the culture medium. Plant Cell Tissue Organ. Cult. 2001, 67, 299–302. [Google Scholar] [CrossRef]
- Bulavin, I.V.; Sidyakin, A.I. Structural and functional characterization of the root of Arabidopsis thaliana in vitro. Biol. Bull. Russ. Acad. Sci. 2023, 50, 1241–1249. [Google Scholar] [CrossRef]
- Ermakov, A.I.; Arasimovich, V.V.; Yarosh, N.P.; Peruvsky, Y.V.; Lukovnikova, G.A.; Ikonnikova, M.I. Methods of biochemical studies of plants. In Methods of Biochemical Studies of Plants; Agropromizdat: Moscow, Russia, 1987. [Google Scholar]
- Love, M.I.; Huber, W.; Anders, S. Moderated Estimation of Fold Change and Dispersion for RNA-Seq Data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Durinck, S.; Moreau, Y.; Kasprzyk, A.; Davis, S.; De Moor, B.; Brazma, A.; Huber, W. BioMart and Bioconductor: A Powerful Link between Biological Databases and Microarray Data Analysis. Bioinformatics 2005, 21, 3439–3440. [Google Scholar] [CrossRef]
- Alexa, A.; Rahnenfuhrer, J. topGO. Bioconductor. 2017. Available online: https://bioconductor.org/packages/release/bioc/html/topGO.html (accessed on 2 November 2024).
- McKinney, W. Data Structures for Statistical Computing in Python. SciPy 2010, 445, 56–61. [Google Scholar]
- Waskom, M. Seaborn: Statistical Data Visualization. J. Open Source Softw. 2021, 6, 3021. [Google Scholar] [CrossRef]
- Ray, D.L.; Johnson, J.C. Validation of reference genes for gene expression analysis in olive (Olea europaea) mesocarp tissue by quantitative real-time RT-PCR. BMC Res. Notes 2014, 18, 304. [Google Scholar] [CrossRef]
- Ahmed, M.; Kim, D.R. Pcr: An R Package for Quality Assessment, Analysis and Testing of QPCR Data. PeerJ 2018, 6, e4473. [Google Scholar] [CrossRef] [PubMed]



| Month | Temperature, °C | Air Humidity, % | Total Precipitation, mm | |||
|---|---|---|---|---|---|---|
| Average | Min | Max | ||||
| January | 3.7 | −12.4 | 17.0 | 77 | 78 | |
| February | 3.6 | −11.9 | 25.2 | 75 | 61 | |
| March | 6.0 | −5.3 | 23.2 | 72 | 54 | |
| April | 10.7 | −5.5 | 27.5 | 67 | 31 | |
| May | 16.0 | 4.0 | 31.6 | 67 | 33 | |
| June | 20.9 | 8.8 | 35.6 | 63 | 41 | |
| July | 24.2 | 11.6 | 37.8 | 58 | 34 | |
| August | 24.4 | 13.0 | 39.0 | 56 | 37 | |
| September | 19.3 | 8.0 | 33.4 | 62 | 41 | |
| October | 14.0 | −0.3 | 32.2 | 70 | 51 | |
| November | 9.0 | −6.3 | 24.7 | 74 | 60 | |
| December | 5.5 | −8.4 | 20.4 | 77 | 87 | |
| Average temperature for the year | Absolute min temperature | Average absolute min temperature | Absolute max temperature | Average absolute max temperature | Average humidity for the year | Total precipitation for the year |
| 13.1 | −12.4 | −7.1 | 39.0 | 34.8 | 68 | 609 |
| Temperature | Cells | ‘Corregiolo’ | ‘Nikitskaya-2’ |
|---|---|---|---|
| −7 °C | Palisade | 0.98 ± 0.04 | 0.30 ± 0.01 |
| Spongy mesophyll | 4.83 ± 0.20 | 4.64 ± 0.28 | |
| −12 °C | Palisade | 5.88 ± 0.55 | 4.30 ± 0.21 |
| Spongy mesophyll | 32.49 ± 1.67 | 19.44 ± 0.86 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gladysheva-Azgari, M.; Slobodova, N.; Sharko, F.; Fatkulin, A.; Tsygankova, S.; Tsiupka, V.; Grebennikova, O.; Bulavin, I.; Boulygina, E.; Tsiupka, S. Freeze-Dependent Physiological and Transcriptional Changes in Olea europaea L. Cultivars with Different Cold Resistances. Int. J. Mol. Sci. 2025, 26, 3934. https://doi.org/10.3390/ijms26093934
Gladysheva-Azgari M, Slobodova N, Sharko F, Fatkulin A, Tsygankova S, Tsiupka V, Grebennikova O, Bulavin I, Boulygina E, Tsiupka S. Freeze-Dependent Physiological and Transcriptional Changes in Olea europaea L. Cultivars with Different Cold Resistances. International Journal of Molecular Sciences. 2025; 26(9):3934. https://doi.org/10.3390/ijms26093934
Chicago/Turabian StyleGladysheva-Azgari, Maria, Natalia Slobodova, Fedor Sharko, Artem Fatkulin, Svetlana Tsygankova, Valentina Tsiupka, Oksana Grebennikova, Iliya Bulavin, Eugenia Boulygina, and Sergei Tsiupka. 2025. "Freeze-Dependent Physiological and Transcriptional Changes in Olea europaea L. Cultivars with Different Cold Resistances" International Journal of Molecular Sciences 26, no. 9: 3934. https://doi.org/10.3390/ijms26093934
APA StyleGladysheva-Azgari, M., Slobodova, N., Sharko, F., Fatkulin, A., Tsygankova, S., Tsiupka, V., Grebennikova, O., Bulavin, I., Boulygina, E., & Tsiupka, S. (2025). Freeze-Dependent Physiological and Transcriptional Changes in Olea europaea L. Cultivars with Different Cold Resistances. International Journal of Molecular Sciences, 26(9), 3934. https://doi.org/10.3390/ijms26093934






