Prognostic Significance of Overexpression of BCL9 and TPX2 in High-Grade Clear Cell Renal Cell Carcinoma: Prognostic Markers for Metastasis and Survival
Abstract
:1. Introduction
2. Results
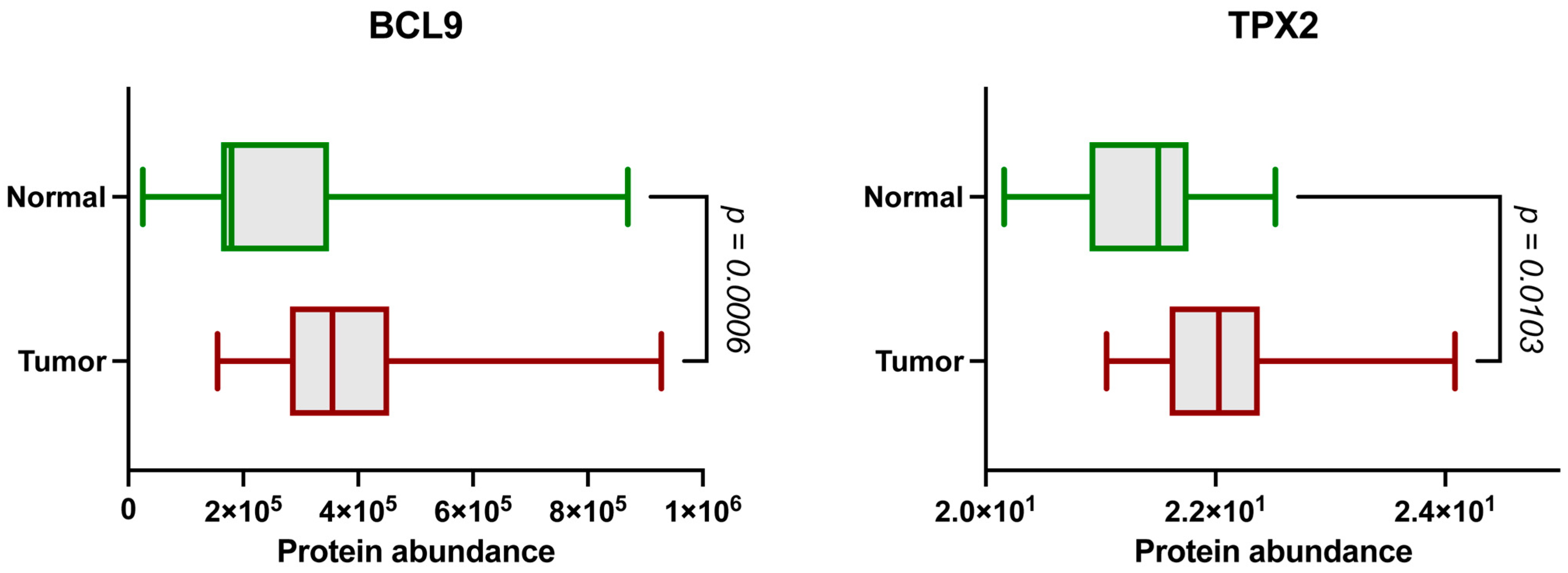
2.1. The BCL9 and TPX2 Protein Levels Are Significantly Elevated in ccRCC
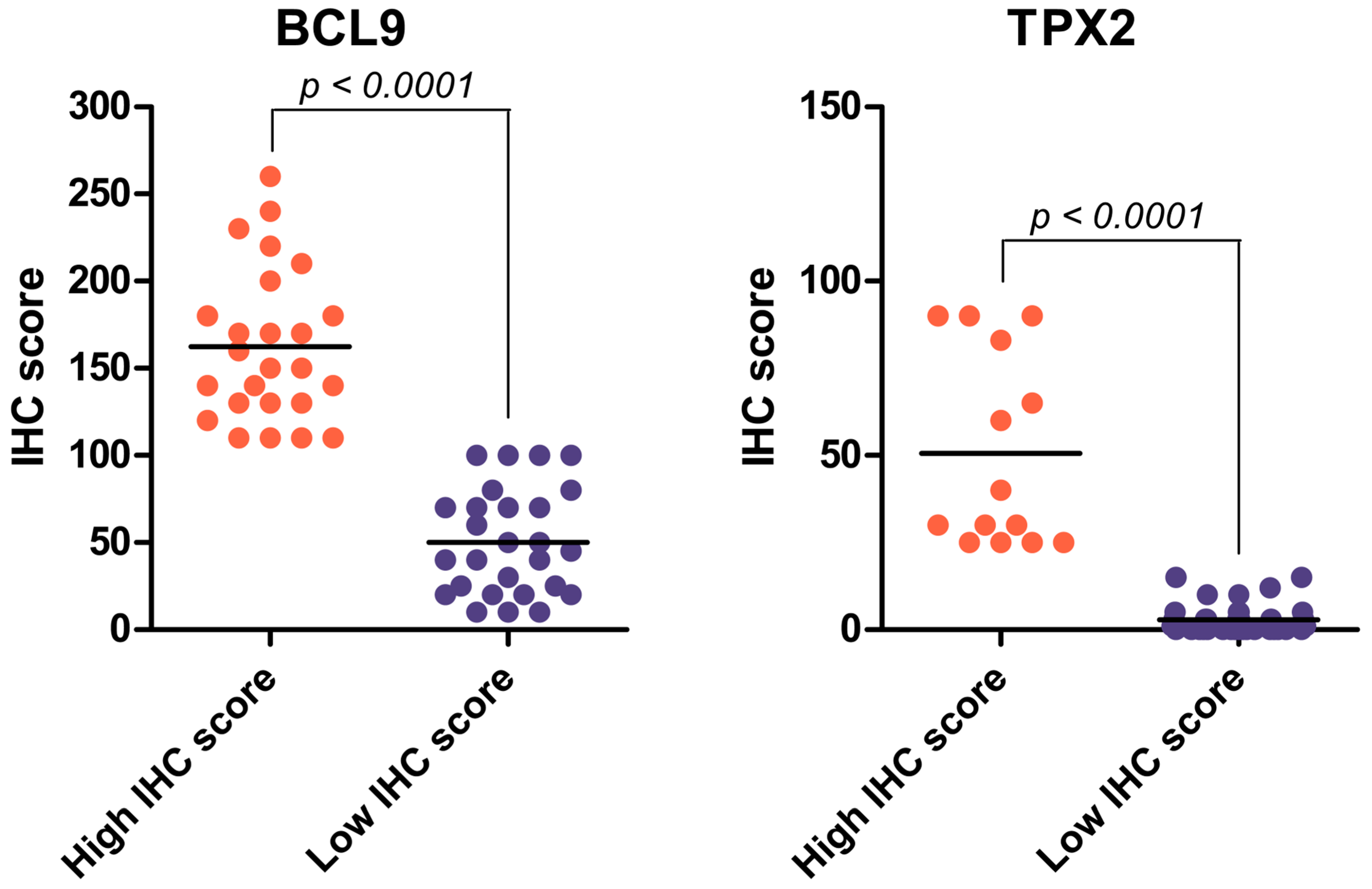
2.2. Immunohistochemical Analysis of BCL9 and TPX2 Levels on Validation Cohort of ccRCC Patients
2.3. BCL9 and TPX2 Protein Levels Allow for Distinguishing Two Distinct Groups Depending on IHC Score
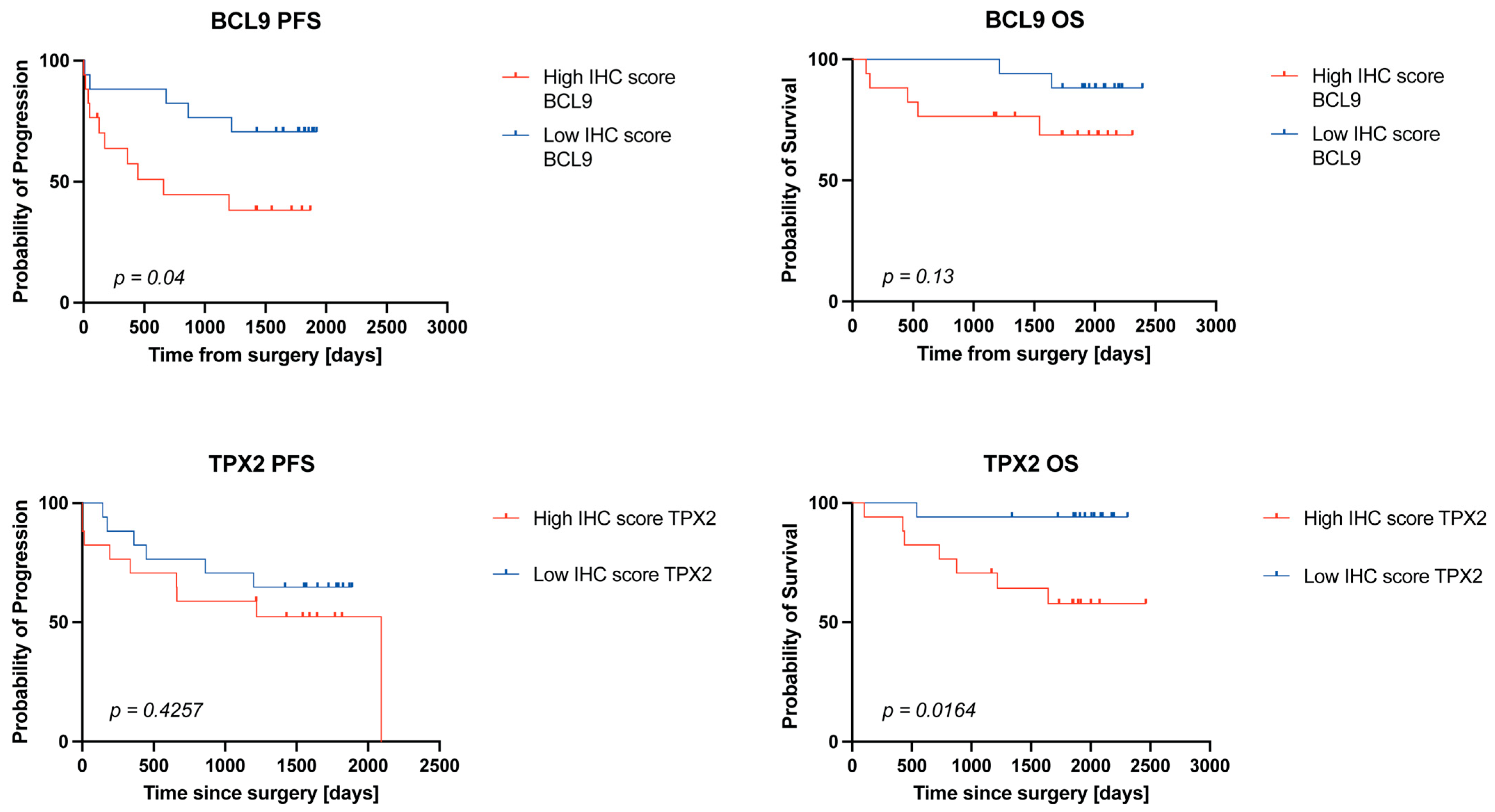
2.4. High BCL9 and TPX2 Protein Levels Are Associated with Worse Survival in ccRCC Patients
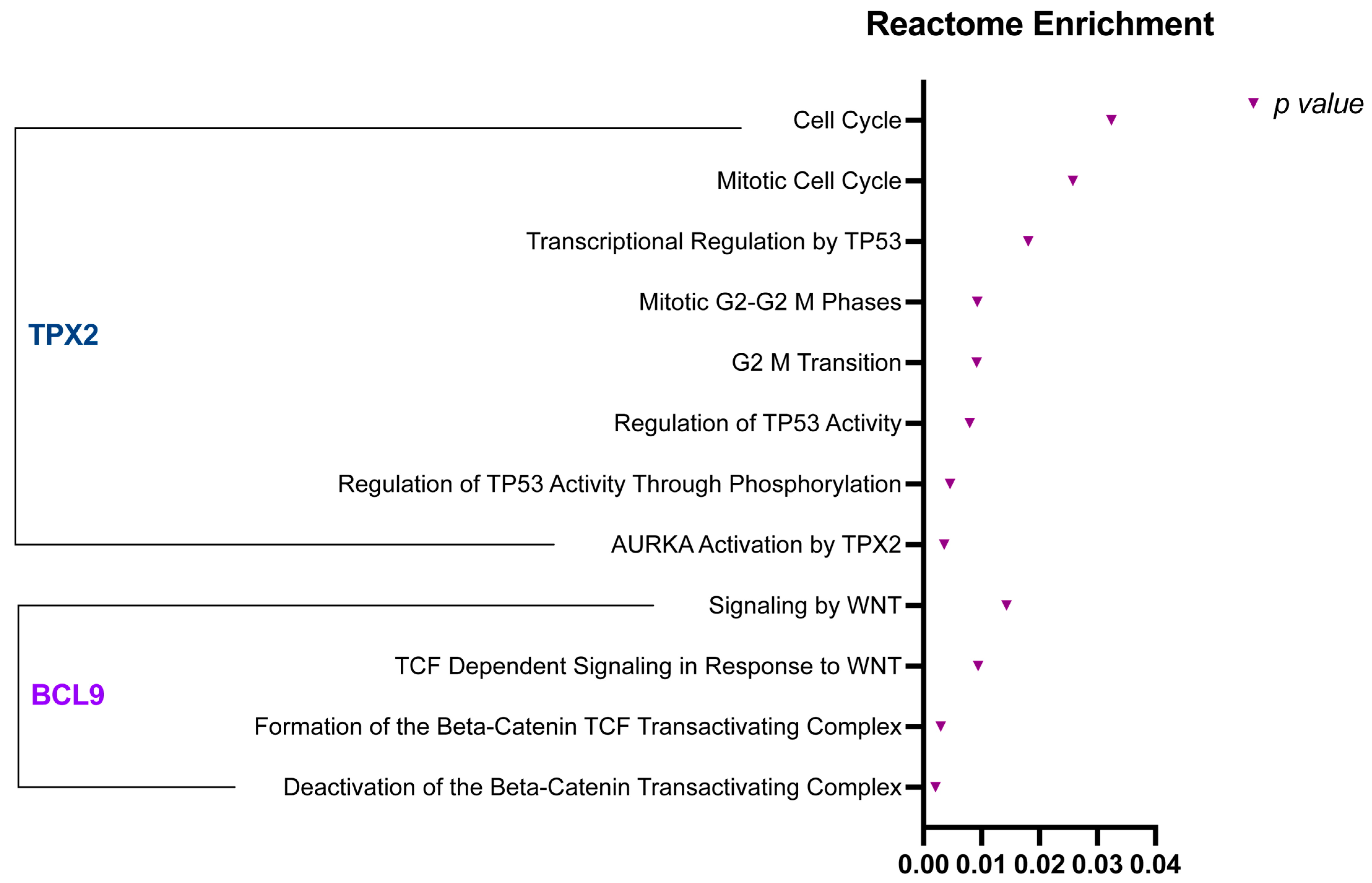
2.5. BCL9 and TPX2 Regulate Wnt Signaling Pathway, Cell Cycle, and Cell Proliferation
3. Discussion
4. Materials and Methods
4.1. Patients and Cohorts
4.1.1. Publicly Available CPTAC Discovery Cohort
4.1.2. IHC Validation Cohort
4.2. Immunohistochemistry and Pathology Evaluation
- Intensity 1 indicates weak staining.
- Intensity 2 indicates moderate staining.
- Intensity 3 indicates intense staining.
4.3. Digital Image Acquisition and Archiving
4.4. Statistical Analysis
4.5. Reactome Pathway Enrichment Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BCL9 | B-cell CLL/lymphoma 9 |
| ccRCC | Clear-cell renal cell carcinoma |
| CI | Confidence interval |
| COM | Calcium oxalate monohydrate |
| CPTAC | Clinical Proteomic Tumor Analysis Consortium |
| EPO | Erythropoietin |
| FFPE | Formalin-fixed paraffin-embedded |
| GO | Gene Ontology |
| HIF | Hypoxia-inducible factor |
| IHC | Immunohistochemistry |
| KSD | Kidney stone disease |
| LC-MS/MS | Liquid chromatography-tandem mass spectrometry |
| NAT | Normal adjacent tissue |
| OS | Overall survival |
| PFS | Progression-free survival |
| TAM | Tumor-associated macrophage |
| TGF | Transforming growth factor |
| TME | Tumor microenvironment |
| TPX2 | Targeting Protein for Xenopus kinesin-like protein 2 |
| VEGF | Vascular endothelial growth factor |
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Jonasch, E.; Gao, J.; Rathmell, W.K. Renal cell carcinoma. BMJ 2014, 349, g4797. [Google Scholar] [CrossRef] [PubMed]
- Jonasch, E.; Walker, C.L.; Rathmell, W.K. Clear cell renal cell carcinoma ontogeny and mechanisms of lethality. Nat. Rev. Nephrol. 2021, 17, 245–261. [Google Scholar] [CrossRef] [PubMed]
- Kotecha, R.R.; Motzer, R.J.; Voss, M.H. Towards individualized therapy for metastatic renal cell carcinoma. Nat. Rev. Clin. Oncol. 2019, 16, 621–633. [Google Scholar] [CrossRef]
- Young, M.D.; Mitchell, T.J.; Vieira Braga, F.A.; Tran, M.G.B.; Stewart, B.J.; Ferdinand, J.R.; Collord, G.; Botting, R.A.; Popescu, D.-M.; Loudon, K.W.; et al. Single cell transcriptomes from human kidneys reveal the cellular identity of renal tumors. Science 2018, 361, 594–599. [Google Scholar] [CrossRef]
- Chevrier, S.; Levine, J.H.; Zanotelli, V.R.T.; Silina, K.; Schulz, D.; Bacac, M.; Ries, C.H.; Ailles, L.; Jewett, M.A.S.; Moch, H.; et al. An Immune Atlas of Clear Cell Renal Cell Carcinoma. Cell 2017, 169, 736–749.e18. [Google Scholar] [CrossRef]
- Hsieh, J.J.; Le, V.H.; Oyama, T.; Ricketts, C.J.; Ho, T.H.; Cheng, E.H. Chromosome 3p Loss-Orchestrated VHL, HIF, and Epigenetic Deregulation in Clear Cell Renal Cell Carcinoma. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2018, 36, 3533–3539. [Google Scholar] [CrossRef]
- Creighton, C.J.; Morgan, M.; Gunaratne, P.H.; Wheeler, D.A.; Gibbs, R.A.; Gordon Robertson, A.; Chu, A.; Beroukhim, R.; Cibulskis, K.; Signoretti, S.; et al. Comprehensive Molecular Characterization Of Clear Cell Renal Cell Carcinoma. Nature 2013, 499, 43–49. [Google Scholar] [CrossRef]
- Latif, F.; Tory, K.; Gnarra, J.; Yao, M.; Duh, F.-M.; Orcutt, M.L.; Stackhouse, T.; Kuzmin, I.; Modi, W.; Geil, L.; et al. Identification of the von Hippel-Lindau Disease Tumor Suppressor Gene. Science 1993, 260, 1317–1320. [Google Scholar] [CrossRef]
- Metelo, A.M.; Noonan, H.; Iliopoulos, O. HIF2a inhibitors for the treatment of VHL disease. Oncotarget 2015, 6, 23036–23037. [Google Scholar] [CrossRef]
- Hodges, H.C.; Kirkland, J.G.; Crabtree, G.R. The Many Roles of BAF (mSWI/SNF) and PBAF Complexes in Cancer. Cold Spring Harb. Perspect. Med. 2016, 6, a026930. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, K.; Brahma, S.; Henikoff, S. Epigenetic pioneering by SWI/SNF family remodelers. Mol. Cell 2024, 84, 194–201. [Google Scholar] [CrossRef]
- Neri, F.; Rapelli, S.; Krepelova, A.; Incarnato, D.; Parlato, C.; Basile, G.; Maldotti, M.; Anselmi, F.; Oliviero, S. Intragenic DNA methylation prevents spurious transcription initiation. Nature 2017, 543, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Linehan, W.M.; Walther, M.M.; Zbar, B. The genetic basis of cancer of the kidney. J. Urol. 2003, 170 Pt 1, 2163–2172. [Google Scholar] [CrossRef]
- Mani, M.; Zhang, Y.; Takada, K.; Gatt, M.E.; Dutta-Simmons, J.; Ikeda, H.; Diaz-Griffero, F.; Pena-Cruz, V.; Bertagnolli, M.; Myeroff, L.L.; et al. BCL9 Promotes Tumor Progression by Conferring Enhanced Proliferative, Metastatic, and Angiogenic Properties to Cancer Cells. Cancer Res. 2009, 69, 7577–7586. [Google Scholar] [CrossRef] [PubMed]
- Steinhart, Z.; Angers, S. Wnt signaling in development and tissue homeostasis. Dev. Camb. Engl. 2018, 145, dev146589. [Google Scholar] [CrossRef]
- Clevers, H.; Nusse, R. Wnt/β-catenin signaling and disease. Cell 2012, 149, 1192–1205. [Google Scholar] [CrossRef]
- Martin-Orozco, E.; Sanchez-Fernandez, A.; Ortiz-Parra, I.; Ayala-San Nicolas, M. WNT Signaling in Tumors: The Way to Evade Drugs and Immunity. Front. Immunol. 2019, 10, 2854. [Google Scholar] [CrossRef]
- de Castro, I.P.; de Cárcer, G.; Malumbres, M. A census of mitotic cancer genes: New insights into tumor cell biology and cancer therapy. Carcinogenesis 2007, 28, 899–912. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Gruss, O.J.; Wittmann, M.; Yokoyama, H.; Pepperkok, R.; Kufer, T.; Silljé, H.; Karsenti, E.; Mattaj, I.W.; Vernos, I. Chromosome-induced microtubule assembly mediated by TPX2 is required for spindle formation in HeLa cells. Nat. Cell Biol. 2002, 4, 871–879. [Google Scholar] [CrossRef] [PubMed]
- Brunet, S.; Sardon, T.; Zimmerman, T.; Wittmann, T.; Pepperkok, R.; Karsenti, E.; Vernos, I. Characterization of the TPX2 domains involved in microtubule nucleation and spindle assembly in Xenopus egg extracts. Mol. Biol. Cell 2004, 15, 5318–5328. [Google Scholar] [CrossRef]
- Neumayer, G.; Belzil, C.; Gruss, O.J.; Nguyen, M.D. TPX2: Of spindle assembly, DNA damage response, and cancer. Cell. Mol. Life Sci. CMLS 2014, 71, 3027–3047. [Google Scholar] [CrossRef] [PubMed]
- Szász, A.M.; Li, Q.; Eklund, A.C.; Sztupinszki, Z.; Rowan, A.; Tőkés, A.-M.; Székely, B.; Kiss, A.; Szendrői, M.; Győrffy, B.; et al. The CIN4 Chromosomal Instability qPCR Classifier Defines Tumor Aneuploidy and Stratifies Outcome in Grade 2 Breast Cancer. PLoS ONE 2013, 8, e56707. [Google Scholar] [CrossRef] [PubMed]
- Polverino, F.; Mastrangelo, A.; Guarguaglini, G. Contribution of AurkA/TPX2 Overexpression to Chromosomal Imbalances and Cancer. Cells 2024, 13, 1397. [Google Scholar] [CrossRef]
- Ma, C.G.; Xu, W.H.; Xu, Y.; Wang, J.; Liu, W.R.; Cao, D.L.; Wang, H.K.; Shi, G.H.; Zhu, Y.P.; Qu, Y.Y.; et al. Identification and validation of novel metastasis-related signatures of clear cell renal cell carcinoma using gene expression databases. Am. J. Transl. Res. 2020, 12, 4108–4126. [Google Scholar]
- Clark, D.J.; Dhanasekaran, S.M.; Petralia, F.; Pan, J.; Song, X.; Hu, Y.; da Veiga Leprevost, F.; Reva, B.; Lih, T.-S.M.; Chang, H.-Y.; et al. Integrated Proteogenomic Characterization of Clear Cell Renal Cell Carcinoma. Cell 2019, 179, 964–983.e31. [Google Scholar] [CrossRef]
- Brack, A.S.; Murphy-Seiler, F.; Hanifi, J.; Deka, J.; Eyckerman, S.; Keller, C.; Aguet, M.; Rando, T.A. BCL9 is an essential component of canonical Wnt signaling that mediates the differentiation of myogenic progenitors during muscle regeneration. Dev. Biol. 2009, 335, 93–105. [Google Scholar] [CrossRef]
- Amir, E.; Seruga, B.; Kwong, R.; Tannock, I.F.; Ocaña, A. Poor correlation between progression-free and overall survival in modern clinical trials: Are composite endpoints the answer? Eur. J. Cancer 2012, 48, 385–388. [Google Scholar] [CrossRef]
- Ocaña, A.; Amir, E.; Vera, F.; Eisenhauer, E.A.; Tannock, I.F. Addition of Bevacizumab to Chemotherapy for Treatment of Solid Tumors: Similar Results but Different Conclusions. J. Clin. Oncol. 2011, 29, 254–256. [Google Scholar] [CrossRef]
- Aguirre-Portolés, C.; Bird, A.W.; Hyman, A.; Cañamero, M.; Pérez de Castro, I.; Malumbres, M. Tpx2 controls spindle integrity, genome stability, and tumor development. Cancer Res. 2012, 72, 1518–1528. [Google Scholar] [CrossRef] [PubMed]
- Joukov, V.; De Nicolo, A. Aurora-PLK1 cascades as key signaling modules in the regulation of mitosis. Sci. Signal. 2018, 11, eaar4195. [Google Scholar] [CrossRef]
- Naso, F.D.; Polverino, F.; Cilluffo, D.; Latini, L.; Stagni, V.; Asteriti, I.A.; Rosa, A.; Soddu, S.; Guarguaglini, G. AurkA/TPX2 co-overexpression in nontransformed cells promotes genome instability through induction of chromosome mis-segregation and attenuation of the p53 signalling pathway. Biochim. Biophys. Acta Mol. Basis Dis. 2024, 1870, 167116. [Google Scholar] [CrossRef]
- Zhang, H.; Zou, J.; Yin, Y.; Zhang, B.; Hu, Y.; Wang, J.; Mu, H. Bioinformatic analysis identifies potentially key differentially expressed genes in oncogenesis and progression of clear cell renal cell carcinoma. PeerJ 2019, 7, e8096. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Xiao, Y.; Li, H.; Zhang, A.-L.; Meng, L.-B.; Feng, L.; Zhao, Z.-H.; Ni, X.-C.; Fan, B.; Zhang, X.-Y.; et al. Identification and Verification of Biomarker in Clear Cell Renal Cell Carcinoma via Bioinformatics and Neural Network Model. BioMed Res. Int. 2020, 2020, 6954793. [Google Scholar] [CrossRef]
- Xu, D.; Xu, Y.; Lv, Y.; Wu, F.; Liu, Y.; Zhu, M.; Chen, D.; Bai, B. Identification of Four Pathological Stage-Relevant Genes in Association with Progression and Prognosis in Clear Cell Renal Cell Carcinoma by Integrated Bioinformatics Analysis. BioMed Res. Int. 2020, 2020, 2137319. [Google Scholar] [CrossRef]
- Wang, Y.; Yan, K.; Lin, J.; Wang, J.; Zheng, Z.; Li, X.; Hua, Z.; Bu, Y.; Shi, J.; Sun, S.; et al. Three-gene risk model in papillary renal cell carcinoma: A robust likelihood-based survival analysis. Aging 2020, 12, 21854–21873. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, J.; Fukushima, S.; Jinnin, M.; Honda, N.; Makino, K.; Sakai, K.; Masuguchi, S.; Inoue, Y.; Ihn, H. Kinesin family member 20A is a novel melanoma-associated antigen. Acta Derm. Venereol. 2012, 92, 593–597. [Google Scholar] [CrossRef]
- Zhang, W.; He, W.; Shi, Y.; Gu, H.; Li, M.; Liu, Z.; Feng, Y.; Zheng, N.; Xie, C.; Zhang, Y. High Expression of KIF20A Is Associated with Poor Overall Survival and Tumor Progression in Early-Stage Cervical Squamous Cell Carcinoma. PLoS ONE 2016, 11, e0167449. [Google Scholar] [CrossRef]
- Xie, C.; Yiu, W.; Mo, Y. Comprehensive bioinformatics analysis of KIF20A as a prognosis biomarker for clear cell renal cell carcinoma. Cell. Mol. Biol. 2024, 70, 116–124. [Google Scholar] [CrossRef]
- Christofides, A.; Strauss, L.; Yeo, A.; Cao, C.; Charest, A.; Boussiotis, V.A. The complex role of tumor-infiltrating macrophages. Nat. Immunol. 2022, 23, 1148–1156. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Tian, X.; Zhu, S.-X.; Xu, W.-H.; Anwaier, A.; Su, J.-Q.; Gan, H.-L.; Qu, Y.-Y.; Zhao, J.-Y.; Zhang, H.-L.; et al. Identification of prognostic and therapeutic biomarkers in type 2 papillary renal cell carcinoma. World J. Surg. Oncol. 2023, 21, 98. [Google Scholar] [CrossRef]
- Deng, R.; Li, J.; Zhao, H.; Zou, Z.; Man, J.; Cao, J.; Yang, L. Identification of potential biomarkers associated with immune infiltration in papillary renal cell carcinoma. J. Clin. Lab. Anal. 2021, 35, e24022. [Google Scholar] [CrossRef]
- Peerapen, P.; Boonmark, W.; Putpeerawit, P.; Thongboonkerd, V. Calcium oxalate crystals trigger epithelial-mesenchymal transition and carcinogenic features in renal cells: A crossroad between kidney stone disease and renal cancer. Exp. Hematol. Oncol. 2022, 11, 62. [Google Scholar] [CrossRef] [PubMed]
- Xiao, M.; Tang, R.; Pan, H.; Yang, J.; Tong, X.; Xu, H.; Guo, Y.; Lei, Y.; Wu, D.; Lei, Y.; et al. TPX2 serves as a novel target for expanding the utility of PARPi in pancreatic cancer through conferring synthetic lethality. Gut 2025, 74, 410–423. [Google Scholar] [CrossRef]
- Marugán, C.; Sanz-Gómez, N.; Ortigosa, B.; Monfort-Vengut, A.; Bertinetti, C.; Teijo, A.; González, M.; de la Vega, A.A.; Lallena, M.J.; Moreno-Bueno, G.; et al. TPX2 overexpression promotes sensitivity to dasatinib in breast cancer by activating YAP transcriptional signaling. Mol. Oncol. 2024, 18, 1531–1551. [Google Scholar] [CrossRef] [PubMed]
- van Gijn, S.E.; Wierenga, E.; Tempel, N.v.D.; Kok, Y.P.; Heijink, A.M.; Spierings, D.C.J.; Foijer, F.; van Vugt, M.A.T.M.; Fehrmann, R.S.N. TPX2/Aurora kinase A signaling as a potential therapeutic target in genomically unstable cancer cells. Oncogene 2019, 38, 852–867. [Google Scholar] [CrossRef]
- Guenther, M.; Surendran, S.A.; Haas, M.; Heinemann, V.; von Bergwelt-Baildon, M.; Engel, J.; Werner, J.; Boeck, S.; Ormanns, S. TPX2 expression as a negative predictor of gemcitabine efficacy in pancreatic cancer. Br. J. Cancer 2023, 129, 175–182. [Google Scholar] [CrossRef]
- Stockwell, S.R.; Scott, D.E.; Fischer, G.; Guarino, E.; Rooney, T.P.C.; Feng, T.-S.; Moschetti, T.; Srinivasan, R.; Alza, E.; Asteian, A.; et al. Selective Aurora A-TPX2 Interaction Inhibitors Have In Vivo Efficacy as Targeted Antimitotic Agents. J. Med. Chem. 2024, 67, 15521–15536. [Google Scholar] [CrossRef]
- Ma, J.; Kang, Z.; Yang, G.; Wang, X.; Si, M.; Wang, Y.; Li, G.; Bai, S.; Zeng, F.; Li, M.; et al. Pyroptosis-Related Subtypes Predict the Response of Clear Cell Renal Cell Carcinoma to Targeted Therapy. Front. Biosci. Landmark Ed. 2023, 28, 334. [Google Scholar] [CrossRef]
- Wang, X.; Wang, J.; Shen, H.; Luo, Z.; Lu, X. Downregulation of TPX2 impairs the antitumor activity of CD8+ T cells in hepatocellular carcinoma. Cell Death Dis 2022, 13, 223. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Dong, H.; Xu, C.; Sun, M.; Gao, H.; Bu, F.; Chen, J. The Wnt-dependent and Wnt-independent functions of BCL9 in development, tumorigenesis, and immunity: Implications in therapeutic opportunities. Genes. Dis. 2023, 11, 701. [Google Scholar] [CrossRef] [PubMed]
- Kramps, T.; Peter, O.; Brunner, E.; Nellen, D.; Froesch, B.; Chatterjee, S.; Murone, M.; Züllig, S.; Basler, K. Wnt/Wingless Signaling Requires BCL9/Legless-Mediated Recruitment of Pygopus to the Nuclear β-Catenin-TCF Complex. Cell 2002, 109, 47–60. [Google Scholar] [CrossRef]
- El-Khoueiry, A.B.; Lakhani, N.J.; Henry, J.T.; Powell, S.F.; Mettu, N.B.; Gakuria, J.; Abbate, F.; Nuciforo, P.; Rotolo, J.; Haras, A.V. Safety and biomarker assessment of ST316, a novel peptide antagonist of ß-catenin, in patients with advanced solid tumors. J. Clin. Oncol. 2025, 43 (Suppl. S4), 286. [Google Scholar] [CrossRef]
- Jiang, M.; Kang, Y.; Sewastianik, T.; Wang, J.; Tanton, H.; Alder, K.; Dennis, P.; Xin, Y.; Wang, Z.; Liu, R.; et al. BCL9 provides multi-cellular communication properties in colorectal cancer by interacting with paraspeckle proteins. Nat. Commun. 2020, 11, 19. [Google Scholar] [CrossRef] [PubMed]
- Elsarraj, H.S.; Hong, Y.; Limback, D.; Zhao, R.; Berger, J.; Bishop, S.C.; Sabbagh, A.; Oppenheimer, L.; Harper, H.E.; Tsimelzon, A.; et al. BCL9/STAT3 regulation of transcriptional enhancer networks promote DCIS progression. npj Breast Cancer 2020, 6, 19. [Google Scholar] [CrossRef]
- Feng, M.; Jin, J.Q.; Xia, L.; Xiao, T.; Mei, S.; Wang, X.; Huang, X.; Chen, J.; Liu, M.; Chen, C.; et al. Pharmacological inhibition of β-catenin/BCL9 interaction overcomes resistance to immune checkpoint blockades by modulating Treg cells. Sci. Adv. 2019, 5, eaau5240. [Google Scholar] [CrossRef]
- Bottino, C.; Castriconi, R.; Pende, D.; Rivera, P.; Nanni, M.; Carnemolla, B.; Cantoni, C.; Grassi, J.; Marcenaro, S.; Reymond, N.; et al. Identification of PVR (CD155) and Nectin-2 (CD112) as Cell Surface Ligands for the Human DNAM-1 (CD226) Activating Molecule. J. Exp. Med. 2003, 198, 557–567. [Google Scholar] [CrossRef]
- Wang, H.; Qi, J.; Zhang, S.; Li, Y.; Tan, S.; Gao, G.F. Binding mode of the side-by-side two-IgV molecule CD226/DNAM-1 to its ligand CD155/Necl-5. Proc. Natl. Acad. Sci. USA 2019, 116, 988–996. [Google Scholar] [CrossRef]
- Feng, M.; Wu, Z.; Zhou, Y.; Wei, Z.; Tian, E.; Mei, S.; Zhu, Y.; Liu, C.; He, F.; Li, H.; et al. BCL9 regulates CD226 and CD96 checkpoints in CD8+ T cells to improve PD-1 response in cancer. Signal Transduct. Target. Ther. 2021, 6, 313. [Google Scholar] [CrossRef]
- Wang, X.; Feng, M.; Xiao, T.; Guo, B.; Liu, D.; Liu, C.; Pei, J.; Liu, Q.; Xiao, Y.; Rosin-Arbesfeld, R.; et al. BCL9/BCL9L promotes tumorigenicity through immune-dependent and independent mechanisms in triple negative breast cancer. Oncogene 2021, 40, 2982–2997. [Google Scholar] [CrossRef]
- Zhang, R.; Gui, Z.; Zhao, J.; Zhao, L. BCL9 is a Risk Factor of Neck Lymph Nodes Metastasis and Correlated with Immune Cell Infiltration in Papillary Thyroid Carcinoma. Int. J. Gen. Med. 2024, 17, 1451–1466. [Google Scholar] [CrossRef]
- Zhao, F.; Yan, F.; Liu, H. New Biomarkers Based on Dendritic Cells for Breast Cancer Treatment and Prognosis Diagnosis. Int. J. Mol. Sci. 2023, 24, 4058. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Feng, M.; Wu, Z.; Shen, S.; Zhu, D. Bcl9 Depletion Modulates Endothelial Cell in Tumor Immune Microenvironment in Colorectal Cancer Tumor. Front. Oncol. 2021, 10, 603702. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Yang, M.; Feng, M.; Wu, Z.; Rosin-Arbesfeld, R.; Dong, J.; Zhu, D. Inhibition of BCL9 Modulates the Cellular Landscape of Tumor-Associated Macrophages in the Tumor Immune Microenvironment of Colorectal Cancer. Front. Pharmacol. 2021, 12, 713331. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Ying, Y.; Bo, S.; Li, G.; Yuan, F. Differentially expressed microRNA-218 modulates the viability of renal cell carcinoma by regulating BCL9. Mol. Med. Rep. 2016, 14, 1829–1834. [Google Scholar] [CrossRef]
- Grigolo, S.; Filgueira, L. Immunotherapy of Clear-Cell Renal-Cell Carcinoma. Cancers 2024, 16, 2092. [Google Scholar] [CrossRef]

| Progression-Free Survival (PFS) | Overall Survival (OS) | |||
|---|---|---|---|---|
| Hazard Ratio (HR) | 95% CI of HR | Hazard Ratio (HR) | 95% CI of HR | |
| BLC9 | 2.906 | 1.030–8.198 | 3.158 | 0.7030–14.19 |
| TPX2 | 1.534 | 0.5352–4.398 | 5.52 | 1.368–22.27 |
| Protein | H-Score Range—High IHC Score Group | H-Score Range—Low IHC Score Group | Reaction |
|---|---|---|---|
| BCL9 | 120–280 | 10–80 | Nuclear |
| TPX2 | 25–220 | 0–15 | Cytoplasmic, membranous |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kasperczak, M.; Kołodziejczak-Guglas, I.; Kasperczak, F.; Wiznerowicz, M.; Antczak, A. Prognostic Significance of Overexpression of BCL9 and TPX2 in High-Grade Clear Cell Renal Cell Carcinoma: Prognostic Markers for Metastasis and Survival. Int. J. Mol. Sci. 2025, 26, 4114. https://doi.org/10.3390/ijms26094114
Kasperczak M, Kołodziejczak-Guglas I, Kasperczak F, Wiznerowicz M, Antczak A. Prognostic Significance of Overexpression of BCL9 and TPX2 in High-Grade Clear Cell Renal Cell Carcinoma: Prognostic Markers for Metastasis and Survival. International Journal of Molecular Sciences. 2025; 26(9):4114. https://doi.org/10.3390/ijms26094114
Chicago/Turabian StyleKasperczak, Michał, Iga Kołodziejczak-Guglas, Filip Kasperczak, Maciej Wiznerowicz, and Andrzej Antczak. 2025. "Prognostic Significance of Overexpression of BCL9 and TPX2 in High-Grade Clear Cell Renal Cell Carcinoma: Prognostic Markers for Metastasis and Survival" International Journal of Molecular Sciences 26, no. 9: 4114. https://doi.org/10.3390/ijms26094114
APA StyleKasperczak, M., Kołodziejczak-Guglas, I., Kasperczak, F., Wiznerowicz, M., & Antczak, A. (2025). Prognostic Significance of Overexpression of BCL9 and TPX2 in High-Grade Clear Cell Renal Cell Carcinoma: Prognostic Markers for Metastasis and Survival. International Journal of Molecular Sciences, 26(9), 4114. https://doi.org/10.3390/ijms26094114





