The Role and Diagnostic Potential of Insulin-like Growth Factor 1 in Diabetic Retinopathy and Diabetic Macular Edema
Abstract
1. The Diabetes Mellitus Epidemic
2. Ocular Complications of Diabetes
3. Pathogenesis and the Role of Insulin-like Growth Factor 1 (IGF-1) in DR and DME
4. The Effects of IGF-1 on Inflammatory Processes in DR and DME
5. Systemic Effects and Clinical Findings of IGF-1 on DR and DME
6. Therapeutic Potential of Targeting IGF-1
7. Conclusions
Funding
Conflicts of Interest
Abbreviations
| DM | Diabetes mellitus |
| DR | Diabetic retinopathy |
| DME | Diabetic macular edema |
| GH | Growth hormone |
| IGF-1 | Insulin-like growth factor-1 |
| US | United States |
| T1D | Type 1 diabetes mellitus |
| T2D | Type 2 diabetes mellitus |
| MODY | Maturity-onset diabetes of the young |
| NPDR | Non-proliferative diabetic retinopathy |
| PDR | Proliferative diabetic retinopathy |
| BRB | Blood–retinal barrier |
| IRMA | Intraretinal microvascular abnormalities |
| VEGF | Vascular endothelial growth factor |
| PI-3K/AkT | Phosphoinositide 3-kinase/protein kinase B |
| HIF-1α | Hypoxia-inducible factor 1-alpha |
| NF-κB | Nuclear factor-kappa B |
| AP-1 | Activator Protein 1 |
| JNK | Jun N-terminal kinase |
| LPA | Lysophosphatidic acid |
| ERK | Extracellular signal-regulated kinase |
| SHPS-1 | Src homology 2 domain-containing protein tyrosine phosphatase substrate 1 |
| IAP | Integrin-associated protein |
| MAPK | Mitogen-activated protein kinase |
| INL | Inner nuclear layer |
| SST | Somatostatin |
| BDNF | Brain-derived neurotrophic factor |
| EGFR | Epidermal growth factor receptor |
| ICAM-1 | Intracellular adhesion molecule-1 |
| LFA-1 | Lymphocyte function-associated antigen |
| IL | Interleukin |
| TNF-α | Tumor necrosis factor alpha |
| TGF-β | Transforming growth factor beta |
| MAC | Membrane attack complex |
| CRP | C-reactive protein |
| MBL | Mannose-binding lectin |
| CCL-2 | C-C motif chemokine ligand 2 |
References
- National Diabetes Statistics Report|Diabetes|CDC. Available online: https://www.cdc.gov/diabetes/php/data-research/?CDC_AAref_Val=https://www.cdc.gov/diabetes/data/statistics-report/index.html (accessed on 17 April 2025).
- Hossain, M.J.; Al-Mamun, M.; Islam, M.R. Diabetes mellitus, the fastest growing global public health concern: Early detection should be focused. Health Sci. Rep. 2024, 7, e2004. [Google Scholar] [CrossRef] [PubMed]
- Parker, E.D.; Lin, J.; Mahoney, T.; Ume, N.; Yang, G.; Gabbay, R.A.; ElSayed, N.A.; Bannuru, R.R. Economic Costs of Diabetes in the U.S. in 2022. Diabetes Care 2024, 47, 26–43. [Google Scholar] [CrossRef] [PubMed]
- Solis-Herrera, C.; Triplitt, C.; Reasner, C.; DeFronzo, R.A.; Cersosimo, E. Classification of Diabetes Mellitus. [Updated 24 February 2018]. In Endotext [Internet]; Feingold, K.R., Ahmed, S.F., Anawalt, B., Blackman, M.R., Boyce, A., Chrousos, G., Corpas, E., de Herder, W.W., Dhatariya, K., Dungan, K., et al., Eds.; MDText.com, Inc.: South Dartmouth, MA, USA, 2000. Available online: https://www.ncbi.nlm.nih.gov/books/NBK279119/ (accessed on 17 April 2025).
- Sapra, A.; Bhandari, P.; Wilhite, A. Diabetes. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Banday, M.Z.; Sameer, A.S.; Nissar, S. Pathophysiology of diabetes: An overview. Avicenna J. Med. 2020, 10, 174. [Google Scholar] [CrossRef] [PubMed]
- Kyrou, I.; Tsigos, C.; Mavrogianni, C.; Cardon, G.; Van Stappen, V.; Latomme, J.; Kivelä, J.; Wikström, K.; Tsochev, K.; Nanasi, A.; et al. Sociodemographic and lifestyle-related risk factors for identifying vulnerable groups for type 2 diabetes: A narrative review with emphasis on data from Europe. BMC Endocr. Disord. 2020, 20, 134. [Google Scholar] [CrossRef]
- Diabetic Nephropathy (Kidney Disease)|Johns Hopkins Medicine. Available online: https://www.hopkinsmedicine.org/health/conditions-and-diseases/diabetes/diabetic-nephropathy-kidney-disease (accessed on 17 April 2025).
- Deshpande, A.D.; Harris-Hayes, M.; Schootman, M. Epidemiology of diabetes and diabetes-related complications. Phys. Ther. 2008, 88, 1254–1264. [Google Scholar] [CrossRef]
- Sayin, N.; Kara, N.; Pekel, G. Ocular complications of diabetes mellitus. World J. Diabetes 2015, 6, 92. [Google Scholar] [CrossRef]
- Lundeen, E.A.; Burke-Conte, Z.; Rein, D.B.; Wittenborn, J.S.; Saaddine, J.; Lee, A.Y.; Flaxman, A.D. Prevalence of Diabetic Retinopathy in the US in 2021. JAMA Ophthalmol. 2023, 141, 747–754. [Google Scholar] [CrossRef]
- Kropp, M.; Golubnitschaja, O.; Mazurakova, A.; Koklesova, L.; Sargheini, N.; Vo, T.-T.K.S.; de Clerck, E.; Polivka, J., Jr.; Potuznik, P.; Polivka, J.; et al. Diabetic retinopathy as the leading cause of blindness and early predictor of cascading complications-risks and mitigation. EPMA J. 2023, 14, 21–42. [Google Scholar] [CrossRef]
- Shukla, U.V.; Tripathy, K. Diabetic Retinopathy. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Threatt, J.; Williamson, J.F.; Huynh, K.; Davis, R.M.; Hermayer, K. Ocular disease, knowledge and technology applications in patients with diabetes. Am. J. Med. Sci. 2013, 345, 266–270. [Google Scholar] [CrossRef]
- Stem, M.S.; Gardner, T.W. Neurodegeneration in the Pathogenesis of Diabetic Retinopathy: Molecular Mechanisms and Therapeutic Implications. Curr. Med. Chem. 2013, 20, 3241. [Google Scholar] [CrossRef]
- Chaum, E.; Drewry, R.D.; Ware, G.T.; Charles, S. Nerve fiber bundle visual field defect resulting from a giant peripapillary cotton-wool spot. J. Neuroophthalmol. 2001, 21, 276–277. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Lo, A.C.Y. Diabetic Retinopathy: Pathophysiology and Treatments. Int. J. Mol. Sci. 2018, 19, 1816. [Google Scholar] [CrossRef]
- Wilkinson-Berka, J.L.; Wraight, C.; Werther, G. The role of growth hormone, insulin-like growth factor and somatostatin in diabetic retinopathy. Curr. Med. Chem. 2006, 13, 3307–3317. [Google Scholar] [CrossRef] [PubMed]
- Pierce, E.A.; Avery, R.L.; Foley, E.D.; Aiello, L.P.; Smith, L.E. Vascular endothelial growth factor/vascular permeability factor expression in a mouse model of retinal neovascularization. Proc. Natl. Acad. Sci. USA 1995, 92, 905–909. [Google Scholar] [CrossRef]
- Aiello, L.P.; Pierce, E.A.; Foley, E.D.; Takagi, H.; Chen, H.; Riddle, L.; Ferrara, N.; King, G.L.; Smith, L.E. Suppression of retinal neovascularization in vivo by inhibition of vascular endothelial growth factor (VEGF) using soluble VEGF-receptor chimeric proteins. Proc. Natl. Acad. Sci. USA 1995, 92, 10457–10461. [Google Scholar] [CrossRef]
- Smith, L.E.H.; Shen, W.; Perruzzi, C.; Soker, S.; Kinose, F.; Xu, X.; Robinson, G.; Driver, S.; Bischoff, J.; Zhang, B.; et al. Regulation of vascular endothelial growth factor-dependent retinal neovascularization by insulin-like growth factor-1 receptor. Nat. Med. 1999, 5, 1390–1395. [Google Scholar] [CrossRef]
- Kondo, T.; Vicent, D.; Suzuma, K.; Yanagisawa, M.; King, G.L.; Holzenberger, M.; Kahn, C.R. Knockout of insulin and IGF-1 receptors on vascular endothelial cells protects against retinal neovascularization. J. Clin. Investig. 2003, 111, 1835. [Google Scholar] [CrossRef] [PubMed]
- Poulaki, V.; Joussen, A.M.; Mitsiades, N.; Mitsiades, C.S.; Iliaki, E.F.; Adamis, A.P. Insulin-like growth factor-I plays a pathogenetic role in diabetic retinopathy. Am. J. Pathol. 2004, 165, 457–469. [Google Scholar] [CrossRef]
- Jacobo, S.M.P.; Kazlauskas, A. Insulin-like Growth Factor 1 (IGF-1) Stabilizes Nascent Blood Vessels. J. Biol. Chem. 2015, 290, 6349. [Google Scholar] [CrossRef]
- Shin, E.S.; Sorenson, C.M.; Sheibani, N. Diabetes and retinal vascular dysfunction. J. Ophthalmic Vis. Res. 2014, 9, 362–373. [Google Scholar]
- Rudraraju, M.; Narayanan, S.P.; Somanath, P.R. Regulation of blood-retinal barrier cell-junctions in diabetic retinopathy. Pharmacol. Res. 2020, 161, 105115. [Google Scholar] [CrossRef]
- Díaz-Coránguez, M.; Ramos, C.; Antonetti, D.A. The inner blood-retinal barrier: Cellular basis and development. Vis. Res. 2017, 139, 123–137. [Google Scholar] [CrossRef]
- Cunha-Vaz, J.; Bernardes, R.; Lobo, C. Blood-retinal barrier. Eur. J. Ophthalmol. 2011, 21 (Suppl. 6), 3–9. [Google Scholar] [CrossRef]
- Morcos, Y.; Hosie, M.J.; Bauer, H.C.; Chan-Ling, T. Immunolocalization of occludin and claudin-1 to tight junctions in intact CNS vessels of mammalian retina. J. Neurocytol. 2001, 30, 107–123. [Google Scholar] [CrossRef] [PubMed]
- Haurigot, V.; Villacampa, P.; Ribera, A.; Llombart, C.; Bosch, A.; Nacher, V.; Ramos, D.; Ayuso, E.; Segovia, J.C.; Bueren, J.A.; et al. Increased intraocular insulin-like growth factor-I triggers blood-retinal barrier breakdown. J. Biol. Chem. 2009, 284, 22961–22969. [Google Scholar] [CrossRef] [PubMed]
- Ruberte, J.; Ayuso, E.; Navarro, M.; Carretero, A.; Nacher, V.; Haurigot, V.; George, M.; Llombart, C.; Casellas, A.; Costa, C.; et al. Increased ocular levels of IGF-1 in transgenic mice lead to diabetes-like eye disease. J. Clin. Investig. 2004, 113, 1149. [Google Scholar] [CrossRef] [PubMed]
- Radhakrishnan, Y.; Shen, X.; Maile, L.A.; Xi, G.; Clemmons, D.R. IGF-I stimulates cooperative interaction between the IGF-I receptor and CSK homologous kinase that regulates SHPS-1 phosphorylation in vascular smooth muscle cells. Mol. Endocrinol. 2011, 25, 1636–1649. [Google Scholar] [CrossRef]
- Ling, Y.; Maile, L.A.; Lieskovska, J.; Badley-Clarke, J.; Clemmons, D.R. Role of SHPS-1 in the regulation of insulin-like growth factor I-stimulated Shc and mitogen-activated protein kinase activation in vascular smooth muscle cells. Mol. Biol. Cell 2005, 16, 3353–3364. [Google Scholar] [CrossRef]
- Xi, G.; Wai, C.; White, M.F.; Clemmons, D.R. Down-regulation of Insulin Receptor Substrate 1 during Hyperglycemia Induces Vascular Smooth Muscle Cell Dedifferentiation. J. Biol. Chem. 2017, 292, 2009–2020. [Google Scholar] [CrossRef]
- Maile, L.A.; Gollahon, K.; Wai, C.; Byfield, G.; Hartnett, M.E.; Clemmons, D. Disruption of the association of integrin-associated protein (IAP) with tyrosine phosphatase non-receptor type substrate-1 (SHPS)-1 inhibits pathophysiological changes in retinal endothelial function in a rat model of diabetes. Diabetologia 2012, 55, 835–844. [Google Scholar] [CrossRef][Green Version]
- Xi, G.; Wai, C.; Clemmons, D. Inhibition of Aberrant IGF-I Signaling in Diabetic Male Rat Retina Prevents and Reverses Changes of Diabetic Retinopathy. J. Diabetes Res. 2019, 2019, 6456032. [Google Scholar] [CrossRef] [PubMed]
- Upreti, S.; Sen, S.; Nag, T.C.; Ghosh, M.P. Insulin like growth factor-1 works synergistically with dopamine to attenuate diabetic retinopathy by downregulating vascular endothelial growth factor. Biomed. Pharmacother. 2022, 149, 112868. [Google Scholar] [CrossRef]
- Toda, N.; Nakanishi-Toda, M. Nitric oxide: Ocular blood flow, glaucoma, and diabetic retinopathy. Prog. Retin. Eye Res. 2007, 26, 205–238. [Google Scholar] [CrossRef]
- Imrie, H.; Viswambharan, H.; Sukumar, P.; Abbas, A.; Cubbon, R.M.; Yuldasheva, N.; Gage, M.; Smith, J.; Galloway, S.; Skromna, A.; et al. Novel Role of the IGF-1 Receptor in Endothelial Function and Repair: Studies in Endothelium-Targeted IGF-1 Receptor Transgenic Mice. Diabetes 2012, 61, 2359–2368. [Google Scholar] [CrossRef] [PubMed]
- Feenstra, D.J.; Yego, E.C.; Mohr, S. Modes of Retinal Cell Death in Diabetic Retinopathy. J. Clin. Exp. Ophthalmol. 2013, 4, 298. [Google Scholar] [PubMed]
- Seigel, G.M.; Lupien, S.B.; Campbell, L.M.; Ishii, D.N. Systemic IGF-I treatment inhibits cell death in diabetic rat retina. J. Diabetes Its Complicat. 2006, 20, 196–204. [Google Scholar] [CrossRef]
- Kermer, P.; Klöcker, N.; Labes, M.; Bähr, M. Insulin-Like Growth Factor-I Protects Axotomized Rat Retinal Ganglion Cells from Secondary Death via PI3-K-Dependent Akt Phosphorylation and Inhibition of Caspase-3 In Vivo. J. Neurosci. 2000, 20, 722–728. [Google Scholar] [CrossRef]
- Rajala, A.; Teel, K.; Bhat, M.A.; Batushansky, A.; Griffin, T.M.; Purcell, L.; Rajala, R.V.S. Insulin-like growth factor 1 receptor mediates photoreceptor neuroprotection. Cell Death Dis. 2022, 13, 613. [Google Scholar] [CrossRef]
- Arroba, A.I.; Mazzeo, A.; Cazzoni, D.; Beltramo, E.; Hernández, C.; Porta, M.; Simó, R.; Valverde, Á.M. Somatostatin protects photoreceptor cells against high glucose–induced apoptosis. Mol. Vis. 2016, 22, 1522. [Google Scholar]
- Wan, J.; Zhao, X.F.; Vojtek, A.; Goldman, D. Retinal injury, growth factors, and cytokines converge on β-catenin and pStat3 signaling to stimulate retina regeneration. Cell Rep. 2014, 9, 285–297. [Google Scholar] [CrossRef]
- Rajala, R.V.S.; Rajala, A. From Insight to Eyesight: Unveiling the Secrets of the Insulin-Like Growth Factor Axis in Retinal Health. Aging Dis. 2024, 15, 1994–2002. [Google Scholar] [CrossRef]
- de Figueiredo, C.S.; Raony, Í.; Medina, S.V.; de Mello Silva, E.; dos Santos, A.A.; Giestal-de-Araujo, E. Insulin-like growth factor-1 stimulates retinal cell proliferation via activation of multiple signaling pathways. Curr. Res. Neurobiol. 2023, 4, 100068. [Google Scholar] [CrossRef]
- Granja, M.G.; Braga, L.E.G.; de Oliveira, R.M.; de Mello Silva, E.; Gonçalves-de-Albuquerque, C.F.; Silva, A.R.; de Castro-Faria-Neto, H.C.; dos Santos, A.A.; Giestal-de-Araujo, E. IGF-1 and IGF-1R modulate the effects of IL-4 on retinal ganglion cells survival: The involvement of M1 muscarinic receptor. Biochem. Biophys. Res. Commun. 2019, 519, 53–60. [Google Scholar] [CrossRef]
- Tang, J.; Kern, T.S. Inflammation in diabetic retinopathy. Prog. Retin. Eye Res. 2011, 30, 343–358. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, K.; Khosrof, S.; Bursell, S.-E.; Rohan, R.; Murata, T.; Clermont, A.C.; Aiello, L.P.; Ogura, Y.; Adamis, A.P. Prevention of leukostasis and vascular leakage in streptozotocin-induced diabetic retinopathy via intercellular adhesion molecule-1 inhibition. Proc. Natl. Acad. Sci. USA 1999, 96, 10836–10841. [Google Scholar] [CrossRef] [PubMed]
- Pflugfelder, S.C.; Stern, M.; Zhang, S.; Shojaei, A. LFA-1/ICAM-1 Interaction as a Therapeutic Target in Dry Eye Disease. J. Ocul. Pharmacol. Ther. 2017, 33, 5. [Google Scholar] [CrossRef] [PubMed]
- Joussen, A.M.; Poulaki, V.; Qin, W.; Kirchhof, B.; Mitsiades, N.; Wiegand, S.J.; Rudge, J.; Yancopoulos, G.D.; Adamis, A.P. Retinal vascular endothelial growth factor induces intercellular adhesion molecule-1 and endothelial nitric oxide synthase expression and initiates early diabetic retinal leukocyte adhesion in vivo. Am. J. Pathol. 2002, 160, 501–509. [Google Scholar] [CrossRef]
- Guo, S.; Wang, H.; Yin, Y. Microglia Polarization From M1 to M2 in Neurodegenerative Diseases. Front. Aging Neurosci. 2022, 14, 815347. [Google Scholar] [CrossRef]
- Pierre, W.C.; Smith, P.L.; Londono, I.; Chemtob, S.; Mallard, C.; Lodygensky, G.A. Neonatal microglia: The cornerstone of brain fate. Brain Behav. Immun. 2017, 59, 333–345. [Google Scholar] [CrossRef]
- Paolicelli, R.C.; Sierra, A.; Stevens, B.; Tremblay, M.-E.; Aguzzi, A.; Ajami, B.; Amit, I.; Audinat, E.; Bechmann, I.; Bennett, M.; et al. Microglia states and nomenclature: A field at its crossroads. Neuron 2022, 110, 3458–3483. [Google Scholar] [CrossRef]
- Altmann, C.; Schmidt, M.H.H. The Role of Microglia in Diabetic Retinopathy: Inflammation, Microvasculature Defects and Neurodegeneration. Int. J. Mol. Sci. 2018, 19, 110. [Google Scholar] [CrossRef] [PubMed]
- Zeng, H.Y.; Green, W.R.; Tso, M.O.M. Microglial activation in human diabetic retinopathy. Arch. Ophthalmol. 2008, 126, 227–232. [Google Scholar] [CrossRef]
- Arroba, A.I.; Alcalde-Estevez, E.; García-Ramírez, M.; Cazzoni, D.; de la Villa, P.; Sánchez-Fernández, E.M.; Mellet, C.O.; Fernández, J.M.G.; Hernández, C.; Simó, R.; et al. Modulation of microglia polarization dynamics during diabetic retinopathy in db/db mice. Biochim. Biophys. Acta 2016, 1862, 1663–1674. [Google Scholar] [CrossRef]
- Li, J.; Yu, S.; Lu, X.; Cui, K.; Tang, X.; Xu, Y.; Liang, X. The phase changes of M1/M2 phenotype of microglia/macrophage following oxygen-induced retinopathy in mice. Inflamm. Res. 2021, 70, 183–192. [Google Scholar] [CrossRef]
- Mohlin, C.; Sandholm, K.; Kvanta, A.; Ekdahl, K.N.; Johansson, K. A model to study complement involvement in experimental retinal degeneration. Upsala J. Med. Sci. 2018, 123, 28. [Google Scholar] [CrossRef] [PubMed]
- Muramatsu, D.; Wakabayashi, Y.; Usui, Y.; Okunuki, Y.; Kezuka, T.; Goto, H. Correlation of complement fragment C5a with inflammatory cytokines in the vitreous of patients with proliferative diabetic retinopathy. Graefes Arch. Clin. Exp. Ophthalmol. 2013, 251, 15–17. [Google Scholar] [CrossRef]
- Gerl, V.B.; Bohl, J.; Pitz, S.; Stoffelns, B.; Pfeiffer, N.; Bhakdi, S. Extensive deposits of complement C3d and C5b-9 in the choriocapillaris of eyes of patients with diabetic retinopathy. Investig. Ophthalmol. Vis. Sci. 2002, 43, 1104–1108. Available online: https://pubmed.ncbi.nlm.nih.gov/11923252/ (accessed on 17 April 2025).
- Koleva-Georgieva, D.N.; Sivkova, N.P.; Terzieva, D. Serum inflammatory cytokines IL-1beta, IL-6, TNF-alpha and VEGF have influence on the development of diabetic retinopathy. Folia Med. 2011, 53, 44–50. [Google Scholar]
- Kinuthia, U.M.; Wolf, A.; Langmann, T. Microglia and Inflammatory Responses in Diabetic Retinopathy. Front. Immunol. 2020, 11, 564077. [Google Scholar] [CrossRef]
- Labandeira-Garcia, J.L.; Costa-Besada, M.A.; Labandeira, C.M.; Villar-Cheda, B.; Rodríguez-Perez, A.I. Insulin-like growth factor-1 and neuroinflammation. Front. Aging Neurosci. 2017, 9, 309933. [Google Scholar] [CrossRef]
- Villacampa, P.; Ribera, A.; Motas, S.; Ramírez, L.; García, M.; de la Villa, P.; Haurigot, V.; Bosch, F. Insulin-like Growth Factor I (IGF-I)-induced Chronic Gliosis and Retinal Stress Lead to Neurodegeneration in a Mouse Model of Retinopathy. J. Biol. Chem. 2013, 288, 17631. [Google Scholar] [CrossRef]
- Rodriguez-de la Rosa, L.; Fernandez-Sanchez, L.; Germain, F.; Murillo-Cuesta, S.; Varela-Nieto, I.; De La Villa, P.; Cuenca, N. Age-related functional and structural retinal modifications in the Igf1-/- null mouse. Neurobiol. Dis. 2012, 46, 476–485. [Google Scholar] [CrossRef]
- Spittau, B.; Wullkopf, L.; Zhou, X.; Rilka, J.; Pfeifer, D.; Krieglstein, K. Endogenous transforming growth factor-beta promotes quiescence of primary microglia in vitro. Glia 2013, 61, 287–300. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Silverman, S.M.; Zhao, L.; Villasmil, R.; Campos, M.M.; Amaral, J.; Wong, W.T.; States, U. Absence of TGFβ signaling in retinal microglia induces retinal degeneration and exacerbates choroidal neovascularization. Elife 2019, 8, e42049. [Google Scholar] [CrossRef] [PubMed]
- Usui-Ouchi, A.; Eade, K.; Giles, S.; Ideguchi, Y.; Ouchi, Y.; Aguilar, E.; Wei, G.; Marra, K.V.; Berlow, R.B.; Friedlander, M. Deletion of Tgfβ signal in activated microglia prolongs hypoxia-induced retinal neovascularization enhancing Igf1 expression and retinal leukostasis. Glia 2022, 70, 1762. [Google Scholar] [CrossRef] [PubMed]
- Sohrabji, F. Estrogen-IGF-1 interactions in neuroprotection: Ischemic stroke as a case study. Front. Neuroendocrinol. 2015, 36, 1–14. [Google Scholar] [CrossRef]
- Garcia-Segura, L.M.; Arevalo, M.A.; Azcoitia, I. Interactions of estradiol and insulin-like growth factor-I signalling in the nervous system: New advances. Prog. Brain Res. 2010, 181, 251–272. [Google Scholar]
- Brywe, K.G.; Mallard, C.; Gustavsson, M.; Hedtjärn, M.; Leverin, A.L.; Wang, X.; Blomgren, K.; Isgaard, J.; Hagberg, H. IGF-I neuroprotection in the immature brain after hypoxia-ischemia, involvement of Akt and GSK3β? Eur. J. Neurosci. 2005, 21, 1489–1502. [Google Scholar] [CrossRef]
- Rodriguez-Perez, A.I.; Borrajo, A.; Diaz-Ruiz, C.; Garrido-Gil, P.; Labandeira-Garcia, J.L. Crosstalk between insulin-like growth factor-1 and angiotensin-II in dopaminergic neurons and glial cells: Role in neuroinflammation and aging. Oncotarget 2016, 7, 30049–30067. [Google Scholar] [CrossRef]
- Poulsen, J.E. The Houssay Phenomenon in Man: Recovery from Retinopathy in a Case of Diabetes with Simmonds’ Disease. Diabetes 1953, 2, 7–12. [Google Scholar] [CrossRef]
- Wright, A.D.; Kohner, E.M.; Oakley, N.W.; Hartog, M.; Joplin, G.F.; Fraser, T.R. Serum Growth Hormone Levels and the Response of Diabetic Retinopathy to Pituitary Ablation. Br. Med. J. 1969, 2, 346. [Google Scholar] [CrossRef] [PubMed]
- Merimee, T.J. A follow-up study of vascular disease in growth-hormone-deficient dwarfs with diabetes. N. Engl. J. Med. 1978, 298, 1217–1222. [Google Scholar] [CrossRef]
- Dills, D.G.; Moss, S.E.; Klein, R.; Klein, B.E.K. Association of elevated IGF-I levels with increased retinopathy in late-onset diabetes. Diabetes 1991, 40, 1725–1730. [Google Scholar] [CrossRef]
- Stojanovic, M.; Popevic, M.; Pekic, S.; Doknic, M.; Miljic, D.; Medic-Stojanoska, M.; Topalov, D.; Stojanovic, J.; Milovanovic, A.; Petakov, M.; et al. Serum Insulin-Like Growth Factor-1 (IGF-1) Age-Specific Reference Values for Healthy Adult Population of Serbia. Acta Endocrinol. 2021, 17, 462. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, J.; Song, C.; Zhu, L.; Yu, Y. Lower prevalence of proliferative diabetic retinopathy in elderly onset patients with diabetes. Diabetes Res. Clin. Pract. 2017, 125, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Huber, A.L.; Angermann, R.; Nowosielski, Y.; Seifarth, C.; Kralinger, M.T.; Zehetner, C. Effects of Intravitreal Aflibercept on the Systemic Insulin-like Growth Factor-I and Vascular Endothelial Growth Factor-A in Patients with Diabetic Retinopathy and Age-Related Macular Degeneration. J. Ophthalmol. 2021, 2021, 7058505. [Google Scholar] [CrossRef] [PubMed]
- Loukovaara, S.; Immonen, I.J.; Koistinen, R.; Rutanen, E.-M.; Hiilesmaa, V.; Loukovaara, M.; Kaaja, R.J. The insulin-like growth factor system and Type 1 diabetic retinopathy during pregnancy. J. Diabetes Complicat. 2005, 19, 297–304. [Google Scholar] [CrossRef]
- Janssen, J.A.M.J.L.; Lamberts, S.W.J. Circulating IGF-I and its protective role in the pathogenesis of diabetic angiopathy. Clin. Endocrinol. 2000, 52, 1–9. [Google Scholar] [CrossRef]
- Raman, P.; Singal, A.K.; Behl, A. Effect of Insulin-Like Growth Factor-1 on Diabetic Retinopathy in Pubertal Age Patients with Type 1 Diabetes. Asia Pac. J. Ophthalmol. 2019, 8, 319. [Google Scholar] [CrossRef]
- Öberg, D.; Salemyr, J.; Örtqvist, E.; Juul, A.; Bang, P. A longitudinal study of serum insulin-like growth factor-I levels over 6 years in a large cohort of children and adolescents with type 1 diabetes mellitus: A marker reflecting diabetic retinopathy. Pediatr. Diabetes 2018, 19, 972–978. [Google Scholar] [CrossRef]
- Feldt-Rasmussen, U.; Klose, M. Adult Growth Hormone Deficiency—Clinical Management. In Endotext [Internet]; MDText.com, Inc.: South Dartmouth, MA, USA, 2022. [Google Scholar]
- Payne, J.F.; Tangpricha, V.; Cleveland, J.; Lynn, M.J.; Ray, R.; Srivastava, S.K. Serum insulin-like growth factor-I in diabetic retinopathy. Mol. Vis. 2011, 17, 2318. [Google Scholar]
- Yorston, D. Anti-VEGF drugs in the prevention of blindness. Community Eye Health 2014, 27, 44. [Google Scholar] [PubMed]
- Boyer, D.S.; Hopkins, J.J.; Sorof, J.; Ehrlich, J.S. Anti-vascular endothelial growth factor therapy for diabetic macular edema. Ther. Adv. Endocrinol. Metab. 2013, 4, 151–169. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Obeid, A.; Aderman, C.M.; Talcott, K.E.; Ali, F.S.; Adam, M.K.; Rovner, B.W.; Hyman, L.; Ho, A.C.; Hsu, J. Loss to Follow-up After Intravitreal Anti-Vascular Endothelial Growth Factor Injections in Patients with Diabetic Macular Edema. Ophthalmol. Retin. 2019, 3, 230–236. [Google Scholar] [CrossRef] [PubMed]
- Blinder, K.J.; Dugel, P.U.; Chen, S.; Jumper, J.M.; Walt, J.G.; Hollander, D.; Scott, L.C. Anti-VEGF treatment of diabetic macular edema in clinical practice: Effectiveness and patterns of use (ECHO Study Report 1). Clin. Ophthalmol. 2017, 11, 393–401. [Google Scholar] [CrossRef]
- Nguyen, Q.D.; Brown, D.M.; Marcus, D.M.; Boyer, D.S.; Patel, S.; Feiner, L.; Gibson, A.; Sy, J.; Rundle, A.C.; Hopkins, J.J.; et al. Ranibizumab for diabetic macular edema: Results from 2 phase III randomized trials: RISE and RIDE. Ophthalmology 2012, 119, 789–801. [Google Scholar] [CrossRef]
- Chawan-Saad, J.; Wu, M.; Wu, A.; Wu, L. Corticosteroids for Diabetic Macular Edema. Taiwan. J. Ophthalmol. 2019, 9, 233–242. [Google Scholar]
- Bailes, J.; Soloviev, M. Insulin-Like Growth Factor-1 (IGF-1) and Its Monitoring in Medical Diagnostic and in Sports. Biomolecules 2021, 11, 1–15. [Google Scholar] [CrossRef]
- Koller, E.A.; Green, L.; Gertner, J.M.; Bost, M.; Malozowski, S.N. Retinal changes mimicking diabetic retinopathy in two nondiabetic, growth hormone-treated patients. J. Clin. Endocrinol. Metab. 1998, 83, 2380–2383. [Google Scholar] [CrossRef]
- Wu, T.E.; Chen, H.S. The role of growth hormone and IGF-1 in retinopathy: A prospective study of retinopathy in patients with acromegaly and impaired fasting glucose. Diabetol. Metab. Syndr. 2022, 14, 38. [Google Scholar] [CrossRef]
- Füchtbauer, L.; Olsson, D.S.; Coopmans, E.C.; Bengtsson, B.; Norrman, L.-L.; Neggers, S.J.C.M.M.; Hellström, A.; Johannsson, G. Increased number of retinal vessels in acromegaly. Eur. J. Endocrinol. 2020, 182, 293–302. [Google Scholar] [CrossRef] [PubMed]
- Frohman, L.A. Physiology of the Growth Hormone Releasing Hormone-Somatostatin-Growth Hormone-Insulin-Like Growth Factor I Axis. In GHRH, GH, and IGF-I; Springer: New York, NY, USA, 1995; pp. 3–10. [Google Scholar] [CrossRef]
- Fang, Y.; Wang, Q.; Li, Y.; Zeng, L.; Liu, J.; Ou, K. On implications of somatostatin in diabetic retinopathy. Neural Regen. Res. 2024, 19, 1984. [Google Scholar] [CrossRef]
- Grant, M.B.; Caballero, S. The potential role of octreotide in the treatment of diabetic retinopathy. Treat. Endocrinol. 2005, 4, 199–203. [Google Scholar] [CrossRef]
- Mallet, B.; Vialettes, B.; Haroche, S.; Escoffier, P.; Gastaut, P.; Taubert, J.P.; Vague, P. Stabilization of severe proliferative diabetic retinopathy by long-term treatment with SMS 201-995. Diabete Metab. 1992, 18, 438–444. [Google Scholar]
- Grant, M.B.; Mames, R.N.; Fitzgerald, C.; Hazariwala, K.M.; Cooper-DeHoff, R.; Caballero, S.; Estes, K.S. The efficacy of octreotide in the therapy of severe nonproliferative and early proliferative diabetic retinopathy: A randomized controlled study. Diabetes Care 2000, 23, 504–509. [Google Scholar] [CrossRef]
- Boehm, B.O.; Lang, G.K.; Jehle, P.M.; Feldmann, B.; Lang, G.E. Octreotide reduces vitreous hemorrhage and loss of visual acuity risk in patients with high-risk proliferative diabetic retinopathy. Horm. Metab. Res. 2001, 33, 300–306. [Google Scholar] [CrossRef] [PubMed]
- Krassas, G.E.; Tzotzas, T.; Papazisis, K.; Pazaitou-Panayiotou, K.; Boboridis, K. The efficacy of somatostatin analogues in the treatment of diabetic retinopathy and thyroid eye disease. Clin. Ophthalmol. 2007, 1, 209. [Google Scholar]
- Plöckinger, U.; Dienemann, D.; Quabbe, H.J. Gastrointestinal side-effects of octreotide during long-term treatment of acromegaly. J. Clin. Endocrinol. Metab. 1990, 71, 1658–1662. [Google Scholar] [CrossRef] [PubMed]
- Witek, P.; Bolanowski, M.; Szamotulska, K.; Wojciechowska-Luźniak, A.; Jawiarczyk-Przybyłowska, A.; Kałużny, M. The Effect of 6 Months’ Treatment with Pasireotide LAR on Glucose Metabolism in Patients with Resistant Acromegaly in Real-World Clinical Settings. Front. Endocrinol. 2021, 12, 633944. [Google Scholar] [CrossRef]
- Hernández, C.; Arroba, A.I.; Bogdanov, P.; Ramos, H.; Simó-Servat, O.; Simó, R.; Valverde, A.M. Effect of Topical Administration of Somatostatin on Retinal Inflammation and Neurodegeneration in an Experimental Model of Diabetes. J. Clin. Med. 2020, 9, 1–18. [Google Scholar] [CrossRef]
- Amato, R.; Giannaccini, M.; Monte, M.D.; Cammalleri, M.; Pini, A.; Raffa, V.; Lulli, M.; Casini, G. Association of the Somatostatin Analog Octreotide with Magnetic Nanoparticles for Intraocular Delivery: A Possible Approach for the Treatment of Diabetic Retinopathy. Front. Bioeng. Biotechnol. 2020, 8, 515624. [Google Scholar] [CrossRef] [PubMed]
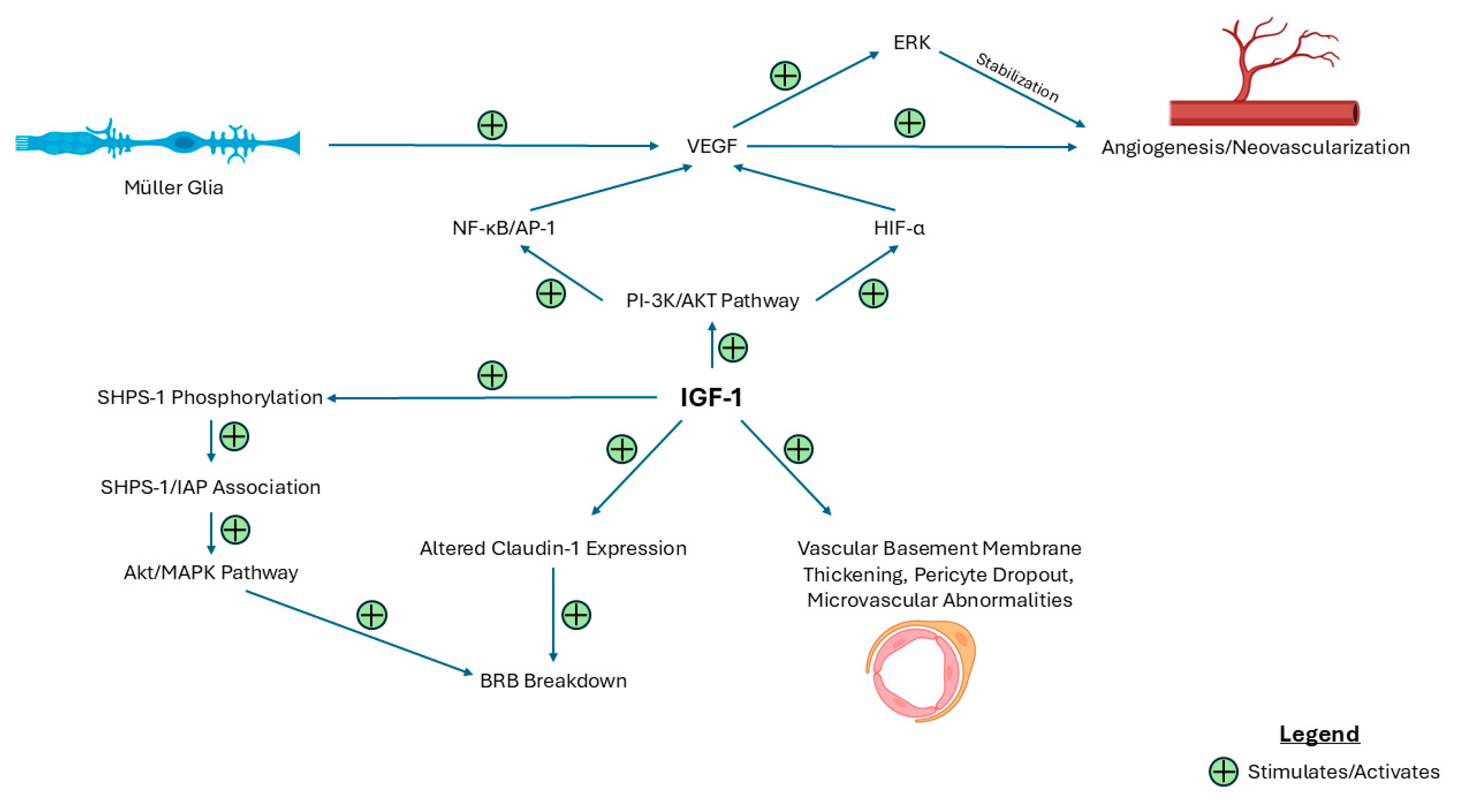
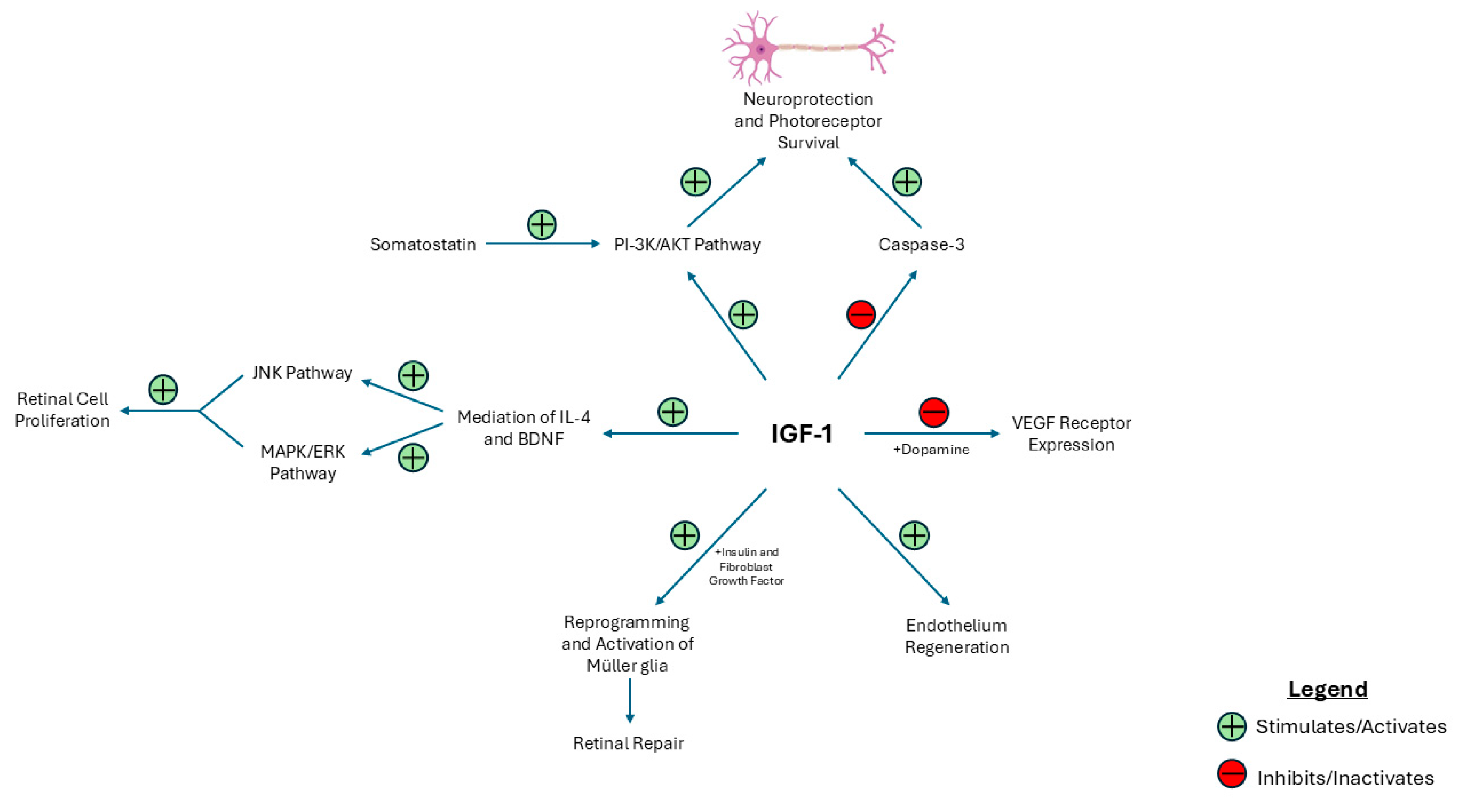
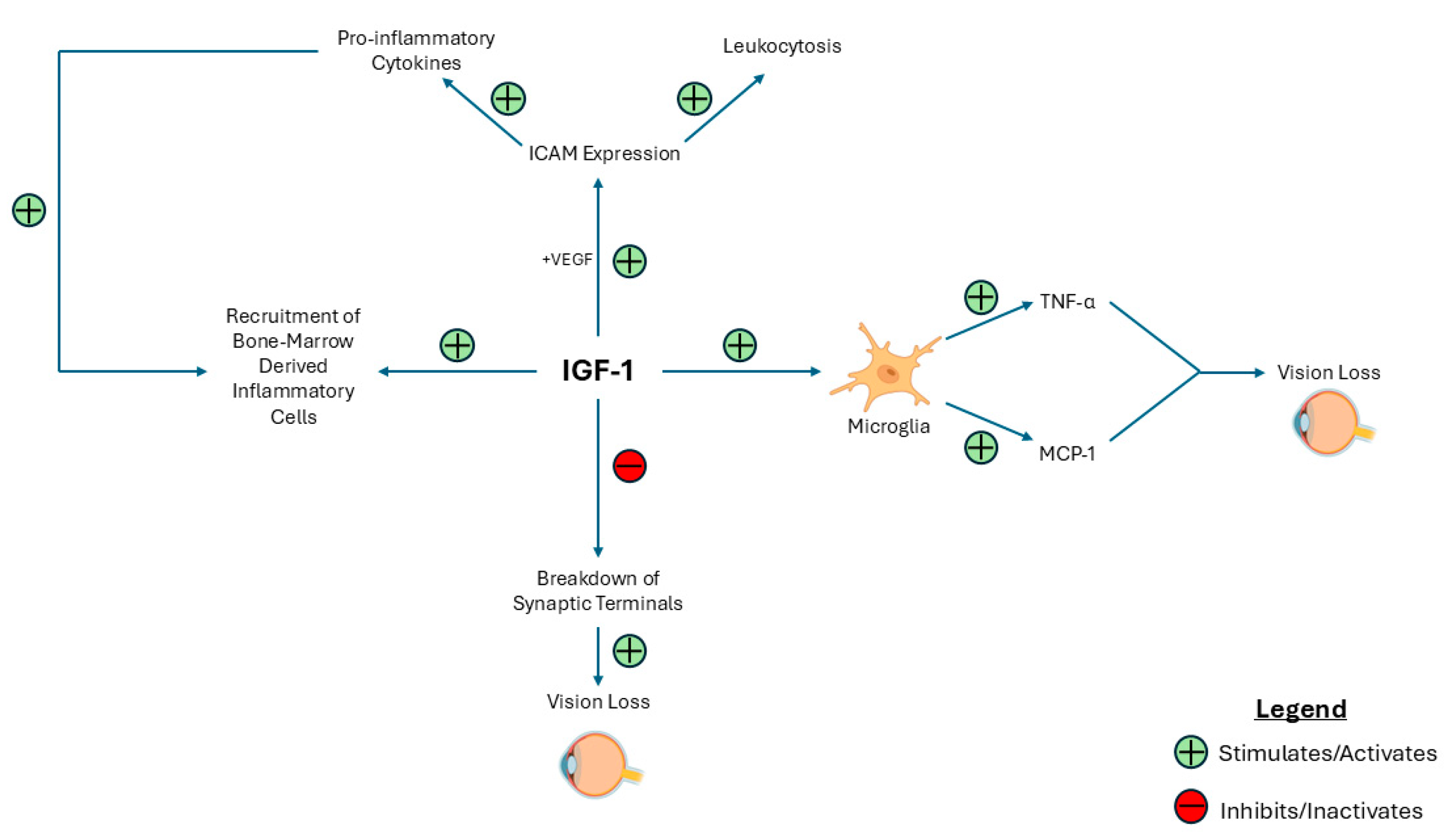
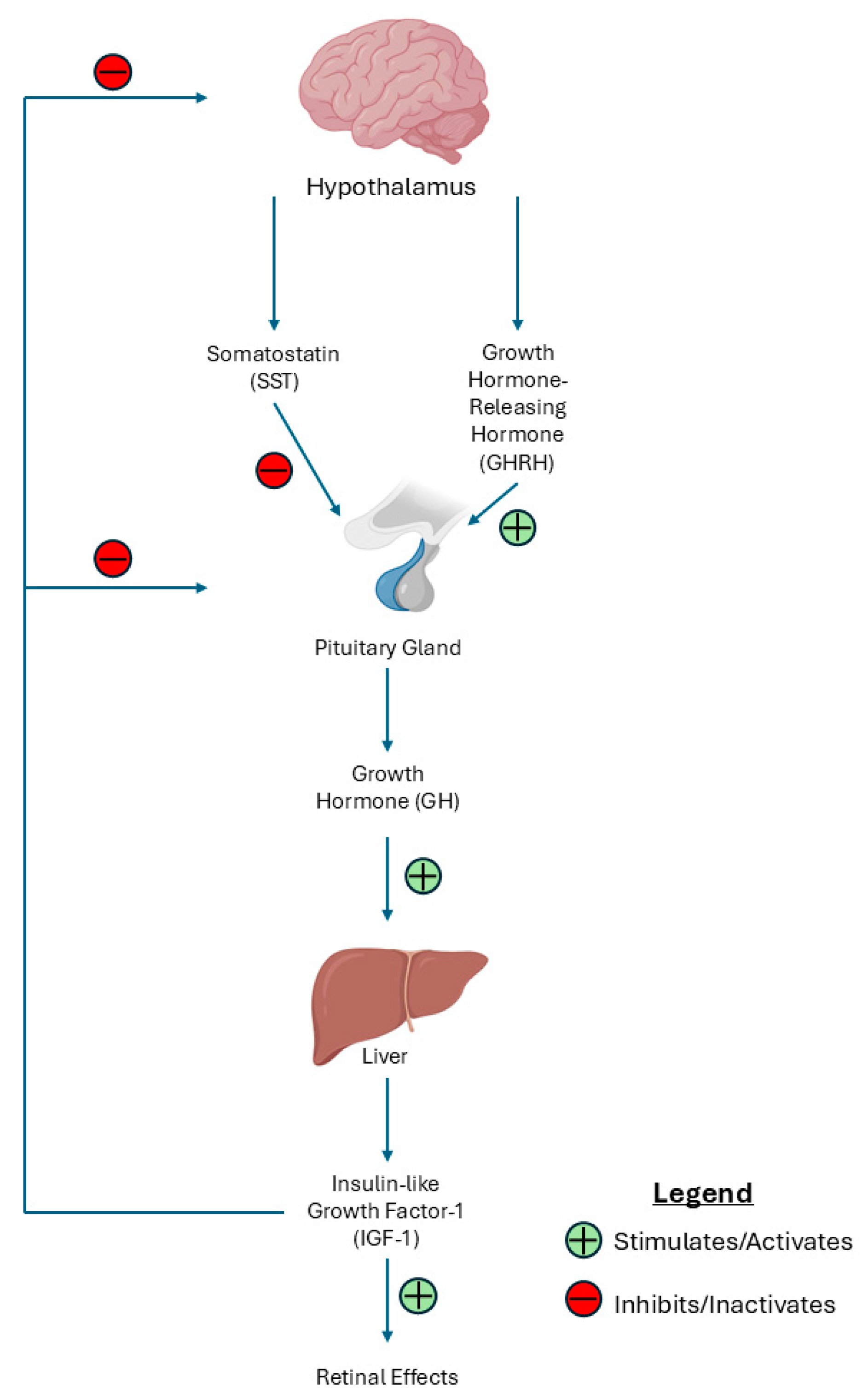
| Key Finding | Evidence |
|---|---|
| IGF-1 promotes vascular changes. | IGF-1 upregulates VEGF expression via PI-3K/AKT and JNK/AP-1 pathways and stabilizes neovasculature. |
| IGF-1 disrupts blood–retinal barrier integrity. | IGF-1 downregulates claudin-1 and increases aberrant activity of AKT and MAPK via SHPS-1 phosphorylation and contributes to structural microvascular abnormalities. |
| IGF-1 promotes retinal cell survival and repair. | Apoptosis is reduced via modulation of the PI-3K/AKT and caspase-3 pathways. Retinal repair and endothelium regeneration are also promoted via direct effects. Retinal cell proliferation is modulated via IL-4 and BDNF. |
| IGF-1 drives retinal inflammation. | The upregulation of proinflammatory cytokines such as TNF-α and MCP-1 and the expression of ICAM-1 contributes to leukostasis and microglial activation. |
| IGF-1 interacts with several signaling molecules. | TGF-β suppresses retinal neovascularization and leukostasis. Estrogen provides neuroprotection via the PI-3K/AKT and MAPK pathways. Dopamine prevents VEGF-1 receptor expression. Angiotensin II promotes the M2 phenotype of microglia. |
| Clinically, the link between IGF-1 and the progression of DR and DME remains controversial. | Conflicting studies report both positive and negative correlations between serum IGF-1 and DR and DME progression. |
| Targets of the GH/IGF-1 axis provide potential therapeutic avenues for the management of DR and DME. | Somatostatin (SST) analogues have shown efficacy in the treatment of DR. Novel delivery methods may prevent unwanted side effects. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malepati, A.; Grant, M.B. The Role and Diagnostic Potential of Insulin-like Growth Factor 1 in Diabetic Retinopathy and Diabetic Macular Edema. Int. J. Mol. Sci. 2025, 26, 3961. https://doi.org/10.3390/ijms26093961
Malepati A, Grant MB. The Role and Diagnostic Potential of Insulin-like Growth Factor 1 in Diabetic Retinopathy and Diabetic Macular Edema. International Journal of Molecular Sciences. 2025; 26(9):3961. https://doi.org/10.3390/ijms26093961
Chicago/Turabian StyleMalepati, Akanksha, and Maria B. Grant. 2025. "The Role and Diagnostic Potential of Insulin-like Growth Factor 1 in Diabetic Retinopathy and Diabetic Macular Edema" International Journal of Molecular Sciences 26, no. 9: 3961. https://doi.org/10.3390/ijms26093961
APA StyleMalepati, A., & Grant, M. B. (2025). The Role and Diagnostic Potential of Insulin-like Growth Factor 1 in Diabetic Retinopathy and Diabetic Macular Edema. International Journal of Molecular Sciences, 26(9), 3961. https://doi.org/10.3390/ijms26093961




