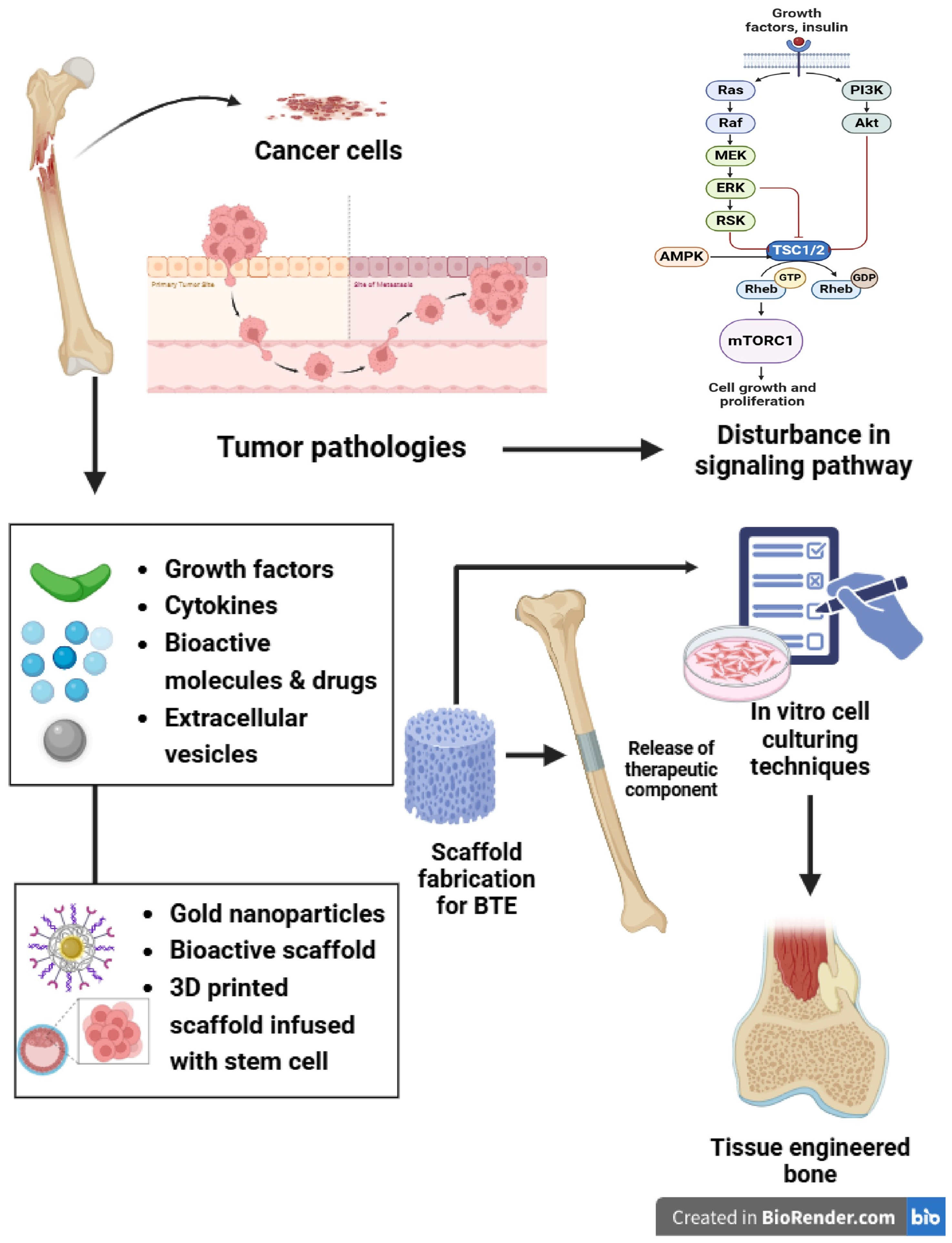
Innovative Approaches in Bone Tissue Engineering: Strategies for Cancer Treatment and Recovery
Abstract
1. Introduction
2. Biomaterials
2.1. Natural Polymers
2.2. Synthetic Polymers
2.3. Bioactive Ceramics
2.4. Biocompatibility
3. Scaffold Fabrication Techniques
3.1. Three-Dimensional Printing
3.2. Electrospinning
3.3. Phase Separation
3.4. Lyophilization
3.5. Calcium Phosphate Scaffold Fabrication
3.6. Polymer Blending
3.7. Functionalization of Scaffolds
4. Advances in BTE
| Modification | Properties | Therapy Model | Reference |
|---|---|---|---|
| Functionalized 3D-printed scaffolds | |||
| PCL/HA/β-TCP | Biocompatibility, osteoconductivity, controlled porosity, compressive strength (4–8 MPa) | Used for critical-sized bone defects in vivo, promotes osteogenesis | [96] |
| GelMA/nHA | Hydrophilicity, high cell adhesion, and proliferation, enhanced mechanical properties. | Applied in cranial defect repair in rats; promotes mineralized bone tissue | [97] |
| PCL-based composites | Enhanced mechanical properties, slow degradation, tailored porosity, FDA-approved | Used in load-bearing bone repair; applied in over 20,000 patients | [98] |
| PEG-DA/PLGA/nHA | Excellent biodegradability and mechanical support (compressive modulus ~12 MPa) | Tested for bone regeneration under in vitro conditions; osteoinductive | [96] |
| PCL/Graphene/HA | Superior mechanical strength, hydrophilicity, enhanced electrical conductivity | Promotes bone tissue formation in large defect models | [98] |
| Ceramic/polymer composites | High compressive strength (~77 MPa for PCL/nHA composites), biodegradability | Applied in orthopedic surgery for repairing fractures and defects | [98] |
| PLA scaffold/gelatin and Polylysine/BMP-2/VEGF | Sequential release of BMP-2/VEGF in spatiotemporal successfully induced angiogenesis and osteogenesis | In vitro cell experiments | [99] |
| Special carrier-loaded 3D-printed scaffolds | |||
| GelMA Hydrogel-Impregnated PCL Scaffolds | Sustained release of VEGF promotes osteogenesis and angiogenesis | In vitro cell experiments | [100] |
| β-TCP scaffold/Gel microspheres/Lipo some DFO | Controlled release of DFO promotes osteogenesis and angiogenesis | Rat femoral defect | [100] |
| PCL scaffold/exosomes/VEGF | Delivery and protection of VEGF promotes osteogenesis and angiogenesis | Rat radial defect | [101] |
| Bionic 3D-printed scaffolds | |||
| β-TCP scaffold/MSCs/ECFCs Hydrogel | Realized central vascularization and Osteogenesis | Rabbit femoral defect | [102] |
| OCP/GelMA hydrogel/HUVECs scaffold | Simulated bone structure; accelerated osteogenesis and angiogenesis | In vitro cell experiments | [103] |
| CDHA/axial vascular pedicle scaffold | Simulated bone structure achieved osteogenesis and angiogenesis | Sheep large bone defect | [104] |
| PLGA/β-TCP/CMs AV bundle scaffold | Combined an AV bundle and rhBMP-2 | Rabbit intramuscular pocket | [105] |
| AKT hollow-channel scaffold | Multi-channel structure achieved osteogenesis and angiogenesis | Rabbit cranial defect; rat muscle implantation | [106] |
| AKT/bio-ceramic/bioactive glass scaffold | Haversian bone-mimicking scaffold promoted osteogenesis and angiogenesis | Rabbit femoral defect | [107] |
5. Conclusions and Future Prospects
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Laversanne, M.; Weiderpass, E.; Soerjomataram, I. The ever-increasing importance of cancer as a leading cause of premature death worldwide. Cancer 2021, 127, 3029–3030. [Google Scholar] [CrossRef]
- Chen, S.; Cao, Z.; Prettner, K.; Kuhn, M.; Yang, J.; Jiao, L.; Wang, Z.; Li, W.; Geldsetzer, P.; Bärnighausen, T.; et al. Estimates and projections of the global economic cost of 29 cancers in 204 countries and territories from 2020 to 2050. JAMA Oncol. 2023, 9, 465–472. [Google Scholar] [CrossRef]
- Available online: https://www.cancer.org/cancer/types/bone-cancer/about/key-statistics.html (accessed on 17 June 2024).
- Siclari, V.A.; Qin, L. Targeting the osteosarcoma cancer stem cell. J. Orthop. Surg. Res. 2010, 5, 78. [Google Scholar] [CrossRef]
- Nakano, K. Challenges of systemic therapy investigations for bone sarcomas. Int. J. Mol. Sci. 2022, 23, 3540. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, H.; Qiang, H.; Li, M.; Cai, Y.; Zhou, X.; Xu, Y.; Yan, Z.; Dong, J.; Gao, Y.; et al. Innovative Biomaterials for Bone Tumor Treatment and Regeneration: Tackling Postoperative Challenges and Charting the Path Forward. Adv. Healthc. Mater. 2024, 1, 2304060. [Google Scholar] [CrossRef]
- Vacanti, J.P.; Langer, R. Tissue engineering: The design and fabrication of living replacement devices for surgical reconstruction and transplantation. Lancet 1999, 354, S32–S34. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Carmeliet, P.; Jain, R.K. Angiogenesis in cancer and other diseases. Nature 2000, 407, 249–257. [Google Scholar] [CrossRef]
- Kolk, A.; Handschel, J.; Drescher, W.; Rothamel, D.; Kloss, F.; Blessmann, M.; Heiland, M.; Wolff, K.D.; Smeets, R. Current trends and future perspectives of bone substitute materials–from space holders to innovative biomaterials. J. Cranio-Maxillofac. Surg. 2012, 40, 706–718. [Google Scholar] [CrossRef] [PubMed]
- Lanza, R.; Langer, R.; Vacanti, J.P.; Atala, A. (Eds.) Principles of Tissue Engineering; Academic Press: Cambridge, MA, USA, 2020; p. 26. [Google Scholar]
- Augustine, R.; Gezek, M.; Nikolopoulos, V.K.; Buck, P.L.; Bostanci, N.S.; Camci-Unal, G. Stem Cells in Bone Tissue Engineering: Progress, Promises and Challenges. Stem Cell Rev. Rep. 2024, 19, 1692–1731. [Google Scholar] [CrossRef]
- Kazimierczak, P.; Przekora, A. Osteoconductive and osteoinductive surface modifications of biomaterials for bone regeneration: A concise review. Coatings 2020, 10, 971. [Google Scholar] [CrossRef]
- Shanmugavadivu, A.; Lekhavadhani, S.; Miranda, P.J.; Selvamurugan, N. Current approaches in tissue engineering-based nanotherapeutics for osteosarcoma treatment. Biomed. Mater. 2024, 2, 351–357. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, Y.; Qiang, H.; Leng, D.; Yang, L.; Hu, X.; Chen, F.; Zhang, T.; Gao, J.; Yu, Z. Exploring the frontiers: The potential and challenges of bioactive scaffolds in osteosarcoma treatment and bone regeneration. Mater. Today Bio 2024, 29, 101276. [Google Scholar] [CrossRef] [PubMed]
- Ni, T.; Liu, M.; Zhang, Y.; Cao, Y.; Pei, R. 3D bioprinting of bone marrow mesenchymal stem cell-laden silk fibroin double network scaffolds for cartilage tissue repair. Bioconjug. Chem. 2020, 31, 1938–1947. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Zhou, F.; Wang, S.; Wang, G.; Bai, L.; Su, J. Bioinspired injectable hydrogels for bone regeneration. J. Adv. Res. 2024. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Feng, X.; Liang, H.; Wang, K.; Song, Y.; Tan, L.; Wang, B.; Luo, R.; Liao, Z.; Li, G.; et al. A novel photothermally controlled multifunctional scaffold for clinical treatment of osteosarcoma and tissue regeneration. Mater. Today 2020, 36, 48–62. [Google Scholar] [CrossRef]
- Krasilnikova, O.; Yakimova, A.; Ivanov, S.; Atiakshin, D.; Kostin, A.A.; Sosin, D.; Shegay, P.; Kaprin, A.D.; Klabukov, I. Gene-Activated Materials in Regenerative Dentistry: Narrative Review of Technology and Study Results. Int. J. Mol. Sci. 2023, 24, 16250. [Google Scholar] [CrossRef]
- Esmaeili, Y.; Bidram, E.; Bigham, A.; Atari, M.; Azadani, R.N.; Tavakoli, M.; Salehi, S.; Mirhaj, M.; Basiri, A.; Mirzavandi, Z.; et al. Exploring the evolution of tissue engineering strategies over the past decade: From cell-based strategies to gene-activated matrix. Alex. Eng. J. 2023, 81, 137–169. [Google Scholar] [CrossRef]
- Henkel, J.; Woodruff, M.A.; Epari, D.R.; Steck, R.; Glatt, V.; Dickinson, I.C.; Choong, P.F.; Schuetz, M.A.; Hutmacher, D.W. Bone regeneration based on tissue engineering conceptions—A 21st century perspective. Bone Res. 2013, 1, 216–248. [Google Scholar] [CrossRef] [PubMed]
- Albrektsson, T.; Johansson, C. Osteoinduction, osteoconduction and osseointegration. Eur. Spine J. 2001, 10 (Suppl. S2), S96–S101. [Google Scholar]
- Giannoudis, P.V.; Dinopoulos, H.; Tsiridis, E. Bone substitutes: An update. Injury 2005, 36, S20–S27. [Google Scholar] [CrossRef] [PubMed]
- Stevens, M.M. Biomaterials for bone tissue engineering. Mater. Today 2008, 11, 18–25. [Google Scholar] [CrossRef]
- O’brien, F.J. Biomaterials & scaffolds for tissue engineering. Mater. Today 2011, 14, 88–95. [Google Scholar]
- Stevens, M.M.; George, J.H. Exploring and engineering the cell surface interface. Science 2005, 310, 1135–1138. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, P.P.; Rodrigues, M.T.; Reis, R.L.; Gomes, M.E. Starch-Based Blends in Tissue Engineering. Biomater. Nat. Adv. Devices Ther. 2016, 14, 244–257. [Google Scholar]
- Pina, S.; Oliveira, J.M.; Reis, R.L. Natural-based nanocomposites for BTE and regenerative medicine: A review. Adv. Mater. 2015, 27, 1143–1169. [Google Scholar] [CrossRef]
- Rodríguez, G.R.; Patrício, T.M.; López, J.D. Natural polymers for bone repair. In Bone Repair Biomaterials; Woodhead Publishing: Cambridge, UK, 2019; pp. 199–232. [Google Scholar]
- Albu, M.G.; Titorencu, I.; Ghica, M.V. Collagen-Based Drug Delivery Systems for Tissue Engineering. In Biomaterials Applications for Nanomedicine; IntechOpen: London, UK, 2011. [Google Scholar]
- Gorgieva, S.; Kokol, V. Collagen-vs. gelatine-based biomaterials and their biocompatibility: Review and perspectives. Biomater. Appl. Nanomed. 2011, 2, 17–52. [Google Scholar]
- Bhattacharjee, P.; Kundu, B.; Naskar, D.; Kim, H.W.; Maiti, T.K.; Bhattacharya, D.; Kundu, S.C. Silk scaffolds in bone tissue engineering: An overview. Acta Biomater. 2017, 63, 1–17. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, K.Q. Silk fiber—Molecular formation mechanism, structure-property relationship and advanced applications. Oligomerization Chem. Biol. Compd. 2014, 3, 69–102. [Google Scholar]
- Vepari, C.; Kaplan, D.L. Silk as a biomaterial. Prog. Polym. Sci. 2007, 32, 991–1007. [Google Scholar] [CrossRef]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and biomedical applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef] [PubMed]
- Moon, R.J.; Martini, A.; Nairn, J.; Simonsen, J.; Youngblood, J. Cellulose nanomaterials review: Structure, properties and nanocomposites. Chem. Soc. Rev. 2011, 40, 3941–3994. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.; Kim, J. Preparation and characterization of novel bacterial cellulose/gelatin scaffold for tissue regeneration using bacterial cellulose hydrogel. J. Nanotechnol. Eng. Med. 2010, 1, 021002. [Google Scholar] [CrossRef]
- Ruiz, G.A.; Corrales, H.F. Chitosan, chitosan derivatives and their biomedical applications. Biol. Act. Appl. Mar. Polysacch. 2017, 11, 87. [Google Scholar]
- Shi, C.; Yuan, Z.; Han, F.; Zhu, C.; Li, B. Polymeric biomaterials for bone regeneration. Ann. Jt. 2016, 1, 9. [Google Scholar] [CrossRef]
- Wang, S.; Copeland, L. Effect of acid hydrolysis on starch structure and functionality: A review. Crit. Rev. Food Sci. Nutr. 2015, 55, 1081–1097. [Google Scholar] [CrossRef]
- Pradhan, S.; Hassani, I.; Clary, J.M.; Lipke, E.A. Polymeric biomaterials for in vitro cancer tissue engineering and drug testing applications. Tissue Eng. Part B Rev. 2016, 22, 470–484. [Google Scholar] [CrossRef] [PubMed]
- Tanvir, M.A.; Khaleque, M.A.; Kim, G.H.; Yoo, W.Y.; Kim, Y.Y. The Role of Bioceramics for Bone Regeneration: History, Mechanisms, and Future Perspectives. Biomimetics 2024, 9, 230. [Google Scholar] [CrossRef] [PubMed]
- LogithKumar, R.; KeshavNarayan, A.; Dhivya, S.; Chawla, A.; Saravanan, S.; Selvamurugan, N. A review of chitosan and its derivatives in bone tissue engineering. Carbohydr. Polym. 2016, 151, 172–188. [Google Scholar] [CrossRef]
- Saravanan, S.; Vimalraj, S.; Thanikaivelan, P.; Banudevi, S.; Manivasagam, G. A review on injectable chitosan/beta glycerophosphate hydrogels for bone tissue regeneration. Int. J. Biol. Macromol. 2019, 121, 38–54. [Google Scholar] [CrossRef] [PubMed]
- Cinici, B.; Yaba, S.; Kurt, M.; Yalcin, H.C.; Duta, L.; Gunduz, O. Fabrication Strategies for Bioceramic Scaffolds in Bone Tissue Engineering with Generative Design Applications. Biomimetics 2024, 9, 409. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.J.; Choi, S.; Sang Cho, Y.; Yang, S.J.; Cho, Y.S.; Kim, K.K. Magnesium ions enhance infiltration of osteoblasts in scaffolds via increasing cell motility. J. Mater. Sci. Mater. Med. 2017, 28, 96. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Feyerabend, F.; Schilling, A.F.; Willumeit-Römer, R.; Luthringer, B.J. Effects of extracellular magnesium extract on the proliferation and differentiation of human osteoblasts and osteoclasts in coculture. Acta Biomater. 2015, 27, 294–304. [Google Scholar] [CrossRef]
- Lu, W.C.; Pringa, E.; Chou, L. Effect of magnesium on the osteogenesis of normal human osteoblasts. Magnes. Res. 2017, 30, 42. [Google Scholar] [CrossRef] [PubMed]
- Zhao, N.; Zhu, D. Collagen self-assembly on orthopedic magnesium biomaterials surface and subsequent bone cell attachment. PLoS ONE 2014, 9, e110420. [Google Scholar] [CrossRef] [PubMed]
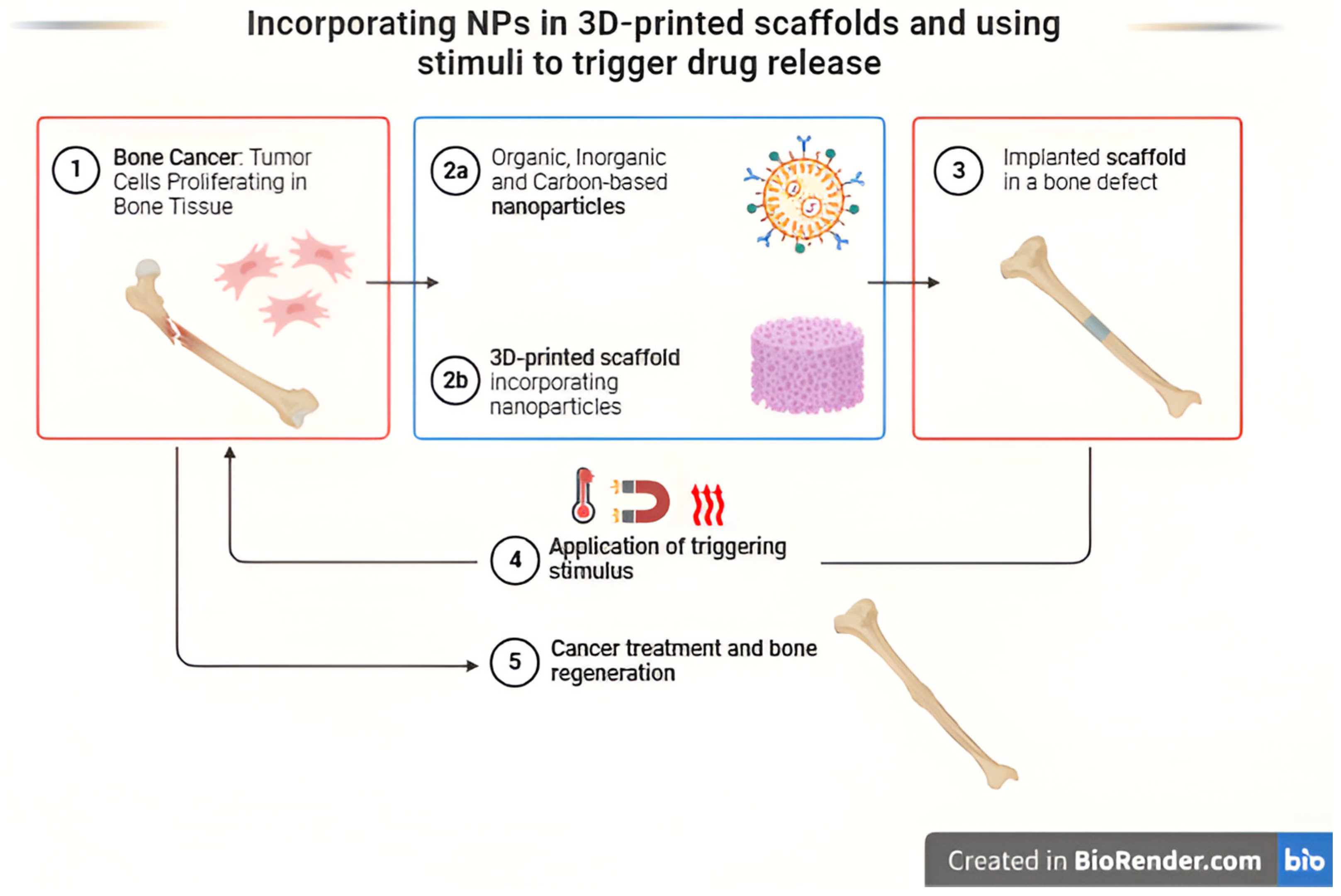
- Percival, K.M.; Paul, V.; Husseini, G.A. Recent Advancements in Bone Tissue Engineering: Integrating Smart Scaffold Technologies and Bio-Responsive Systems for Enhanced Regeneration. Int. J. Mol. Sci. 2024, 25, 6012. [Google Scholar] [CrossRef]
- Al Sawaftah, N.M.; Pitt, W.G.; Husseini, G.A. Incorporating nanoparticles in 3D printed scaffolds for bone cancer therapy. Bioprinting 2023, 31, e00322. [Google Scholar] [CrossRef]
- Mattioli-Belmonte, M.; De Maria, C.; Vitale-Brovarone, C.; Baino, F.; Dicarlo, M.; Vozzi, G. Pressure-activated microsyringe (PAM) fabrication of bioactive glass–poly (lactic-co-glycolic acid) composite scaffolds for bone tissue regeneration. J. Tissue Eng. Regen. Med. 2017, 11, 1986–1997. [Google Scholar] [CrossRef]
- Wang, Z.; Huang, C.; Wang, J.; Zou, B. Development of a novel aqueous hydroxyapatite suspension for stereolithography applied to bone tissue engineering. Ceram. Int. 2019, 45, 3902–3909. [Google Scholar] [CrossRef]
- Lyu, S.; Huang, C.; Yang, H.; Zhang, X. Electrospun Fibers as a Scaffolding Platform for Bone Tissue Repair. J. Orthop. Res. 2013, 31, 1382–1389. [Google Scholar] [CrossRef]
- Aydin, M.S.; Sahin, M.; Dogan, Z.; Kiziltas, G. Microstructural Characterization of PCL-HA Bone Scaffolds Based on Nonsolvent-Induced Phase Separation. ACS Omega 2023, 8, 47595–47605. [Google Scholar] [CrossRef]
- Parfenov, V.A.; Mironov, V.A.; Koudan, E.V.; Nezhurina, E.K.; Karalkin, P.A.; Pereira, F.D.; Petrov, S.V.; Krokhmal, A.A.; Aydemir, T.; Vakhrushev, I.V.; et al. Fabrication of calcium phosphate 3D scaffolds for bone repair using magnetic levitational assembly. Sci. Rep. 2020, 10, 4013. [Google Scholar] [CrossRef] [PubMed]
- Heo, D.N.; Hospodiuk, M.; Ozbolat, I.T. Synergistic interplay between human MSCs and HUVECs in 3D spheroids laden in collagen/fibrin hydrogels for bone tissue engineering. Acta Biomater. 2019, 95, 348–356. [Google Scholar] [CrossRef]
- Al-Hammadi, A.S.; Saidin, S.; Ramlee, M.H. Simulation Analyses Related to Human Bone Scaffold: Utilisation of Solidworks® Software in 3D Modelling and Mechanical Simulation Analyses. J. Hum. Centered Technol. 2022, 1, 97–104. [Google Scholar] [CrossRef]
- Chen, Y.; Pan, X.; Li, Z. Analysis of mechanical properties in scaffolds using melt electrospinning. Mater. Sci. Eng. C 2020, 116, 111229. [Google Scholar]
- Li, S.; Cui, S.; Liu, Y. Simpleware for laser-assisted bioprinting in bone tissue research. J. Appl. Biomat Funct. Mater. 2022, 20, 1–10. [Google Scholar]
- Amini, A.R.; Laurencin, C.T.; Nukavarapu, S.P. Bone tissue engineering: Recent advances and challenges. Crit. Rev. Biomed. Eng. 2012, 40, 363–408. [Google Scholar] [CrossRef] [PubMed]
- Kargozar, S.; Hashemian, S.J.; Soleimani, M.; Milan, P.B.; Askari, M.; Khalaj, V.; Samadikuchaksaraie, A.; Hamzehlou, S.; Katebi, A.R.; Latifi, N.; et al. Acceleration of bone regeneration in bioactive glass/gelatin composite scaffolds seeded with bone marrow-derived mesenchymal stem cells over-expressing bone morphogenetic protein-7. Mater. Sci. Eng. C 2017, 75, 688–698. [Google Scholar] [CrossRef] [PubMed]
- Awad, H.A.; O’Keefe, R.J.; Mao, J.J. Bone tissue engineering. In Principles of Tissue Engineering; Academic Press: Cambridge, MA, USA, 2020; pp. 1511–1519. [Google Scholar]
- Chocholata, P.; Kulda, V.; Babuska, V. Fabrication of scaffolds for bone-tissue regeneration. Materials 2019, 12, 568. [Google Scholar] [CrossRef]
- Nyberg, E.; Rindone, A.; Dorafshar, A.; Grayson, W.L. Comparison of 3D-printed poly-ɛ-caprolactone scaffolds functionalized with tricalcium phosphate, hydroxyapatite, bio-oss, or decellularized bone matrix. Tissue Eng. Part A 2017, 23, 503–514. [Google Scholar] [CrossRef]
- Li, M.; Sun, D.; Zhang, J.; Wang, Y.; Wei, Q.; Wang, Y. Application and development of 3D bioprinting in cartilage tissue engineering. Biomater. Sci. 2022, 10, 5430–5458. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Ma, X.; Zhu, W.; Wang, P.; Miller, K.L.; Stupin, J.; Koroleva-Maharajh, A.; Hairabedian, A.; Chen, S. Scanningless and continuous 3D bioprinting of human tissues with decellularized extracellular matrix. Biomaterials 2019, 194, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Kelly, B.E.; Bhattacharya, I.; Heidari, H.; Shusteff, M.; Spadaccini, C.M.; Taylor, H.K. Volumetric additive manufacturing via tomographic reconstruction. Science 2019, 363, 1075–1079. [Google Scholar] [CrossRef]
- Hull, C.W. Apparatus for Production of Three-Dimensional Objects by Stereolithography. U.S. Patent US 4,575,330, 11 March 1986. [Google Scholar]
- Hart, A.J.; Rao, A. How to print a 3D object all at once. Science 2019, 363, 1042–1043. [Google Scholar] [CrossRef]
- Liang, K.; Carmone, S.; Brambilla, D.; Leroux, J.C. 3D-printing of a wearable personalized oral delivery device: A first-in-human study. Sci. Adv. 2018, 4, eaat2544. [Google Scholar] [CrossRef]
- Lee, A.R.; Hudson, A.R.; Shiwarski, D.J.; Tashman, J.W.; Hinton, T.J.; Yerneni, S.; Bliley, J.M.; Campbell, P.G.; Feinberg, A.W. 3D bioprinting of collagen to rebuild components of the human heart. Science 2019, 365, 482–487. [Google Scholar] [CrossRef] [PubMed]
- Murphy, S.V.; De Coppi, P.; Atala, A. Opportunities and challenges of translational 3D bioprinting. Nat. Biomed. Eng. 2020, 4, 370–380. [Google Scholar] [CrossRef]
- Li, J.; Pumera, M. 3D-printing of functional microrobots. Chem. Soc. Rev. 2021, 50, 2794–2838. [Google Scholar] [CrossRef]
- Ouyang, L.; Armstrong, J.P.; Lin, Y.; Wojciechowski, J.P.; Lee-Reeves, C.; Hachim, D.; Zhou, K.; Burdick, J.A.; Stevens, M.M. Expanding and optimizing 3D bioprinting capabilities using complementary network bioinks. Sci. Adv. 2020, 6, eabc5529. [Google Scholar] [CrossRef] [PubMed]
- Urciuolo, A.; Poli, I.; Brandolino, L.; Raffa, P.; Scattolini, V.; Laterza, C.; Giobbe, G.G.; Zambaiti, E.; Selmin, G.; Magnussen, M.; et al. Intravital three-dimensional bioprinting. Nat. Biomed. Eng. 2020, 4, 901–915. [Google Scholar] [CrossRef]
- Tibbits, S. The Emergence of “4D-Printing”; TED Talk: New york, NY, USA, 2013; Volume 10. [Google Scholar]
- Ding, Z.; Yuan, C.; Peng, X.; Wang, T.; Qi, H.J.; Dunn, M.L. Direct 4D-printing via active composite materials. Sci. Adv. 2017, 3, e1602890. [Google Scholar] [CrossRef] [PubMed]
- Gillaspie, E.A.; Matsumoto, J.S.; Morris, N.E.; Downey, R.J.; Shen, K.R.; Allen, M.S.; Blackmon, S.H. From 3-dimensional printing to 5-dimensional printing: Enhancing thoracic surgical planning and resection of complex tumors. Ann. Thorac. Surg. 2016, 101, 1958–1962. [Google Scholar] [CrossRef]
- Augustin, H.G.; Koh, G.Y. Organotypic vasculature: From descriptive heterogeneity to functional pathophysiology. Science 2017, 357, eaal2379. [Google Scholar] [CrossRef]
- Jia, F.; Ruan, L.; Du, C.; Liu, Y.; Cai, X.; Dou, R.; Zhang, J.; Liu, X.; Chen, J.; Zhang, X.; et al. The nanoformula of zoledronic acid and calcium carbonate targets osteoclasts and reverses osteoporosis. Biomaterials 2023, 296, 122059. [Google Scholar] [CrossRef]
- Zhou, J.; Zhang, Z.; Joseph, J.; Zhang, X.; Ferdows, B.E.; Patel, D.N.; Chen, W.; Banfi, G.; Molinaro, R.; Cosco, D.; et al. Biomaterials and nanomedicine for bone regeneration: Progress and future prospects. Exploration 2021, 1, 20210011. [Google Scholar] [CrossRef] [PubMed]
- Kuss, M.A.; Harms, R.; Wu, S.; Wang, Y.; Untrauer, J.B.; Carlson, M.A.; Duan, B. Short-term hypoxic preconditioning promotes prevascularization in 3D bioprinted bone constructs with stromal vascular fraction derived cells. RSC Adv. 2017, 7, 29312–29320. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.W.; Shen, Y.F.; Ho, C.C.; Yu, J.; Wu, Y.H.; Wang, K.; Shih, C.T.; Shie, M.Y. Osteogenic and angiogenic potentials of the cell-laden hydrogel/mussel-inspired calcium silicate complex hierarchical porous scaffold fabricated by 3D bioprinting. Mater. Sci. Eng. C 2018, 91, 679–687. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.W.; Lee, S.J.; Ko, I.K.; Kengla, C.; Yoo, J.J.; Atala, A. A 3D bioprinting system to produce human-scale tissue constructs with structural integrity. Nat. Biotechnol. 2016, 34, 312–319. [Google Scholar] [CrossRef]
- Rukavina, P.; Koch, F.; Wehrle, M.; Tröndle, K.; Björn Stark, G.; Koltay, P.; Zimmermann, S.; Zengerle, R.; Lampert, F.; Strassburg, S.; et al. In vivo evaluation of bioprinted prevascularized bone tissue. Biotechnol. Bioeng. 2020, 117, 3902–3911. [Google Scholar] [CrossRef]
- Lin, H.; Tang, Y.; Lozito, T.P.; Oyster, N.; Wang, B.; Tuan, R.S. Efficient in vivo bone formation by BMP-2 engineered human mesenchymal stem cells encapsulated in a projection stereolithographically fabricated hydrogel scaffold. Stem Cell Res. Ther. 2019, 10, 254. [Google Scholar] [CrossRef] [PubMed]
- Cunniffe, G.M.; Gonzalez-Fernandez, T.; Daly, A.; Sathy, B.N.; Jeon, O.; Alsberg, E.; Kelly, D.J. Three-dimensional bioprinting of polycaprolactone reinforced gene activated bioinks for bone tissue engineering. Tissue Eng. Part A 2017, 23, 891–900. [Google Scholar] [CrossRef] [PubMed]
- Tertuliano, O.A.; Greer, J.R. The nanocomposite nature of bone drives its strength and damage resistance. Nat. Mater. 2016, 15, 1195–1202. [Google Scholar] [CrossRef]
- Miri, A.K.; Khalilpour, A.; Cecen, B.; Maharjan, S.; Shin, S.R.; Khademhosseini, A. Multiscale bioprinting of vascularized models. Biomaterials 2019, 198, 204–216. [Google Scholar] [CrossRef] [PubMed]
- Devillard, C.D.; Mandon, C.A.; Lambert, S.A.; Blum, L.J.; Marquette, C.A. Bioinspired multi-activities 4D-printing objects: A new approach toward complex tissue engineering. Biotechnol. J. 2018, 13, 1800098. [Google Scholar] [CrossRef]
- Kumar, P.R.; Tech, M.; Roy, S.U.; Hegde, H.A.; Bharti, S.H.; Kumar, M.A. 4D and 5D-printing: Healthcare’s new edge. 3D Print Technol. Nanomed. 2019, 143, 143–163. [Google Scholar]
- Akhtar, M.N.; Haleem, A.; Javaid, M.; Mathur, S.; Vaish, A.; Vaishya, R. Artificial intelligence-based orthopaedic perpetual design. J. Clin. Orthop. Trauma 2024, 49, 102356. [Google Scholar] [CrossRef] [PubMed]
- Zeijderveld, J.V. 5D-printing: A new branch of additive manufacturing. In Sculpteo Report [Internet]; Sculpteo: Villejuif, France, 2018. [Google Scholar]
- Haleem, A.; Javaid, M. Expected applications of five-dimensional (5D) printing in the medical field. Curr. Med. Res. Pract. 2019, 9, 208. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, M.; Wu, R.; Guo, J.; Sun, A.; Li, Z.; Ye, R.; Xu, G.; Cheng, Y. From materials to clinical use: Advances in 3D-printed scaffolds for cartilage tissue engineering. Phys. Chem. Chem. Phys. 2023, 25, 24244–24263. [Google Scholar] [CrossRef]
- Dong, J.; Ding, H.; Wang, Q.; Wang, L. A 3D-Printed Scaffold for Repairing Bone Defects. Polymers 2024, 16, 706. [Google Scholar] [CrossRef]
- Gharibshahian, M.; Salehi, M.; Beheshtizadeh, N.; Kamalabadi-Farahani, M.; Atashi, A.; Nourbakhsh, M.S.; Alizadeh, M. Recent advances on 3D-printed PCL-based composite scaffolds for bone tissue engineering. Front. Bioeng. Biotechnol. 2023, 11, 1168504. [Google Scholar] [CrossRef]
- Cui, H.; Zhu, W.; Holmes, B.; Zhang, L.G. Biologically inspired smart release system based on 3D bioprinted perfused scaffold for vascularized tissue regeneration. Adv. Sci. 2016, 3, 1600058. [Google Scholar] [CrossRef]
- Augustine, R.; Nikolopoulos, V.K.; Camci-Unal, G. Hydrogel-Impregnated Self-Oxygenating Electrospun Scaffolds for Bone Tissue Engineering. Bioengineering 2023, 10, 854. [Google Scholar] [CrossRef]
- Zha, Y.; Li, Y.; Lin, T.; Chen, J.; Zhang, S.; Wang, J. Progenitor cell-derived exosomes endowed with VEGF plasmids enhance osteogenic induction and vascular remodeling in large segmental bone defects. Theranostics 2021, 11, 397. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhu, L.X.; Wang, Z.; Lou, A.J.; Yang, Y.X.; Guo, Y.; Liu, S.; Zhang, C.; Zhang, Z.; Hu, H.S.; et al. Development of a centrally vascularized tissue engineering bone graft with the unique core-shell composite structure for large femoral bone defect treatment. Biomaterials 2018, 175, 44–60. [Google Scholar] [CrossRef]
- Anada, T.; Pan, C.C.; Stahl, A.M.; Mori, S.; Fukuda, J.; Suzuki, O.; Yang, Y. Vascularized bone-mimetic hydrogel constructs by 3D bioprinting to promote osteogenesis and angiogenesis. Int. J. Mol. Sci. 2019, 20, 1096. [Google Scholar] [CrossRef] [PubMed]
- Vidal, L.; Kampleitner, C.; Krissian, S.; Brennan, M.A.; Hoffmann, O.; Raymond, Y.; Maazouz, Y.; Ginebra, M.P.; Rosset, P.; Layrolle, P. Regeneration of segmental defects in metatarsus of sheep with vascularized and customized 3D-printed calcium phosphate scaffolds. Sci. Rep. 2020, 10, 7068. [Google Scholar] [CrossRef]
- Li, B.; Ruan, C.; Ma, Y.; Huang, Z.; Huang, Z.; Zhou, G.; Zhang, J.; Wang, H.; Wu, Z.; Qiu, G. Fabrication of vascularized bone flaps with sustained release of recombinant human bone morphogenetic protein-2 and arteriovenous bundle. Tissue Eng. Part A 2018, 24, 1413–1422. [Google Scholar] [CrossRef]
- Feng, C.; Zhang, W.; Deng, C.; Li, G.; Chang, J.; Zhang, Z.; Jiang, X.; Wu, C. 3D-printing of lotus root-like biomimetic materials for cell delivery and tissue regeneration. Adv. Sci. 2017, 4, 1700401. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Lin, R.; Wang, X.; Xue, J.; Deng, C.; Feng, C.; Zhuang, H.; Ma, J.; Qin, C.; Wan, L.; et al. 3D-printing of Haversian bone–mimicking scaffolds for multicellular delivery in bone regeneration. Sci. Adv. 2020, 6, eaaz6725. [Google Scholar] [CrossRef]
- Damiri, F.; Fatimi, A.; Musuc, A.M.; Santos, A.C.; Paszkiewicz, S.; Idumah, C.I.; Singh, S.; Varma, R.S.; Berrada, M. Nano-hydroxyapatite (nHAp) scaffolds for bone regeneration: Preparation, characterization and biological applications. J. Drug Deliv. Sci. Technol. 2024, 18, 105601. [Google Scholar] [CrossRef]
- Nika, D.L.; Balandin, A.A. Phonons and thermal transport in graphene and graphene-based materials. Rep. Prog. Phys. 2017, 80, 036502. [Google Scholar] [CrossRef]
- Peng, Z.; Zhao, T.; Zhou, Y.; Li, S.; Li, J.; Leblanc, R.M. Bone tissue engineering via carbon?based nanomaterials. Adv. Healthc. Mater. 2020, 9, 1901495. [Google Scholar] [CrossRef] [PubMed]
- Lavanya, K.; Chandran, S.V.; Balagangadharan, K.; Selvamurugan, N.J. Temperature-and pH-responsive chitosan-based injectable hydrogels for bone tissue engineering. Mater. Sci. Eng. C 2020, 111, 110862. [Google Scholar] [CrossRef] [PubMed]
- Dasari, A.; Xue, J.; Deb, S. Magnetic nanoparticles in bone tissue engineering. Nanomaterials 2022, 12, 757. [Google Scholar] [CrossRef] [PubMed]
- Sankaranarayanan, A.; Ramprasad, A.; Ganesh, S.S.; Ganesh, H.; Ramanathan, B.; Shanmugavadivu, A.; Selvamurugan, N. Nanogels for bone tissue engineering-from synthesis to application. Nanoscale 2023, 15, 10206–10222. [Google Scholar] [CrossRef] [PubMed]
- She, J.; Liu, J.; Mu, Y.; Lv, S.; Tong, J.; Liu, L.; He, T.; Wang, J.; Wei, D. Recent advances in collagen-based hydrogels: Materials, preparation and applications. React. Funct. Polym. 2024, 12, 106136. [Google Scholar] [CrossRef]
- Wu, E.; Huang, L.; Shen, Y.; Wei, Z.; Li, Y.; Wang, J.; Chen, Z. Application of gelatin-based composites in bone tissue engineering. Heliyon 2024, 10, e36258. [Google Scholar] [CrossRef] [PubMed]
- Han, D.; Wang, W.; Gong, J.; Ma, Y.; Li, Y. Controlled delivery of mesenchymal stem cells via biodegradable scaffolds for fracture healing. Nanomedicine 2024, 18, 1–18. [Google Scholar] [CrossRef]
- Kumar, B.; Singh, N.; Kumar, P. A review on sources, modification techniques, properties and potential applications of alginate-based modified polymers. Eur. Polym. J. 2024, 16, 113078. [Google Scholar] [CrossRef]
- Beig, B.; Liaqat, U.; Niazi, M.F.; Douna, I.; Zahoor, M.; Niazi, M.B. Current challenges and innovative developments in hydroxyapatite-based coatings on metallic materials for bone implantation: A review. Coatings 2020, 10, 1249. [Google Scholar] [CrossRef]
- Dubey, S.; Mishra, R.; Roy, P.; Singh, R.P. 3-D macro/microporous-nanofibrous bacterial cellulose scaffolds seeded with BMP-2 preconditioned mesenchymal stem cells exhibit remarkable potential for bone tissue engineering. Int. J. Biol. Macromol. 2021, 167, 934–946. [Google Scholar] [CrossRef] [PubMed]
- Deng, H.; Wang, J.; An, R. Hyaluronic acid-based hydrogels: As an exosome delivery system in bone regeneration. Front. Pharmacol. 2023, 14, 1131001. [Google Scholar] [CrossRef] [PubMed]
- Koohkan, R.; Hooshmand, T.; Mohebbi-Kalhori, D.; Tahriri, M.; Marefati, M.T. Synthesis, characterization, and in vitro biological evaluation of copper-containing magnetic bioactive glasses for hyperthermia in bone defect treatment. ACS Biomater. Sci. Eng. 2018, 4, 1797–1811. [Google Scholar] [CrossRef]
- Eslami, H.; Lisar, H.A.; Kashi, T.S.; Tahriri, M.; Ansari, M.; Rafiei, T.; Bastami, F.; Shahin-Shamsabadi, A.; Abbas, F.M.; Tayebi, L. Poly (lactic-co-glycolic acid)(PLGA)/TiO2 nanotube bioactive composite as a novel scaffold for bone tissue engineering: In vitro and in vivo studies. Biologicals 2018, 53, 51–62. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Wang, Y.; Zhou, Y.; Chen, J.; Wan, Q. The application of polycaprolactone in three-dimensional printing scaffolds for bone tissue engineering. Polymers 2021, 13, 2754. [Google Scholar] [CrossRef] [PubMed]
- Habibi, P.; Bagheri, F.; Tork, N. Polyethylene glycol: Novel applications in tissue engineering and carriers of antimicrobial and anticancer agents. Nano Micro Biosyst. 2024, 3, 1–7. [Google Scholar]
- Alavi, M.S.; Memarpour, S.; Pazhohan-Nezhad, H.; Salimi Asl, A.; Moghbeli, M.; Shadmanfar, S.; Saburi, E. Applications of poly (lactic acid) in bone tissue engineering: A review article. Artif. Organs 2023, 47, 1423–1430. [Google Scholar] [CrossRef] [PubMed]
- Ramanathan, S.; Lin, Y.C.; Thirumurugan, S.; Hu, C.C.; Duann, Y.F.; Chung, R.J. Poly (methyl methacrylate) in Orthopedics: Strategies, Challenges, and Prospects in Bone Tissue Engineering. Polymers 2024, 16, 367. [Google Scholar] [CrossRef] [PubMed]
- Annunziata, M.; Nastri, L.; Cecoro, G.; Guida, L. The use of poly-d, l-lactic acid (PDLLA) devices for bone augmentation techniques: A systematic review. Molecules 2017, 22, 2214. [Google Scholar] [CrossRef] [PubMed]
- Szczepańczyk, P.; Szlachta, M.; Złocista-Szewczyk, N.; Chłopek, J.; Pielichowska, K. Recent developments in polyurethane-based materials for bone tissue engineering. Polymers 2021, 13, 946. [Google Scholar] [CrossRef]

| Polymer | Source | Advantages | Disadvantages | References |
|---|---|---|---|---|
| Natural polymer | ||||
| Collagen | Ovine, porcine, equine, and bovine | Biocompatibility promotes cell adhesion and growth, biodegradable. | Relatively weak mechanical stiffness, risk of immunogenic reactions, potential disease transmission. | [30] |
| Gelatin | Denaturation and hydrolysis of natural collagen | Biocompatible, biodegradable, cell-binding properties, low cost, gelling properties | Low mechanical properties and fast degradation rate. | [31] |
| Silk | Arthropods (Lepidoptera larvae such as silkworms; arachnids such as spiders, mites, and some scorpions; and some flies) | Biocompatible, biodegradable, flexible processability, high mechanical strength, thermally stable | Slow degradation rate (2–4 years) | [32,33,34] |
| Alginate | Brown seaweed | Biocompatible, biodegradable, relatively low cost, easy gelatin by ionic cross-linking, and easy chemical modification with adhesion via RGD ligands | Poor cell-material interaction due to inherent lack of cell adhesivity and low protein adsorption | [35] |
| Cellulose | Wood, plants, tunicates, and algae | Biodegradable, biocompatible, high mechanical performance | Low cell-binding properties | [36,37] |
| Chitosan | Exoskeleton of crustaceans and mollusks, insect cuticles, and fungi | Bioactive, biocompatible, biodegradable, antibacterial, and nonimmunogenic properties; the ability for cell ingrowth | Relatively weak mechanical strength and stability | [38,39] |
| Starch | Corn, potato, wheat, and tapioca | Biocompatible, biodegradable, low cost | Lack of processability, low surface area | [27,40] |
| Technique | Materials Used | Working Principle | Advantages | Disadvantages | Schematic |
|---|---|---|---|---|---|
| Fused deposition modeling (FDM)/Fused filament fabrication (FFF) [51] | Acrylonitrile butadiene styrene (ABS), PLA, PCL, polyethylene terephthalate glycol (PET-G), tricalcium phosphate (TCP), nylon | FDM works by extruding a thermoplastic or composite filament layer by layer to create customized, porous scaffolds for bone tissue engineering, enabling cell infiltration, nutrient diffusion, and osteogenesis. |
|
| 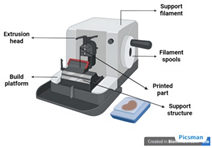 |
| Pressure-assisted microsyringes (PAMs) [52] | A semi-liquid mixture of polymers and solvents (solution, paste, or dispersion) | Pressure-assisted microsyringes (PAMs) work by extruding bio-inks or biomaterials through a microsyringe nozzle under controlled pressure to fabricate 3D scaffolds layer by layer. |
|
|  |
| Stereolithography (SLA) [53] | Photo-curable liquid resin | Uses light sources from UV to visible light to crosslink or polymerize the ink for the development of 3D structures. |
|
| 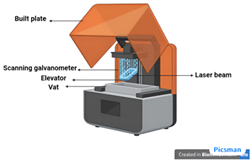 |
| Laser-assisted method [51] | Ink solution, laser energy-absorbing powders | Based on laser-induced forward transfer (LIFT) effect. A NIR or UV pulsed laser is used that transfers energy into a liquid photopolymerizable material. Photopolymerization occurs, and the product is created LbL. |
|
|  |
| Binder jetting [52] | Binder fluid, powder bed | Binder jetting involves depositing a liquid binder onto a powder material, layer by layer, to create scaffolds. The powder typically includes bioactive ceramics or polymers, and the binder fuses the particles. Post-processing like sintering enhances the scaffold’s mechanical properties for bone regeneration. |
|
| 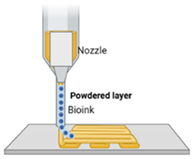 |
| Inkjet printing [51] | Ink–drug solution, substrate–polymer-based films | Two-step process: (1) formation of electrostatically charged ink droplets and directing them toward predefined locations on the substrate and (2) droplet and substrate get to interact. |
|
| 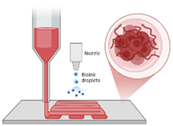 |
| List of Software | Method of Fabrication | Materials | Application | Reference |
|---|---|---|---|---|
| SolidWorks | 3D-printing | Polycaprolactone (PCL) | Designing load-bearing scaffolds for critical-sized bone defects | [58] |
| Autodesk Fusion 360 | Selective Laser Sintering (SLS) | Poly(L-lactide) (PLLA) | Fabrication of scaffolds for large bone defect repair, particularly in long bones | [59] |
| MATLAB | Electrospinning | Poly (lactic-co-glycolic acid) (PLGA) | Developing nanofiber structures for bone cell attachment and proliferation | [60] |
| ANSYS | Bioprinting | Hydrogel + Mesenchymal Stem Cells | Enhancing osteogenesis by creating stem cell-laden scaffolds for bone regeneration | [61] |
| Rhino + Grasshopper | Stereolithography (SLA) | Hydroxyapatite (HA) | Creating high-resolution scaffolds for craniofacial bone reconstruction | [62] |
| BioCAD | Direct Ink Writing | Collagen + Bioactive Glass | Printing composite scaffolds for repairing segmental bone defects | [63] |
| Blender | Fused Deposition Modeling (FDM) | Polyethylene Glycol (PEG) | Producing scaffolds for filling irregular bone defects in orthopedic surgery | [64] |
| Mimics | Computer-Aided Design (CAD) | Calcium Phosphate (CaP) | Generating patient-specific scaffolds for complex bone reconstruction surgeries. | [65] |
| COMSOL Multiphysics | Melt Electrospinning | Polycaprolactone (PCL) + Gelatin | Optimizing mechanical properties of scaffolds for vertebral bone repair | [66] |
| Simpleware | Laser-Assisted Bioprinting | Bioink + Osteoblast cells | Fabricating osteoblast-laden scaffolds for in vitro studies of bone formation | [67] |
| Biomaterials for BTE | Cutting-Edge Techniques in BTE | Emerging Molecules in BTE | Oncological Patient | Reference |
|---|---|---|---|---|
| Natural Biomaterials | ||||
| Collagen | Three-dimensional bioprinting | Bone Morphogenetic Proteins (BMPs) | Patients with bone defects post-tumor resection or bone metastasis (e.g., breast cancer) | [114] |
| Gelatin | Decellularized scaffolds | Vascular Endothelial Growth Factor (VEGF) | Patients with bone defects caused by metastatic cancers (e.g., lung, prostate) | [115] |
| Chitosan | Controlled drug Delivery Systems | Mesenchymal Stem Cells (MSCs) | Patients undergoing chemotherapy with compromised immune systems (e.g., lymphoma) | [116] |
| Alginate | decellularized scaffolds | Gene Therapy | Patients with metastatic bone disease needing bone regeneration (e.g., multiple myeloma) | [117] |
| Hydroxyapatite | Three-dimensional bioprinting | BMPs and VEGF combined | Patients with large bone defects from tumor excision (e.g., osteosarcoma) | [118] |
| Bacterial cellulose | Three-dimensional bioprinting | BMP-2 | Patients with bone defects post-tumor resection (e.g., osteosarcoma) | [119] |
| Hyaluronic acid (HA) | Hydrogel formation | HA | Post-operative patients requiring tissue repair (e.g., colorectal cancer) | [120] |
| Cerium oxide-containing beads | Antioxidant activity enhancement | Cerium Oxides (Ce3+/Ce4+) | Patients with bone defects and oxidative stress (e.g., breast cancer) | [121] |
| Synthetic Biomaterials | ||||
| Poly (lactic-co-glycolic acid) (PLGA) | Cell-aligned HDGs | Bone Morphogenetic Proteins (BMPs): BMP-2 and BMP-7 | Patients with bone defects post-tumor resection or bone metastasis (e.g., breast cancer) | [122] |
| Polycaprolactone (PCL) | Decellularized scaffolds | Vascular Endothelial Growth Factor (VEGF) | Patients with bone defects due to metastases (e.g., prostate, lung cancers) | [123] |
| Polyethylene glycol (PEG) | Controlled drug delivery systems | Mesenchymal Stem Cells (MSCs) | Patients undergoing chemotherapy with bone regeneration needs (e.g., lymphoma, leukemia) | [124] |
| Polylactic acid (PLA) | Three-dimensional bioprinting | Gene Therapy | Patients with bone loss from tumor resection or radiation (e.g., head and neck cancers) | [125] |
| Polymethyl methacrylate (PMMA) | Electrospinning | BMPs and VEGF combined | Patients with critical bone defects after excision of bone tumors (e.g., osteosarcoma) | [126] |
| Poly-D, L-lactic acid (PDLLA) | Bioactive coatings | Growth Factors (BMP-2, TGF-β) | Patients with post-surgical bone loss due to cancer resection (e.g., bone metastasis) | [127] |
| Polyurethane (PU) | In situ gelation | Calcium Phosphate Compounds | Patients with bone damage from radiation therapy or metastatic bone disease (e.g., multiple myeloma) | [128] |
| Nanomaterials and Smart Materials | ||||
| Nano-hydroxyapatite (nHA) | Electrospinning, nanocomposite formation, AI-based predictive models for scaffold design | BMP-2, VEGF, MSCs | Patients with bone-related disorders like tumors, metastases, and osteoporosis. | [108] |
| Graphene-based nanomaterials | Three-dimensional bioprinting, nanocoating, machine learning for material optimization | VEGF, BMP-2, TGF-β, IGF-1 | Patients undergoing chemotherapy with bone metastasis and osteosarcoma. | [109] |
| Carbon nanotubes (CNTs) | Functionalization, composite formation, AI and ML algorithms for optimizing CNT loading in scaffolds | BMP-2, VEGF, FGF | Patients with bone defects due to cancer treatment (e.g., osteosarcoma, multiple myeloma) | [110] |
| Silver nanoparticles (AgNPs) | Nanofabrication, controlled drug release | BMP-2, VEGF, Anticancer Drugs | Patients with bone infections or metastasis (e.g., lung cancer with bone metastases) | [14] |
| Mesoporous silica nanoparticles (MSNs) | drug delivery systems, surface modification, CRISPR-based gene editing. | BMP-2, TGF-β, IGF-1 | Patients with bone regeneration issues after cancer surgery or metastasis (e.g., colorectal cancer) | [14] |
| Thermoresponsive hydrogels | Thermoresponsive drug delivery, injectable hydrogels | BMP-2, VEGF, MSCs | Patients with bone fractures after tumor excision or chemotherapy (e.g., lymphoma, leukemia) | [111] |
| Magnetic nanoparticles (MNPs) | Magnetic targeting, magnetically induced hyperthermia, AI-assisted magnetic field optimization | VEGF, BMP-2, Growth Factors | Patients with bone defects post-cancer treatment or bone metastases. | [112] |
| Polymeric nanogels | Nanogel formation, drug delivery systems, CRISPR/Cas9-based gene editing to modify stem cell behavior for bone regeneration | BMP-2, VEGF, TGF-β, FGF | Patients with critical bone defects after tumor surgery or metastasis (e.g., ovarian cancer) | [113] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khobragade, S.S.; Deshmukh, M.; Vyas, U.; Ingle, R.G. Innovative Approaches in Bone Tissue Engineering: Strategies for Cancer Treatment and Recovery. Int. J. Mol. Sci. 2025, 26, 3937. https://doi.org/10.3390/ijms26093937
Khobragade SS, Deshmukh M, Vyas U, Ingle RG. Innovative Approaches in Bone Tissue Engineering: Strategies for Cancer Treatment and Recovery. International Journal of Molecular Sciences. 2025; 26(9):3937. https://doi.org/10.3390/ijms26093937
Chicago/Turabian StyleKhobragade, Samiksha S., Manish Deshmukh, Ujwal Vyas, and Rahul G. Ingle. 2025. "Innovative Approaches in Bone Tissue Engineering: Strategies for Cancer Treatment and Recovery" International Journal of Molecular Sciences 26, no. 9: 3937. https://doi.org/10.3390/ijms26093937
APA StyleKhobragade, S. S., Deshmukh, M., Vyas, U., & Ingle, R. G. (2025). Innovative Approaches in Bone Tissue Engineering: Strategies for Cancer Treatment and Recovery. International Journal of Molecular Sciences, 26(9), 3937. https://doi.org/10.3390/ijms26093937





