In Vitro Biotransformation of Ziziphi Spinosae Semen Saponins by Gut Microbiota from Healthy and Insomniac Groups
Abstract
1. Introduction
2. Results and Discussion
2.1. Identification Compounds in ZSSS
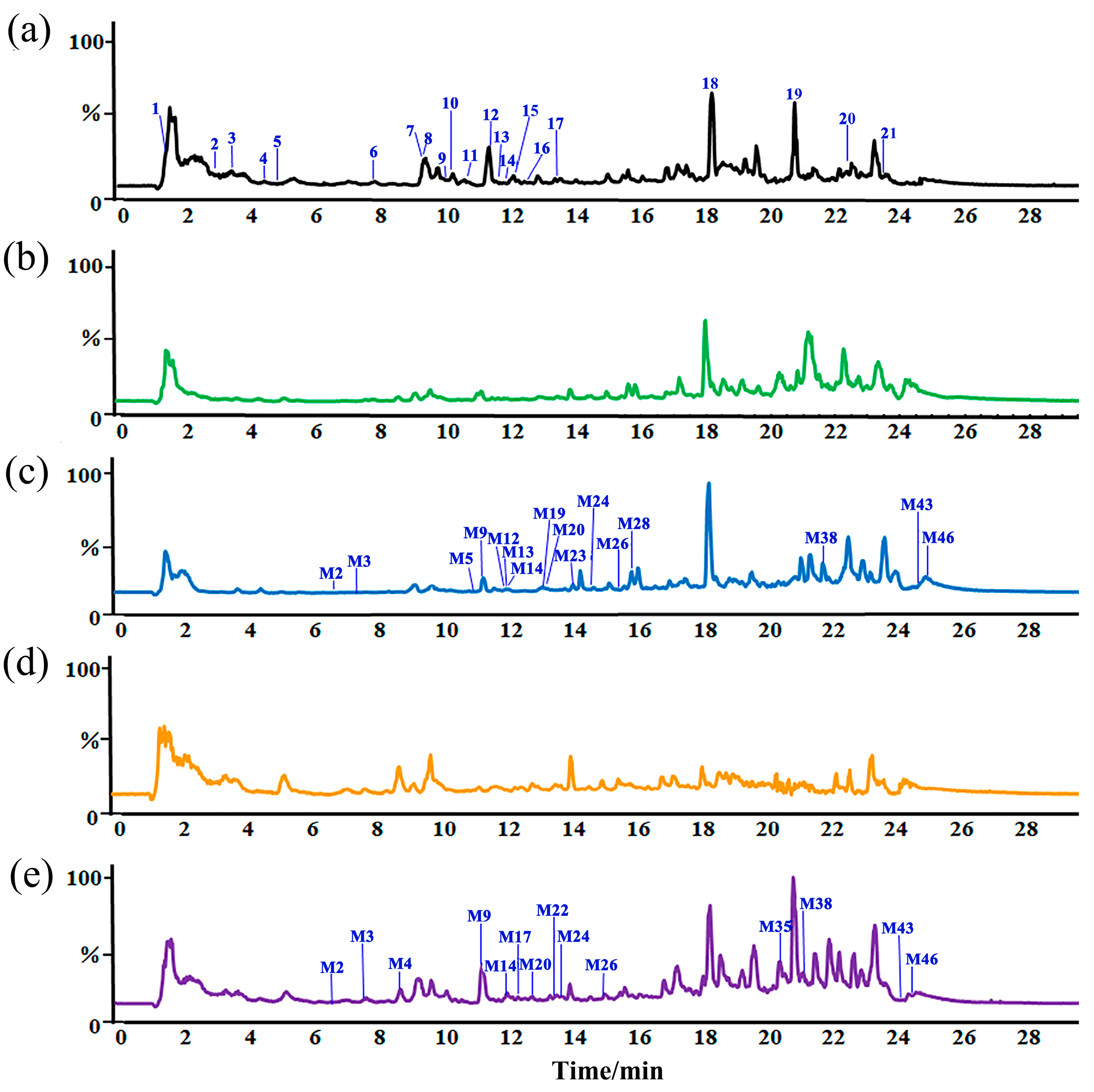
2.2. Metabolic Differences of ZSSS in Gut Microbiota from Normal Patients and Humans with Insomnia
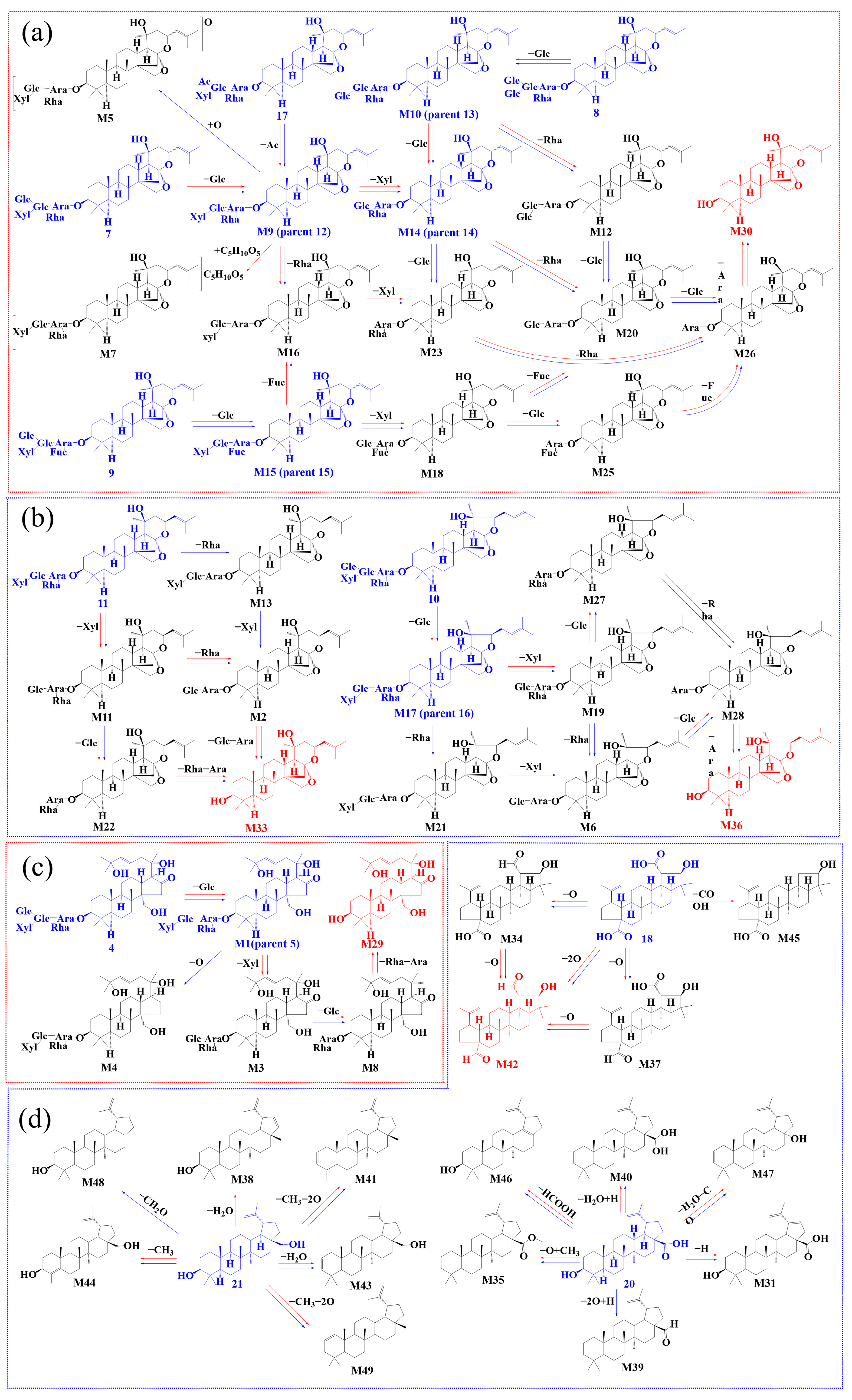
2.3. Biotransformation Pathway
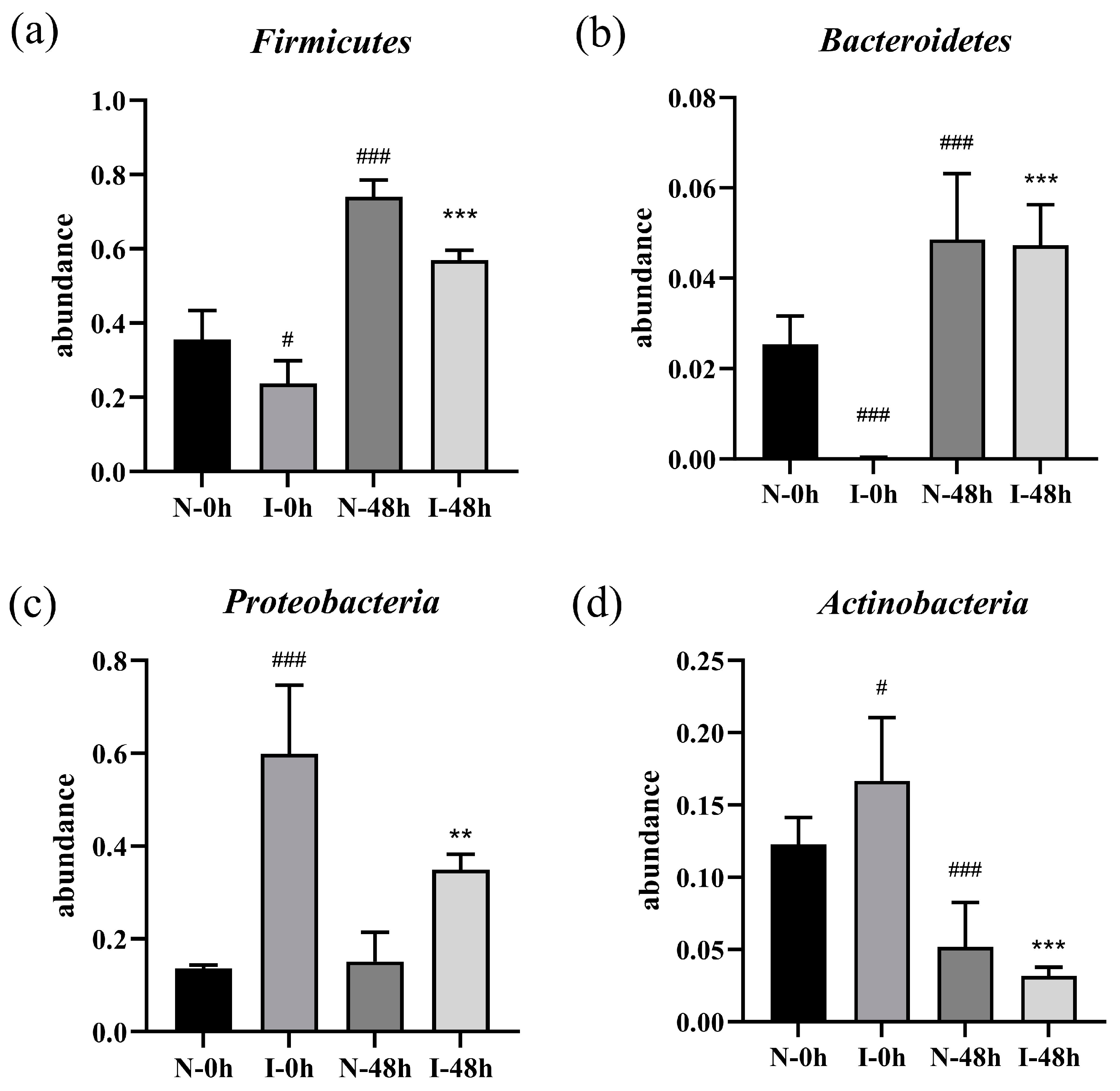
2.4. Effect of ZSSS on the Microbial Communities
2.5. SCFAs Production During Fermentation In Vitro
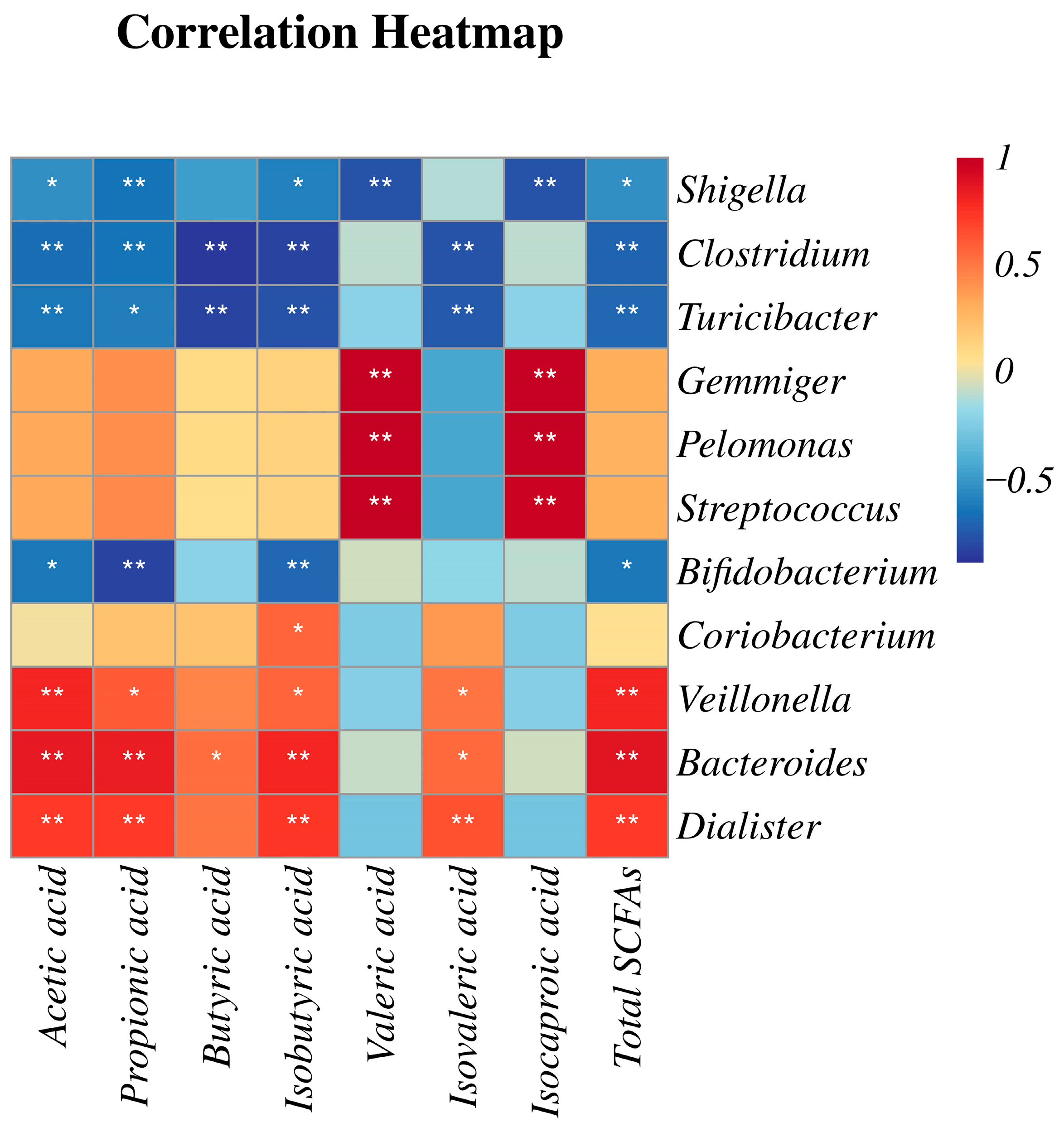
2.6. Correlation Analysis of Gut Microflora and SCFAs
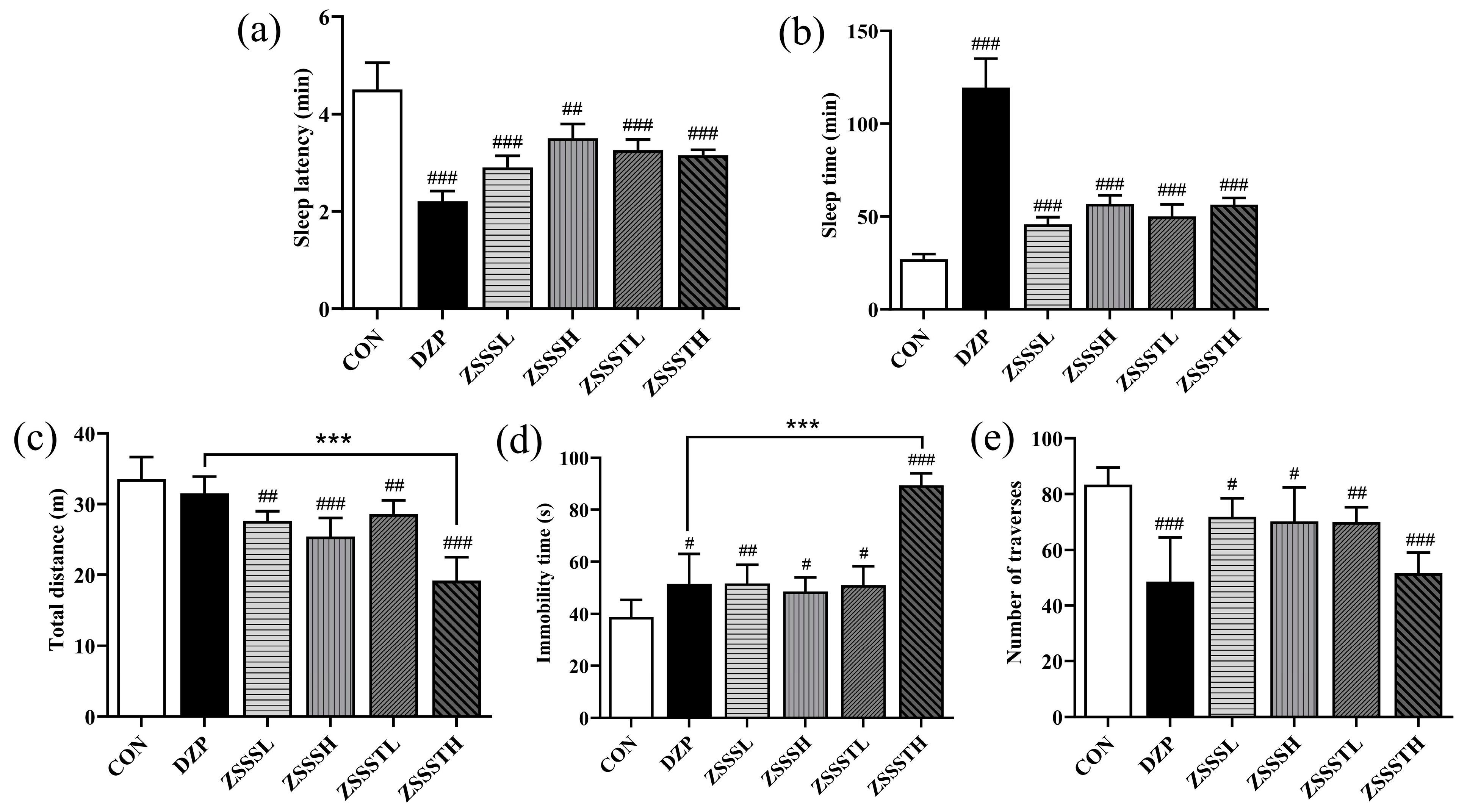
2.7. ZSSS Pharmacodynamics Analysis
2.8. Molecular Docking Studies
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Preparation of ZSSS
3.3. Fecal Sample Collection and In Vitro Fermentation of ZSSS
3.4. Sample Preparation
3.5. UHPLC-Q-Orbitrap-MS Analysis
3.6. 16S rRNA Sequencing Analysis
3.7. Determination of the SCFAs in Fecal Samples
3.8. Animals and Treatment
3.9. Behavioral Test
3.9.1. Open-Field Behavior Test
3.9.2. Evaluation of Sleep Onset and Sleep Duration
3.10. Molecular Docking
3.11. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Shergis, J.L.; Ni, X.J.; Sarris, J.; Zhang, A.L.; Guo, X.F.; Xue, C.C.; Lu, C.J.; Hugel, H. Ziziphus spinosa seeds for insomnia: A review of chemistry and psychopharmacology. Phytomedicine 2017, 34, 38–43. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.D.; Ho, C.T.; Bai, N.S. Ziziphi Spinosae Semen: An updated review on pharmacological activity, quality control, and application. J. Food Biochem. 2022, 46, e14153. [Google Scholar] [CrossRef] [PubMed]
- Song, P.P.; Zhang, Y.; Ma, G.J.; Zhang, Y.Q.; Zhou, A.M.; Xie, J.B. Gastrointestinal Absorption and Metabolic Dynamics of Jujuboside A, A Saponin Derived from the Seed of Ziziphus jujuba. J. Agric. Food Chem. 2017, 65, 8331–8339. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.Y.; Yang, X.X.; Le, Q.Y.; Zhang, S.S.; Chen, R.J.; Xiang, Z. Development and validation of an UPLC-MS/MS method for determination of jujuboside B in rat plasma and its application in pharmacokinetic and bioavailability studies. Anal. Methods 2015, 7, 4049–4054. [Google Scholar] [CrossRef]
- Zimmermann, M.; Zimmermann-Kogadeeva, M.; Wegmann, R.; Goodman, A.L. Mapping human microbiome drug metabolism by gut bacteria and their genes. Nature 2019, 570, 462–467. [Google Scholar] [CrossRef]
- Levy, M.; Kolodziejczyk, A.A.; Thaiss, C.A.; Elinav, E. Dysbiosis and the immune system. Nat. Rev. Immunol. 2017, 17, 219–232. [Google Scholar] [CrossRef]
- Zhang, Y.; Xie, J.B.; Zhang, Y.Q.; Zhang, M.C. Degradation kinetics of jujuboside A by rat intestinal flora and identification of the metabolites by HPLC-MS/MS. Int. J. Food Prop. 2014, 17, 1841–1849. [Google Scholar] [CrossRef]
- Shen, C.Y.; Wan, L.; Zhu, J.J.; Jiang, J.G. Targets and underlying mechanisms related to the sedative and hypnotic activities of saponin extracts from semen Ziziphus jujube. Food Funct. 2022, 11, 3895–3903. [Google Scholar] [CrossRef]
- Chen, C.Y.C. Insights into the suanzaoren mechanism-From constructing the 3D structure of GABA-A receptor to its binding interaction analysis. J. Chin. Inst. Eng. 2008, 39, 663–671. [Google Scholar] [CrossRef]
- Wang, Q.H.; Chen, B.; Sheng, D.S.; Yang, J.J.; Fu, S.J.; Wang, J.W.; Zhao, C.Y.; Wang, Y.H.; Gai, X.Z.; Wang, J.F.; et al. Multiomics Analysis Reveals Aberrant Metabolism and Immunity Linked Gut Microbiota with Insomnia. Microbiol. Spectr. 2022, 10, e0099822. [Google Scholar] [CrossRef]
- Zhao, X.S.; Xie, L.W.; Wu, H.F.; Kong, W.J.; Yang, M.H. Analysis of six bioactive components in Semen Ziziphi Spinosae by UPLC-ELSD and UPLC-Q/TOF-MS. Anal. Methods 2014, 6, 5856–5864. [Google Scholar] [CrossRef]
- Yan, Y.; Liu, J.X.; Zhang, M.; Zhang, Y.J.; Shi, B.Y.; Qin, X.M.; Du, C.H. A strategy to explore the quality markers of Ziziphi Spinosae semen by combining metabolic in vivo study with network pharmacology. Biomed. Chromatogr. 2022, 37, e5530. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.X.; Li, M.; Qiao, L.R.; Yao, Z.H.; Li, C.; Shen, X.Y.; Wang, Y.; Yu, K.; Yao, X.S.; Dai, Y. Rapid characterization of Ziziphi Spinosae Semen by UPLC/Qtof MS with novel informatics platform and its application in evaluation of two seeds from Ziziphus species. J. Pharm. Biomed. Anal. 2016, 122, 59–80. [Google Scholar] [CrossRef]
- Kang, Y.B.; Kang, X.; Cai, Y. The gut microbiome as a target for adjuvant therapy in insomnia disorder. Clin. Res. Hepatol. Gastroenterol. 2022, 46, 101834. [Google Scholar] [CrossRef]
- Jiang, W.X.; Guo, J.; Xue, R.; Zhu, K.C.; Li, Z.X.; Chen, M.C.; Huang, C.G. Anti-depressive activities and biotransformation of timosaponin B-III and its derivatives. Nat. Prod. Res. 2014, 28, 1446–1453. [Google Scholar] [CrossRef]
- Zhou, G.S.; Peng, Y.; Zhao, L.J.; Wang, M.Y.; Li, X.B. Biotransformation of total saponins in Siraitia Fructus by human intestinal microbiota of normal and type 2 diabetic patients: Comprehensive metabolite identification and metabolic profile elucidation using LC-Q-TOF/MS. J. Agric. Food Chem. 2017, 65, 1518–1524. [Google Scholar] [CrossRef]
- Bogert, B.V.D.; Erkus, O.; Boekhorst, J.; Goffau, M.D.; Smid, E.J.; Zoetendal, E.G.; Kleerebezem, M. Diversity of human small intestinal Streptococcus and Veillonella populations. FEMS Microbiol. Ecol. 2013, 85, 376–388. [Google Scholar] [CrossRef]
- Li, Q.; Wang, B.; Qiu, H.Y.; Yan, X.J.; Cheng, L.; Wang, Q.Q.; Chen, S.L. Chronic jet lag exacerbates Jejunal and colonic microenvironment in mice. Front. Cell Infect. Microbiol. 2021, 11, 648175. [Google Scholar] [CrossRef]
- Kinoshita, C.; Okamoto, Y.; Aoyama, K.; Nakaki, T. MicroRNA: A Key Player for the Interplay of Circadian Rhythm Abnormalities, Sleep Disorders and Neurodegenerative Diseases. Clocks Sleep 2020, 2, 282–307. [Google Scholar] [CrossRef]
- Lyall, L.M.; Wyse, C.A.; Graham, N.; Ferguson, A.; Lyall, D.M.; Cullen, B.; Morales, C.A.C.; Biello, S.M.; Mackay, D.; Ward, J.; et al. Association of disrupted circadian rhythmicity with mood disorders, subjective wellbeing, and cognitive function: A cross-sectional study of 91105 participants from the UK Biobank. Lancet Psychiatry 2018, 5, 507–514. [Google Scholar] [CrossRef]
- Silva, Y.P.; Bernardi, A.; Frozza, R.L. The role of short-chain fatty acids from gut microbiota in gut-brain communication. Front. Endocrinol. 2020, 11, 25. [Google Scholar] [CrossRef] [PubMed]
- Schnupf, P.; Sansonetti, P.J. Shigella pathogenesis: New insights through advanced methodologies. Microbiol. Spectr. 2019, 7, 15–39. [Google Scholar] [CrossRef]
- Tian, P.J.; Hou, Y.F.; Wang, Z.; Jiang, J.N.; Qian, X.; Qu, Z.H.; Zhao, J.X.; Wang, G.; Chen, W. Probiotics Administration Alleviates Cognitive Impairment and Circadian Rhythm Disturbance Induced by Sleep Deprivation. Food Sci. Hum. Wellness 2024, 13, 1951–1961. [Google Scholar] [CrossRef]
- Chi, L.D.; Khan, I.; Lin, Z.B.; Zhang, J.W.; Lee, M.Y.S.; Leong, W.; Hsiao, W.L.W.; Zheng, Y. Fructo-oligosaccharides from Morinda officinalis remodeled gut microbiota and alleviated depression features in a stress rat model. Phytomedicine 2020, 67, 153157. [Google Scholar] [CrossRef]
- Yang, X.Q.; Xiao, H.S.; Zeng, Y.; Huang, L.L.; Ji, K.; Deng, D.Y.; Yang, W.Y.; Liu, L. Tianwang buxin granules influence the intestinal flora in perimenopausal insomnia. BioMed Res. Int. 2021, 2021, 9979511. [Google Scholar] [CrossRef]
- Ríos-Covián, D.; Ruas-Madiedo, P.; Margolles, A.; Gueimonde, M.; Reyes-Gavilán, C.G.L.; de Salazar, N. Intestinal short chain fatty acids and their link with diet and human health. Front. Microbiol. 2016, 7, 185. [Google Scholar] [CrossRef]
- Li, Z.X.; Song, Y.G.; Xu, W.Z.; Chen, J.B.; Zhou, R.; Yang, M.; Zhu, G.H.; Luo, X.Q.; Ai, Z.F.; Liu, Y.L.; et al. Pulsatilla chinensis saponins improve SCFAs regulating GPR43-NLRP3 signaling pathway in the treatment of ulcerative colitis. J. Ethnopharmacol. 2023, 308, 116215. [Google Scholar] [CrossRef]
- Leone, V.; Gibbons, S.M.; Martinez, K.; Hutchison, A.L.; Huang, E.Y.; Cham, C.M.; Pierre, J.F.; Heneghan, A.F.; Nadimpalli, A.; Hubert, N.; et al. Effects of diurnal variation of gut microbes and high-fat feeding on host circadian clock function and metabolism. Cell Host Microbe 2015, 17, 681–689. [Google Scholar] [CrossRef]
- Pant, K.; Yadav, A.K.; Gupta, P.; Islam, R.; Saraya, A.; Venugopal, S.K. Butyrate induces ROS-mediated apoptosis by modulating miR-22/SIRT-1 pathway in hepatic cancer cells. Redox Biol. 2017, 12, 340–349. [Google Scholar] [CrossRef]
- Liu, L.X.; Li, Q.Q.; Yang, Y.Q.; Guo, A.W. Biological function of short-chain fatty acids and its regulation on intestinal health of poultry. Front. Vet. Sci. 2021, 8, 736739. [Google Scholar] [CrossRef]
- Zhang, Y.Y.; Yu, K.F.; Chen, H.Z.; Su, Y.; Zhu, W.Y. Caecal infusion of the short-chain fatty acid propionate affects the microbiota and expression of inflammatory cytokines in the colon in a fistula pig model. Micro. Biotechnol. 2018, 11, 859–868. [Google Scholar] [CrossRef] [PubMed]
- Louis, P.; Hold, G.L.; Flint, H.J. The gut microbiota, bacterial metabolites and colorectal cancer. Nat. Rev. Microbiol. 2014, 12, 661–672. [Google Scholar] [CrossRef] [PubMed]
- Fu, K.; Ma, C.; Wang, C.; Zhou, H.L.; Gong, L.H.; Zhang, Y.Y.; Li, Y.X. Forsythiaside A alleviated carbon tetrachloride-induced liver fibrosis by modulating gut microbiota composition to increase short-chain fatty acids and restoring bile acids metabolism disorder. Biomed. Pharmacother. 2022, 151, 113185. [Google Scholar] [CrossRef] [PubMed]
- Sen, P.; Molinero-Perez, A.; O’Riordan, K.J.; McCafferty, C.P.; O’Halloran, K.D.; Cryan, J.F. Microbiota and sleep: Awakening the gut feeling. Trends Mol. Med. 2021, 27, 935–945. [Google Scholar] [CrossRef]
- Bian, Z.H.; Zhang, W.M.; Tang, J.Y.; Fei, Q.Q.; Hu, M.M.; Chen, X.W.; Su, L.L.; Fei, C.H.; Ji, D.; Mao, C.Q.; et al. Mechanisms Underlying the Action of Ziziphi Spinosae Semen in the Treatment of Insomnia: A Study Involving Network Pharmacology and Experimental Validation. Front. Pharmacol. 2021, 24, 752211. [Google Scholar] [CrossRef]
- Li, Q.; Du, C.H.; Zhang, M.; Yan, Y.; Gao, Y.; Qin, X.M. Investigation of effective components screening of Ziziphi Spinosae Semen based on serum pharmacochemistry and network pharmacology. Chin. Tradit. Herbal. Drugs 2017, 48, 1936–1943. [Google Scholar]
- Yan, Y.; Fu, C.; Cui, X.F.; Pei, X.P.; Li, A.P.; Qin, X.M.; Du, C.H. Metabolic profile and underlying antioxidant improvement of Ziziphi Spinosae Folium by human intestinal bacteria. Food Chem. 2020, 320, 126651. [Google Scholar] [CrossRef]
- Wang, H.S.; Ren, P.F.; Mang, L.; Shen, N.; Chen, J.R.; Zhang, Y.W. In vitro fermentation of novel microwave-synthesized non-digestible oligosaccharides and their impact on the composition and metabolites of human gut microbiota. J. Funct. Foods 2019, 55, 156–166. [Google Scholar] [CrossRef]
- Yu, X.D.; Zheng, R.B.; Xie, J.H.; Su, J.Y.; Huang, X.Q.; Wang, Y.H.; Zheng, Y.F.; Mo, Z.Z.; Wu, X.L.; Wu, D.; et al. Biological evaluation and molecular docking of baicalin and scutellarin as Helicobacter pylori urease inhibitors. J. Ethnopharmacol. 2015, 162, 69–78. [Google Scholar] [CrossRef]
- Masiulis, S.; Desai, R.; Uchański, T.; Martin, I.S.; Laverty, D.; Karia, D.; Malinauskas, T.; Zivanov, J.; Pardon, E.; Kotecha, A.; et al. GABAA receptor signalling mechanisms revealed by structural pharmacology. Nature 2019, 565, 454–459. [Google Scholar] [CrossRef]
- Gomes, I.S.; Santana, C.A.; Marcolino, L.S.; Lima, L.H.F.; Melo-Minardi, R.C.; Dias, R.S.; Paula, S.O.; Silveira, S.A. Computational prediction of potential inhibitors for SARS-COV-2 main protease based on machine learning, docking, MM-PBSA calculations, and metadynamics. PLoS ONE 2022, 17, e0267471. [Google Scholar] [CrossRef] [PubMed]
- Buey, B.; Forcén, A.; Grasa, L.; Layunta, E.; Mesonero, J.E.; Latorre, E. Gut Microbiota-Derived Short-Chain Fatty Acids: Novel Regulators of Intestinal Serotonin Transporter. Life 2023, 13, 1085. [Google Scholar] [CrossRef] [PubMed]
- Feng, W.Y.; Yang, Z.H.; Liu, Y.X.; Chen, R.; Song, Z.H.; Pan, G.Y.; Wang, Y.H.; Guo, Z.H.; Ding, X.Y.; Chen, L.; et al. Gut microbiota: A new target of traditional Chinese medicine for insomnia. Biomed. Pharmacother. 2023, 160, 114344. [Google Scholar] [CrossRef] [PubMed]


| Treatment | Time (h) | SCFAs (mmol/L) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Acetic Acid | Propionic Acid | Isobutyric Acid | Butyric Acid | Isovaleric Acid | Valeric Acid | Isocaproic Acid | Total SCFAs | ||
| N | 0 | 7.52 ± 0.79 fg | 0.30 ± 0.05 c | 0.10 ± 0.01 abcd | 0.09 ± 0.01 ab | 0.14 ± 0.02 a | 0.14 ± 0.01 abc | 0.01 ± 0.01 a | 8.29 ± 0.82 fg |
| 6 | 8.01 ± 0.14 fg | 0.96 ± 0.12 e | 0.14 ± 0.03 bcd | 0.89 ± 0.04 e | 0.17 ± 0.03 a | 0.15 ± 0.06 bcd | 0.01 ± 0.01 a | 10.33 ± 0.29 gh | |
| 24 | 13.63 ± 0.44 i | 1.69 ± 0.01 f | 0.22 ± 0.10 d | 1.70 ± 0.13 f | 0.59 ± 0.10 de | 0.18 ± 0.06 cd | 0.04 ± 0.01 b | 18.04 ± 0.39 j | |
| 48 | 20.43 ± 1.15 j | 1.98 ± 0.10 g | 0.60 ± 0.11 f | 2.79 ± 0.26 g | 0.85 ± 0.06 f | 0.21 ± 0.03 d | 0.06 ± 0.03 c | 26.87 ± 1.56 k | |
| CN | 0 | 6.91 ± 0.79 ef | 0.22 ± 0.01 bc | ND | 0.22 ± 0.01 ab | ND | ND | ND | 7.34 ± 0.38 ef |
| 6 | 6.84 ± 0.14 ef | 0.72 ± 0.05 d | 0.12 ± 0.01 abcd | 0.30 ± 0.01 bc | 0.12 ± 0.02 a | 0.08 ± 0.01 a | ND | 8.19 ± 0.44 f | |
| 24 | 8.85 ± 0.46 gh | 0.94 ± 0.07 e | 0.37 ± 0.04 e | 0.52 ± 0.01 cd | 0.22 ± 0.04 ab | 0.09 ± 0.01 ab | ND | 10.98 ± 0.46 h | |
| 48 | 10.54 ± 1.15 h | 0.97 ± 0.14 e | 0.50 ± 0.03 ef | 0.54 ± 0.06 d | 0.43 ± 0.02 c | 0.12 ± 0.02 abc | ND | 13.10 ± 1.64 i | |
| I | 0 | 2.30 ± 0.39 ab | ND | 0.17 ± 0.05 cd | 0.01 ± 0.01 a | 0.30 ± 0.01 b | ND | ND | 2.79 ± 0.06 abc |
| 6 | 3.70 ± 0.40 bc | ND | 0.05 ± 0.04 abc | 0.11 ± 0.05 ab | 0.57 ± 0.06 de | ND | ND | 4.43 ± 0.16 bcd | |
| 24 | 3.90 ± 0.31 bcd | 0.20 ± 0.04 bc | 0.11 ± 0.02 abcd | 0.10 ± 0.04 ab | 0.52 ± 0.02 cd | ND | ND | 4.83 ± 0.08 cd | |
| 48 | 10.68 ± 1.56 h | 0.35 ± 0.01 c | 0.14 ± 0.04 abcd | 0.10 ± 0.04 ab | 0.65 ± 0.01 e | ND | ND | 11.92 ± 0.43 hi | |
| CI | 0 | 1.37 ± 0.02 a | ND | ND | ND | ND | ND | ND | 1.37 ± 0.05 a |
| 6 | 2.67 ± 0.16 abc | ND | ND | ND | ND | ND | ND | 2.67 ± 0.65 ab | |
| 24 | 4.28 ± 0.06 cd | ND | ND | ND | ND | ND | ND | 4.28 ± 0.26 bcd | |
| 48 | 5.58 ± 0.50 de | 0.11 ± 0.01 ab | 0.03 ± 0.02 ab | 0.01 ± 0.01 ab | ND | ND | ND | 5.87 ± 0.62 de | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cui, X.; Zhang, S.; He, L.; Duan, H.; Xie, Y.; Pei, X.; Yan, Y.; Du, C. In Vitro Biotransformation of Ziziphi Spinosae Semen Saponins by Gut Microbiota from Healthy and Insomniac Groups. Int. J. Mol. Sci. 2025, 26, 4011. https://doi.org/10.3390/ijms26094011
Cui X, Zhang S, He L, Duan H, Xie Y, Pei X, Yan Y, Du C. In Vitro Biotransformation of Ziziphi Spinosae Semen Saponins by Gut Microbiota from Healthy and Insomniac Groups. International Journal of Molecular Sciences. 2025; 26(9):4011. https://doi.org/10.3390/ijms26094011
Chicago/Turabian StyleCui, Xiaofang, Shengmei Zhang, Ling He, Huizhu Duan, Yujun Xie, Xiangping Pei, Yan Yan, and Chenhui Du. 2025. "In Vitro Biotransformation of Ziziphi Spinosae Semen Saponins by Gut Microbiota from Healthy and Insomniac Groups" International Journal of Molecular Sciences 26, no. 9: 4011. https://doi.org/10.3390/ijms26094011
APA StyleCui, X., Zhang, S., He, L., Duan, H., Xie, Y., Pei, X., Yan, Y., & Du, C. (2025). In Vitro Biotransformation of Ziziphi Spinosae Semen Saponins by Gut Microbiota from Healthy and Insomniac Groups. International Journal of Molecular Sciences, 26(9), 4011. https://doi.org/10.3390/ijms26094011






