Essential Oil Fumigation Modulates Nutrient Content in Selected Mushrooms During Postharvest Storage
Abstract
:1. Introduction
2. Results and Discussion
2.1. Effects of Essential Oil Fumigation on Free Sugar and Free Sugar Alcohol Content
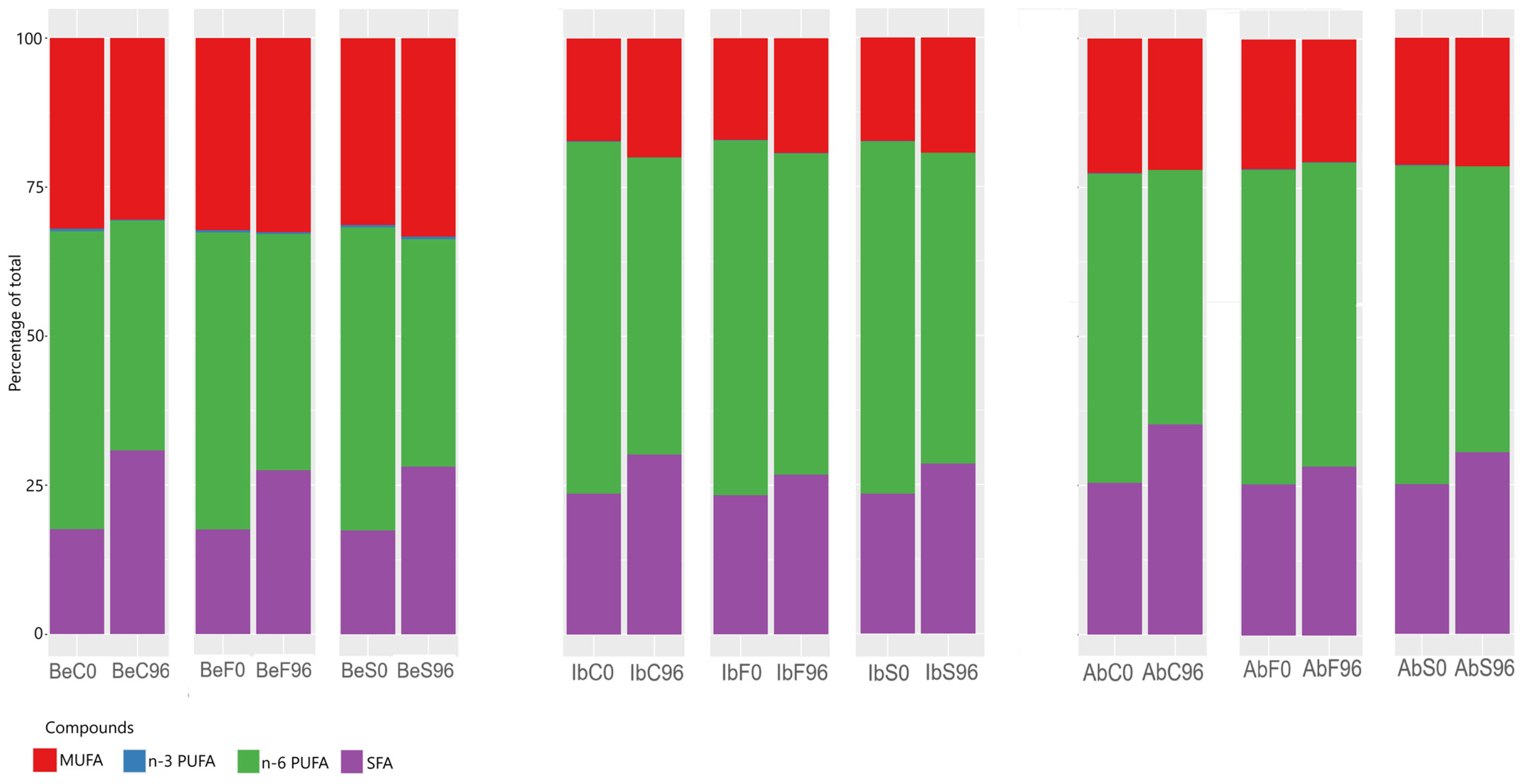
2.2. Effects of Essential Oil Fumigation on Total and Free Fatty Acid Composition
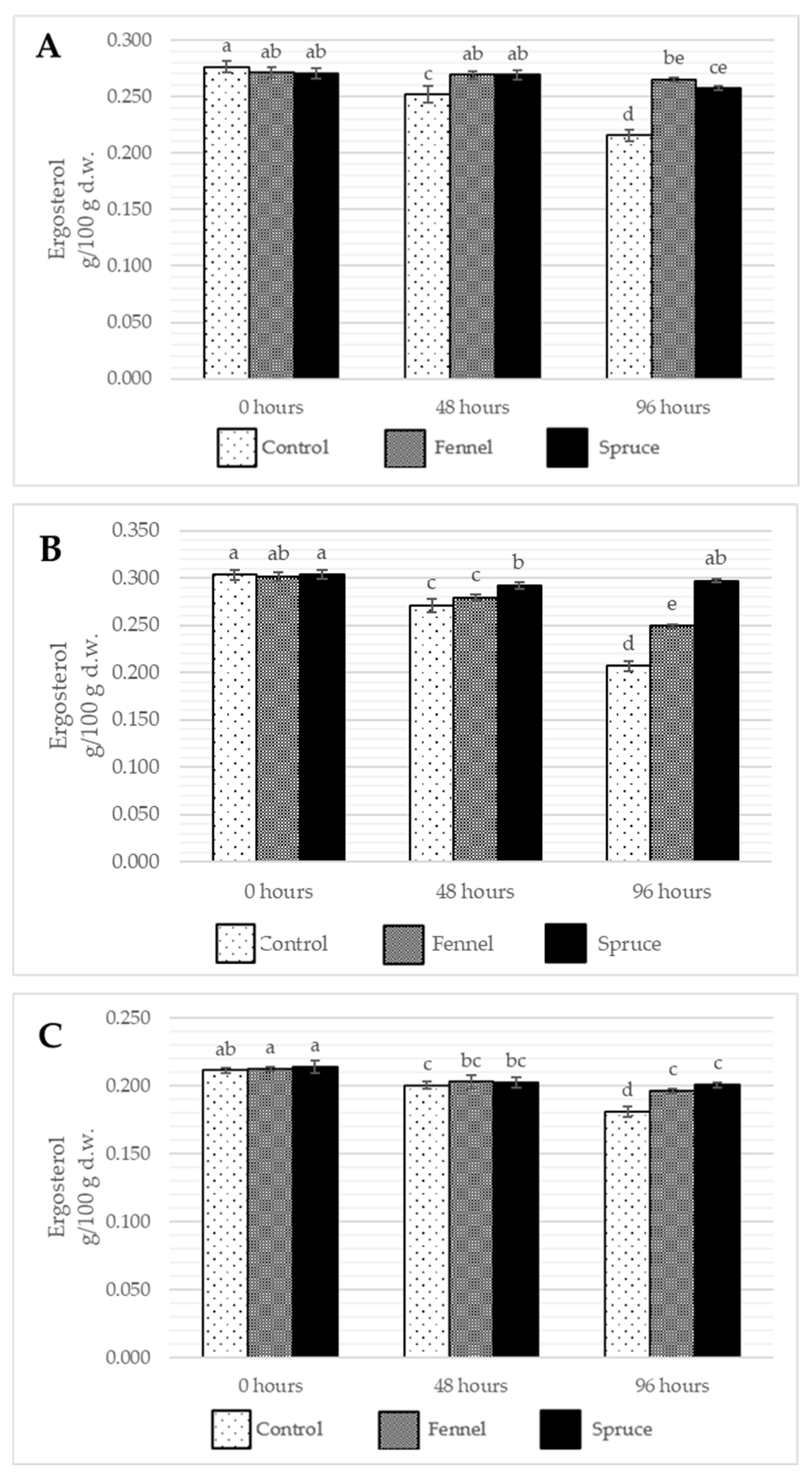
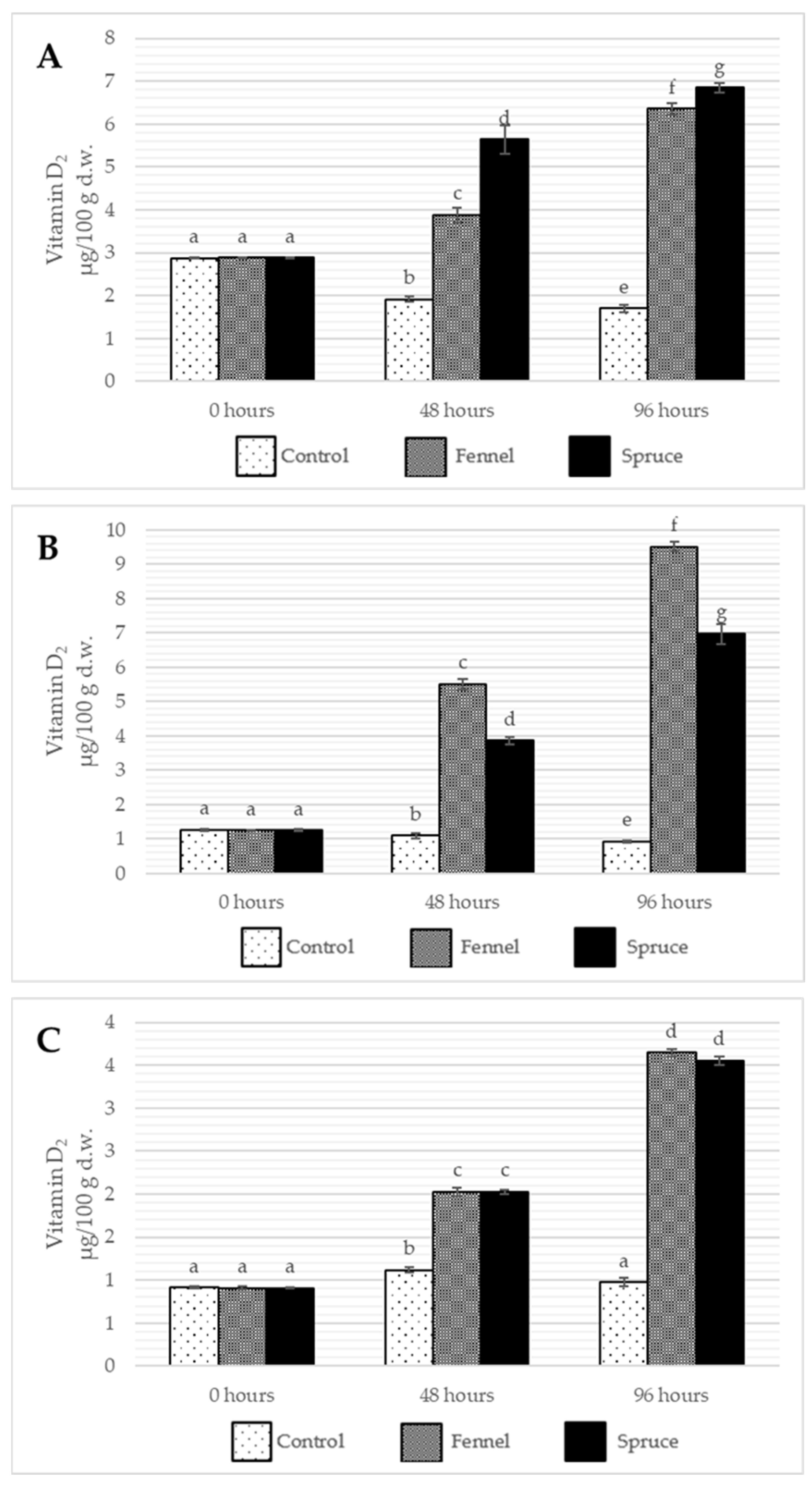
2.3. Effects of Essential Oil Fumigation on Tocopherol, Ergosterol, and Vitamin D2 Content
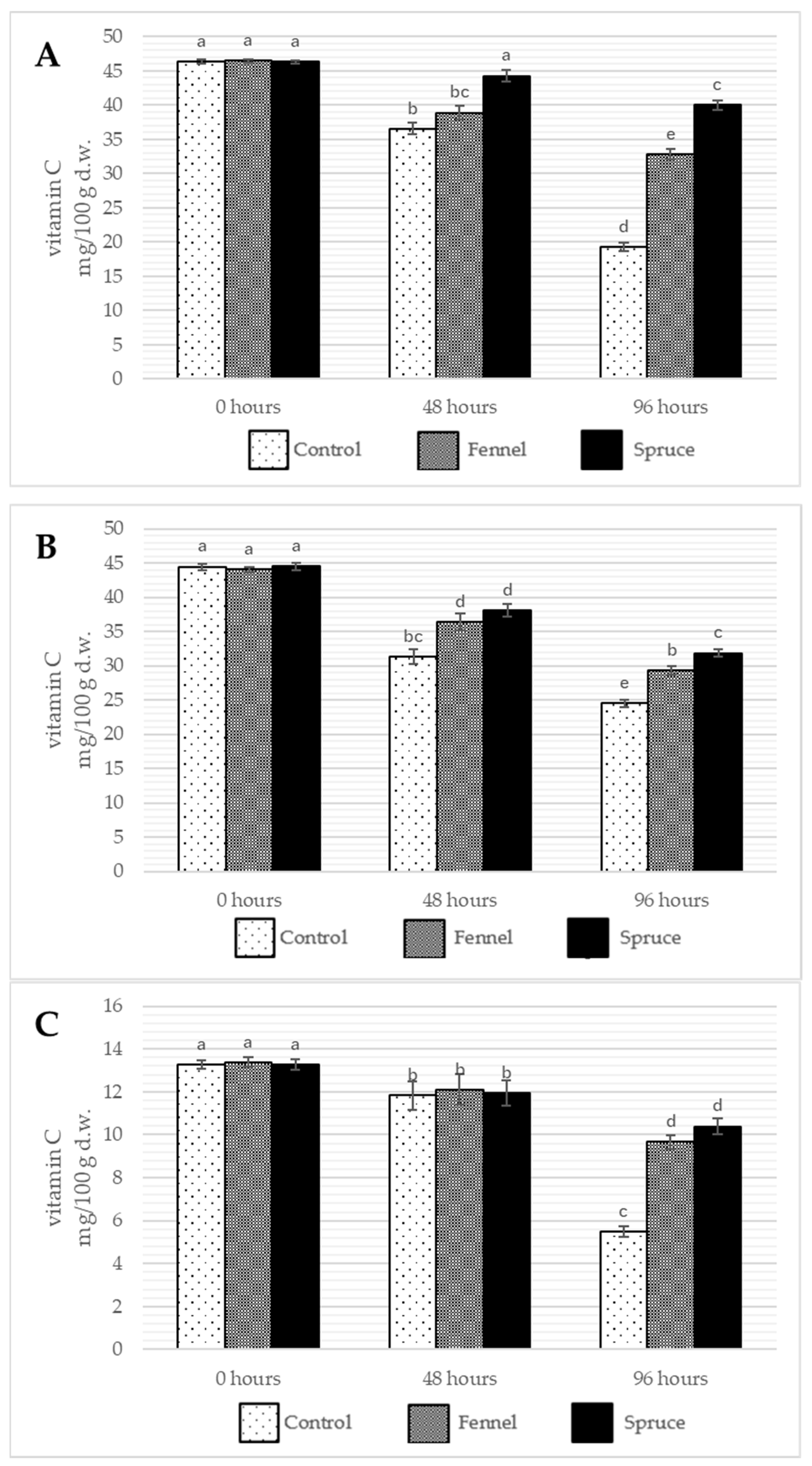
2.4. Effects of Essential Oil Fumigation on Water-Soluble Vitamins
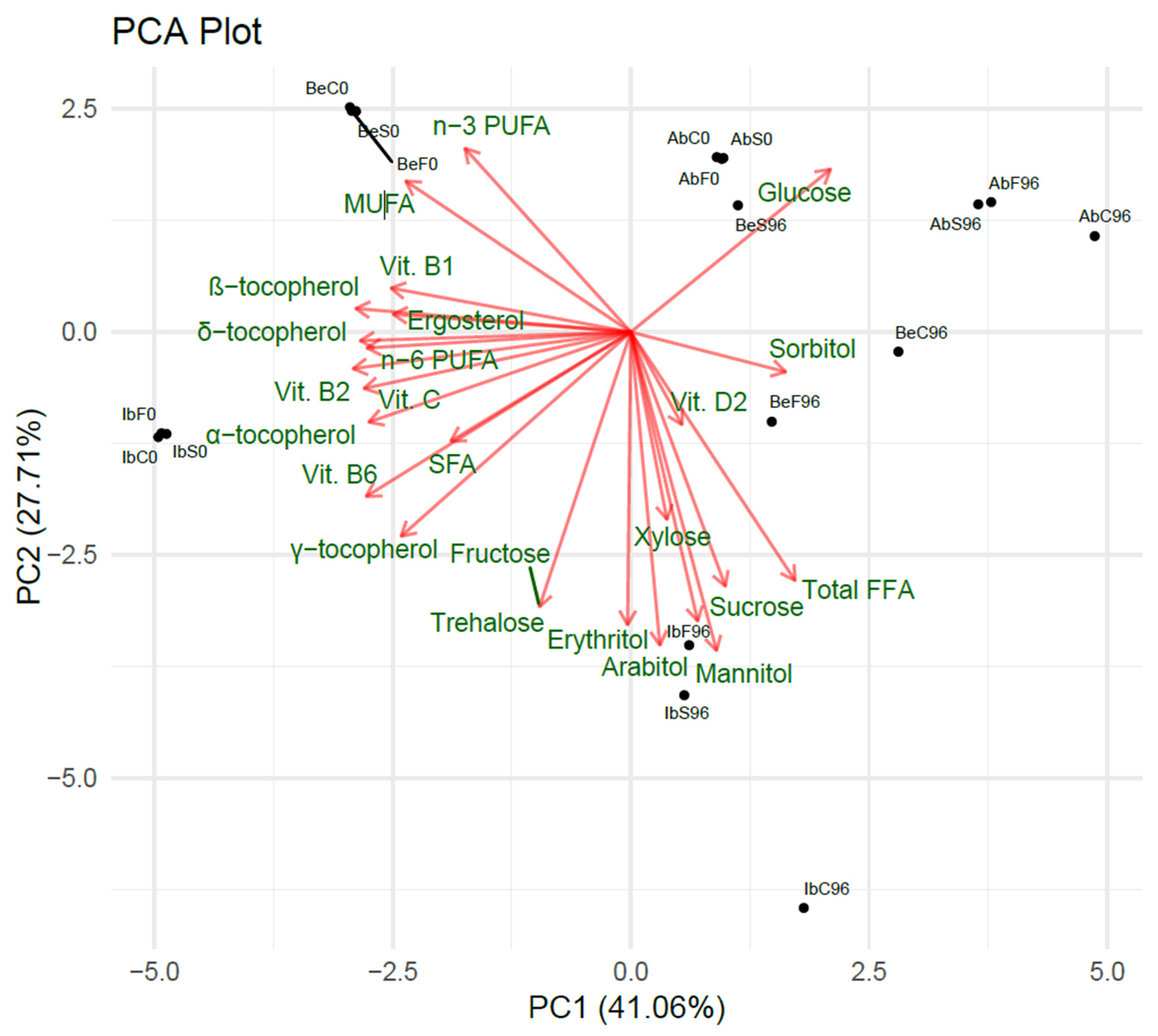
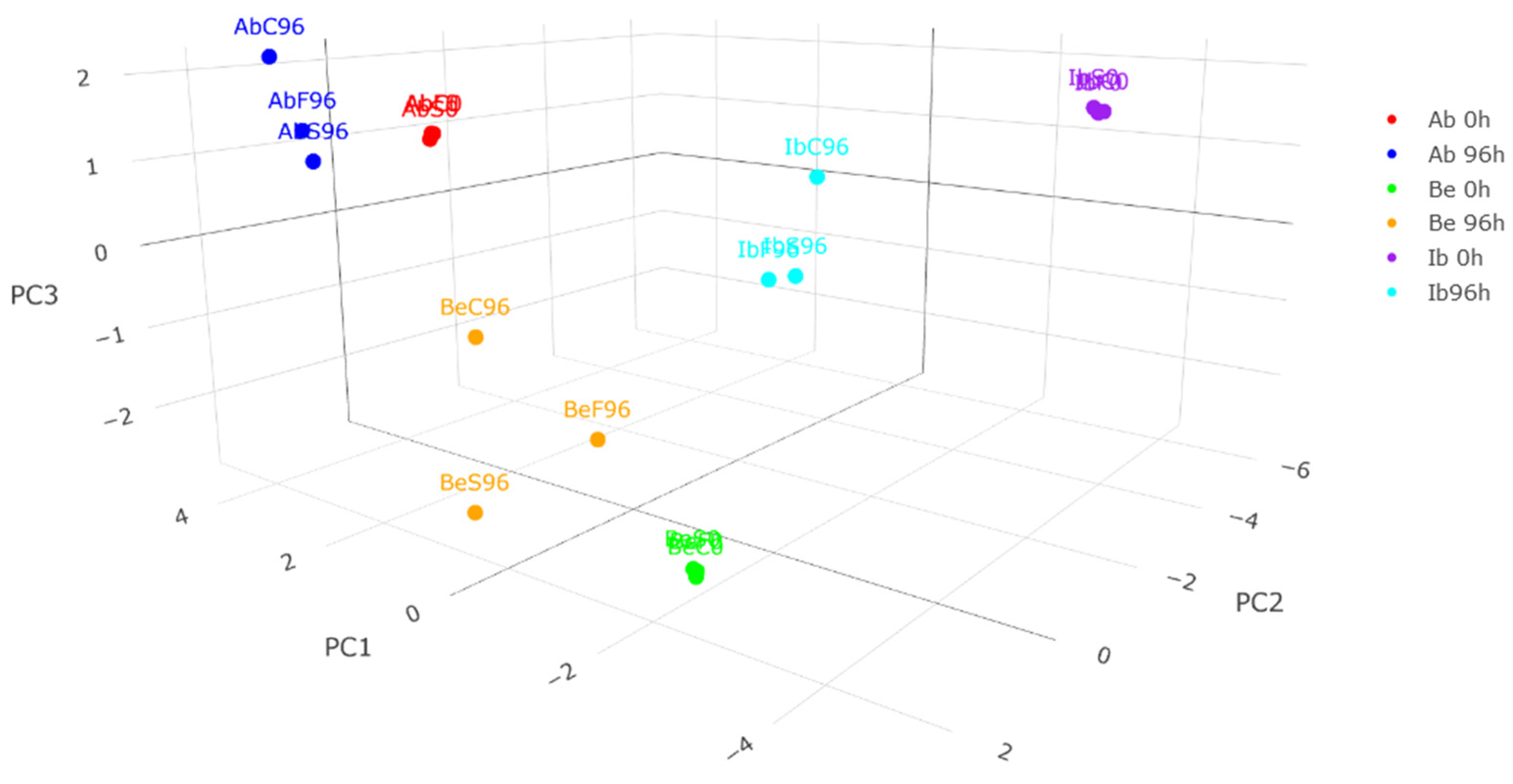
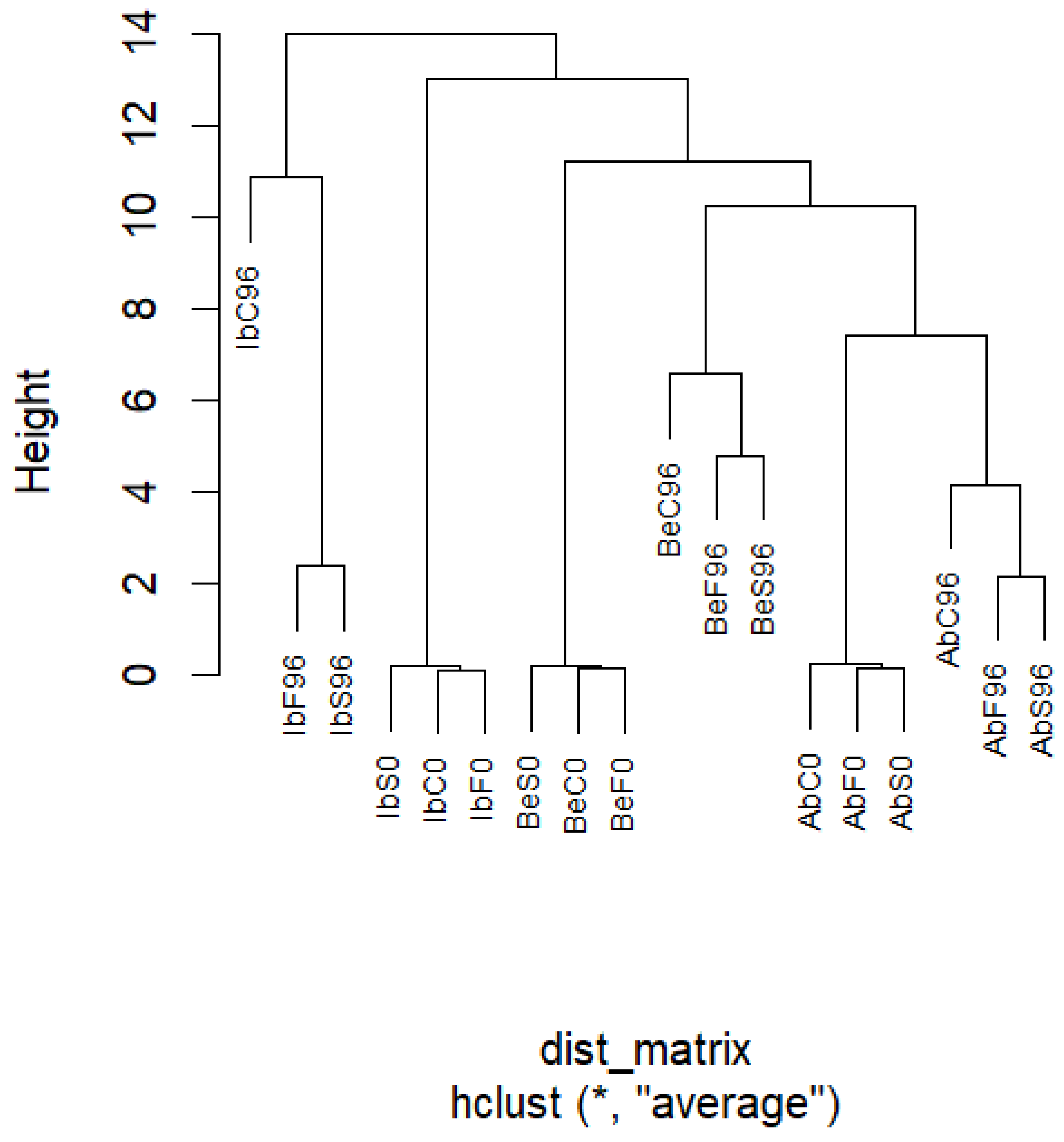
2.5. Principal Component Analysis (PCA) of Samples’ Metabolic Profiles
2.5.1. Two-Dimensional Feature Analysis
- Vitamin B2, vitamin B6, β- and δ-tocopherol, and vitamin C had the most substantial impact on PC1;
- Mannitol, arabitol, trehalose, erythritol, fructose, sucrose, and total FFA had the most substantial impact on PC2.
2.5.2. Three-Dimensional Sample Distribution Analysis
2.5.3. Hierarchical Clustering Analysis
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Samples and Fumigation Process
3.3. Free Sugar and Free Sugar Alcohol Content
3.4. Free Fatty Acid and Total Fatty Acid Composition
3.5. Content of α-, β-, γ-, and δ-Tocopherols
3.6. Ergocalciferol (Vitamin D2) and Ergosterol Content
3.7. Content of Ascorbic Acid (Vitamin C)
3.8. Content of Vitamins B1, B2, and B6
3.9. Statistical Analysis
3.10. Visualisation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tan, Y.; Zeng, N.K.; Xu, B. Chemical Profiles and Health-Promoting Effects of Porcini Mushroom (Boletus Edulis): A Narrative Review. Food Chem. 2022, 390, 133199. [Google Scholar] [CrossRef]
- Phillips, J.M.; Ooi, S.L.; Pak, S.C. Health-Promoting Properties of Medicinal Mushrooms and Their Bioactive Compounds for the COVID-19 Era—An Appraisal: Do the Pro-Health Claims Measure Up? Molecules 2022, 27, 2302. [Google Scholar] [CrossRef]
- Mahajan, P.V.; Oliveira, F.A.R.; Macedo, I. Effect of Temperature and Humidity on the Transpiration Rate of the Whole Mushrooms. J. Food Eng. 2008, 84, 281–288. [Google Scholar] [CrossRef]
- Perumal, A.B.; Sellamuthu, P.S.; Nambiar, R.B.; Sadiku, E.R. Effects of Essential Oil Vapour Treatment on the Postharvest Disease Control and Different Defence Responses in Two Mango (Mangifera indica L.) Cultivars. Food Bioproc Technol. 2017, 10, 1131–1141. [Google Scholar] [CrossRef]
- Wang, C.Y.; Wang, S.Y.; Yin, J.J.; Parry, J.; Yu, L.L. Enhancing Antioxidant, Antiproliferation, and Free Radical Scavenging Activities in Strawberries with Essential Oils. J. Agric. Food Chem. 2007, 55, 6527–6532. [Google Scholar] [CrossRef]
- Spisni, E.; Petrocelli, G.; Imbesi, V.; Spigarelli, R.; Azzinnari, D.; Sarti, M.D.; Campieri, M.; Valerii, M.C. Antioxidant, Anti-Inflammatory, and Microbial-Modulating Activities of Essential Oils: Implications in Colonic Pathophysiology. Int. J. Mol. Sci. 2020, 21, 4152. [Google Scholar] [CrossRef] [PubMed]
- Shahat, A.A.; Ibrahim, A.Y.; Hendawy, S.F.; Omer, E.A.; Hammouda, F.M.; Abdel-Rahman, F.H.; Saleh, M.A. Chemical Composition, Antimicrobial and Antioxidant Activities of Essential Oils from Organically Cultivated Fennel Cultivars. Molecules 2011, 16, 1366–1377. [Google Scholar] [CrossRef] [PubMed]
- Schoss, K.; Kočevar Glavač, N.; Kreft, S. Volatile Compounds in Norway Spruce (Picea abies) Significantly Vary with Season. Plants 2023, 12, 188. [Google Scholar] [CrossRef]
- Gao, M.; Feng, L.; Jiang, T. Browning Inhibition and Quality Preservation of Button Mushroom (Agaricus bisporus) by Essential Oils Fumigation Treatment. Food Chem. 2014, 149, 107–113. [Google Scholar] [CrossRef]
- Aly, A.A.; Mohammed, M.K.; Maraei, R.W.; Abdalla, A.E.; Abouel-Yazeed, A.M. Improving the Nutritional Quality and Bio-Ingredients of Stored White Mushrooms Using Gamma Irradiation and Essential Oils Fumigation. Radiochim. Acta 2023, 111, 387–399. [Google Scholar] [CrossRef]
- Jiang, T.; Luo, Z.; Ying, T. Fumigation with Essential Oils Improves Sensory Quality and Enhanced Antioxidant Ability of Shiitake Mushroom (Lentinus edodes). Food Chem. 2015, 172, 692–698. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, Y.; Wang, K.; Cheng, M.; Zhao, P.; Lu, J.; Xi, X.; Wang, X.; Han, X.; Wang, J. Evaluation of the Postharvest Quality of Agaricus Bisporus Packed Using PVA/SG-Based Active Packaging Film Containing Tea Tree Essential Oil. J. Food Meas. Charact. 2024, 18, 4820–4831. [Google Scholar] [CrossRef]
- Nasiri, M.; Barzegar, M.; Sahari, M.A.; Niakousari, M. Application of Tragacanth Gum Impregnated with Satureja Khuzistanica Essential Oil as a Natural Coating for Enhancement of Postharvest Quality and Shelf Life of Button Mushroom (Agaricus bisporus). Int. J. Biol. Macromol. 2018, 106, 218–226. [Google Scholar] [CrossRef]
- Karimirad, R.; Behnamian, M.; Dezhsetan, S.; Sonnenberg, A. Chitosan Nanoparticles-Loaded Citrus Aurantium Essential Oil: A Novel Delivery System for Preserving the Postharvest Quality of Agaricus Bisporus. J. Sci. Food Agric. 2018, 98, 5112–5119. [Google Scholar] [CrossRef] [PubMed]
- Garg, N.; Singh, V.K. Carbohydrate Metabolism During Fruit Spoilage. In Biotechnology in Horticulture: Methods and Applications; NIPA: New Delhi, India, 2013. [Google Scholar]
- Yu, L.; Liu, H.; Shao, X.; Yu, F.; Wei, Y.; Ni, Z.; Xu, F.; Wang, H. Effects of Hot Air and Methyl Jasmonate Treatment on the Metabolism of Soluble Sugars in Peach Fruit during Cold Storage. Postharvest Biol. Technol. 2016, 113, 8–16. [Google Scholar] [CrossRef]
- Fernandez, O.; Béthencourt, L.; Quero, A.; Sangwan, R.S.; Clément Christophe, C. Trehalose and Plant Stress Responses: Friend or Foe? Trends Plant Sci. 2010, 15, 409–417. [Google Scholar] [CrossRef]
- Shen, B.; Hohmann, S.; Jensen, R.G.; Bohnert, H.J. Roles of Sugar Alcohols in Osmotic Stress Adaptation. Replacement of Glycerol by Mannitol and Sorbitol in Yeast 1. Plant Physiol. 1999, 121, 45–52. [Google Scholar] [CrossRef]
- Chen, Y.-Z.; Zhang, B.W.; Yang, J.; Zou, C.S.; Li, T.; Zhang, G.C.; Chen, G.S. Detoxification, Antioxidant, and Digestive Enzyme Activities and Gene Expression Analysis of Lymantria dispar Larvae under Carvacrol. J. Asia Pac. Entomol. 2021, 24, 208–216. [Google Scholar] [CrossRef]
- Jiang, L.L.; Wang, J.B.; Wang, W.H.; Lei, B.; Feng, J.T.; Wu, H.; Ma, Z.Q. Effects of Three Essential Oil Fumigation Treatments on the Postharvest Control of Botrytis Cinerea and Their Efficacy as Preservatives of Cherry Tomatoes. Plant Dis. 2023, 107, 1874–1882. [Google Scholar] [CrossRef]
- Gałgowska, M.; Pietrzak-Fiećko, R. Evaluation of the Nutritional and Health Values of Selected Polish Mushrooms Considering Fatty Acid Profiles and Lipid Indices. Molecules 2022, 27, 6193. [Google Scholar] [CrossRef]
- Kapoor, B.; Kapoor, D.; Gautam, S.; Singh, R.; Bhardwaj, S. Dietary Polyunsaturated Fatty Acids (PUFAs): Uses and Potential Health Benefits. Curr. Nutr. Rep. 2021, 10, 232–242. [Google Scholar] [CrossRef]
- Maki, K.C.; Dicklin, M.R.; Kirkpatrick, C.F. Saturated Fats and Cardiovascular Health: Current Evidence and Controversies. J. Clin. Lipidol. 2021, 15, 765–772. [Google Scholar] [CrossRef]
- Legrand, P.; Rioux, V. The Complex and Important Cellular and Metabolic Functions of Saturated Fatty Acids. Lipids 2010, 45, 941–946. [Google Scholar] [CrossRef]
- Chaula, D.; Jacobsen, C.; Laswai, H.S.; Chove, B.E.; Dalsgaard, A.; Mdegela, R.; Hyldig, G. Changes in Fatty Acids during Storage of Artisanal-Processed Freshwater Sardines (Rastrineobola argentea). Food Sci. Nutr. 2023, 11, 3040–3047. [Google Scholar] [CrossRef]
- Baysal, T.; Demirdöven, A. Lipoxygenase in Fruits and Vegetables: A Review. Enzym. Microb. Technol. 2007, 40, 491–496. [Google Scholar] [CrossRef]
- Farhoosh, R.; Einafshar, S.; Sharayei, P. The Effect of Commercial Refining Steps on the Rancidity Measures of Soybean and Canola Oils. Food Chem. 2009, 115, 933–938. [Google Scholar] [CrossRef]
- Emebu, S.; Osaikhuiwuomwan, O.; Mankonen, A.; Udoye, C.; Okieimen, C.; Janáčová, D. Influence of Moisture Content, Temperature, and Time on Free Fatty Acid in Stored Crude Palm Oil. Sci. Rep. 2022, 12, 9846. [Google Scholar] [CrossRef]
- Marçal, S.; Sousa, A.S.; Taofiq, O.; Antunes, F.; Morais, A.M.M.B.; Freitas, A.C.; Barros, L.; Ferreira, I.C.F.R.; Pintado, M. Impact of Postharvest Preservation Methods on Nutritional Value and Bioactive Properties of Mushrooms. Trends Food Sci. Technol. 2021, 110, 418–431. [Google Scholar] [CrossRef]
- Cardoso, R.V.C.; Fernandes, Â.; Barreira, J.C.M.; Verde, S.C.; Antonio, A.L.; Gonzaléz-Paramás, A.M.; Barros, L.; Ferreira, I.C.F.R. Effectiveness of Gamma and Electron Beam Irradiation as Preserving Technologies of Fresh Agaricus Bisporus Portobello: A Comparative Study. Food Chem. 2019, 278, 760–766. [Google Scholar] [CrossRef]
- Heleno, S.A.; Ferreira, R.C.; Antonio, A.L.; Queiroz, M.J.R.P.; Barros, L.; Ferreira, I.C.F.R. Nutritional Value, Bioactive Compounds and Antioxidant Properties of Three Edible Mushrooms from Poland. Food Biosci. 2015, 11, 48–55. [Google Scholar] [CrossRef]
- Wei, A.; Shibamoto, T. Antioxidant/Lipoxygenase Inhibitory Activities and Chemical Compositions of Selected Essential Oils. J. Agric. Food Chem. 2010, 58, 7218–7225. [Google Scholar] [CrossRef]
- Mäkinen, M. Lipid Hydroperoxides: Effects of Tocopherols and Ascorbic Acid on Their Formation and Decomposition. Ph.D. Thesis, University of Helsinki, Helsinki, Finland, 2002. [Google Scholar]
- Ju, J.; Picinich, S.C.; Yang, Z.; Zhao, Y.; Suh, N.; Kong, A.N.; Yang, C.S. Cancer-Preventive Activities of Tocopherols and Tocotrienols. Carcinogenesis 2009, 31, 533–542. [Google Scholar] [CrossRef]
- Lisa, S.A.; Obaid, M.; Khan, S.; Chowdhury, K. Tocopherol Content of Vegetable Oils/Fats and Their Oxidative Deterioration During Storage AND. World J. Pharm. Pharm. Sci. 2015, 4, 1537–1548. [Google Scholar]
- Šunić, L.; Ilić, Z.S.; Stanojević, L.; Milenković, L.; Stanojević, J.; Kovač, R.; Milenković, A.; Cvetković, D. Comparison of the Essential Oil Content, Constituents and Antioxidant Activity from Different Plant Parts during Development Stages of Wild Fennel (Foeniculum vulgare Mill.). Horticulturae 2023, 9, 364. [Google Scholar] [CrossRef]
- Stevanovic, F. &; Francezon, N.; Stevanovic, T. Chemical Composition of Essential Oil and Hydrosol from Picea Mariana Bark Residue. Bioresources 2017, 12, 2635–2645. [Google Scholar]
- Radulescu, V.; Chifiriuc, M.; Oprea, E. Chemical Composition and Antimicrobial Activity of Essential Oil from Shoots Spruce (Picea abies L). Rev. Chim 2011, 62, 69–74. [Google Scholar]
- Munné-Bosch, S.; Alegre, L. The Function of Tocopherols and Tocotrienols in Plants. CRC Crit. Rev. Plant Sci. 2002, 21, 31–57. [Google Scholar] [CrossRef]
- Rangsinth, P.; Sharika, R.; Pattarachotanant, N.; Duangjan, C.; Wongwan, C.; Sillapachaiyaporn, C.; Nilkhet, S.; Wongsirojkul, N.; Prasansuklab, A.; Tencomnao, T.; et al. Potential Beneficial Effects and Pharmacological Properties of Ergosterol, a Common Bioactive Compound in Edible Mushrooms. Foods 2023, 12, 2529. [Google Scholar] [CrossRef]
- Jiang, Q.; Zhang, M.; Mujumdar, A.S. UV Induced Conversion during Drying of Ergosterol to Vitamin D in Various Mushrooms: Effect of Different Drying Conditions. Trends Food Sci. Technol. 2020, 105, 200–210. [Google Scholar] [CrossRef]
- Laird, E.; Ward, M.; McSorley, E.; Strain, J.J.; Wallace, J. Vitamin D and Bone Health; Potential Mechanisms. Nutrients 2010, 2, 693–724. [Google Scholar] [CrossRef]
- Wu, Z.; Liu, D.; Deng, F. The Role of Vitamin D in Immune System and Inflammatory Bowel Disease. J. Inflamm. Res. 2022, 15, 3167–3185. [Google Scholar] [CrossRef] [PubMed]
- Yap, P.S.X.; Yusoff, K.; Lim, S.H.E.; Chong, C.M.; Lai, K.S. Membrane Disruption Properties of Essential Oils-a Double-Edged Sword? Processes 2021, 9, 595. [Google Scholar] [CrossRef]
- Ferreira, V.R.F.; Militani, I.A.; de Almeida, K.J.; Lunguinho, A.d.S.; Saczk, A.A.; Ionta, M.; da Silva, G.Á.F.; Felix, F.S.; Nelson, D.L.; Cardoso, M. das G. Antioxidant and Cytotoxic Activity of Essential Oils and Their Principal Components: Spectrophotometric, Voltammetric, and Theoretical Investigation of the Chelating Effect of Eugenol and Carvacrol. ACS Food Sci. Technol. 2023, 3, 350–360. [Google Scholar] [CrossRef]
- Guo, Y.; Chen, X.; Gong, P.; Wang, R.; Han, A.; Deng, Z.; Qi, Z.; Long, H.; Wang, J.; Yao, W.; et al. Advances in the Role and Mechanisms of Essential Oils and Plant Extracts as Natural Preservatives to Extend the Postharvest Shelf Life of Edible Mushrooms. Foods 2023, 12, 801. [Google Scholar] [CrossRef]
- Said, H.M. Intestinal Absorption of Water-Soluble Vitamins in Health and Disease. Biochem. J. 2011, 437, 357–372. [Google Scholar] [CrossRef]
- Giannakourou, M.C.; Taoukis, P.S. Effect of Alternative Preservation Steps and Storage on Vitamin c Stability in Fruit and Vegetable Products: Critical Review and Kinetic Modelling Approaches. Foods 2021, 10, 2630. [Google Scholar] [CrossRef]
- Bernaś, E.; Jaworska, G. Vitamins Profile as an Indicator of the Quality of Frozen Agaricus Bisporus Mushrooms. J. Food Compos. Anal. 2016, 49, 1–8. [Google Scholar] [CrossRef]
- Geransayeh, M.; Sepahvand, S.; Abdossi, V.; Nezhad, R.A. Effect of Thymol Treatment on Decay, Postharvest Life and Quality of Strawberry (Fragaria ananassa) Fruit Cv. “Gaviota”. Int. J. Agron. Agric. Res. 2015, 6, 151–162. [Google Scholar]
- Santoro, K.; Maghenzani, M.; Chiabrando, V.; Bosio, P.; Gullino, M.L.; Spadaro, D.; Giacalone, G. Thyme and Savory Essential Oil Vapor Treatments Control Brown Rot and Improve the Storage Quality of Peaches and Nectarines, but Could Favor Gray Mold. Foods 2018, 7, 7. [Google Scholar] [CrossRef]
- Namiota, M.; Bonikowski, R. The Current State of Knowledge about Essential Oil Fumigation for Quality of Crops during Postharvest. Int. J. Mol. Sci. 2021, 22, 13351. [Google Scholar] [CrossRef]
- Freire, R.S.; Morais, S.M.; Catunda, F.E.A.; Pinheiro, D.C.S.N. Synthesis and Antioxidant, Anti-Inflammatory and Gastroprotector Activities of Anethole and Related Compounds. Bioorg Med. Chem. 2005, 13, 4353–4358. [Google Scholar] [CrossRef]
- Ahmed Salim, E.R. Salim-Eisa Method for Modification of Evaporation Test (British Pharmacopeia) by Sudanese Essential Oils. J. Appl. Biotechnol. Bioeng. 2017, 3, 2. [Google Scholar] [CrossRef]
- Aninowski, M.; Leszczyńska, J.; Bonikowski, R.; Ponder, A.; Ewelina, H.; Małgorzata, G. Kamil Szymczak Suitability of Selected Apple Varieties for People with Allergies and Diabetes. Nutrients 2024, 16, 13. [Google Scholar] [CrossRef]
- Piatek, P.; Lewkowicz, N.; Michlewska, S.; Wieczorek, M.; Bonikowski, R.; Parchem, K.; Lewkowicz, P.; Namiecinska, M. Natural Fish Oil Improves the Differentiation and Maturation of Oligodendrocyte Precursor Cells to Oligodendrocytes in Vitro after Interaction with the Blood–Brain Barrier. Front. Immunol. 2022, 13, 932383. [Google Scholar] [CrossRef]
- Cyran, M.R.; Dynkowska, W.M.; Ceglińska, A.; Bonikowski, R. Improving Rye Bread Antioxidant Capacity by Bread-Making Methodology: Contribution of Phosphate-Buffered Saline- and Methanol-Soluble Phenolic Phytochemicals with Different Molecular Profiles. J. Cereal Sci. 2021, 100, 103262. [Google Scholar] [CrossRef]
- Sasaki, K.; Hatate, H.; Tanaka, R. Determination of 13 Vitamin B and the Related Compounds Using HPLC with UV Detection and Application to Food Supplements. Chromatographia 2020, 83, 839–851. [Google Scholar] [CrossRef]
| Sugar | Boletus edulis | Imleria badia | Agaricus bisporus | ||||
|---|---|---|---|---|---|---|---|
| 0 h | 96 h | 0 h | 96 h | 0 h | 96 h | ||
| Glucose | Control | 52.6 ± 3.98 a | 123.07 ± 22.92 c | 10.49 ± 0.84 ab | 16.92 ± 1.41 c | 37.71 ± 1.56 a | 134.21 ± 6.99 b |
| Fennel | 49.16 ± 2.39 a | 68.58 ± 5.03 b | 11.09 ± 0.99 ab | 12.83 ± 1.46 b | 36.89 ± 1.11 a | 119.68 ± 5.73 bc | |
| Spruce | 57.02 ± 5.13 ab | 135.57 ± 8.06 c | 9.96 ± 1.40 a | 8.89 ± 0.80 a | 38.21 ± 1.60 a | 108.86 ± 5.31 c | |
| Fructose | Control | 0.16 ± 0.03 a | 4.02 ± 0.35 b | 7.57 ± 0.86 a | 10.56 ± 0.77 b | 0.10 ± 0.01 a | 1.10 ± 0.13 b |
| Fennel | 0.16 ± 0.01 a | 7.29 ± 0.34 c | 7.25 ± 0.49 a | 2.38 ± 0.29 c | 0.09 ± 0.01 a | 0.07 ± 0.02 a | |
| Spruce | 0.17 ± 0.01 a | 2.58 ± 0.19 d | 7.31 ± 0.43 a | 4.45 ± 0.39 d | 0.10 ± 0.01 a | 0.09 ± 0.01 a | |
| Xylose | Control | 15.21 ± 1.05 a | 49.46 ± 3.68 b | 10.58 ± 0.67 a | 47.88 ± 2.59 b | 0.33 ± 0.04 a | 3.43 ± 0.75 bc |
| Fennel | 16.25 ± 0.92 a | 54.35 ± 2.97 b | 10.61 ± 0.77 a | 11.67 ± 1.37 a | 0.33 ± 0.06 a | 4.01 ± 0.20 b | |
| Spruce | 15.63 ± 1.10 a | 27.31 ± 2.61 c | 10.62 ± 0.67 a | 34.68 ± 3.08 c | 0.34 ± 0.05 a | 2.80 ± 0.15 c | |
| Trehalose | Control | 106.14 ± 5.92 ab | 721.57 ± 11.86 c | 557.85 ± 15.47 a | 813.14 ± 25.41 b | 177.06 ± 3.27 a | 287.04 ± 20.58 b |
| Fennel | 114.08 ± 5.54 ab | 650.52 ± 18.62 c | 555.04 ± 23.37 a | 756.32 ± 28.02 b | 174.77 ± 5.65 a | 216.43 ± 8.69 c | |
| Spruce | 117.31 ± 7.57 a | 102.54 ± 6.12 b | 556.67 ± 19.16 a | 429.24 ± 25.05 c | 173.64 ± 4.26 a | 290.37 ± 11.71 b | |
| Sucrose | Control | nd | 19.92 ± 3.24 a | 0.68 ± 0.06 a | 51.03 ± 3.69 b | nd | 1.38 ± 0.15 a |
| Fennel | nd | 32.49 ± 3.61 b | 0.67 ± 0.07 a | 15.37 ± 2.44 c | nd | 0.82 ± 0.07 b | |
| Spruce | nd | 18.01 ± 1.49 a | 0.69 ± 0.07 a | 17.07 ± 2.51 c | nd | 0.88 ± 0.13 b | |
| Total sugars | Control | 174.11 ± 7.95 a | 918.05 ± 19.78 c | 587.16 ± 17.67 a | 939.51 ± 29.38 b | 215.20 ± 2.67 a | 425.79 ± 27.53 b |
| Fennel | 179.65 ± 6.84 ab | 813.24 ± 23.64 d | 584.66 ± 21.84 a | 798.57 ± 32.46 c | 212.09 ± 6.45 a | 340.27 ± 11.77 c | |
| Spruce | 190.13 ± 7.10 b | 286.01 ± 1.05 e | 585.25 ± 19.69 a | 494.34 ± 19.99 d | 212.29 ± 5.74 a | 402.11 ± 10.67 b | |
| Sugar Alcohol | Boletus edulis | Imleria badia | Agaricus bisporus | ||||
|---|---|---|---|---|---|---|---|
| 0 h | 96 h | 0 h | 96 h | 0 h | 96 h | ||
| Sorbitol | Control | 6.06 ± 0.08 a | 15.49 ± 1.85 b | nd | nd | 15.48 ± 0.52 a | 98.72 ± 8.66 b |
| Fennel | 6.02 ± 0.05 a | 41.94 ± 4.54 c | nd | nd | 17.28 ± 0.53 a | 85.84 ± 3.65 b | |
| Spruce | 6.12 ± 0.06 a | 22.50 ± 3.02 d | nd | nd | 15.73 ± 0.71 a | 27.98 ± 7.64 c | |
| Mannitol | Control | 33.73 ± 1.57 a | 238.32 ± 7.99 b | 222.14 ± 6.96 a | 974.01 ± 65.50 b | 86.38 ± 3.50 a | 258.03 ± 6.28 b |
| Fennel | 33.45 ± 3.49 a | 295.38 ± 7.64 c | 222.37 ± 7.00 a | 745.50 ± 23.63 c | 85.68 ± 4.00 a | 322.25 ± 15.83 c | |
| Spruce | 34.19 ± 2.57 a | 221.30 ± 10.41 b | 224.10 ± 5.88 a | 613.60 ± 14.24 d | 85.55 ± 3.44 a | 225.43 ± 15.01 d | |
| Arabitol | Control | 2.41 ± 0.18 a | 26.77 ± 4.49 b | 20.76 ± 1.06 a | 147.97 ± 9.17 b | 1.86 ± 0.12 a | 4.5 ± 0.27 b |
| Fennel | 2.77 ± 0.03 a | 13.28 ± 2.00 c | 20.41 ± 0.86 a | 120.57 ± 10.06 c | 1.80 ± 0.16 a | 3.23 ± 0.38 c | |
| Spruce | 2.69 ± 0.08 a | 13.45 ± 1.92 c | 20.37 ± 0.61 a | 146.27 ± 7.04 b | 1.85 ± 0.17 a | 2.72 ± 0.19 c | |
| Trehalose | Control | nd | 3.84 ± 0.27 a | 0.39 ± 0.16 a | 145.96 ± 12.35 b | 3.16 ± 0.09 a | 13.74 ± 2.51 bc |
| Fennel | nd | 3.80 ± 0.27 a | 0.41 ± 0.19 a | 126.85 ± 8.45 b | 3.22 ± 0.08 a | 14.89 ± 1.74 b | |
| Spruce | nd | 3.02 ± 0.20 a | 0.43 ± 0.15 a | 141.64 ± 7.51 b | 3.24 ± 0.05 a | 10.99 ± 1.87 c | |
| Total sugar alcohols | Control | 42.20 ± 1.43 a | 284.42 ± 12.70 b | 243.29 ± 7.04 a | 1267.94 ± 86.83 b | 106.88 ± 3.94 a | 375.00 ± 12.05 b |
| Fennel | 42.24 ± 3.56 a | 354.39 ± 8.24 c | 243.19 ± 6.08 a | 992.92 ± 5.95 c | 107.98 ± 4.61 a | 426.21 ± 17.41 c | |
| Spruce | 43.00 ± 2.37 a | 260.27 ± 11.85 b | 244.90 ± 5.82 a | 901.50 ± 28.21 c | 106.37 ± 4.01 a | 267.13 ± 9.42 d | |
| Boletus edulis | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0 h | 96 h | |||||||||
| Control | Fennel | Spruce | % | Control | % | Fennel | % | Spruce | % | |
| Lauric Acid (C12:0) | 0.79 ± 0.08 a | 0.70 ± 0.08 a | 0.71 ± 0.10 a | 0.06 ± 0.01 | 0.25 ± 0.02 b | 0.05 ± 0.00 | 0.38 ± 0.03 c | 0.06 ± 0.01 | 0.17 ± 0.08 d | 0.03 ± 0.02 |
| Myristic Acid (C14:0) | 2.35 ± 0.07 a | 2.42 ± 0.12 a | 2.42 ± 0.17 a | 0.21 ± 0.02 | 2.11 ± 0.15 a | 0.43 ± 0.04 | 0.93 ± 0.16 b | 0.15 ± 0.03 | 0.99 ± 0.21 b | 0.16 ± 0.03 |
| Pentadecanoic Acid (C15:0) | 2.90 ± 0.28 a | 2.89 ± 0.41 a | 2.81 ± 0.46 a | 0.25 ± 0.04 | 1.98 ± 0.12 b | 0.40 ± 0.02 | 2.52 ± 0.12 ab | 0.41 ± 0.02 | 2.81 ± 0.83 a | 0.47 ± 0.03 |
| Palmitic Acid (C16:0) | 117.57 ± 6.70 a | 116.50 ± 5.03 a | 116.97 ± 7.67 a | 10.38 ± 0.67 | 99.74 ± 2.06 b | 20.28 ± 0.82 | 113.42 ± 2.85 a | 18.52 ± 0.66 | 109.14 ± 6.61 ab | 18.08 ± 0.11 |
| Palmitoleic Acid (C16:1 n-7c) | 11.01 ± 1.64 a | 12.27 ± 1.46 a | 11.55 ± 0.84 a | 1.03 ± 0.08 | 7.55 ± 0.36 b | 1.54 ± 0.10 | 5.51 ± 0.28 c | 0.90 ± 0.06 | 6.37 ± 0.42 bc | 1.05 ± 0.07 |
| Hexadecadienoic Acid (C16:2 n-6c,9c) | 3.82 ± 0.22 a | 3.78 ± 0.11 a | 3.75 ± 0.15 a | 0.33 ± 0.01 | 2.09 ± 0.13 b | 0.42 ± 0.02 | 1.87 ± 0.23 b | 0.30 ± 0.05 | 0.20 ± 0.04 c | 0.03 ± 0.01 |
| Margaric Acid (C17:0) | 11.87 ± 1.27 a | 11.59 ± 1.07 a | 12.37 ± 1.11 a | 1.10 ± 0.10 | 8.53 ± 0.44 b | 1.73 ± 0.06 | 4.50 ± 0.18 c | 0.74 ± 0.04 | 7.11 ± 0.10 d | 1.18 ± 0.03 |
| Heptadecenoic Acid (C17:1 n-7c) | 1.28 ± 0.17 a | 1.41 ± 0.23 a | 1.26 ± 0.13 a | 0.11 ± 0.01 | nd | — | nd | — | nd | — |
| Stearic Acid (C18:0) | 34.80 ± 3.72 a | 35.50 ± 2.21 a | 36.54 ± 1.86 a | 3.24 ± 0.18 | 27.88 ± 3.18 b | 5.67 ± 0.62 | 34.25 ± 2.74 a | 5.59 ± 0.58 | 34.08 ± 8.99 a | 5.65 ± 0.35 |
| Oleic Acid (C18:1 n-9c) | 330.00 ± 17.02 a | 333.22 ± 15.94 a | 326.48 ± 13.94 a | 28.97 ± 1.26 | 136.00 ± 9.47 b | 27.66 ± 2.21 | 189.41 ± 9.64 c | 30.92 ± 1.01 | 190.33 ± 9.65 c | 31.52 ± 1.05 |
| Linoleic Acid (C18:2 n-6c,9c) | 549.88 ± 13.74 a | 552.48 ± 15.27 a | 566.02 ± 12.75 a | 50.23 ± 0.94 | 187.33 ± 16.33 b | 38.10 ± 2.38 | 240.05 ± 13.94 c | 39.19 ± 1.40 | 299.74 ± 12.25 c | 38.05 ± 1.12 |
| α-Linolenic Acid (C18:3 n-3c,6c,9c) | 4.81 ± 0.46 a | 4.59 ± 0.36 a | 4.66 ± 0.24 a | 0.41 ± 0.02 | 0.94 ± 0.12 b | 0.19 ± 0.03 | 1.67 ± 0.24 c | 0.27 ± 0.05 | 2.64 ± 0.32 d | 0.44 ± 0.04 |
| Arachidic Acid (C20:0) | 19.51 ± 2.42 a | 21.93 ± 2.24 a | 18.97 ± 1.17 a | 1.68 ± 0.11 | 8.85 ± 2.31 b | 1.80 ± 0.44 | 9.14 ± 0.46 bc | 1.49 ± 0.04 | 12.18 ± 0.88 c | 2.02 ± 0.13 |
| Gondoic Acid (C20:1 n-9c) | 14.09 ± 0.75 a | 14.99 ± 0.69 a | 13.97 ± 0.54 a | 1.24 ± 0.04 | 6.32 ± 0.34 b | 1.29 ± 0.07 | 4.86 ± 0.19 c | 0.79 ± 0.05 | 4.30 ± 0.36 c | 0.71 ± 0.04 |
| Eicosadienoic Acid (C20:2 n-6c,11c) | 1.71 ± 0.07 a | 1.68 ± 0.15 a | 1.83 ± 0.16 a | 0.16 ± 0.01 | 0.10 ± 0.02 b | 0.02 ± 0.00 | 0.84 ± 0.06 c | 0.14 ± 0.01 | 0.14 ± 0.10 c | 0.07 ± 0.04 |
| Heneicosanoic Acid (C21:0) | 1.93 ± 0.46 a | 1.61 ± 0.12 a | 1.71 ± 0.12 a | 0.15 ± 0.01 | 0.20 ± 0.05 b | 0.04 ± 0.01 | 0.65 ± 0.13 c | 0.11 ± 0.02 | 0.11 ± 0.10 c | 0.10 ± 0.02 |
| Behenic Acid (C22:0) | 3.99 ± 0.65 a | 3.52 ± 0.36 ab | 3.47 ± 0.24 ab | 0.31 ± 0.02 | 1.85 ± 0.20 c | 0.38 ± 0.03 | 2.53 ± 0.33 cd | 0.41 ± 0.07 | 2.73 ± 0.35 bd | 0.45 ± 0.05 |
| Docosadienoic Acid (C22:2 n-6c,13c) | 1.46 ± 0.13 a | 1.48 ± 0.15 a | 1.42 ± 0.10 a | 0.13 ± 0.01 | nd | — | nd | — | 0.42 ± 0.04 b | 0.07 ± 0.00 |
| SFA | 195.69 ± 6.50 a | 196.67 ± 10.01 a | 195.97 ± 4.18 a | 17.39 ± 0.37 | 151.38 ± 2.56 b | 30.79 ± 0.47 | 168.33 ± 2.23 c | 27.48 ± 1.11 | 169.77 ± 10.27 c | 28.12 ± 0.38 |
| MUFA | 356.39 ± 17.16 a | 361.90 ± 14.01 a | 353.27 ± 13.67 a | 31.35 ± 1.25 | 149.88 ± 9.45 b | 30.48 ± 2.25 | 199.78 ± 9.50 c | 32.61 ± 1.01 | 201.00 ± 9.92 c | 33.29 ± 1.07 |
| PUFA | 561.69 ± 14.09 a | 564.00 ± 15.03 a | 577.69 ± 12.71 a | 51.26 ± 0.94 | 190.45 ± 16.37 b | 38.73 ± 2.38 | 244.43 ± 13.67 c | 39.90 ± 1.37 | 233.01 ± 12.41 c | 38.59 ± 1.09 |
| Total | 1113.77 ± 11.07 a | 1122.57 ± 7.59 a | 1126.92 ± 7.16 a | 491.71 ± 14.56 b | 612.54 ± 17.25 c | 603.78 ± 28.48 c | ||||
| UFA/SFA | 4.69 ± 0.13 a | 4.72 ± 0.25 a | 4.75 ± 0.12 a | 2.25 ± 0.05 b | 2.64 ± 0.15 c | 2.56 ± 0.05 c | ||||
| Imleria badia | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0 h | 96 h | |||||||||
| Control | Fennel | Spruce | % | Control | % | Fennel | % | Spruce | % | |
| Lauric Acid (C12:0) | 0.93 ± 0.06 a | 0.89 ± 0.08 a | 0.92 ± 0.11 a | 0.07 ± 0.01 | 0.61 ± 0.06 b | 0.23 ± 0.03 | 0.17 ± 0.02 c | 0.02 ± 0.00 | 0.59 ± 0.03 b | 0.07 ± 0.00 |
| Myristic Acid (C14:0) | 3.18 ± 0.13 a | 3.19 ± 0.24 a | 3.39 ± 0.41 a | 0.23 ± 0.02 | 1.73 ± 0.24 b | 0.76 ± 0.10 | 1.23 ± 0.15 c | 0.12 ± 0.01 | 0.92 ± 0.04 d | 0.10 ± 0.01 |
| Pentadecanoic Acid (C15:0) | 10.52 ± 1.38 a | 10.48 ± 1.01 a | 10.34 ± 0.41 a | 0.76 ± 0.06 | 5.71 ± 0.85 b | 19.47 ± 0.52 | 4.84 ± 1.01 b | 0.48 ± 0.10 | 8.98 ± 0.12 a | 0.99 ± 0.02 |
| Palmitic Acid (C16:0) | 225.86 ± 13.03 a | 220.33 ± 8.97 a | 221.44 ± 9.96 a | 16.29 ± 0.52 | 146.24 ± 6.70 b | 0.27 ± 0.03 | 178.86 ± 9.12 a | 17.89 ± 0.85 | 171.20 ± 7.02 ab | 18.80 ± 0.59 |
| Palmitoleic Acid (C16:1 n-7c) | 4.56 ± 0.17 a | 4.43 ± 0.32 a | 4.36 ± 0.33 a | 0.33 ± 0.03 | 2.03 ± 0.21 b | 0.06 ± 0.00 | 0.80 ± 0.06 c | 0.08 ± 0.01 | 3.13 ± 0.12 d | 0.34 ± 0.01 |
| Margaric Acid (C17:0) | 1.65 ± 0.13 a | 1.72 ± 0.17 a | 1.66 ± 0.14 a | 0.12 ± 0.01 | 0.47 ± 0.03 b | 8.94 ± 0.43 | 1.03 ± 0.10 c | 0.10 ± 0.01 | 1.61 ± 0.09 a | 0.18 ± 0.01 |
| Stearic Acid (C18:0) | 76.60 ± 7.97 ab | 77.20 ± 8.97 a | 79.31 ± 7.58 a | 5.53 ± 0.65 | 67.14 ± 3.71 a | 19.70 ± 0.88 | 74.37 ± 4.31 a | 7.44 ± 0.44 | 69.09 ± 0.78 a | 7.59 ± 0.04 |
| Oleic Acid (C18:1 n-9c) | 229.24 ± 13.03 a | 225.51 ± 9.40 a | 230.73 ± 13.40 a | 16.54 ± 0.78 | 147.97 ± 3.94 b | 49.85 ± 0.54 | 190.48 ± 7.84 c | 19.06 ± 0.74 | 173.03 ± 5.81 c | 19.00 ± 0.48 |
| Linoleic Acid (C18:2 n-6c,9c) | 812.75 ± 17.25 a | 816.51 ± 18.98 a | 814.06 ± 21.63 a | 58.63 ± 1.22 | 374.36 ± 0.85 b | 0.01 ± 0.01 | 537.50 ± 13.19 c | 53.77 ± 1.46 | 474.70 ± 9.92 d | 52.13 ± 1.06 |
| α-Linolenic Acid (C18:3 n-3c,6c,9c) | 1.33 ± 0.07 a | 1.36 ± 0.06 a | 1.33 ± 0.06 a | 0.10 ± 0.00 | 0.10 ± 0.06 b | 0.23 ± 0.03 | 1.04 ± 0.07 c | 0.10 ± 0.01 | 0.02 ± 0.01 b | 0.00 ± 0.00 |
| Gondoic Acid (C20:1 n-9c) | 4.92 ± 0.08 a | 4.91 ± 0.11 a | 4.91 ± 0.11 a | 0.35 ± 0.00 | nd | — | 0.85 ± 0.08 b | 0.09 ± 0.01 | 0.02 ± 0.01 c | 0.00 ± 0.00 |
| Eicosadienoic Acid (C20:2 n-6c,11c) | 5.43 ± 0.24 a | 5.26 ± 0.95 a | 5.21 ± 0.41 a | 0.39 ± 0.03 | nd | — | 0.71 ± 0.03 b | 0.07 ± 0.00 | nd | — |
| Heneicosanoic Acid (C21:0) | 1.44 ± 0.11 a | 1.43 ± 0.05 a | 1.40 ± 0.05 a | 0.10 ± 0.00 | 0.27 ± 0.04 b | 0.04 ± 0.01 | 1.36 ± 0.17 c | 0.14 ± 0.02 | 1.09 ± 0.09 c | 0.12 ± 0.01 |
| Behenic Acid (C22:0) | 7.22 ± 1.01 a | 6.48 ± 1.01 a | 6.84 ± 0.94 a | 0.52 ± 0.08 | 4.43 ± 0.25 b | 0.59 ± 0.04 | 5.92 ± 0.29 a | 0.59 ± 0.03 | 6.20 ± 0.26 a | 0.68 ± 0.03 |
| Erucic Acid (C22:1 n-9c) | 0.18 ± 0.01 a | 0.18 ± 0.01 a | 0.19 ± 0.02 a | 0.01 ± 0.00 | nd | — | 0.16 ± 0.02 a | 0.02 ± 0.00 | nd | — |
| Nervonic Acid (C24:1 n-9c) | 0.37 ± 0.05 a | 0.40 ± 0.03 a | 0.37 ± 0.03 a | 0.03 ± 0.00 | nd | — | 0.23 ± 0.02 b | 0.02 ± 0.00 | nd | — |
| SFA | 327.41 ± 14.07 a | 321.73 ± 13.81 a | 325.30 ± 9.67 a | 23.62 ± 1.03 | 226.60 ± 9.22 b | 30.17 ± 0.64 | 267.78 ± 8.26 c | 26.79 ± 0.74 | 259.68 ± 7.95 c | 28.52 ± 0.60 |
| MUFA | 239.26 ± 9.79 a | 235.43 ± 9.78 a | 240.57 ± 13.35 a | 17.26 ± 0.81 | 150.00 ± 3.95 b | 19.97 ± 0.89 | 192.52 ± 7.89 c | 19.26 ± 0.75 | 176.17 ± 5.71 c | 19.35 ± 0.46 |
| PUFA | 819.51 ± 20.87 a | 823.13 ± 19.25 a | 820.60 ± 21.29 a | 59.12 ± 1.20 | 374.43 ± 9.87 b | 49.86 ± 0.54 | 539.24 ± 13.19 c | 53.95 ± 1.46 | 474.71 ± 9.91 d | 52.13 ± 1.06 |
| Total | 1386.18 ± 8.83 a | 1380.30 ± 12.91 a | 1386.47 ± 15.06 a | 751.02 ± 14.89 b | 999.54 ± 3.37 c | 910.57 ± 14.09 d | ||||
| UFA/SFA | 3.24 ± 0.18 a | 3.29 ± 0.16 a | 3.26 ± 0.14 a | 2.32 ± 0.07 b | 2.73 ± 0.10 c | 2.51 ± 0.07 bc | ||||
| Agaricus bisporus | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0 h | 96 h | |||||||||
| Control | Fennel | Spruce | % | Control | % | Fennel | % | Spruce | % | |
| Lauric Acid (C12:0) | 0.87 ± 0.06 a | 0.91 ± 0.03 a | 0.86 ± 0.10 a | 0.08 ± 0.01 | 0.63 ± 0.04 b | 0.12 ± 0.01 | 0.55 ± 0.03 bc | 0.07 ± 0.00 | 0.43 ± 0.04 c | 0.06 ± 0.01 |
| Myristic Acid (C14:0) | 9.42 ± 0.13 ab | 9.77 ± 0.15 a | 9.14 ± 0.15 a | 0.91 ± 0.03 | 6.84 ± 0.50 b | 1.27 ± 0.13 | 6.31 ± 0.29 bc | 0.86 ± 0.05 | 5.73 ± 0.24 c | 0.87 ± 0.03 |
| Pentadecanoic Acid (C15:0) | 23.16 ± 1.38 a | 22.31 ± 0.30 a | 24.01 ± 0.42 b | 2.23 ± 0.12 | 17.31 ± 1.50 b | 3.20 ± 0.34 | 18.38 ± 1.07 b | 2.50 ± 0.12 | 19.43 ± 1.33 a | 2.94 ± 0.13 |
| Palmitic Acid (C16:0) | 117.51 ± 13.03 a | 112.20 ± 3.14 a | 111.03 ± 3.10 a | 11.31 ± 0.28 | 89.23 ± 3.38 b | 16.51 ± 0.90 | 97.94 ± 3.40 b | 13.31 ± 0.19 | 95.92 ± 1.92 b | 14.54 ± 0.60 |
| Palmitoleic Acid (C16:1 n-7c) | 3.36 ± 0.33 ab | 3.94 ± 0.09 a | 4.04 ± 0.10 a | 0.32 ± 0.04 | 1.23 ± 0.44 b | 0.23 ± 0.08 | 2.41 ± 0.28 c | 0.33 ± 0.04 | 3.52 ± 0.18 a | 0.53 ± 0.04 |
| Margaric Acid (C17:0) | 8.13 ± 0.50 a | 8.97 ± 0.19 a | 9.02 ± 0.24 a | 0.78 ± 0.05 | 6.05 ± 0.52 b | 1.12 ± 0.11 | 6.69 ± 0.41 c | 0.91 ± 0.07 | 8.23 ± 0.32 a | 1.25 ± 0.06 |
| Stearic Acid (C18:0) | 69.44 ± 4.53 a | 70.35 ± 3.46 a | 63.41 ± 0.10 ab | 6.68 ± 0.34 | 49.33 ± 4.05 c | 9.12 ± 0.50 | 56.22 ± 2.96 bc | 7.64 ± 0.40 | 50.06 ± 2.20 c | 7.59 ± 0.09 |
| Oleic Acid (C18:1 n-9c) | 208.92 ± 12.90 a | 195.82 ± 6.15 a | 186.88 ± 0.35 a | 20.11 ± 1.07 | 106.42 ± 6.06 b | 19.69 ± 1.06 | 134.48 ± 6.45 c | 18.28 ± 0.84 | 122.68 ± 5.83 c | 18.59 ± 0.35 |
| Linoleic Acid (C18:2 n-6c,9c) | 536.76 ± 17.39 a | 539.15 ± 15.06 a | 538.16 ± 3.10 a | 51.65 ± 1.63 | 230.69 ± 10.21 b | 42.68 ± 0.84 | 373.85 ± 13.67 c | 50.81 ± 1.58 | 315.90 ± 11.17 d | 47.87 ± 0.34 |
| α-Linolenic Acid (C18:3 n-3c,6c,9c) | 1.21 ± 0.04 a | 1.26 ± 0.05 a | 1.25 ± 0.10 a | 0.12 ± 0.00 | nd | — | 0.73 ± 0.10 b | 0.10 ± 0.01 | nd | — |
| Arachidic Acid (C20:0) | 24.37 ± 2.13 a | 24.42 ± 2.71 a | 24.97 ± 0.10 a | 2.34 ± 0.19 | 16.32 ± 0.93 b | 3.02 ± 0.09 | 15.60 ± 0.81 b | 2.12 ± 0.11 | 13.56 ± 0.09 b | 2.05 ± 0.17 |
| Gondoic Acid (C20:1 n-9c) | 22.35 ± 2.41 a | 23.46 ± 2.60 a | 24.69 ± 0.25 a | 2.15 ± 0.26 | 11.73 ± 1.74 b | 2.17 ± 0.29 | 14.68 ± 1.01 c | 2.00 ± 0.10 | 16.44 ± 0.84 c | 2.49 ± 0.06 |
| Eicosadienoic Acid (C20:2 n-6c,11c) | 2.13 ± 0.12 a | 2.28 ± 0.13 a | 2.31 ± 0.22 a | 0.20 ± 0.01 | 0.19 ± 0.03 b | 0.04 ± 0.02 | 1.10 ± 0.12 c | 0.15 ± 0.01 | 0.78 ± 0.09 b | 0.12 ± 0.07 |
| Behenic Acid (C22:0) | 11.29 ± 0.59 a | 11.27 ± 0.26 a | 11.12 ± 0.50 a | 1.09 ± 0.06 | 4.67 ± 0.60 b | 0.86 ± 0.09 | 6.76 ± 0.80 c | 0.92 ± 0.09 | 7.49 ± 0.66 c | 1.14 ± 0.06 |
| Erucic Acid (C22:1 n-9c) | 0.13 ± 0.02 a | 0.16 ± 0.05 a | 0.19 ± 0.02 a | 0.01 ± 0.00 | nd | — | 0.10 ± 0.01 b | 0.01 ± 0.01 | nd | — |
| Nervonic Acid (C24:1 n-9c) | 0.08 ± 0.01 a | 0.08 ± 0.01 a | 0.09 ± 0.03 a | 0.01 ± 0.00 | nd | — | nd | — | nd | — |
| SFA | 264.19 ± 7.97 a | 260.21 ± 5.85 a | 253.55 ± 3.47 a | 25.42 ± 0.48 | 190.37 ± 4.22 b | 35.22 ± 4.22 | 208.43 ± 7.43 c | 28.33 ± 0.75 | 200.84 ± 3.32 bc | 30.44 ± 0.47 |
| MUFA | 234.84 ± 13.03 a | 223.46 ± 8.55 a | 215.89 ± 3.18 a | 22.60 ± 1.15 | 119.38 ± 7.54 b | 22.08 ± 1.23 | 151.63 ± 6.99 c | 20.61 ± 0.84 | 142.64 ± 6.46 d | 21.62 ± 0.34 |
| PUFA | 540.10 ± 17.25 a | 542.69 ± 15.16 a | 541.72 ± 14.00 a | 51.98 ± 1.62 | 230.82 ± 10.31 b | 42.70 ± 0.85 | 375.67 ± 0.16 c | 51.06 ± 1.59 | 316.41 ± 11.51 d | 47.95 ± 0.34 |
| Total | 1039.13 ± 17.89 a | 1026.35 ± 12.52 a | 1011.17 ± 7.98 a | 540.57 ± 15.88 b | 735.74 ± 15.75 c | 659.90 ± 20.96 d | ||||
| UFA/SFA | 2.93 ± 0.07 a | 2.94 ± 0.04 a | 2.99 ± 0.09 a | 1.84 ± 0.06 b | 2.53 ± 0.09 c | 2.29 ± 0.05 d | ||||
| Boletus edulis | ||||
|---|---|---|---|---|
| 0 h | 96 h | Relative Change | ||
| Lauric Acid (C12:0) | Control | 0.23 ± 0.04 a | 0.25 ± 0.03 a | — |
| Fennel | 0.27 ± 0.08 ab | 0.38 ± 0.04 b | — | |
| Spruce | 0.30 ± 0.07 ab | 0.23 ± 0.03 a | — | |
| Myristic Acid (C14:0) | Control | 0.53 ± 0.08 a | 2.11 ± 0.15 b | +298% |
| Fennel | 0.53 ± 0.10 a | 1.62 ± 0.19 c | +205% | |
| Spruce | 0.56 ± 0.10 a | 0.99 ± 0.22 d | +77% | |
| Pentadecanoic Acid (C15:0) | Control | nd | 0.58 ± 0.05 a | — |
| Fennel | nd | 0.84 ± 0.10 b | — | |
| Spruce | nd | nd | — | |
| Palmitic Acid (C16:0) | Control | 6.54 ± 0.29 a | 10.17 ± 0.27 ab | — |
| Fennel | 7.40 ± 0.67 ab | 12.47 ± 2.09 ab | — | |
| Spruce | 7.58 ± 0.60 ab | 8.63 ± 0.68 b | — | |
| Palmitoleic Acid (C16:1 n-7c) | Control | 0.69 ± 0.14 a | 2.94 ± 0.08 b | +326% |
| Fennel | 0.71 ± 0.12 a | 0.82 ± 0.09 a | — | |
| Spruce | 0.73 ± 0.05 a | 1.27 ± 0.08 c | +74% | |
| Palmitelaidic Acid (C16:1 n-7t) | Control | nd | 0.55 ± 0.10 a | — |
| Fennel | nd | 0.90 ± 0.07 b | — | |
| Spruce | nd | 1.45 ± 0.12 c | — | |
| Margaric Acid (C17:0) | Control | nd | 6.53 ± 0.44 | — |
| Fennel | nd | nd | — | |
| Spruce | nd | nd | — | |
| Stearic Acid (C18:0) | Control | 7.98 ± 0.60 a | 13.47 ± 0.90 b | +69% |
| Fennel | 8.01 ± 0.36 a | 11.33 ± 0.97 c | +41% | |
| Spruce | 7.98 ± 0.22 a | 8.62 ± 0.58 b | +8% | |
| Oleic Acid (C18:1 n-9c) | Control | 34.28 ± 2.02 a | 69.34 ± 2.22 b | +102% |
| Fennel | 34.02 ± 1.97 a | 56.07 ± 2.80 c | +65% | |
| Spruce | 33.33 ± 3.56 a | 48.33 ± 0.93 c | +45% | |
| Linoleic Acid (C18:2 n-6c,9c) | Control | 75.79 ± 3.60 ab | 108.66 ± 4.67 c | +43% |
| Fennel | 74.73 ± 2.46 a | 92.70 ± 5.69 d | +24% | |
| Spruce | 74.82 ± 4.33 ab | 88.33 ± 3.45 bd | — | |
| Arachidic Acid (C20:0) | Control | 3.41 ± 0.18 a | 5.50 ± 0.42 b | +61% |
| Fennel | 3.43 ± 0.21 a | 4.83 ± 0.19 bc | +41% | |
| Spruce | 3.44 ± 0.22 a | 4.30 ± 0.15 ac | — | |
| Gondoic Acid (C20:1 n-9c) | Control | 1.70 ± 0.13 a | 2.38 ± 0.20 b | — |
| Fennel | 1.74 ± 0.10 a | 2.23 ± 0.11 b | — | |
| Spruce | 1.70 ± 0.10 a | 2.18 ± 0.07 b | — | |
| Total | Control | 131.16 ± 4.73 a | 222.48 ± 7.80 b | +69% |
| Fennel | 130.83 ± 2.56 a | 184.20 ± 2.79 c | +41% | |
| Spruce | 130.43 ± 2.86 a | 164.33 ± 3.32 d | +26% | |
| Imleria badia | ||||
|---|---|---|---|---|
| 0 h | 96 h | Relative Change | ||
| Lauric Acid (C12:0) | Control | 0.40 ± 0.06 a | 0.46 ± 0.05 a | — |
| Fennel | 0.40 ± 0.03 a | 0.47 ± 0.03 a | — | |
| Spruce | 0.40 ± 0.07 a | 0.47 ± 0.05 a | — | |
| Myristic Acid (C14:0) | Control | 1.74 ± 0.12 a | 1.99 ± 0.08 a | — |
| Fennel | 1.77 ± 0.14 a | 1.95 ± 0.07 a | — | |
| Spruce | 1.72 ± 0.05 a | 1.82 ± 0.08 a | — | |
| Pentadecanoic Acid (C15:0) | Control | 0.68 ± 0.07 a | 2.93 ± 0.07 b | +331% |
| Fennel | 0.69 ± 0.07 a | 1.39 ± 0.11 c | +48% | |
| Spruce | 0.68 ± 0.05 a | 3.03 ± 0.15 b | +345% | |
| Palmitic Acid (C16:0) | Control | 30.31 ± 3.94 a | 42.81 ± 1.38 b | +41% |
| Fennel | 30.20 ± 0.96 a | 37.21 ± 3.03 c | +23% | |
| Spruce | 30.35 ± 1.11 a | 49.38 ± 3.83 d | +63% | |
| Palmitoleic Acid (C16:1 n-7c) | Control | 1.05 ± 0.07 a | 2.47 ± 0.18 b | +135% |
| Fennel | 1.06 ± 0.14 a | 2.41 ± 0.17 b | +127% | |
| Spruce | 1.13 ± 0.06 a | 4.13 ± 0.15 c | +265% | |
| Palmitelaidic Acid (C16:1 n-7t) | Control | 0.27 ± 0.07 a | 0.37 ± 0.05 b | +37% |
| Fennel | 0.23 ± 0.06 a | 0.28 ± 0.04 b | +22% | |
| Spruce | 0.26 ± 0.04 a | 0.62 ± 0.07 c | +138% | |
| Margaric Acid (C17:0) | Control | nd | 0.32 ± 0.04 ab | — |
| Fennel | nd | 0.21 ± 0.05 b | — | |
| Spruce | nd | 0.53 ± 0.07 a | — | |
| Stearic Acid (C18:0) | Control | 10.51 ± 0.69 a | 28.93 ± 2.30 b | +175% |
| Fennel | 10.57 ± 0.65 a | 17.06 ± 2.51 c | +59% | |
| Spruce | 10.73 ± 0.82 a | 27.42 ± 3.19 b | +156% | |
| Oleic Acid (C18:1 n-9c) | Control | 50.41 ± 3.01 a | 98.78 ± 6.32 b | +96% |
| Fennel | 50.49 ± 3.20 a | 78.48 ± 6.19 c | +55% | |
| Spruce | 50.60 ± 3.19 a | 98.44 ± 6.98 b | +94% | |
| Linoleic Acid (C18:2 n-6c,9c) | Control | 85.07 ± 4.54 a | 209.40 ± 9.51 b | +146% |
| Fennel | 87.08 ± 3.40 a | 117.70 ± 8.51 c | +35% | |
| Spruce | 84.43 ± 3.33 a | 187.00 ± 4.42 d | +121% | |
| Total | Control | 181.53 ± 5.77 a | 388.45 ± 1.93 b | +114% |
| Fennel | 182.18 ± 3.92 a | 257.15 ± 7.72 c | +41% | |
| Spruce | 180.31 ± 6.21 a | 372.84 ± 4.25 d | +107% | |
| Agaricus bisporus | ||||
|---|---|---|---|---|
| 0 h | 96 h | Relative Change | ||
| Myristic Acid (C14:0) | Control | 3.10 ± 0.12 a | 3.70 ± 0.13 a | — |
| Fennel | 3.02 ± 0.12 a | 3.69 ± 0.17 a | — | |
| Spruce | 3.04 ± 0.06 a | 3.15 ± 0.06 a | — | |
| Pentadecanoic Acid (C15:0) | Control | 9.15 ± 0.14 a | 11.35 ± 0.38 b | +24% |
| Fennel | 9.04 ± 0.07 a | 9.70 ± 0.29 c | +7% | |
| Spruce | 9.05 ± 0.08 a | 9.80 ± 0.20 b | +8% | |
| Palmitic Acid (C16:0) | Control | 23.89 ± 2.18 a | 32.01 ± 2.43 b | +34% |
| Fennel | 22.46 ± 1.01 a | 25.33 ± 1.07 c | +13% | |
| Spruce | 22.96 ± 1.47 a | 24.77 ± 1.94 d | +8% | |
| Palmitoleic Acid (C16:1 n-7c) | Control | 0.72 ± 0.07 ab | 0.88 ± 0.03 a | — |
| Fennel | 0.73 ± 0.04 ab | 0.74 ± 0.03 ab | — | |
| Spruce | 0.75 ± 0.06 ab | 0.68 ± 0.02 b | — | |
| Margaric Acid (C17:0) | Control | nd | 1.47 ± 0.14a b | — |
| Fennel | nd | 0.59 ± 0.14 b | — | |
| Spruce | nd | 0.52 ± 0.15 a | — | |
| Stearic Acid (C18:0) | Control | 17.53 ± 0.30 a | 24.54 ± 1.88 b | +40% |
| Fennel | 17.56 ± 0.39 a | 19.23 ± 0.31 c | +10% | |
| Spruce | 17.18 ± 0.18 a | 18.45 ± 0.51 b | +7% | |
| Oleic Acid (C18:1 n-9c) | Control | 33.98 ± 2.21 a | 54.51 ± 3.58 b | +61% |
| Fennel | 32.44 ± 1.29 a | 40.63 ± 1.58 c | +25% | |
| Spruce | 32.79 ± 1.13 a | 40.80 ± 2.22 c | +24% | |
| Linoleic Acid (C18:2 n-6c,9c) | Control | 85.07 ± 4.54 a | 179.40 ± 9.51 b | +111% |
| Fennel | 86.08 ± 3.40 a | 117.70 ± 8.51 c | +37% | |
| Spruce | 84.43 ± 3.33 a | 143.66 ± 3.16 d | +70% | |
| Arachidic Acid (C20:0) | Control | 6.16 ± 0.45 a | 10.14 ± 0.79 b | +65% |
| Fennel | 5.86 ± 0.60 a | 8.68 ± 0.68 bc | +48% | |
| Spruce | 6.05 ± 0.48 a | 7.66 ± 0.46 c | +27% | |
| Gondoic Acid (C20:1 n-9c) | Control | 2.49 ± 0.21 ab | 10.56 ± 0.69 c | +324% |
| Fennel | 2.26 ± 0.16 a | 2.79 ± 0.13 b | +23% | |
| Spruce | 2.22 ± 0.13 a | 3.47 ± 0.32 d | +56% | |
| Behenic Acid (C22:0) | Control | 1.49 ± 0.26 a | 2.54 ± 0.24 c | +70% |
| Fennel | 1.50 ± 0.36 a | 2.31 ± 0.17 bc | +54% | |
| Spruce | 1.54 ± 0.30 ab | 2.19 ± 0.17 abc | +42% | |
| Total | Control | 180.13 ± 5.86 a | 331.09 ± 7.99 b | +84% |
| Fennel | 180.94 ± 6.20 a | 231.37 ± 7.25 c | +28% | |
| Spruce | 180.01 ± 3.23 a | 255.17 ± 1.66 d | +42% | |
| Boletus edulis | Imleria badia | Agaricus bisporus | |||||
|---|---|---|---|---|---|---|---|
| 0 h | 96 h | 0 h | 96 h | 0 h | 96 h | ||
| α-tocopherol | Control | 0.262 ± 0.013 a | 0.016 ± 0.002 b | 0.553 ± 0.014 a | 0.392 ± 0.015 b | 0.148 ± 0.005 a | 0.019 ± 0.002 b |
| Fennel | 0.261 ± 0.021 a | 0.075 ± 0.006 c | 0.542 ± 0.021 a | 0.066 ± 0.007 c | 0.146 ± 0.007 a | 0.007 ± 0.003 b | |
| Spruce | 0.267 ± 0.022 a | 0.182 ± 0.008 d | 0.544 ± 0.011 a | 0.135 ± 0.007 d | 0.149 ± 0.005 a | 0.015 ± 0.003 b | |
| β-tocopherol | Control | 0.347 ± 0.010 a | 0.019 ± 0.001 b | 0.716 ± 0.005 a | 0.056 ± 0.003 b | 0.230 ± 0.011 a | 0.027 ± 0.002 b |
| Fennel | 0.338 ± 0.009 a | 0.051 ± 0.006 c | 0.718 ± 0.014 a | 0.053 ± 0.008 b | 0.219 ± 0.002 a | 0.060 ± 0.002 c | |
| Spruce | 0.336 ± 0.013 a | 0.010 ± 0.003 b | 0.714 ± 0.005 a | 0.043 ± 0.004 b | 0.221 ± 0.006 a | 0.014 ± 0.003 b | |
| γ-tocopherol | Control | 0.738 ± 0.003 a | 0.260 ± 0.033 b | 1.493 ± 0.010 a | 1.035 ± 0.015 b | 0.231 ± 0.002 a | 0.105 ± 0.003 b |
| Fennel | 0.744 ± 0.006 a | 0.473 ± 0.011 c | 1.492 ± 0.009 a | 1.481 ± 0.024 a | 0.238 ± 0.014 a | 0.120 ± 0.002 b | |
| Spruce | 0.764 ± 0.014 a | 0.268 ± 0.024 b | 1.493 ± 0.009 a | 0.978 ± 0.028 b | 0.235 ± 0.007 a | 0.137 ± 0.004 b | |
| δ-tocopherol | Control | 0.475 ± 0.016 ab | 0.158 ± 0.004 c | 1.027 ± 0.020 a | 0.123 ± 0.007 b | 0.107 ± 0.004 a | 0.089 ± 0.003 a |
| Fennel | 0.489 ± 0.010 a | 0.154 ± 0.007 c | 1.035 ± 0.020 a | 0.117 ± 0.006 b | 0.106 ± 0.002 a | 0.103 ± 0.001 a | |
| Spruce | 0.457 ± 0.017 b | 0.289 ± 0.004 d | 1.035 ± 0.025 a | 0.093 ± 0.003 c | 0.105 ± 0.006 a | 0.094 ± 0.005 a | |
| Sum of tocopherols | Control | 1.822 ± 0.027 a | 0.453 ± 0.037 b | 3.788 ± 0.022 a | 1.605 ± 0.011 b | 0.716 ± 0.013 a | 0.241 ± 0.007 b |
| Fennel | 1.832 ± 0.023 a | 0.753 ± 0.022 c | 3.788 ± 0.035 a | 1.717 ± 0.031 c | 0.710 ± 0.017 a | 0.290 ± 0.008 c | |
| Spruce | 1.824 ± 0.008 a | 0.750 ± 0.020 c | 3.786 ± 0.019 a | 1.248 ± 0.038 d | 0.711 ± 0.007 a | 0.260 ± 0.004 bc | |
| Boletus edulis | Imleria badia | Agaricus bisporus | |||||
|---|---|---|---|---|---|---|---|
| 0 h | 96 h | 0 h | 96 h | 0 h | 96 h | ||
| Vitamin B1 | Control | 6.60 ± 0.372 a | 4.50 ± 2.7 b | 5.94 ± 0.27 a | 2.86 ± 0.16 b | 3.66 ± 0.15 a | 2.71 ± 0.12 b |
| Fennel | 6.63 ± 0.382 a | 6.27 ± 1.8 ac | 5.99 ± 0.32 a | 4.78 ± 0.23 c | 3.63 ± 0.13 a | 3.30 ± 0.14 ac | |
| Spruce | 6.53 ± 0.208 a | 5.56 ± 3.26 c | 5.89 ± 0.35 a | 4.85 ± 0.16 c | 3.70 ± 0.13 a | 2.99 ± 0.12 bc | |
| Vitamin B2 | Control | 30.09 ± 2.084 a | 22.80 ± 19.65 b | 32.92 ± 2.33 a | 23.69 ± 1.83 b | 21.90 ± 1.29 a | 18.82 ± 1.33 ab |
| Fennel | 30.11 ± 2.032 a | 20.21 ± 11.29 b | 33.40 ± 1.90 a | 20.04 ± 1.71 b | 22.08 ± 1.11 a | 18.06 ± 1.51 b | |
| Spruce | 29.75 ± 3.595 a | 23.83 ± 13.45 b | 32.30 ± 2.05 a | 30.41 ± 1.57 a | 22.11 ± 1.00 a | 19.15 ± 1.04 ab | |
| Vitamin B6 | Control | 0.117 ± 0.007 a | 0.067 ± 0.001 b | 0.166 ± 0.004 a | 0.138 ± 0.002 b | 0.074 ± 0.005 a | 0.035 ± 0.003 b |
| Fennel | 0.113 ± 0.004 a | 0.094 ± 0.002 c | 0.166 ± 0.004 a | 0.113 ± 0.003 c | 0.073 ± 0.005 a | 0.051 ± 0.003 c | |
| Spruce | 0.116 ± 0.003 a | 0.066 ± 0.001 b | 0.166 ± 0.003 a | 0.115 ± 0.001 c | 0.073 ± 0.004 a | 0.041 ± 0.002 b | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grzyb, M.; Szymczak, K.; Bonikowski, R. Essential Oil Fumigation Modulates Nutrient Content in Selected Mushrooms During Postharvest Storage. Int. J. Mol. Sci. 2025, 26, 3939. https://doi.org/10.3390/ijms26093939
Grzyb M, Szymczak K, Bonikowski R. Essential Oil Fumigation Modulates Nutrient Content in Selected Mushrooms During Postharvest Storage. International Journal of Molecular Sciences. 2025; 26(9):3939. https://doi.org/10.3390/ijms26093939
Chicago/Turabian StyleGrzyb, Małgorzata, Kamil Szymczak, and Radosław Bonikowski. 2025. "Essential Oil Fumigation Modulates Nutrient Content in Selected Mushrooms During Postharvest Storage" International Journal of Molecular Sciences 26, no. 9: 3939. https://doi.org/10.3390/ijms26093939
APA StyleGrzyb, M., Szymczak, K., & Bonikowski, R. (2025). Essential Oil Fumigation Modulates Nutrient Content in Selected Mushrooms During Postharvest Storage. International Journal of Molecular Sciences, 26(9), 3939. https://doi.org/10.3390/ijms26093939






