Bisphenol a Disrupts Steroidogenesis and Induces Apoptosis in Human Granulosa Cells Cultured In Vitro
Abstract
1. Introduction
2. Results
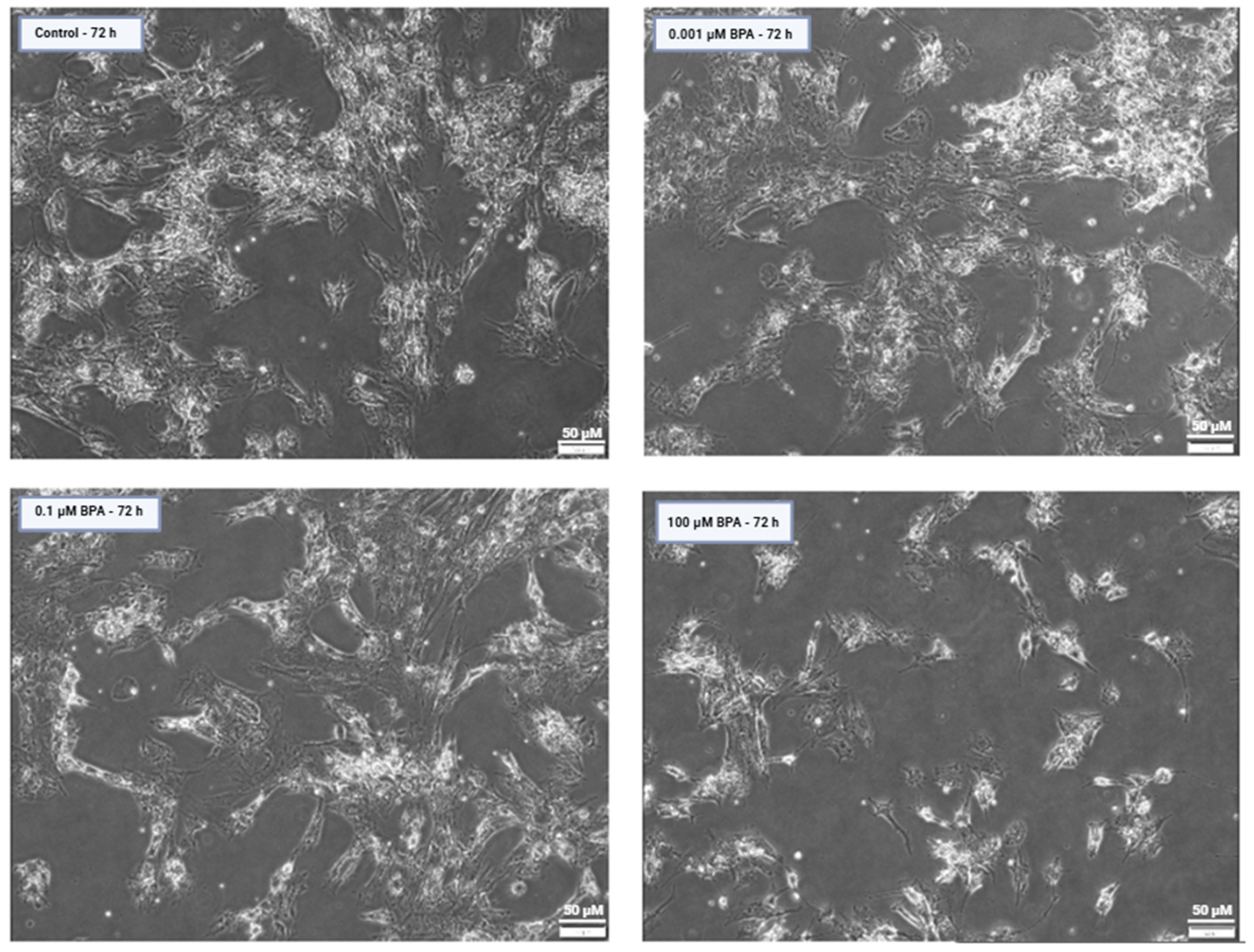
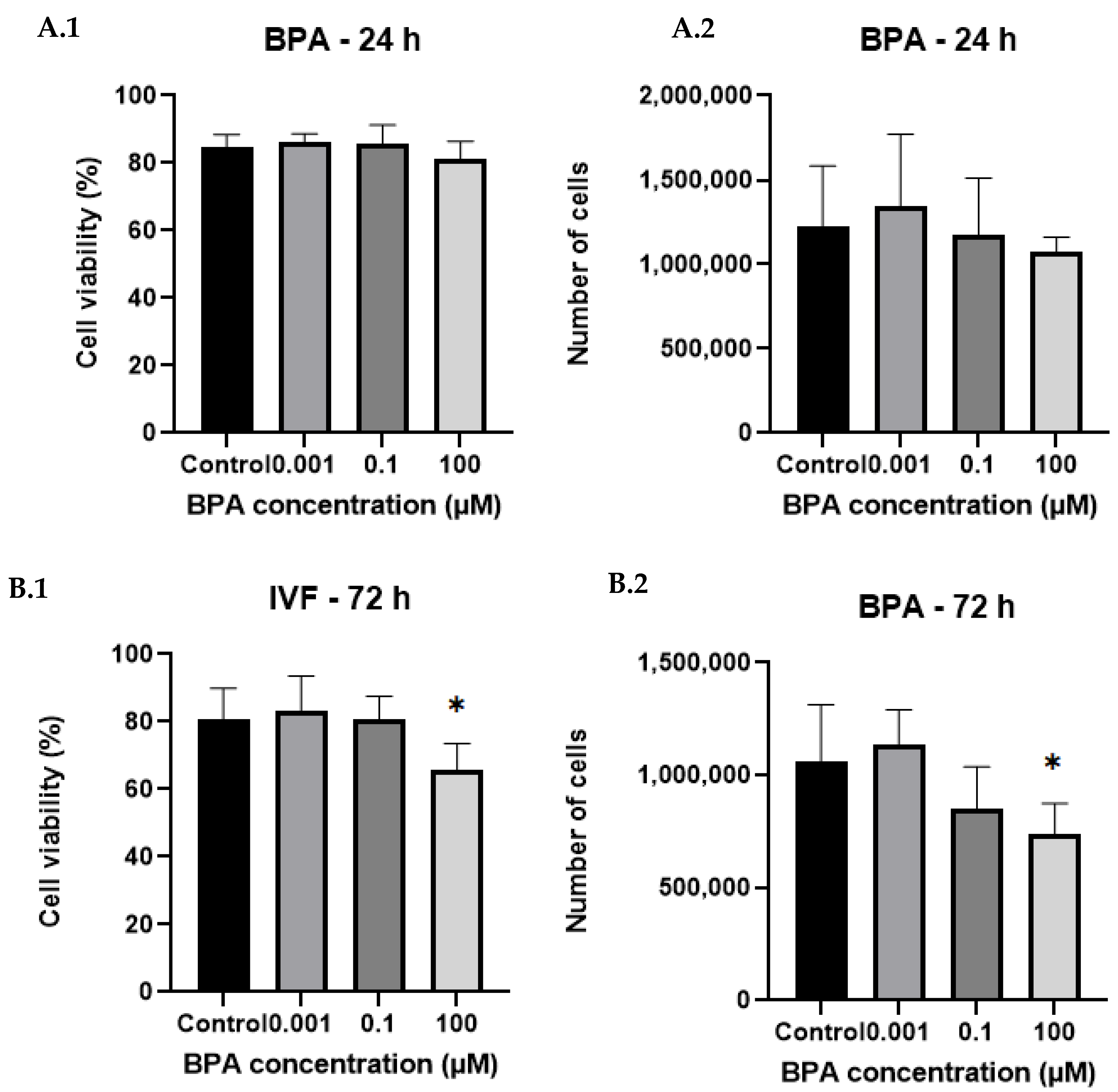
2.1. Cell Counts and Viability After BPA Exposure
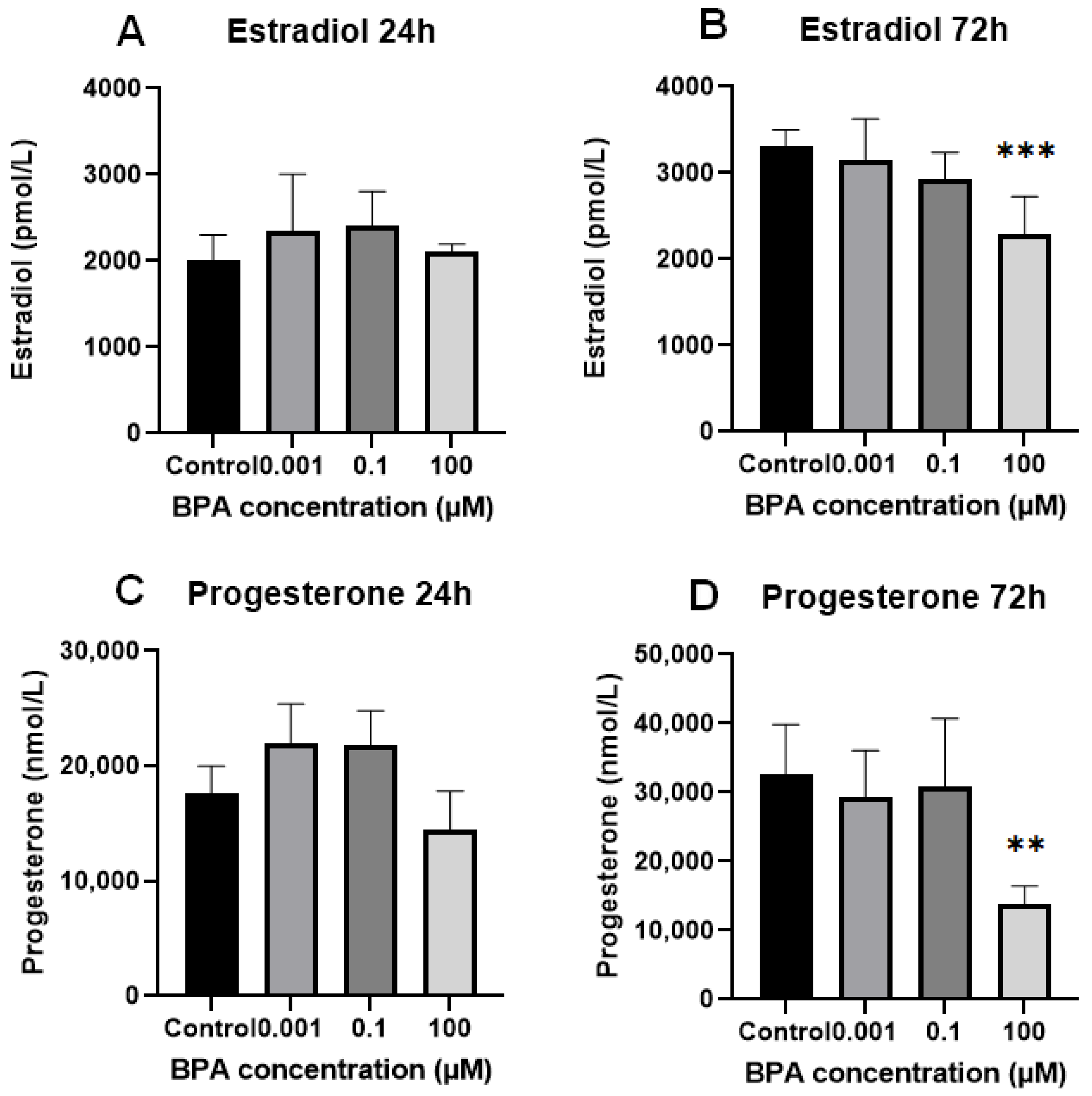
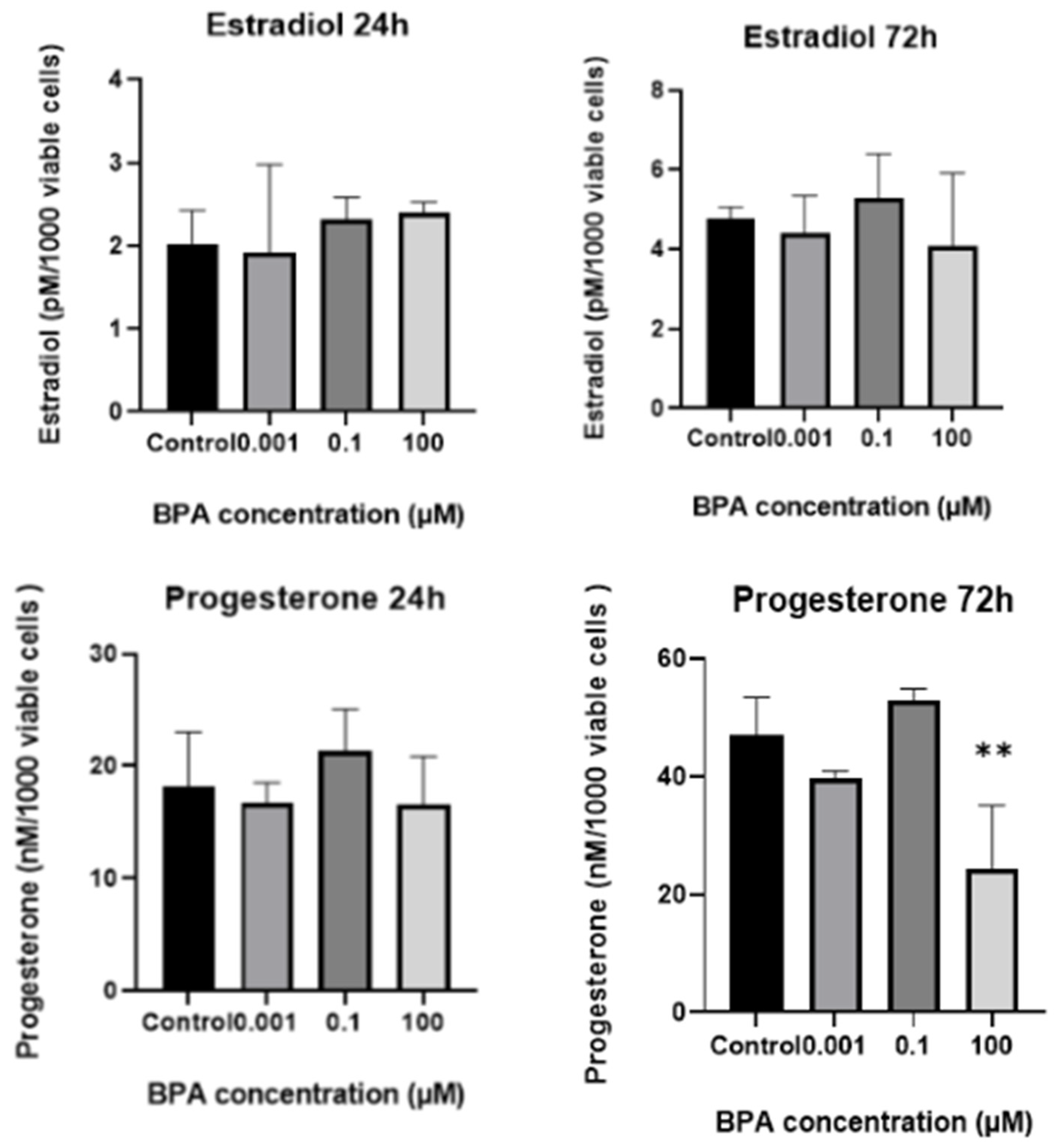
2.2. In Vitro Progesterone and Estradiol Synthesis: Concentrations in Spent Culture Medium
2.3. Differential Expression of Genes Associated with Steroidogenesis and Apoptosis in GCs After BPA Exposure
2.4. Differential Expression of Steroidogenesis and Apoptosis-Related Genes in GCs Based on Exposure to Different BPA Concentrations
2.5. Functional Annotation of Upregulated Genes in GCs Exposed to Different Concentrations of BPA
2.6. Functional Annotation of Downregulated Genes in GCs Exposed to Different Concentrations of BPA
2.7. Protein Expression
3. Discussion
4. Materials and Methods
4.1. Sample Collection
4.2. Isolation of Human Granulosa Cells from Follicular Fluid
4.3. Cell Culture
4.4. Cell Viability and Counts
4.5. Progesterone and Estradiol Assays in Spent Culture Medium
4.6. Real-Time Polymerase Chain Reaction (qPCR) and Gene Expression
4.7. Western Blot and Protein Expression
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vandenberg, L.N.; Colborn, T.; Hayes, T.B.; Heindel, J.J.; Jacobs, D.R.; Lee, D.-H.; Shioda, T.; Soto, A.M.; vom Saal, F.S.; Welshons, W.V.; et al. Hormones and Endocrine-Disrupting Chemicals: Low-Dose Effects and Nonmonotonic Dose Responses. Endocr. Rev. 2012, 33, 378–455. [Google Scholar] [CrossRef] [PubMed]
- Geens, T.; Aerts, D.; Berthot, C.; Bourguignon, J.-P.; Goeyens, L.; Lecomte, P.; Maghuin-Rogister, G.; Pironnet, A.-M.; Pussemier, L.; Scippo, M.-L.; et al. A Review of Dietary and Non-Dietary Exposure to Bisphenol-A. Food Chem. Toxicol. 2012, 50, 3725–3740. [Google Scholar] [CrossRef]
- Charkiewicz, A.E.; Omeljaniuk, W.J.; Nikliński, J. Bisphenol A—What Do We Know? A Global or Local Approach at the Public Health Risk Level. Int. J. Mol. Sci. 2024, 25, 6229. [Google Scholar] [CrossRef] [PubMed]
- Huo, X.; Chen, D.; He, Y.; Zhu, W.; Zhou, W.; Zhang, J. Bisphenol-A and Female Infertility: A Possible Role of Gene-Environment Interactions. Int. J. Environ. Res. Public Health 2015, 12, 11101–11116. [Google Scholar] [CrossRef]
- Acconcia, F.; Pallottini, V.; Marino, M. Molecular Mechanisms of Action of BPA. Dose-Response 2015, 13, 1559325815610582. [Google Scholar] [CrossRef]
- MacKay, H.; Abizaid, A. A Plurality of Molecular Targets: The Receptor Ecosystem for Bisphenol-A (BPA). Horm. Behav. 2018, 101, 59–67. [Google Scholar] [CrossRef]
- Liu, X.; Sakai, H.; Nishigori, M.; Suyama, K.; Nawaji, T.; Ikeda, S.; Nishigouchi, M.; Okada, H.; Matsushima, A.; Nose, T.; et al. Receptor-Binding Affinities of Bisphenol A and Its Next-Generation Analogs for Human Nuclear Receptors. Toxicol. Appl. Pharmacol. 2019, 377, 114610. [Google Scholar] [CrossRef]
- Rubin, B.S. Bisphenol A: An Endocrine Disruptor with Widespread Exposure and Multiple Effects. J. Steroid Biochem. Mol. Biol. 2011, 127, 27–34. [Google Scholar] [CrossRef]
- Mahalingam, S.; Ther, L.; Gao, L.; Wang, W.; Ziv-Gal, A.; Flaws, J.A. The Effects of in Utero Bisphenol A Exposure on Ovarian Follicle Numbers and Steroidogenesis in the F1 and F2 Generations of Mice. Reprod. Toxicol. 2017, 74, 150–157. [Google Scholar] [CrossRef]
- Huang, M.; Huang, M.; Li, X.; Liu, S.; Fu, L.; Jiang, X.; Yang, M. Bisphenol A Induces Apoptosis through GPER-Dependent Activation of the ROS/Ca2+-ASK1-JNK Pathway in Human Granulosa Cell Line KGN. Ecotoxicol. Environ. Saf. 2021, 208, 111429. [Google Scholar] [CrossRef]
- Celar Sturm, D.; Virant-Klun, I. Negative Effects of Endocrine Disruptor Bisphenol A on Ovarian Granulosa Cells and the Protective Role of Folic Acid. Reproduction 2023, 165, R117–R134. [Google Scholar] [CrossRef] [PubMed]
- Sabry, R.; Nguyen, M.; Younes, S.; Favetta, L.A. BPA and Its Analogs Increase Oxidative Stress Levels in in Vitro Cultured Granulosa Cells by Altering Anti-Oxidant Enzymes Expression. Mol. Cell. Endocrinol. 2022, 545, 111574. [Google Scholar] [CrossRef] [PubMed]
- Meli, R.; Monnolo, A.; Annunziata, C.; Pirozzi, C.; Ferrante, M.C. Oxidative Stress and BPA Toxicity: An Antioxidant Approach for Male and Female Reproductive Dysfunction. Antioxidants 2020, 9, 405. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-K.; Ko, D.-H.; Lee, W.; Kim, K.-R.; Chun, S.; Song, J.; Min, W.-K. Body Fluid Concentrations of Bisphenol A and Their Association with in Vitro Fertilization Outcomes. Hum. Fertil. 2021, 24, 199–207. [Google Scholar] [CrossRef]
- Parikh, F.R.; Uttamchandani, S.; Athalye, A.; Sinkar, P.; Velumani, A.S.; Panpalia, M.; Makwana, P.; Velumani, A. Bisphenol a (BPA) Levels in the Serum of Indian Women Collected at the Time of Oocyte Retrieval May Predict BPA Levels in Their Follicular Fluid (FF). Fertil. Steril. 2018, 110, e171. [Google Scholar] [CrossRef]
- Paoli, D.; Pallotti, F.; Dima, A.P.; Albani, E.; Alviggi, C.; Causio, F.; Dioguardi, C.C.; Conforti, A.; Ciriminna, R.; Fabozzi, G.; et al. Phthalates and Bisphenol A: Presence in Blood Serum and Follicular Fluid of Italian Women Undergoing Assisted Reproduction Techniques. Toxics 2020, 8, 91. [Google Scholar] [CrossRef]
- Raimondo, S.; Chiusano, M.L.; Gentile, M.; Gentile, T.; Cuomo, F.; Gentile, R.; Danza, D.; Siani, L.; Crescenzo, C.; Palmieri, M.; et al. Comparative Analysis of the Bioaccumulation of Bisphenol A in the Blood Serum and Follicular Fluid of Women Living in Two Areas with Different Environmental Impacts. Front. Endocrinol. 2024, 15, 1392550. [Google Scholar] [CrossRef]
- Poormoosavi, S.M.; Behmanesh, M.A.; Janati, S.; Najafzadehvarzi, H. Level of Bisphenol A in Follicular Fluid and Serum and Oocyte Morphology in Patients Undergoing IVF Treatment. J. Fam. Reprod. Health 2019, 13, 154–159. [Google Scholar] [CrossRef]
- Stelzer, G.; Rosen, N.; Plaschkes, I.; Zimmerman, S.; Twik, M.; Fishilevich, S.; Stein, T.I.; Nudel, R.; Lieder, I.; Mazor, Y.; et al. The GeneCards Suite: From Gene Data Mining to Disease Genome Sequence Analyses. Curr. Protoc. Bioinforma. 2016, 54, 1.30.1–1.30.33. [Google Scholar] [CrossRef]
- Protasoni, M.; López-Polo, V.; Stephan-Otto Attolini, C.; Brandariz, J.; Herranz, N.; Mateo, J.; Ruiz, S.; Fernandez-Capetillo, O.; Kovatcheva, M.; Serrano, M. Cyclophilin D Plays a Critical Role in the Survival of Senescent Cells. EMBO J. 2024, 43, 5972–6000. [Google Scholar] [CrossRef]
- Peng, J.Z.; Xue, L.; Chen, J.; Chen, B.S.; Yang, Y.Q. Influence of Cyclophilin D Protein Expression Level on Endothelial Cell Oxidative Damage Resistance. Genet. Mol. Res. 2015, 14, 4258–4268. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.-Y.; Fu, X.; Fan, S.; Guo, P.; Su, D.; Dong, J.-T. Oestrogen Causes ATBF1 Protein Degradation through the Oestrogen-Responsive E3 Ubiquitin Ligase EFP. Biochem. J. 2012, 444, 581–590. [Google Scholar] [CrossRef] [PubMed]
- Thackray, V.G. Fox Tales: Regulation of Gonadotropin Gene Expression by Forkhead Transcription Factors. Mol. Cell. Endocrinol. 2014, 385, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Walsh, J.G.; Cullen, S.P.; Sheridan, C.; Lüthi, A.U.; Gerner, C.; Martin, S.J. Executioner Caspase-3 and Caspase-7 Are Functionally Distinct Proteases. Proc. Natl. Acad. Sci. USA 2008, 105, 12815–12819. [Google Scholar] [CrossRef] [PubMed]
- Nakatsumi, H.; Yonehara, S. Identification of Functional Regions Defining Different Activity in Caspase-3 and Caspase-7 within Cells. J. Biol. Chem. 2010, 285, 25418–25425. [Google Scholar] [CrossRef]
- Thomsen, N.D.; Koerber, J.T.; Wells, J.A. Structural Snapshots Reveal Distinct Mechanisms of Procaspase-3 and -7 Activation. Proc. Natl. Acad. Sci. USA 2013, 110, 8477–8482. [Google Scholar] [CrossRef]
- Peng, T.; Tao, X.; Xia, Z.; Hu, S.; Xue, J.; Zhu, Q.; Pan, X.; Zhang, Q.; Li, S. Pathogen Hijacks Programmed Cell Death Signaling by Arginine ADPR-Deacylization of Caspases. Mol. Cell 2022, 82, 1806–1820.e8. [Google Scholar] [CrossRef]
- Liu, Y.; Zeng, H.; Hou, Y.; Li, Z.; Li, L.; Song, X.; Ding, J.; Shao, F.; Xu, Y. Calmodulin Binding Activates Chromobacterium CopC Effector to ADP-Riboxanate Host Apoptotic Caspases. mBio 2022, 13, e0069022. [Google Scholar] [CrossRef]
- Nicholson, D.W.; Ali, A.; Thornberry, N.A.; Vaillancourt, J.P.; Ding, C.K.; Gallant, M.; Gareau, Y.; Griffin, P.R.; Labelle, M.; Lazebnik, Y.A. Identification and Inhibition of the ICE/CED-3 Protease Necessary for Mammalian Apoptosis. Nature 1995, 376, 37–43. [Google Scholar] [CrossRef]
- Kourmaeva, E.; Sabry, R.; Favetta, L.A. Bisphenols A and F, but Not S, Induce Apoptosis in Bovine Granulosa Cells via the Intrinsic Mitochondrial Pathway. Front. Endocrinol. 2022, 13, 1028438. [Google Scholar] [CrossRef]
- Qi, J.; Liu, L.; Yang, J.; Gao, X.; Zhang, W. Bisphenol A Decreases Progesterone Synthesis in Human Ovarian Granulosa Cells. Birth Defects Res. 2020, 112, 1843–1849. [Google Scholar] [CrossRef] [PubMed]
- Kwintkiewicz, J.; Nishi, Y.; Yanase, T.; Giudice, L.C. Peroxisome Proliferator–Activated Receptor-γ Mediates Bisphenol A Inhibition of FSH-Stimulated IGF-1, Aromatase, and Estradiol in Human Granulosa Cells. Environ. Health Perspect. 2010, 118, 400. [Google Scholar] [CrossRef]
- Xu, J.; Osuga, Y.; Yano, T.; Morita, Y.; Tang, X.; Fujiwara, T.; Takai, Y.; Matsumi, H.; Koga, K.; Taketani, Y.; et al. Bisphenol A Induces Apoptosis and G2-to-M Arrest of Ovarian Granulosa Cells. Biochem. Biophys. Res. Commun. 2002, 292, 456–462. [Google Scholar] [CrossRef] [PubMed]
- Grasselli, F.; Baratta, L.; Baioni, L.; Bussolati, S.; Ramoni, R.; Grolli, S.; Basini, G. Bisphenol A Disrupts Granulosa Cell Function. Domest. Anim. Endocrinol. 2010, 39, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Liu, C.; Chen, M.; Yan, J.; Wang, C.; Zuo, Z.; He, C. The Interference Effects of Bisphenol A on the Synthesis of Steroid Hormones in Human Ovarian Granulosa Cells. Environ. Toxicol. 2021, 36, 665–674. [Google Scholar] [CrossRef]
- Fehlberg, S.; Gregel, C.M.; Göke, A.; Göke, R. Bisphenol A Diglycidyl Ether-induced Apoptosis Involves Bax/Bid-dependent Mitochondrial Release of Apoptosis-inducing Factor (AIF), Cytochrome c and Smac/DIABLO. Br. J. Pharmacol. 2003, 139, 495–500. [Google Scholar] [CrossRef]
- Ke, F.S.; Holloway, S.; Uren, R.T.; Wong, A.W.; Little, M.H.; Kluck, R.M.; Voss, A.K.; Strasser, A. The BCL-2 Family Member BID Plays a Role during Embryonic Development in Addition to Its BH3-only Protein Function by Acting in Parallel to BAX, BAK and BOK. EMBO J. 2022, 41, e110300. [Google Scholar] [CrossRef]
- Kodanch, S.M.; Mukherjee, S.; Prabhu, N.B.; Kabekkodu, S.P.; Bhat, S.K.; Rai, P.S. Altered Mitochondrial Homeostasis on Bisphenol-A Exposure and Its Association in Developing Polycystic Ovary Syndrome: A Comprehensive Review. Reprod. Toxicol. 2024, 130, 108700. [Google Scholar] [CrossRef]
- Téteau, O.; Jaubert, M.; Desmarchais, A.; Papillier, P.; Binet, A.; Maillard, V.; Elis, S. Bisphenol A and S Impaired Ovine Granulosa Cell Steroidogenesis. Reproduction 2020, 159, 571–583. [Google Scholar] [CrossRef]
- Bujnakova Mlynarcikova, A.; Scsukova, S. Simultaneous Effects of Endocrine Disruptor Bisphenol A and Flavonoid Fisetin on Progesterone Production by Granulosa Cells. Environ. Toxicol. Pharmacol. 2018, 59, 66–73. [Google Scholar] [CrossRef]
- Samardzija, D.; Pogrmic-Majkic, K.; Fa, S.; Stanic, B.; Jasnic, J.; Andric, N. Bisphenol A Decreases Progesterone Synthesis by Disrupting Cholesterol Homeostasis in Rat Granulosa Cells. Mol. Cell. Endocrinol. 2018, 461, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Mansur, A.; Adir, M.; Yerushalmi, G.; Hourvitz, A.; Gitman, H.; Yung, Y.; Orvieto, R.; Machtinger, R. Does BPA Alter Steroid Hormone Synthesis in Human Granulosa Cells in Vitro? Hum. Reprod. 2016, 31, 1562–1569. [Google Scholar] [CrossRef] [PubMed]
- Mlynarcíková, A.; Kolena, J.; Ficková, M.; Scsuková, S. Alterations in Steroid Hormone Production by Porcine Ovarian Granulosa Cells Caused by Bisphenol A and Bisphenol A Dimethacrylate. Mol. Cell. Endocrinol. 2005, 244, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Bujnakova Mlynarcikova, A.; Scsukova, S. Bisphenol Analogs AF, S and F: Effects on Functional Characteristics of Porcine Granulosa Cells. Reprod. Toxicol. 2021, 103, 18–27. [Google Scholar] [CrossRef]
- Lebachelier de la Riviere, M.-E.; Wu, L.; Gayet, M.; Bousquet, M.; Buron, C.; Vignault, C.; Téteau, O.; Desmarchais, A.; Maillard, V.; Uzbekova, S.; et al. Cumulative and Potential Synergistic Effects of Seven Different Bisphenols on Human Granulosa Cells in Vitro? Environ. Pollut. 2023, 330, 121818. [Google Scholar] [CrossRef]
- Mansur, A.; Israel, A.; Combelles, C.M.H.; Adir, M.; Racowsky, C.; Hauser, R.; Baccarelli, A.A.; Machtinger, R. Bisphenol-A Exposure and Gene Expression in Human Luteinized Membrana Granulosa Cells in Vitro. Hum. Reprod. 2017, 32, 409–417. [Google Scholar] [CrossRef]
- Bujnakova Mlynarcikova, A.; Scsukova, S. Bisphenol Analogs AF and S: Effects on Cell Status and Production of Angiogenesis-Related Factors by COV434 Human Granulosa Cell Line. Toxicol. Appl. Pharmacol. 2021, 426, 115634. [Google Scholar] [CrossRef]
- Zhou, W.; Liu, J.; Liao, L.; Han, S.; Liu, J. Effect of Bisphenol A on Steroid Hormone Production in Rat Ovarian Theca-Interstitial and Granulosa Cells. Mol. Cell. Endocrinol. 2008, 283, 12–18. [Google Scholar] [CrossRef]
- Prossnitz, E.R.; Barton, M. Estrogen Biology: New Insights into GPER Function and Clinical Opportunities. Mol. Cell. Endocrinol. 2014, 389, 71–83. [Google Scholar] [CrossRef]
- Pivonello, C.; Muscogiuri, G.; Nardone, A.; Garifalos, F.; Provvisiero, D.P.; Verde, N.; de Angelis, C.; Conforti, A.; Piscopo, M.; Auriemma, R.S.; et al. Bisphenol A: An Emerging Threat to Female Fertility. Reprod. Biol. Endocrinol. 2020, 18, 22. [Google Scholar] [CrossRef]
- Ruiz, T.F.R.; Grigio, V.; Ferrato, L.J.; de Souza, L.G.; Colleta, S.J.; Amaro, G.M.; Góes, R.M.; Vilamaior, P.S.L.; Leonel, E.C.R.; Taboga, S.R. Impairment of Steroidogenesis and Follicle Development after Bisphenol A Exposure during Pregnancy and Lactation in the Ovaries of Mongolian Gerbils Aged Females. Mol. Cell. Endocrinol. 2023, 566–567, 111892. [Google Scholar] [CrossRef] [PubMed]
- Richter, C.A.; Taylor, J.A.; Ruhlen, R.L.; Welshons, W.V.; Vom Saal, F.S. Estradiol and Bisphenol A Stimulate Androgen Receptor and Estrogen Receptor Gene Expression in Fetal Mouse Prostate Mesenchyme Cells. Environ. Health Perspect. 2007, 115, 902–908. [Google Scholar] [CrossRef] [PubMed]
- Apparao, K.B.C.; Lovely, L.P.; Gui, Y.; Lininger, R.A.; Lessey, B.A. Elevated Endometrial Androgen Receptor Expression in Women with Polycystic Ovarian Syndrome1. Biol. Reprod. 2002, 66, 297–304. [Google Scholar] [CrossRef] [PubMed]
- Catteau-Jonard, S.; Jamin, S.P.; Leclerc, A.; Gonzalès, J.; Dewailly, D.; di Clemente, N. Anti-Mullerian Hormone, Its Receptor, FSH Receptor, and Androgen Receptor Genes Are Overexpressed by Granulosa Cells from Stimulated Follicles in Women with Polycystic Ovary Syndrome. J. Clin. Endocrinol. Metab. 2008, 93, 4456–4461. [Google Scholar] [CrossRef]
- Hulchiy, M.; Nybacka, Å.; Sahlin, L.; Hirschberg, A.L. Endometrial Expression of Estrogen Receptors and the Androgen Receptor in Women with Polycystic Ovary Syndrome: A Lifestyle Intervention Study. J. Clin. Endocrinol. Metab. 2016, 101, 561–571. [Google Scholar] [CrossRef]
- Lan, Z.-J.; Xu, X.; Chung, A.C.-K.; Cooney, A.J. Extra-Germ Cell Expression of Mouse Nuclear Receptor Subfamily 6, Group A, Member 1 (NR6A1). Biol. Reprod. 2009, 80, 905–912. [Google Scholar] [CrossRef]
- Shimada, N.; Suzuki, T.; Inoue, S.; Kato, K.; Imatani, A.; Sekine, H.; Ohara, S.; Shimosegawa, T.; Sasano, H. Systemic Distribution of Estrogen-Responsive Finger Protein (Efp) in Human Tissues. Mol. Cell. Endocrinol. 2004, 218, 147–153. [Google Scholar] [CrossRef]
- Harrington, W.R.; Sengupta, S.; Katzenellenbogen, B.S. Estrogen Regulation of the Glucuronidation Enzyme UGT2B15 in Estrogen Receptor-Positive Breast Cancer Cells. Endocrinology 2006, 147, 3843–3850. [Google Scholar] [CrossRef]
- Javadov, S.; Kuznetsov, A. Mitochondrial Permeability Transition and Cell Death: The Role of Cyclophilin D. Front. Physiol. 2013, 4, 76. [Google Scholar] [CrossRef]
- Schubert, A.; Grimm, S. Cyclophilin D, a Component of the Permeability Transition-Pore, Is an Apoptosis Repressor. Cancer Res. 2004, 64, 85–93. [Google Scholar] [CrossRef]
- Karamyshev, A.L.; Karamysheva, Z.N. Lost in Translation: Ribosome-Associated mRNA and Protein Quality Controls. Front. Genet. 2018, 9, 431. [Google Scholar] [CrossRef] [PubMed]
- Ahsan, N.; Shariq, M.; Surolia, A.; Raj, R.; Khan, M.F.; Kumar, P. Multipronged Regulation of Autophagy and Apoptosis: Emerging Role of TRIM Proteins. Cell. Mol. Biol. Lett. 2024, 29, 13. [Google Scholar] [CrossRef] [PubMed]
- Baines, C.P.; Kaiser, R.A.; Purcell, N.H.; Blair, N.S.; Osinska, H.; Hambleton, M.A.; Brunskill, E.W.; Sayen, M.R.; Gottlieb, R.A.; Dorn, G.W.; et al. Loss of Cyclophilin D Reveals a Critical Role for Mitochondrial Permeability Transition in Cell Death. Nature 2005, 434, 658–662. [Google Scholar] [CrossRef] [PubMed]
- Amanakis, G.; Murphy, E. Cyclophilin D: An Integrator of Mitochondrial Function. Front. Physiol. 2020, 11, 595. [Google Scholar] [CrossRef]
- Lim, H.C.; Xie, L.; Zhang, W.; Li, R.; Chen, Z.-C.; Wu, G.-Z.; Cui, S.-S.; Tan, E.K.; Zeng, L. Ribosomal S6 Kinase 2 (RSK2) Maintains Genomic Stability by Activating the Atm/P53-Dependent DNA Damage Pathway. PLoS ONE 2013, 8, e74334. [Google Scholar] [CrossRef]
- Gassman, N.R. Induction of Oxidative Stress by Bisphenol A and Its Pleiotropic Effects. Environ. Mol. Mutagen. 2017, 58, 60–71. [Google Scholar] [CrossRef]
- Xu, G.; Huang, M.; Hu, J.; Liu, S.; Yang, M. Bisphenol A and Its Structural Analogues Exhibit Differential Potential to Induce Mitochondrial Dysfunction and Apoptosis in Human Granulosa Cells. Food Chem. Toxicol. 2024, 188, 114713. [Google Scholar] [CrossRef]
- Mukherjee, U.; Das, S.; Ghosh, S.; Maitra, S. Reproductive Toxicity of Bisphenol A, at Environmentally Relevant Concentrations, on Ovarian Redox Balance, Maturational Response, and Intra-Oocyte Signalling Events in Labeo bata. Sci. Total Environ. 2024, 906, 167415. [Google Scholar] [CrossRef]
- Vandenberg, L.N. Non-Monotonic Dose Responses in Studies of Endocrine Disrupting Chemicals: Bisphenol a as a Case Study. Dose-Response 2014, 12, 259–276. [Google Scholar] [CrossRef]
- Birnbaum, L.S. Environmental Chemicals: Evaluating Low-Dose Effects. Environ. Health Perspect. 2012, 120, a143–a144. [Google Scholar] [CrossRef]
- Ferrero, H.; Delgado-Rosas, F.; Garcia-Pascual, C.M.; Monterde, M.; Zimmermann, R.C.; Simon, C.; Pellicer, A.; Gomez, R. Efficiency and Purity Provided by the Existing Methods for the Isolation of Luteinized Granulosa Cells: A Comparative Study. Hum. Reprod. 2012, 27, 1781–1789. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Gao, G.; Li, S.; Xiao, N.; Zhang, Y.; Luo, H. Development of an Optimal Protocol for Isolation and Purification of Human Granulosa Cells in Patients with Different Ovarian Reserves. Exp. Ther. Med. 2021, 22, 938. [Google Scholar] [CrossRef] [PubMed]
- Jančar, N.; Kopitar, A.N.; Ihan, A.; Klun, I.V.; Bokal, E.V. Effect of Apoptosis and Reactive Oxygen Species Production in Human Granulosa Cells on Oocyte Fertilization and Blastocyst Development. J. Assist. Reprod. Genet. 2007, 24, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Aghadavod, E.; Zarghami, N.; Farzadi, L.; Zare, M.; Barzegari, A.; Movassaghpour, A.A.; Nouri, M. Isolation of Granulosa Cells from Follicular Fluid; Applications in Biomedical and Molecular Biology Experiments. Adv. Biomed. Res. 2015, 4, 250. [Google Scholar] [CrossRef]
- Velthut-Meikas, A.; Simm, J.; Tuuri, T.; Tapanainen, J.S.; Metsis, M.; Salumets, A. Research Resource: Small RNA-Seq of Human Granulosa Cells Reveals miRNAs in FSHR and Aromatase Genes. Mol. Endocrinol. 2013, 27, 1128–1141. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Pavlidis, P. Using ANOVA for Gene Selection from Microarray Studies of the Nervous System. Methods 2003, 31, 282–289. [Google Scholar] [CrossRef]
- Gilda, J.E.; Gomes, A.V. Stain Free Total Protein Staining Is a Superior Loading Control to β-Actin for Western Blots. Anal. Biochem. 2013, 440, 186–188. [Google Scholar] [CrossRef]
- Taylor, S.C.; Posch, A. The Design of a Quantitative Western Blot Experiment. BioMed Res. Int. 2014, 2014, 361590. [Google Scholar] [CrossRef]
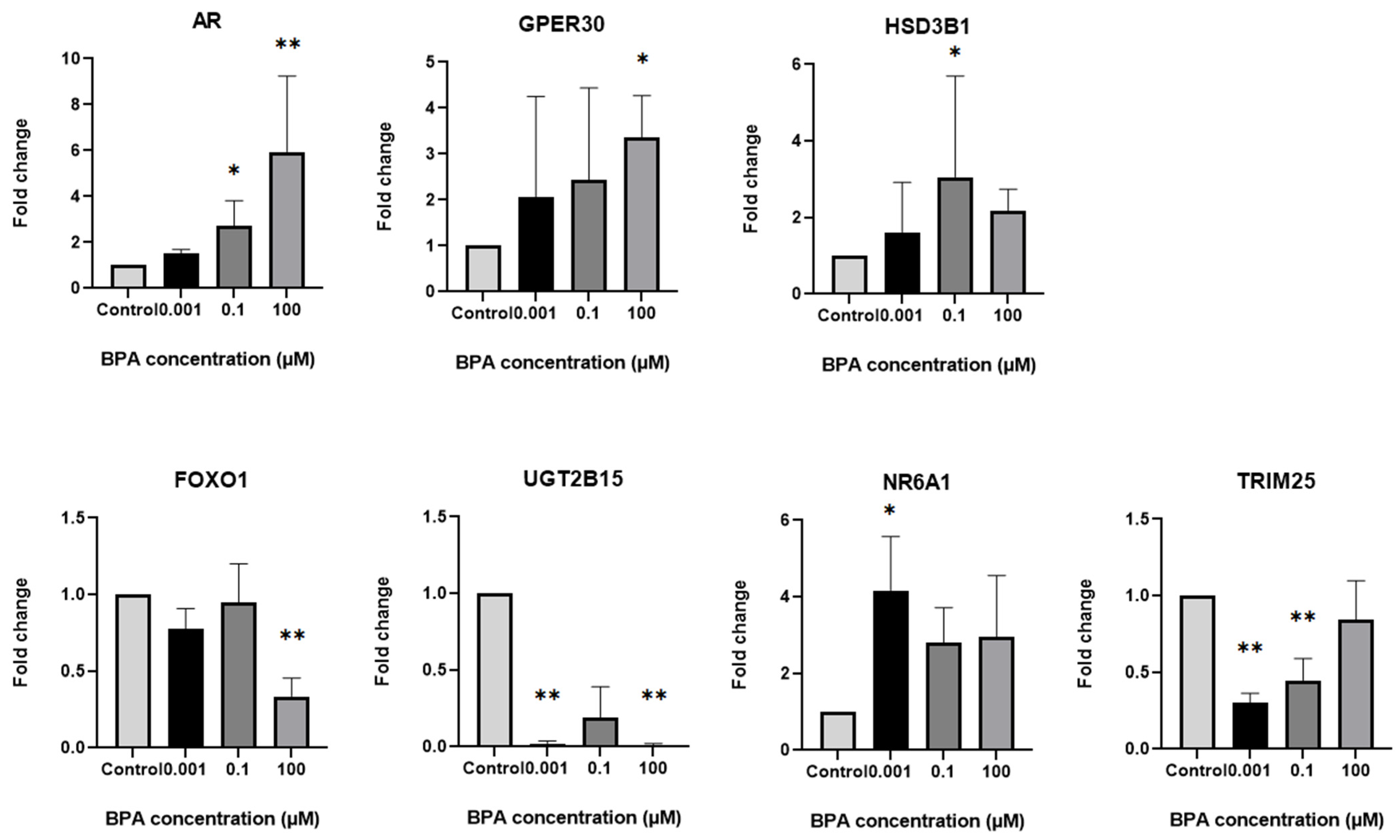
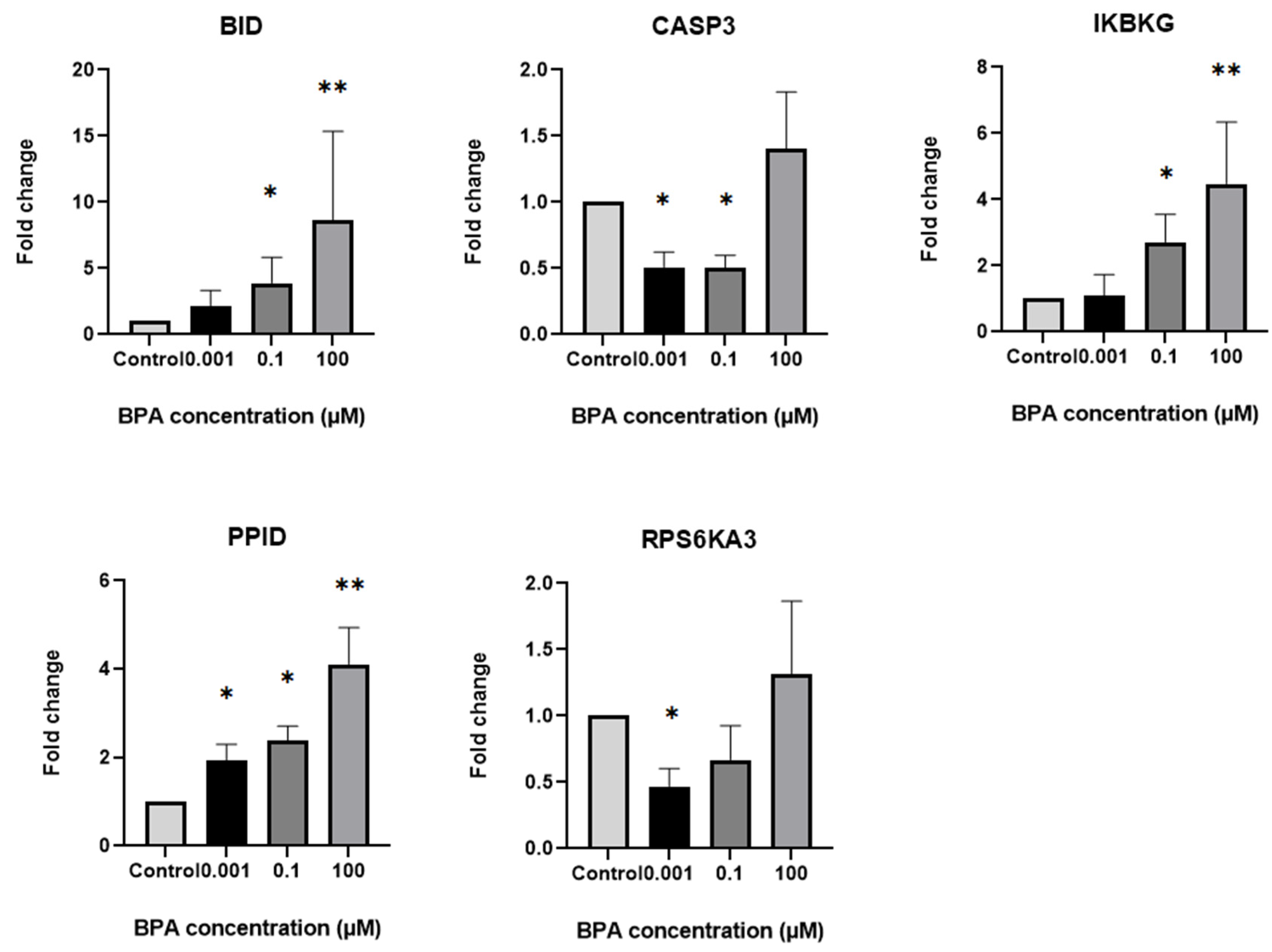


| Genes | Concentrations of BPA | ||
|---|---|---|---|
| 0.001 µM | 0.1 µM | 100 µM | |
| Steroidogenesis | |||
| NR6A1 (Nuclear Receptor Subfamily 6 Group A Member 1) | ↑ | ||
| TRIM25 (Tripartite Motif Containing 25) | ↓ | ↓ | |
| UGT2B15 (UDP Glucuronosyltransferase Family 2 Member B15) | ↓ | ↓ | |
| HSD3B1 (Hydroxy-Delta-5-Steroid Dehydrogenase, 3 Beta- And Steroid Delta-Isomerase 1) | ↑ | ||
| AR (Androgen Receptor) | ↑ | ↑ | |
| GPER30 (G Protein-Coupled Estrogen Receptor 30) | ↑ | ||
| FOXO1 (Forkhead Box O1) | ↓ | ||
| Apoptosis | |||
| PPID (Peptidylprolyl Isomerase D) | ↑ | ↑ | ↑ |
| CASP3 (Caspase 3) | ↓ | ↓ | |
| RPS6KA3 (Ribosomal Protein S6 Kinase A3) | ↓ | ||
| BID (BH3 Interacting Domain Death Agonist) | ↑ | ↑ | |
| IKBKG (Inhibitor of Nuclear Factor Kappa B Kinase Regulatory Subunit Gamma) | ↑ | ↑ | |
| Antibody | Company | Mono/Polyclonal | Source | Dilution for WB |
|---|---|---|---|---|
| Anti-RTR antibody | Abcam, Cambridge, UK | Polyclonal | Rabbit | 1:250 |
| Anti-G-protein coupled receptor 30 antibody | Abcam, Cambridge, UK | Monoclonal | Rabbit | 1:500 |
| Anti-UGT2B15 antibody | Abcam, Cambridge, UK | Monoclonal | Rabbit | 1:1000 |
| Anti-Bid antibody | Abcam, Cambridge, UK | Monoclonal | Rabbit | 1:2500 |
| Anti-HSD3B1 antibody | Abcam, Cambridge, UK | Monoclonal | Mouse | 1:2000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Celar Šturm, D.; Režen, T.; Jančar, N.; Virant-Klun, I. Bisphenol a Disrupts Steroidogenesis and Induces Apoptosis in Human Granulosa Cells Cultured In Vitro. Int. J. Mol. Sci. 2025, 26, 4081. https://doi.org/10.3390/ijms26094081
Celar Šturm D, Režen T, Jančar N, Virant-Klun I. Bisphenol a Disrupts Steroidogenesis and Induces Apoptosis in Human Granulosa Cells Cultured In Vitro. International Journal of Molecular Sciences. 2025; 26(9):4081. https://doi.org/10.3390/ijms26094081
Chicago/Turabian StyleCelar Šturm, Dominika, Tadeja Režen, Nina Jančar, and Irma Virant-Klun. 2025. "Bisphenol a Disrupts Steroidogenesis and Induces Apoptosis in Human Granulosa Cells Cultured In Vitro" International Journal of Molecular Sciences 26, no. 9: 4081. https://doi.org/10.3390/ijms26094081
APA StyleCelar Šturm, D., Režen, T., Jančar, N., & Virant-Klun, I. (2025). Bisphenol a Disrupts Steroidogenesis and Induces Apoptosis in Human Granulosa Cells Cultured In Vitro. International Journal of Molecular Sciences, 26(9), 4081. https://doi.org/10.3390/ijms26094081







