Autosomal Dominant Polycystic Kidney Disease-Related Multifocal Renal Cell Carcinoma: A Narrative Iconographic Review
Abstract
1. Introduction
2. ADPKD Severity and ESRD
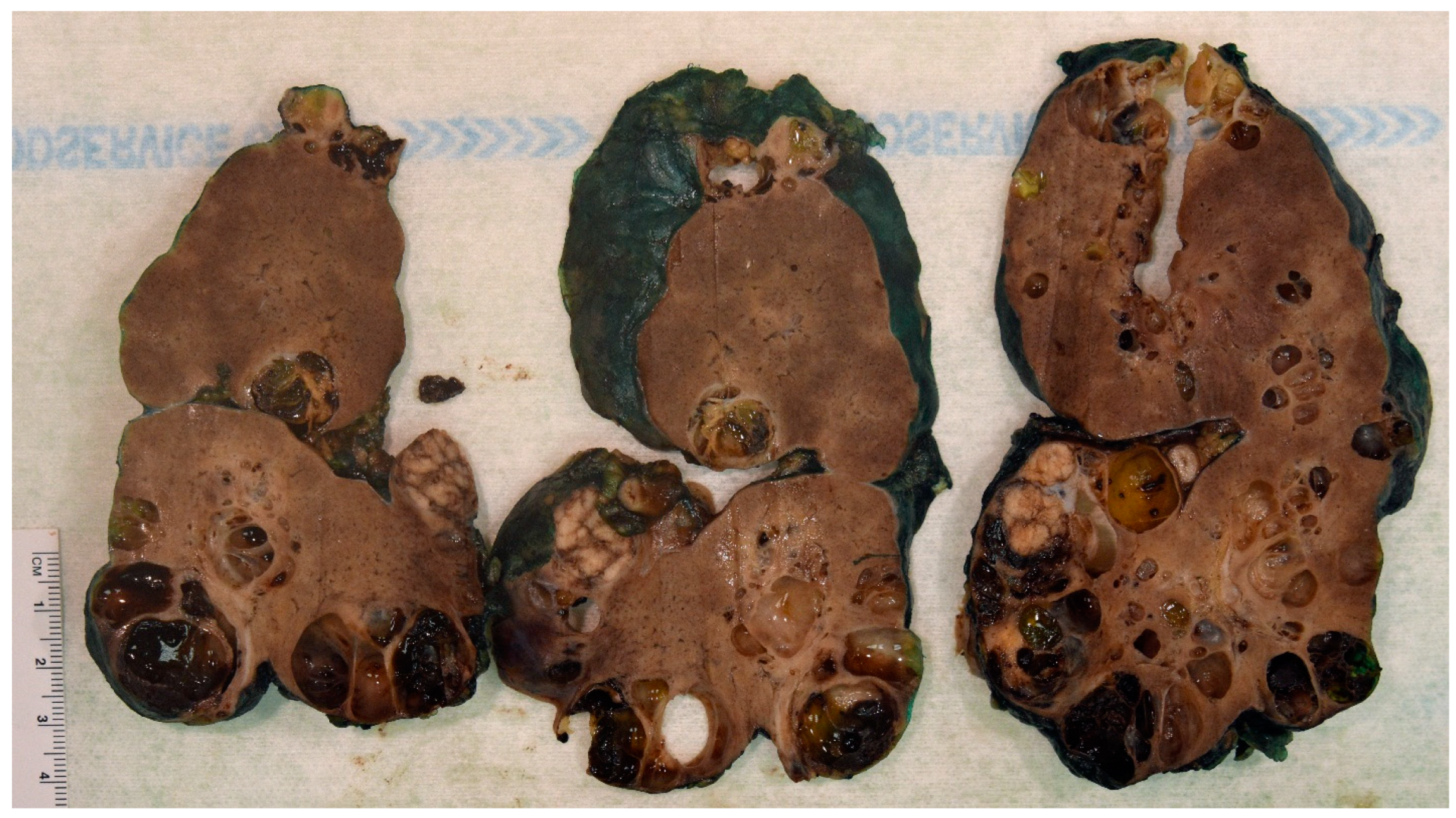
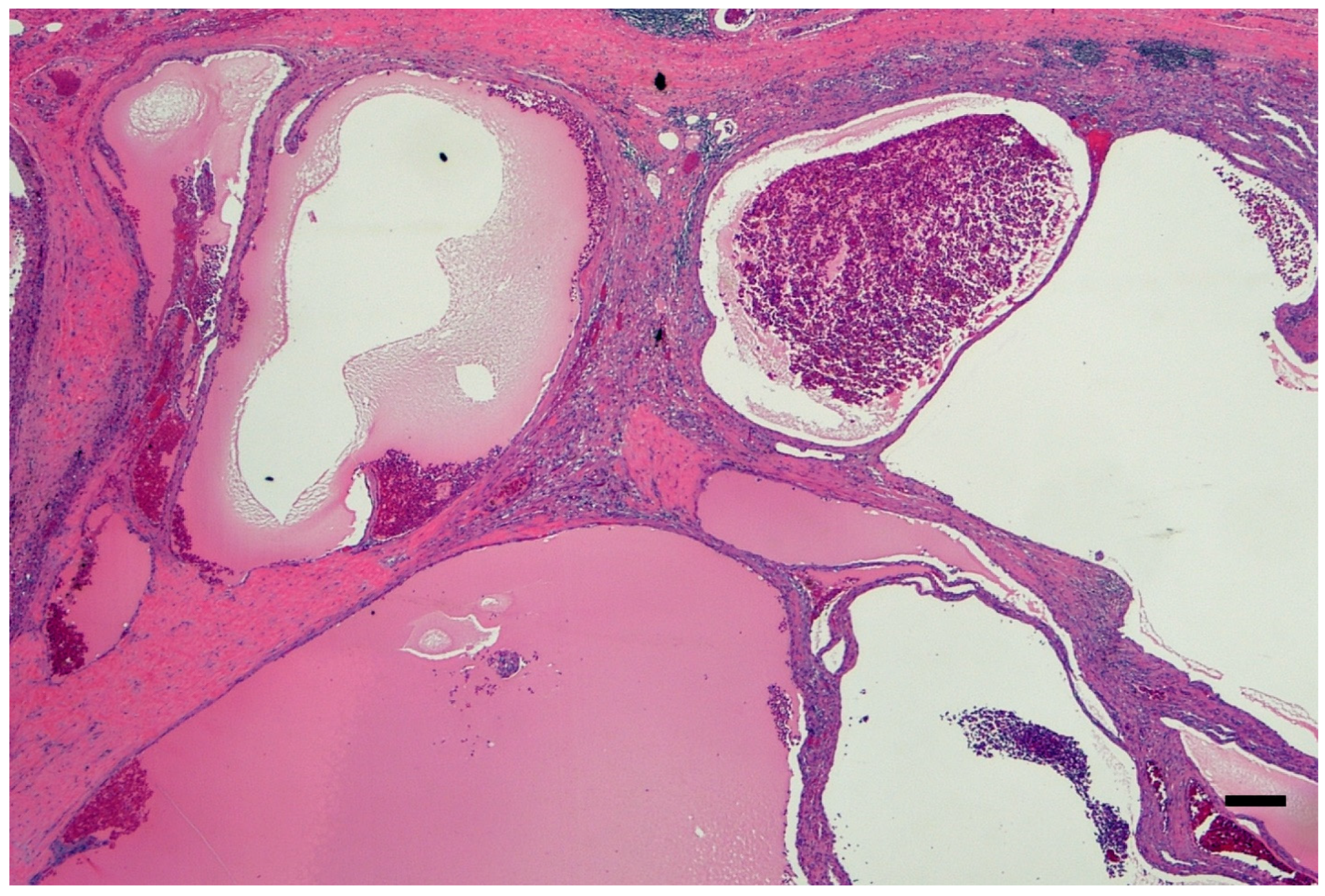
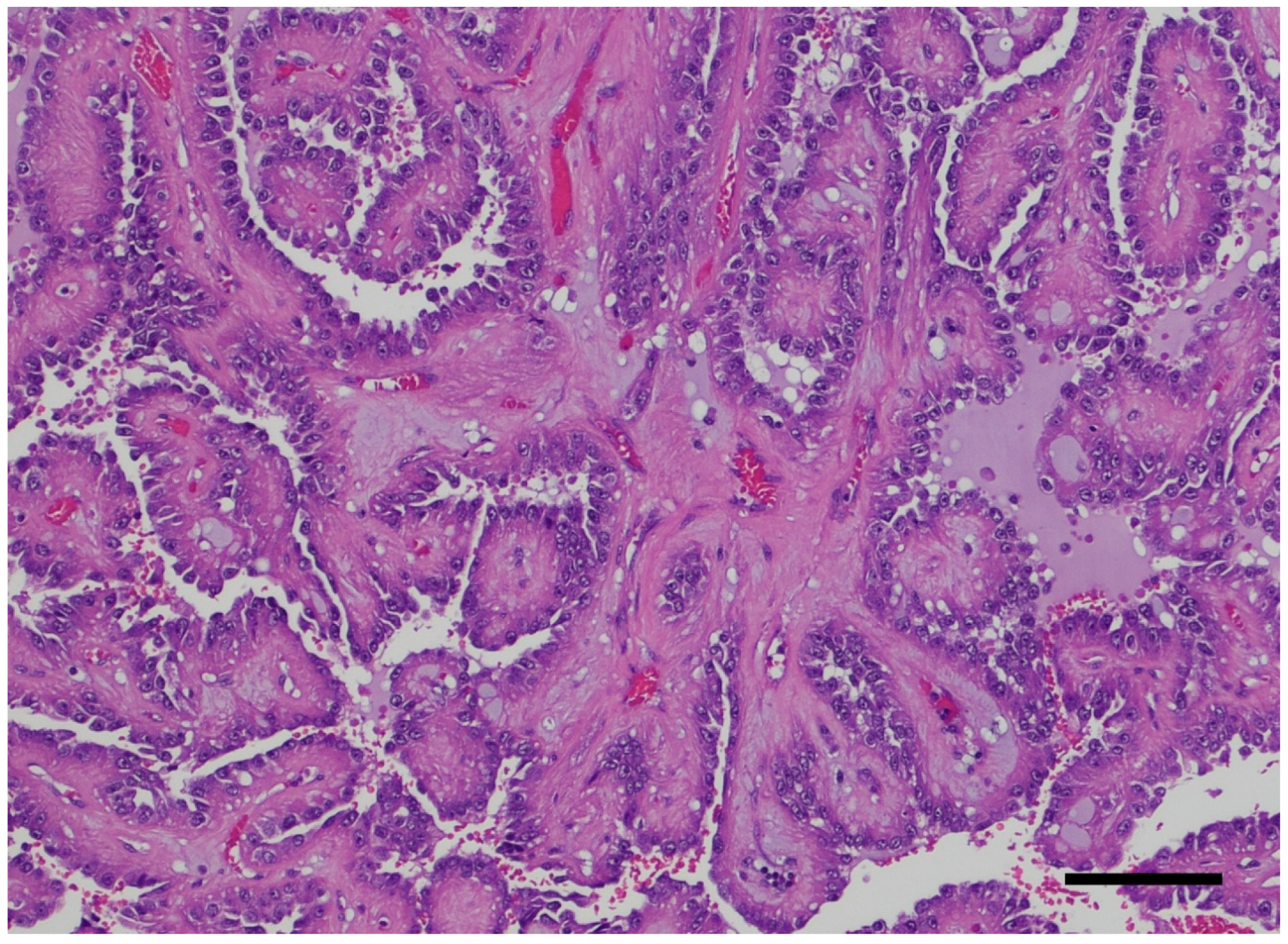
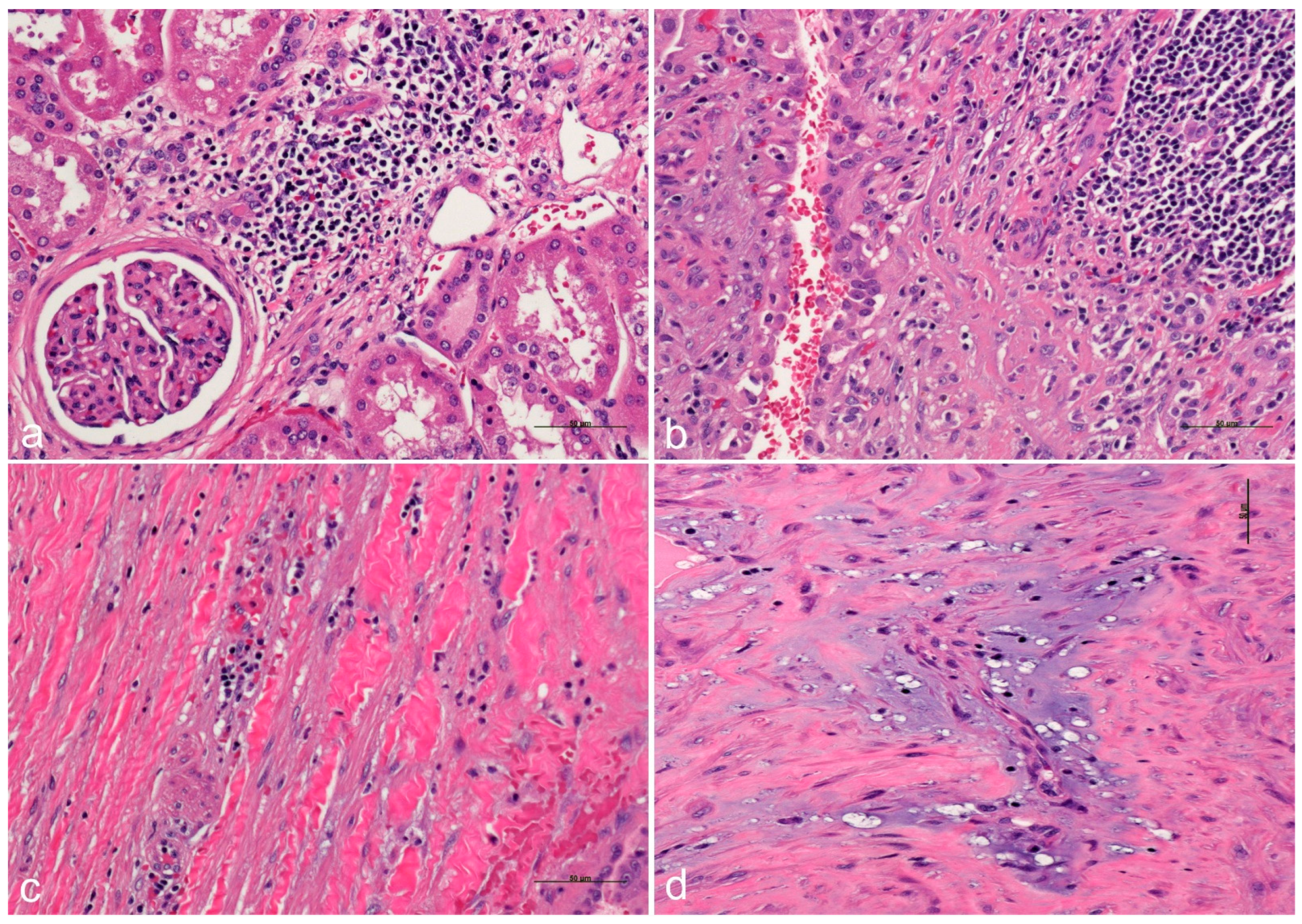
3. Pathology Workup
4. ADPKD-RCC Conundrum
5. Role of Inflammation as Promoter in ADPKD-Related RCC
6. “Disguising” Epidemiology
7. Therapeutical Options
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Sergi, C. Pathology of Childhood and Adolescence, 1st ed.; Springer: Berlin/Heidelberg, Germany, 2020. [Google Scholar]
- Johnson, C.A.; Gissen, P.; Sergi, C. Molecular pathology and genetics of congenital hepatorenal fibrocystic syndromes. J. Med. Genet. 2003, 40, 311–319. [Google Scholar] [CrossRef]
- van de Laarschot, L.F.M.; Te Morsche, R.H.M.; Hoischen, A.; Venselaar, H.; Roelofs, H.M.; Cnossen, W.R.; Banales, J.M.; Roepman, R.; Drenth, J.P.H. Novel GANAB variants associated with polycystic liver disease. Orphanet J. Rare Dis. 2020, 15, 302. [Google Scholar] [CrossRef]
- Cantero, M.D.R.; Cantiello, H.F. Polycystin-2 (TRPP2): Ion channel properties and regulation. Gene 2022, 827, 146313. [Google Scholar] [CrossRef]
- Chang, A.R.; Moore, B.S.; Luo, J.Z.; Sartori, G.; Fang, B.; Jacobs, S.; Abdalla, Y.; Taher, M.; Carey, D.J.; Triffo, W.J.; et al. Exome Sequencing of a Clinical Population for Autosomal Dominant Polycystic Kidney Disease. JAMA 2022, 328, 2412–2421. [Google Scholar] [CrossRef]
- Mehta, Y.R.; Lewis, S.A.; Leo, K.T.; Chen, L.; Park, E.; Raghuram, V.; Chou, C.L.; Yang, C.R.; Kikuchi, H.; Khundmiri, S.; et al. “ADPKD-omics”: Determinants of cyclic AMP levels in renal epithelial cells. Kidney Int. 2022, 101, 47–62. [Google Scholar] [CrossRef]
- Cornec-Le Gall, E.; Alam, A.; Perrone, R.D. Autosomal dominant polycystic kidney disease. Lancet 2019, 393, 919–935. [Google Scholar] [CrossRef]
- Shao, A.; Chan, S.C.; Igarashi, P. Role of transcription factor hepatocyte nuclear factor-1beta in polycystic kidney disease. Cell Signal. 2020, 71, 109568. [Google Scholar] [CrossRef]
- Gimpel, C.; Bergmann, C.; Mekahli, D. The wind of change in the management of autosomal dominant polycystic kidney disease in childhood. Pediatr. Nephrol. 2022, 37, 473–487. [Google Scholar] [CrossRef]
- Liebau, M.C.; Mekahli, D. Translational research approaches to study pediatric polycystic kidney disease. Mol. Cell Pediatr. 2021, 8, 20. [Google Scholar] [CrossRef]
- Ars, E.; Bernis, C.; Fraga, G.; Furlano, M.; Martinez, V.; Martins, J.; Ortiz, A.; Perez-Gomez, M.V.; Rodriguez-Perez, J.C.; Sans, L.; et al. Consensus document on autosomal dominant polycystic kindey disease from the Spanish Working Group on Inherited Kindey Diseases. Review 2020. Nefrologia 2022, 42, 367–389. [Google Scholar] [CrossRef]
- Capuano, I.; Buonanno, P.; Riccio, E.; Amicone, M.; Pisani, A. Therapeutic advances in ADPKD: The future awaits. J. Nephrol. 2022, 35, 397–415. [Google Scholar] [CrossRef]
- Yu, Z.; Shen, X.; Hu, C.; Zeng, J.; Wang, A.; Chen, J. Molecular Mechanisms of Isolated Polycystic Liver Diseases. Front. Genet. 2022, 13, 846877. [Google Scholar] [CrossRef]
- Lanktree, M.B.; Haghighi, A.; di Bari, I.; Song, X.; Pei, Y. Insights into Autosomal Dominant Polycystic Kidney Disease from Genetic Studies. Clin. J. Am. Soc. Nephrol. 2021, 16, 790–799. [Google Scholar] [CrossRef]
- Meguro, S.; Koguchi, T.; Hakozaki, Y.; Onagi, A.; Matsuoka, K.; Hoshi, S.; Hata, J.; Sato, Y.; Akaihata, H.; Kataoka, M.; et al. Concurrent Reduced Expression of Contiguous PKD1, TSC2 and NTHL1 Leading to Kidney Diseases and Multiple Diverse Renal Cancers. Cancer Genom. Proteom. 2023, 20, 40–50. [Google Scholar] [CrossRef]
- Tagliaferri, A.R.; Costanzo, C. Delayed Metastasis of Clear Cell Renal Carcinoma to the Colon in the Setting of Benign Kidney Disease. Cureus 2022, 14, e22659. [Google Scholar] [CrossRef]
- Zhang, X.; Li, X. Abnormal Iron and Lipid Metabolism Mediated Ferroptosis in Kidney Diseases and Its Therapeutic Potential. Metabolites 2022, 12, 58. [Google Scholar] [CrossRef]
- Costa, E.; Giuliani, A.; Corradi, V.; Caprara, C.; Rigato, M.; Milan Manani, S.; Tantillo, I.; Ronco, C.; Gastaldon, F.; Zanella, M. Lymphocytic leukopenia in two patients affected by polycystic kidney disease waiting for renal transplantation. G Ital. Nefrol. 2022, 39. Available online: https://www.ncbi.nlm.nih.gov/pubmed/35470994 (accessed on 19 March 2025).
- Econimo, L.; Schaeffer, C.; Zeni, L.; Cortinovis, R.; Alberici, F.; Rampoldi, L.; Scolari, F.; Izzi, C. Autosomal Dominant Tubulointerstitial Kidney Disease: An Emerging Cause of Genetic CKD. Kidney Int. Rep. 2022, 7, 2332–2344. [Google Scholar] [CrossRef]
- Abbas, M.; Patzel, M.; Thurn, A.; Brinkmann, O.A.; Bettendorf, O. Incidental occurrence of papillary renal cell carcinoma in the native kidney with autosomal dominant polycystic kidney disease after renal transplantation: A case report. Mol. Clin. Oncol. 2021, 15, 223. [Google Scholar] [CrossRef]
- Hakozaki, Y.; Uchiyama, K.; Yanai, A.; Yamada, D.; Kamijo, Y.; Ishibashi, Y. Sarcomatoid renal cell carcinoma with autosomal dominant polycystic kidney disease: A case report and literature review. CEN Case Rep. 2021, 10, 199–207. [Google Scholar] [CrossRef]
- Millet-Boureima, C.; He, S.; Le, T.B.U.; Gamberi, C. Modeling Neoplastic Growth in Renal Cell Carcinoma and Polycystic Kidney Disease. Int. J. Mol. Sci. 2021, 22, 3918. [Google Scholar] [CrossRef] [PubMed]
- Pagliarini, R.; Podrini, C. Metabolic Reprogramming and Reconstruction: Integration of Experimental and Computational Studies to Set the Path Forward in ADPKD. Front. Med. 2021, 8, 740087. [Google Scholar] [CrossRef] [PubMed]
- Santoni, M.; Piva, F.; Cimadamore, A.; Giulietti, M.; Battelli, N.; Montironi, R.; Cosmai, L.; Porta, C. Exploring the Spectrum of Kidney Ciliopathies. Diagnostics 2020, 10, 1099. [Google Scholar] [CrossRef] [PubMed]
- Shim, K.E.; Lee, C.; Kim, J.U.; Choi, G.H.; Kwak, K.M.; Kim, S.H.; Kim, H.; Yoon, J.W.; Shin, T.Y.; Jeong, C.W.; et al. Comprehensive analysis of mutations of renal cell carcinoma in an autosomal dominant polycystic kidney disease patient. Medicine 2020, 99, e20071. [Google Scholar] [CrossRef]
- Podrini, C.; Cassina, L.; Boletta, A. Metabolic reprogramming and the role of mitochondria in polycystic kidney disease. Cell Signal. 2020, 67, 109495. [Google Scholar] [CrossRef]
- Crane, A.; Eltemamy, M.; Shoskes, D. Transplant immunosuppressive drugs in urology. Transl. Androl. Urol. 2019, 8, 109–117. [Google Scholar] [CrossRef]
- Trott, J.F.; Hwang, V.J.; Ishimaru, T.; Chmiel, K.J.; Zhou, J.X.; Shim, K.; Stewart, B.J.; Mahjoub, M.R.; Jen, K.Y.; Barupal, D.K.; et al. Arginine reprogramming in ADPKD results in arginine-dependent cystogenesis. Am. J. Physiol. Renal Physiol. 2018, 315, F1855–F1868. [Google Scholar] [CrossRef]
- Sahoo, N.; Patra, S.; Senapati, S.; Mishra, T.S. Multicentric papillary and chromophobe renal cell carcinomas in a patient with autosomal dominant polycystic kidney disease: Report of a rare case. Indian. J. Pathol. Microbiol. 2017, 60, 405–408. [Google Scholar] [CrossRef]
- Nezu, K.; Sakai, T.; Kuromoto, A.; Kanno, H.; Sato, M.; Numahata, K.; Hoshi, S. A Case of Autosomal Dominant Polycystic Kidney Disease (ADPKD) with Metastases from Bilateral Small Renal Cell Carcinoma. Hinyokika Kiyo. 2016, 62, 313–316. Available online: https://www.ncbi.nlm.nih.gov/pubmed/27452494 (accessed on 19 March 2025).
- Patel, S.; Rajalakshmi, B.R.; Manjunath, G.V. Histopathologic Findings in Autopsies with Emphasis on Interesting and Incidental Findings-A Pathologist’s Perspective. J. Clin. Diagn. Res. 2016, 10, EC08–EC12. [Google Scholar] [CrossRef]
- Xu, L.; Rong, Y.; Wang, W.; Lian, H.; Gan, W.; Yan, X.; Li, X.; Guo, H. Percutaneous radiofrequency ablation with contrast-enhanced ultrasonography for solitary and sporadic renal cell carcinoma in patients with autosomal dominant polycystic kidney disease. World J. Surg. Oncol. 2016, 14, 193. [Google Scholar] [CrossRef][Green Version]
- Zhang, W.; Tan, A.Y.; Blumenfeld, J.; Liu, G.; Michaeel, A.; Zhang, T.; Robinson, B.D.; Salvatore, S.P.; Kapur, S.; Donahue, S.; et al. Papillary renal cell carcinoma with a somatic mutation in MET in a patient with autosomal dominant polycystic kidney disease. Cancer Genet. 2016, 209, 11–20. [Google Scholar] [CrossRef]
- Ito, K.; Asano, T.; Tominaga, S.; Yoshii, H.; Sawazaki, H.; Asano, T. Erythropoietin production in renal cell carcinoma and renal cysts in autosomal dominant polycystic kidney disease in a chronic dialysis patient with polycythemia: A case report. Oncol. Lett. 2014, 8, 2032–2036. [Google Scholar] [CrossRef][Green Version]
- Jilg, C.A.; Drendel, V.; Bacher, J.; Pisarski, P.; Neeff, H.; Drognitz, O.; Schwardt, M.; Glasker, S.; Malinoc, A.; Erlic, Z.; et al. Autosomal dominant polycystic kidney disease: Prevalence of renal neoplasias in surgical kidney specimens. Nephron Clin. Pract. 2013, 123, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Na, K.Y.; Kim, H.S.; Park, Y.K.; Chang, S.G.; Kim, Y.W. Multifocal renal cell carcinoma of different histological subtypes in autosomal dominant polycystic kidney disease. Korean J. Pathol. 2012, 46, 382–386. [Google Scholar] [CrossRef] [PubMed]
- Patel, P.; Horsfield, C.; Compton, F.; Taylor, J.; Koffman, G.; Olsburgh, J. Native nephrectomy in transplant patients with autosomal dominant polycystic kidney disease. Ann. R. Coll. Surg. Engl. 2011, 93, 391–395. [Google Scholar] [CrossRef] [PubMed]
- Hajj, P.; Ferlicot, S.; Massoud, W.; Awad, A.; Hammoudi, Y.; Charpentier, B.; Durrbach, A.; Droupy, S.; Benoit, G. Prevalence of renal cell carcinoma in patients with autosomal dominant polycystic kidney disease and chronic renal failure. Urology 2009, 74, 631–634. [Google Scholar] [CrossRef]
- Chang, Y.L.; Chung, H.J.; Chen, K.K. Bilateral renal cell carcinoma in a patient with autosomal dominant polycystic kidney disease. J. Chin. Med. Assoc. 2007, 70, 403–405. [Google Scholar] [CrossRef]
- Hama, Y.; Kaji, T.; Ito, K.; Hayakawa, M.; Tobe, M.; Kosuda, S. Erythropoietin-producing renal cell carcinoma arising from autosomal dominant polycystic kidney disease. Br. J. Radiol. 2005, 78, 269–271. [Google Scholar] [CrossRef]
- Grantham, J.J. Polycystic kidney disease: Neoplasia in disguise. Am. J. Kidney Diseases. 1990, 15, 110–116. [Google Scholar] [CrossRef]
- Renda, A.; de Werra, C.; Donzelli, I.; Perone, M.; Micco, R.D.; Orabona, G. Multifocal and multicentric tumors. Multiple Primary Malignancies; Springer: Berlin/Heidelberg, Germany, 2009; pp. 129–142. [Google Scholar]
- Ishikawa, I.; Saito, Y.; Onouchi, Z.; Kitada, H.; Suzuki, S.; Kurihara, S.; Yuri, T.; Shinoda, A. Development of acquired cystic disease and adenocarcinoma of the kidney in glomerulonephritic chronic hemodialysis patients. Clin. Nephrol. 1980, 14, 1–6. [Google Scholar]
- Nargund, A.M.; Pham, C.G.; Dong, Y.; Wang, P.I.; Osmangeyoglu, H.U.; Xie, Y.; Aras, O.; Han, S.; Oyama, T.; Takeda, S. The SWI/SNF protein PBRM1 restrains VHL-loss-driven clear cell renal cell carcinoma. Cell Rep. 2017, 18, 2893–2906. [Google Scholar] [CrossRef] [PubMed]
- Drusian, L.; Boletta, A. mTORC1-driven accumulation of the oncometabolite fumarate as a potential critical step in renal cancer progression. Mol. Cell. Oncol. 2019, 6, 1537709. [Google Scholar] [CrossRef]
- Neumann, H.P.; Zbar, B. Renal cysts, renal cancer and von Hippel-Lindau disease. Kidney Int. 1997, 51, 16–26. [Google Scholar] [CrossRef] [PubMed]
- Mandriota, S.J.; Turner, K.J.; Davies, D.R.; Murray, P.G.; Morgan, N.V.; Sowter, H.M.; Wykoff, C.C.; Maher, E.R.; Harris, A.L.; Ratcliffe, P.J.; et al. HIF activation identifies early lesions in VHL kidneys: Evidence for site-specific tumor suppressor function in the nephron. Cancer Cell 2002, 1, 459–468. [Google Scholar] [CrossRef]
- Perretta-Tejedor, N.; Jafree, D.J.; Long, D.A. Endothelial-epithelial communication in polycystic kidney disease: Role of vascular endothelial growth factor signalling. Cell Signal. 2020, 72, 109624. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.L.; Woolf, A.S.; Long, D.A. Angiogenesis and autosomal dominant polycystic kidney disease. Pediatr. Nephrol. 2013, 28, 1749–1755. [Google Scholar] [CrossRef]
- Huang, J.L.; Woolf, A.S.; Kolatsi-Joannou, M.; Baluk, P.; Sandford, R.N.; Peters, D.J.; McDonald, D.M.; Price, K.L.; Winyard, P.J.; Long, D.A. Vascular endothelial growth factor C for polycystic kidney diseases. J. Am. Soc. Nephrol. 2016, 27, 69–77. [Google Scholar] [CrossRef]
- Ogunlade, O.; Connell, J.J.; Huang, J.L.; Zhang, E.; Lythgoe, M.F.; Long, D.A.; Beard, P. In vivo three-dimensional photoacoustic imaging of the renal vasculature in preclinical rodent models. Am. J. Physiol. Ren. Physiol. 2018, 314, F1145–F1153. [Google Scholar] [CrossRef]
- Alitalo, K. The lymphatic vasculature in disease. Nat. Med. 2011, 17, 1371–1380. [Google Scholar] [CrossRef]
- Jafree, D.J.; Moulding, D.; Kolatsi-Joannou, M.; Perretta Tejedor, N.; Price, K.L.; Milmoe, N.J.; Walsh, C.L.; Correra, R.M.; Winyard, P.J.; Harris, P.C. Spatiotemporal dynamics and heterogeneity of renal lymphatics in mammalian development and cystic kidney disease. Elife 2019, 8, e48183. [Google Scholar] [CrossRef]
- Sergi, C. EPAS 1, congenital heart disease, and high altitude: Disclosures by genetics, bioinformatics, and experimental embryology. Biosci. Rep. 2019, 39, BSR20182197. [Google Scholar] [CrossRef] [PubMed]
- Hagenbuchner, J.; Rupp, M.; Salvador, C.; Meister, B.; Kiechl-Kohlendorfer, U.; Muller, T.; Geiger, K.; Sergi, C.; Obexer, P.; Ausserlechner, M.J.; et al. Nuclear FOXO3 predicts adverse clinical outcome and promotes tumor angiogenesis in neuroblastoma. Oncotarget 2016, 7, 77591–77606. [Google Scholar] [CrossRef]
- Persad, R.; Huynh, H.Q.; Hao, L.; Ha, J.R.; Sergi, C.; Srivastava, R.; Persad, S. Angiogenic remodeling in pediatric EoE is associated with increased levels of VEGF-A, angiogenin, IL-8, and activation of the TNF-alpha-NFkappaB pathway. J. Pediatr. Gastroenterol. Nutr. 2012, 55, 251–260. [Google Scholar] [CrossRef]
- Dimke, H.; Sparks, M.A.; Thomson, B.R.; Frische, S.; Coffman, T.M.; Quaggin, S.E. Tubulovascular cross-talk by vascular endothelial growth factor a maintains peritubular microvasculature in kidney. J. Am. Soc. Nephrol. 2015, 26, 1027–1038. [Google Scholar] [CrossRef] [PubMed]
- Raza, A.; Franklin, M.J.; Dudek, A.Z. Pericytes and vessel maturation during tumor angiogenesis and metastasis. Am. J. Hematol. 2010, 85, 593–598. [Google Scholar] [CrossRef] [PubMed]
- Qian, C.N.; Huang, D.; Wondergem, B.; The, B.T. Complexity of tumor vasculature in clear cell renal cell carcinoma. Cancer 2009, 115, 2282–2289. [Google Scholar] [CrossRef]
- Schraml, P.; Athelogou, M.; Hermanns, T.; Huss, R.; Moch, H. Specific immune cell and lymphatic vessel signatures identified by image analysis in renal cancer. Mod. Pathol. 2019, 32, 1042–1052. [Google Scholar] [CrossRef]
- Chen, F.; Zhang, Y.; Şenbabaoğlu, Y.; Ciriello, G.; Yang, L.; Reznik, E.; Shuch, B.; Micevic, G.; De Velasco, G.; Shinbrot, E. Multilevel genomics-based taxonomy of renal cell carcinoma. Cell Rep. 2016, 14, 2476–2489. [Google Scholar] [CrossRef]
- Clifford, S.C.; Walsh, S.; Hewson, K.; Green, E.K.; Brinke, A.; Green, P.M.; Gianelli, F.; Eng, C.; Maher, E.R. Genomic organization and chromosomal localization of the human CUL2 gene and the role of von Hippel-Lindau tumor suppressor-binding protein (CUL2 and VBP1) mutation and loss in renal-cell carcinoma development. Genes Chromosomes Cancer 1999, 26, 20–28. [Google Scholar] [CrossRef]
- Buchholz, B.; Eckardt, K.U. Role of oxygen and the HIF-pathway in polycystic kidney disease. Cell Signal. 2020, 69, 109524. [Google Scholar] [CrossRef] [PubMed]
- Schödel, J.; Ratcliffe, P.J. Mechanisms of hypoxia signalling: New implications for nephrology. Nat. Rev. Nephrol. 2019, 15, 641–659. [Google Scholar] [CrossRef]
- Brugarolas, J. Molecular genetics of clear-cell renal cell carcinoma. J. Clin. Oncol. 2014, 32, 1968–1976. [Google Scholar] [CrossRef]
- Kim, E.; Zschiedrich, S. Renal cell carcinoma in von hippel–lindau disease—From tumor genetics to novel therapeutic strategies. Front. Pediatr. 2018, 6, 16. [Google Scholar] [CrossRef] [PubMed]
- Banumathy, G.; Cairns, P. Signaling pathways in renal cell carcinoma. Cancer Biol. Ther. 2010, 10, 658–664. [Google Scholar] [CrossRef]
- Linehan, W.M.; Srinivasan, R.; Schmidt, L.S. The genetic basis of kidney cancer: A metabolic disease. Nat. Rev. Urol. 2010, 7, 277–285. [Google Scholar] [CrossRef]
- Haase, V. The VHL/HIF oxygen-sensing pathway and its relevance to kidney disease. Kidney Int. 2006, 69, 1302–1307. [Google Scholar] [CrossRef]
- Regan, R.J.; Abercrombie, G.F.; Lee, H.A. Polycystic renal disease--occurrence of malignant change and role of nephrectomy in potential transplant recipients. Br. J. Urol. 1977, 49, 85–91. [Google Scholar] [CrossRef]
- Armstrong, H.K.; Bording-Jorgensen, M.; Santer, D.M.; Zhang, Z.; Valcheva, R.; Rieger, A.M.; Sung-Ho Kim, J.; Dijk, S.I.; Mahmood, R.; Ogungbola, O.; et al. Unfermented β-fructan Fibers Fuel Inflammation in Select Inflammatory Bowel Disease Patients. Gastroenterology 2022, 164, 228–240. [Google Scholar] [CrossRef]
- Sergi, C.M.; Chiu, B. Targeting NLRP3 inflammasome in an animal model for Coronavirus Disease 2019 (COVID-19) caused by the Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2). J. Med. Virol. 2021, 93, 669–670. [Google Scholar] [CrossRef]
- Alipour, M.; Lou, Y.; Zimmerman, D.; Bording-Jorgensen, M.W.; Sergi, C.; Liu, J.J.; Wine, E. A balanced IL-1beta activity is required for host response to Citrobacter rodentium infection. PLoS ONE 2013, 8, e80656. [Google Scholar] [CrossRef]
- Paim-Marques, L.B.; Cavalcante, A.; Castro, C.; Muskardin, T.L.W.; de Oliveira, J.B.; Niewold, T.B.; Appenzeller, S. Novel mutation in the NRLP3 manifesting as an intermediate phenotype of cryopyrinopathies. Rheumatol. Int. 2021, 41, 219–225. [Google Scholar] [CrossRef]
- Huang, W.; Wang, X.; Xie, F.; Zhang, H.; Liu, D. Serum NLRP3: A biomarker for identifying high-risk septic patients. Cytokine 2022, 149, 155725. [Google Scholar] [CrossRef]
- Verma, A.; Pittala, S.; Alhozeel, B.; Shteinfer-Kuzmine, A.; Ohana, E.; Gupta, R.; Chung, J.H.; Shoshan-Barmatz, V. The role of the mitochondrial protein VDAC1 in inflammatory bowel disease: A potential therapeutic target. Mol. Ther. 2022, 30, 726–744. [Google Scholar] [CrossRef]
- Tezcan, G.; Garanina, E.E.; Alsaadi, M.; Gilazieva, Z.E.; Martinova, E.V.; Markelova, M.I.; Arkhipova, S.S.; Hamza, S.; McIntyre, A.; Rizvanov, A.A.; et al. Therapeutic Potential of Pharmacological Targeting NLRP3 Inflammasome Complex in Cancer. Front. Immunol. 2020, 11, 607881. [Google Scholar] [CrossRef]
- Shen, S.; Duan, J.; Hu, J.; Qi, Y.; Kang, L.; Wang, K.; Chen, J.; Wu, X.; Xu, B.; Gu, R. Colchicine alleviates inflammation and improves diastolic dysfunction in heart failure rats with preserved ejection fraction. Eur. J. Pharmacol. 2022, 929, 175126. [Google Scholar] [CrossRef]
- Jeltema, D.; Wang, J.; Cai, J.; Kelley, N.; Yang, Z.; He, Y. A Single Amino Acid Residue Defines the Difference in NLRP3 Inflammasome Activation between NEK7 and NEK6. J. Immunol. 2022, 208, 2029–2036. [Google Scholar] [CrossRef]
- Sun, Z.; Gong, W.; Zhang, Y.; Jia, Z. Physiological and Pathological Roles of Mammalian NEK7. Front. Physiol. 2020, 11, 606996. [Google Scholar] [CrossRef]
- Bertoni, A.; Penco, F.; Mollica, H.; Bocca, P.; Prigione, I.; Corcione, A.; Cangelosi, D.; Schena, F.; Del Zotto, G.; Amaro, A.; et al. Spontaneous NLRP3 inflammasome-driven IL1-beta secretion is induced in severe COVID-19 patients and responds to anakinra treatment. J. Allergy Clin. Immunol. 2022, 4, 796–805. [Google Scholar] [CrossRef]
- Aymonnier, K.; Ng, J.; Fredenburgh, L.E.; Zambrano-Vera, K.; Munzer, P.; Gutch, S.; Fukui, S.; Desjardins, M.; Subramaniam, M.; Baron, R.M.; et al. Inflammasome activation in neutrophils of patients with severe COVID-19. Blood Adv. 2022, 6, 2001–2013. [Google Scholar] [CrossRef]
- Lopez-Rodriguez, A.B.; Decouty-Perez, C.; Farre-Alins, V.; Palomino-Antolin, A.; Narros-Fernandez, P.; Egea, J. Activation of NLRP3 Is Required for a Functional and Beneficial Microglia Response after Brain Trauma. Pharmaceutics 2022, 14, 1550. [Google Scholar] [CrossRef]
- Raptis, V.; Loutradis, C.; Boutou, A.K.; Faitatzidou, D.; Sioulis, A.; Ferro, C.J.; Papagianni, A.; Sarafidis, P.A. Serum Copeptin, NLPR3, and suPAR Levels among Patients with Autosomal-Dominant Polycystic Kidney Disease with and without Impaired Renal Function. Cardiorenal Med. 2020, 10, 440–451. [Google Scholar] [CrossRef]
- Sergi, C.; Shen, F.; Liu, S.M. Insulin/IGF-1R, SIRT1, and FOXOs Pathways-An Intriguing Interaction Platform for Bone and Osteosarcoma. Fron. Endocrinol. 2019, 10, 93. [Google Scholar] [CrossRef]
- Chandler, C.; Liu, T.; Buckanovich, R.; Coffman, L.G. The double edge sword of fibrosis in cancer. Transl. Res. 2019, 209, 55–67. [Google Scholar] [CrossRef]
- Piersma, B.; Hayward, M.K.; Weaver, V.M. Fibrosis and cancer: A strained relationship. Biochim. Biophys. Acta Rev. Cancer 2020, 1873, 188356. [Google Scholar] [CrossRef]
- Denton, M.D.; Magee, C.C.; Ovuworie, C.; Mauiyyedi, S.; Pascual, M.; Colvin, R.B.; Cosimi, A.B.; Tolkoff-Rubin, N. Prevalence of renal cell carcinoma in patients with ESRD pre-transplantation: A pathologic analysis. Kidney Int. 2002, 61, 2201–2209. [Google Scholar] [CrossRef]
- van den Tweel, J.G.; Wittekind, C. The medical autopsy as quality assurance tool in clinical medicine: Dreams and realities. Virchows Arch. 2016, 468, 75–81. [Google Scholar] [CrossRef]
- Johnson Chacko, L.; Wertjanz, D.; Sergi, C.; Dudas, J.; Fischer, N.; Eberharter, T.; Hoermann, R.; Glueckert, R.; Fritsch, H.; Rask-Andersen, H.; et al. Growth and cellular patterning during fetal human inner ear development studied by a correlative imaging approach. BMC Dev. Biol. 2019, 19, 11. [Google Scholar] [CrossRef]
- Sergi, C.; Mikuz, G. External quality assurance as a revalidation method for pathologists in pediatric histopathology: Comparison of four international programs. BMC Clin. Pathol. 2008, 8, 11. [Google Scholar] [CrossRef] [PubMed]
- Torres, V.E.; Harris, P.C.; Pirson, Y. Autosomal dominant polycystic kidney disease. Lancet 2007, 369, 1287–1301. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, T.; Wallace, D.P.; Magenheimer, B.S.; Hempson, S.J.; Grantham, J.J.; Calvet, J.P. Calcium restriction allows cAMP activation of the B-Raf/ERK pathway, switching cells to a cAMP-dependent growth-stimulated phenotype. J. Biol. Chem. 2004, 279, 40419–40430. [Google Scholar] [CrossRef] [PubMed]
- Raina, R.; Houry, A.; Rath, P.; Mangat, G.; Pandher, D.; Islam, M.; Khattab, A.G.; Kalout, J.K.; Bagga, S. Clinical Utility and Tolerability of Tolvaptan in the Treatment of Autosomal Dominant Polycystic Kidney Disease (ADPKD). Drug Healthc. Patient Saf. 2022, 14, 147–159. [Google Scholar] [CrossRef]
- Grantham, J.J. Autosomal dominant polycystic kidney disease. N. Engl. J. Med. 2008, 359, 1477–1485. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, T.; Fujiki, H.; Yamamura, Y.; Nakamura, S.; Mori, T. Tolvaptan, an Orally Active Vasopressin V2-Receptor Antagonist—Pharmacology and Clinical Trials. Cardiovasc. Drug Rev. 2007, 25, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Mekahli, D.; Guay-Woodford, L.M.; Cadnapaphornchai, M.A.; Greenbaum, L.A.; Litwin, M.; Seeman, T.; Dandurand, A.; Shi, L.; Sikes, K.; Shoaf, S.E.; et al. Tolvaptan for Children and Adolescents with Autosomal Dominant Polycystic Kidney Disease: Randomized Controlled Trial. Clin. J. Am. Soc. Nephrol. 2023, 18, 36–46. [Google Scholar] [CrossRef]
- Capuano, I.; Buonanno, P.; Riccio, E.; Rizzo, M.; Pisani, A. Tolvaptan vs. somatostatin in the treatment of ADPKD: A review of the literature. Clin. Nephrol. 2022, 97, 131–140. [Google Scholar] [CrossRef]
- Park, H.C.; Oh, Y.K.; Polycystic Kidney Disease Study, G. Practical Issues in the Management of Polycystic Kidney Disease: Blood Pressure and Water Balance. Electrolyte Blood Press. 2022, 20, 10–16. [Google Scholar] [CrossRef]
- Hallows, K.R.; Li, H.; Saitta, B.; Sepehr, S.; Huang, P.; Pham, J.; Wang, J.; Mancino, V.; Chung, E.J.; Pinkosky, S.L.; et al. Beneficial effects of bempedoic acid treatment in polycystic kidney disease cells and mice. Front. Mol. Biosci. 2022, 9, 1001941. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sergi, C.M.; Guerra, L.; Hager, J. Autosomal Dominant Polycystic Kidney Disease-Related Multifocal Renal Cell Carcinoma: A Narrative Iconographic Review. Int. J. Mol. Sci. 2025, 26, 3965. https://doi.org/10.3390/ijms26093965
Sergi CM, Guerra L, Hager J. Autosomal Dominant Polycystic Kidney Disease-Related Multifocal Renal Cell Carcinoma: A Narrative Iconographic Review. International Journal of Molecular Sciences. 2025; 26(9):3965. https://doi.org/10.3390/ijms26093965
Chicago/Turabian StyleSergi, Consolato M., Luis Guerra, and Josef Hager. 2025. "Autosomal Dominant Polycystic Kidney Disease-Related Multifocal Renal Cell Carcinoma: A Narrative Iconographic Review" International Journal of Molecular Sciences 26, no. 9: 3965. https://doi.org/10.3390/ijms26093965
APA StyleSergi, C. M., Guerra, L., & Hager, J. (2025). Autosomal Dominant Polycystic Kidney Disease-Related Multifocal Renal Cell Carcinoma: A Narrative Iconographic Review. International Journal of Molecular Sciences, 26(9), 3965. https://doi.org/10.3390/ijms26093965






