Protocatechuic Acid Ameliorates Cisplatin-Induced Inflammation and Apoptosis in Mouse Proximal Tubular Cells
Abstract
1. Introduction
2. Results
2.1. Effect of PCA Treatment on % Cell Viability in Cisplatin-Treated BUMPT Cells
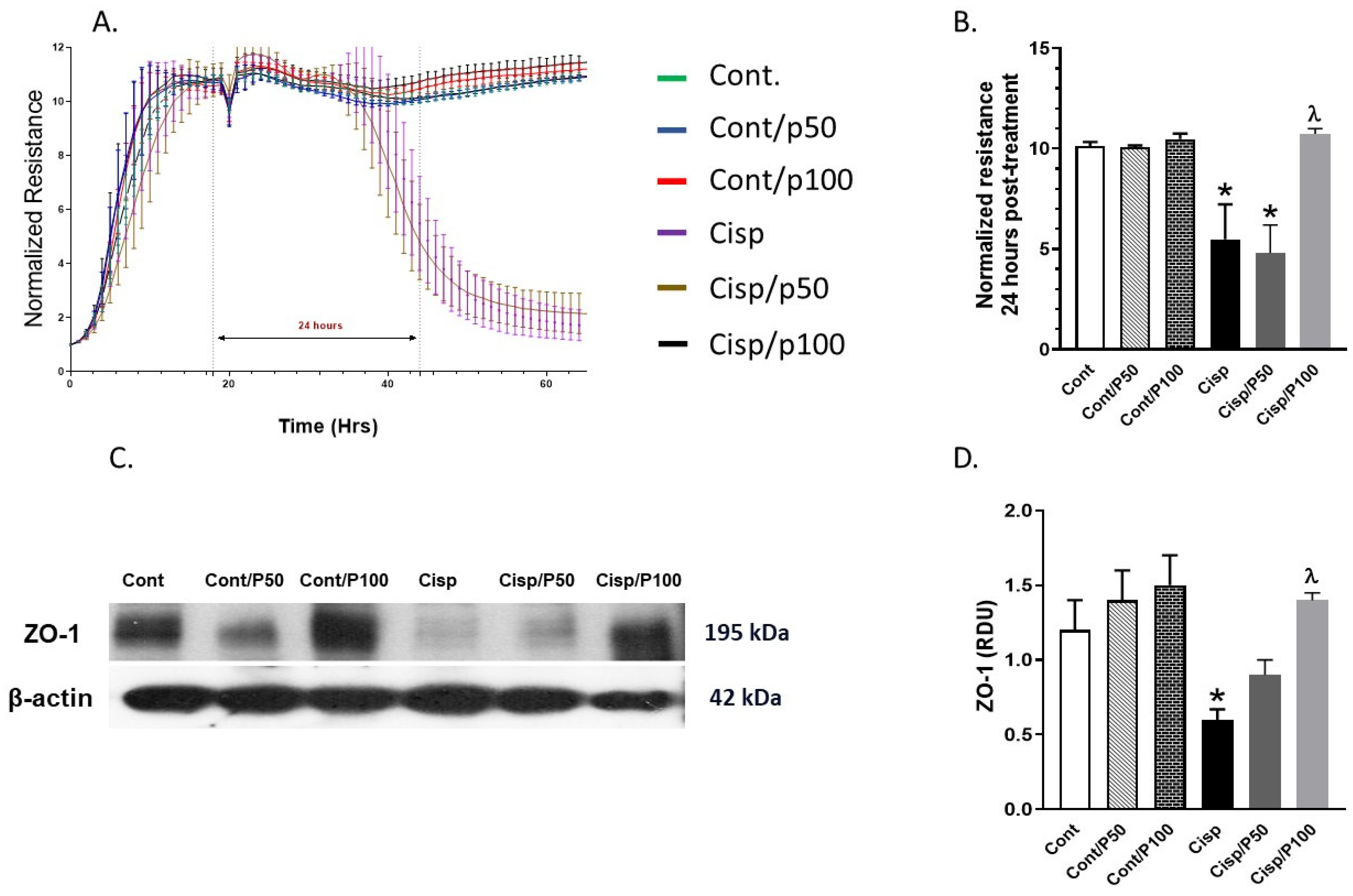
2.2. Effect of PCA on Cisplatin-Induced Changes in Tight Junction Protein (ZO-1) and ECIS
2.3. The Effect of PCA on Cisplatin-Induced Changes in ROS Production and TBARS Concentration in BUMPT Cells
2.4. Effect of PCA on Cisplatin-Induced Changes in IL-6 and p-NF-κB in BUMPT Cells
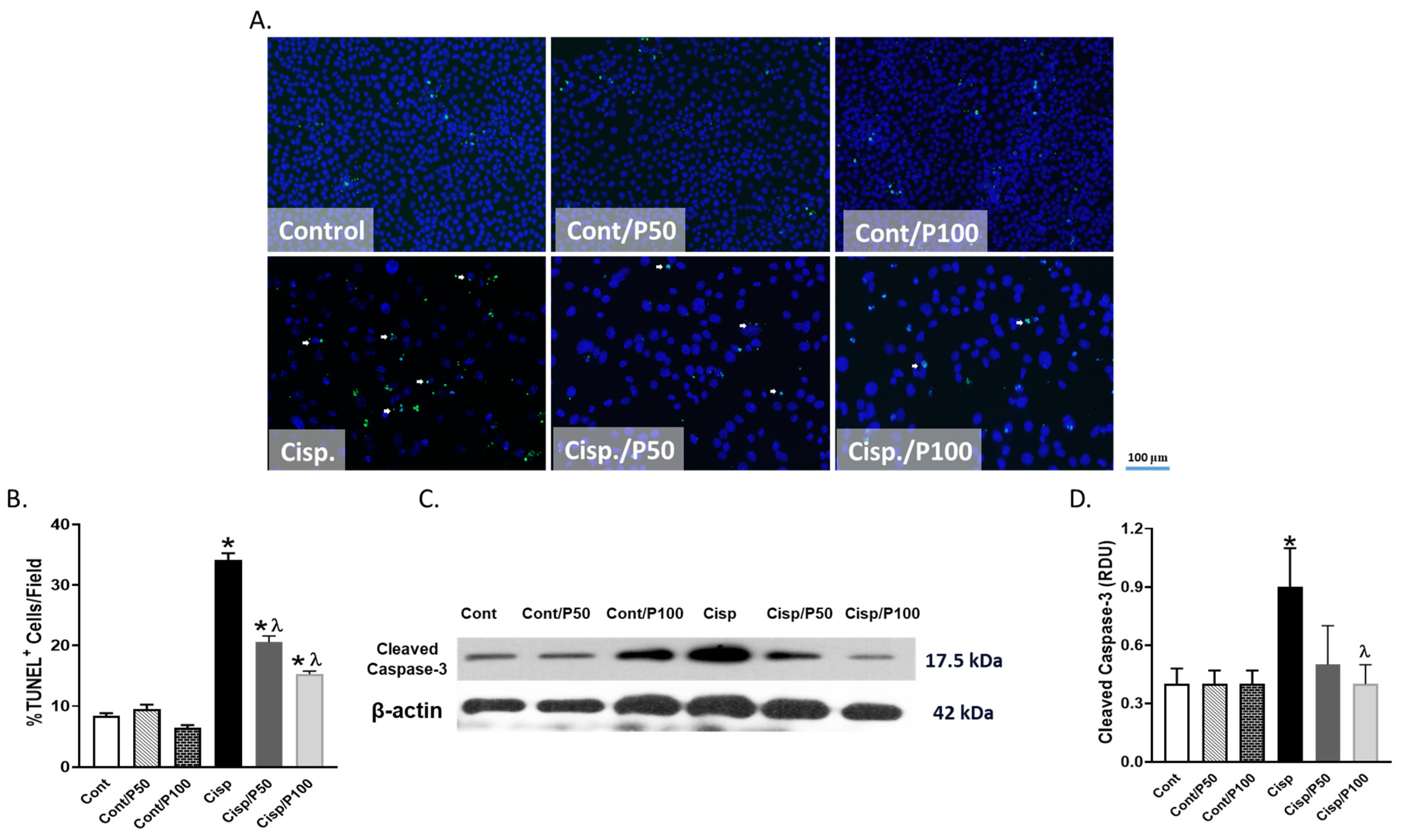
2.5. Effect of PCA on Cisplatin-Induced Apoptosis in BUMPT Cells
3. Discussion
4. Materials and Methods
4.1. Cells and Experimental Groups
4.2. 3-[4,5-Dimethylthiazol-2-yl]-2,5 Diphenyl Tetrazolium Bromide (MTT) Assay
4.3. Quantification of Barrier Function
4.4. Western Blotting Assessments of Caspase-3 and ZO-1 Expression
4.5. Immunocytochemistry
4.6. Quantification of Thio-Barbituric Acid Reactive Species (TBARS)
4.7. Reactive Oxygen Species (ROS) Assessments
4.8. Quantification of Phosphorylated Nuclear Factor Kappa Beta (p-NF-κB)
4.9. TUNEL Assay
4.10. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Romani AMP. Cisplatin in cancer treatment. Biochem. Pharmacol. 2022, 206, 115323. [Google Scholar] [CrossRef]
- Lu, Y.; Cederbaum, A.I. Cisplatin-Induced Hepatotoxicity Is Enhanced by Elevated Expression of Cytochrome P450 2E1. Toxicol. Sci. 2005, 89, 515–523. [Google Scholar] [CrossRef]
- Suddek, G.M. Sunitinib improves chemotherapeutic efficacy and ameliorates cisplatin-induced nephrotoxicity in experimental animals. Cancer Chemother. Pharmacol. 2011, 67, 1035–1044. [Google Scholar] [CrossRef]
- Duan, Z.; Cai, G.; Li, J.; Chen, X. Cisplatin-induced renal toxicity in elderly people. Ther. Adv. Med. Oncol. 2020, 12, 1758835920923430. [Google Scholar] [CrossRef] [PubMed]
- Saad, K.M.; Abdelrahman, R.S.; Said, E. Mechanistic perspective of protective effects of nilotinib against cisplatin-induced testicular injury in rats: Role of JNK/caspase-3 signaling inhibition. Environ. Toxicol. Pharmacol. 2020, 76, 103334. [Google Scholar] [CrossRef]
- Taghizadeh, F.; Hosseinimehr, S.J.; Zargari, M.; Malekshah, A.K.; Mirzaei, M.; Amiri, F. Alleviation of cisplatin-induced hepatotoxicity by gliclazide: Involvement of oxidative stress and caspase-3 activity. Pharmacol. Res. Perspect. 2021, 9, e00788. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, M.A.; Albahlol, I.A.; Wani, F.A.; Tammam, A.A.-E.; Kelleni, M.T.; Sayeed, M.U.; El-Fadeal, N.M.A.; Mohamed, A.A. Resveratrol protects against cisplatin-induced ovarian and uterine toxicity in female rats by attenuating oxidative stress, inflammation and apoptosis. Chem.-Biol. Interact. 2021, 338, 109402. [Google Scholar] [CrossRef]
- Burns, C.V.; Edwin, S.B.; Szpunar, S.; Forman, J. Cisplatin-induced nephrotoxicity in an outpatient setting. Pharmacotherapy 2021, 41, 184–190. [Google Scholar] [CrossRef]
- McSweeney, K.R.; Gadanec, L.K.; Qaradakhi, T.; Ali, B.A.; Zulli, A.; Apostolopoulos, V. Mechanisms of Cisplatin-Induced Acute Kidney Injury: Pathological Mechanisms, Pharmacological Interventions, and Genetic Mitigations. Cancers 2021, 13, 1572. [Google Scholar] [CrossRef]
- Oh, G.S.; Kim, H.J.; Shen, A.; Lee, S.B.; Khadka, D.; Pandit, A.; So, H.S. Cisplatin-induced Kidney Dysfunction and Perspectives on Improving Treatment Strategies. Electrolyte Blood Press 2014, 12, 55–65. [Google Scholar] [CrossRef]
- Ansari, M.A. Sinapic acid modulates Nrf2/HO-1 signaling pathway in cisplatin-induced nephrotoxicity in rats. Biomed. Pharmacother. 2017, 93, 646–653. [Google Scholar] [CrossRef] [PubMed]
- Motwani, S.S.; Sandhu, S.K.; Kitchlu, A. Cisplatin Nephrotoxicity: Novel Insights Into Mechanisms and Preventative Strategies. Semin. Nephrol. 2022, 42, 151341. [Google Scholar] [CrossRef]
- Ozkok, A.; Edelstein, C.L. Pathophysiology of cisplatin-induced acute kidney injury. BioMed Res. Int. 2014, 2014, 967826. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Tang, Z.; Huang, M.; Tao, N.; Feng, B.; Huang, S. Antioxidant efficacy of extracts produced from pickled and dried mustard in rapeseed and peanut oils. J. Food Sci. 2012, 77, C394–C400. [Google Scholar] [CrossRef] [PubMed]
- Dawes, H.M.; Keene, J.B. Phenolic composition of kiwifruit juice. J. Agric. Food Chem. 1999, 47, 2398–2403. [Google Scholar] [CrossRef]
- Huang, W.-Y.; Zhang, H.-C.; Liu, W.-X.; Li, C.-Y. Survey of antioxidant capacity and phenolic composition of blueberry, blackberry, and strawberry in Nanjing. J. Zhejiang Univ. Sci. B 2012, 13, 94–102. [Google Scholar] [CrossRef]
- Palafox-Carlos, H.; Gil-Chávez, J.; Sotelo-Mundo, R.R.; Namiesnik, J.; Gorinstein, S.; González-Aguilar, G.A. Antioxidant interactions between major phenolic compounds found in ‘Ataulfo’mango pulp: Chlorogenic, gallic, protocatechuic and vanillic acids. Molecules 2012, 17, 12657–12664. [Google Scholar] [CrossRef]
- Khan, A.K.; Rashid, R.; Fatima, N.; Mahmood, S.; Mir, S.; Khan, S.; Jabeen, N.; Murtaza, G. Pharmacological Activities of Protocatechuic Acid. Acta Pol. Pharm. 2015, 72, 643–650. Available online: https://ptfarm.pl/pub/File/Acta_Poloniae/2015/4/643.pdf (accessed on 1 November 2023).
- Song, J.; He, Y.; Luo, C.; Feng, B.; Ran, F.; Xu, H.; Ci, Z.; Xu, R.; Han, L.; Zhang, D. New progress in the pharmacology of protocatechuic acid: A compound ingested in daily foods and herbs frequently and heavily. Pharmacol. Res. 2020, 161, 105109. [Google Scholar] [CrossRef]
- Saad, K.M.; Salles, E.L.; Naeini, S.E.; Baban, B.; Abdelmageed, M.E.; Abdelaziz, R.R.; Suddek, G.M.; Elmarakby, A.A. Reno-protective effect of protocatechuic acid is independent of sex-related differences in murine model of UUO-induced kidney injury. Pharmacol. Rep. 2024, 76, 98–111. [Google Scholar] [CrossRef]
- Mirzaei, S.; Hushmandi, K.; Zabolian, A.; Saleki, H.; Torabi, S.M.R.; Ranjbar, A.; SeyedSaleh, S.; Sharifzadeh, S.O.; Khan, H.; Ashrafizadeh, M.; et al. Elucidating Role of Reactive Oxygen Species (ROS) in Cisplatin Chemotherapy: A Focus on Molecular Pathways and Possible Therapeutic Strategies. Molecules 2021, 26, 2382. [Google Scholar] [CrossRef] [PubMed]
- Dasari, S.; Tchounwou, P.B. Cisplatin in cancer therapy: Molecular mechanisms of action. Eur. J. Pharmacol. 2014, 740, 364–378. [Google Scholar] [CrossRef]
- Marullo, R.; Werner, E.; Degtyareva, N.; Moore, B.; Altavilla, G.; Ramalingam, S.S.; Doetsch, P.W. Cisplatin Induces a Mitochondrial-ROS Response That Contributes to Cytotoxicity Depending on Mitochondrial Redox Status and Bioenergetic Functions. PLoS ONE 2013, 8, e81162. [Google Scholar] [CrossRef]
- Sun, C.; Guo, E.; Zhou, B.; Shan, W.; Huang, J.; Weng, D.; Wu, P.; Wang, C.; Wang, S.; Zhang, W.; et al. A reactive oxygen species scoring system predicts cisplatin sensitivity and prognosis in ovarian cancer patients. BMC Cancer 2019, 19, 1061. [Google Scholar] [CrossRef] [PubMed]
- Soni, H.; Kaminski, D.; Gangaraju, R.; Adebiyi, A. Cisplatin-induced oxidative stress stimulates renal Fas ligand shedding. Ren. Fail. 2018, 40, 314–322. [Google Scholar] [CrossRef]
- Li, P.; Li, X.; Wu, W.; Hou, M.; Yin, G.; Wang, Z.; Du, Z.; Ma, Y.; Lou, Q.; Wei, Y. Tim-3 protects against cisplatin nephrotoxicity by inhibiting NF-κB-mediated inflammation. Cell Death Discov. 2023, 9, 218. [Google Scholar] [CrossRef] [PubMed]
- Yamabe, N.; Park, J.Y.; Lee, S.; Cho, E.-J.; Lee, S.; Kang, K.S.; Hwang, G.S.; Kim, S.-N.; Kim, H.Y.; Shibamoto, T.l. Protective effects of protocatechuic acid against cisplatin-induced renal damage in rats. J. Funct. Foods 2015, 19, 20–27. [Google Scholar] [CrossRef]
- Gao, L.; Wu, W.F.; Dong, L.; Ren, G.L.; Li, H.D.; Yang, Q.; Li, X.F.; Xu, T.; Li, Z.; Wu, B.M.; et al. Protocatechuic Aldehyde Attenuates Cisplatin-Induced Acute Kidney Injury by Suppressing Nox-Mediated Oxidative Stress and Renal Inflammation. Front. Pharmacol. 2016, 7, 479. [Google Scholar] [CrossRef] [PubMed]
- Tsao, S.-M.; Hsia, T.-C.; Yin, M.-C. Protocatechuic Acid Inhibits Lung Cancer Cells by Modulating FAK, MAPK, and NF-κB Pathways. Nutr. Cancer 2014, 66, 1331–1341. [Google Scholar] [CrossRef] [PubMed]
- Kaewmool, C.; Kongtawelert, P.; Phitak, T.; Pothacharoen, P.; Udomruk, S. Protocatechuic acid inhibits inflammatory responses in LPS-activated BV2 microglia via regulating SIRT1/NF-κB pathway contributed to the suppression of microglial activation-induced PC12 cell apoptosis. J. Neuroimmunol. 2020, 341, 577164. [Google Scholar] [CrossRef]
- Nam, Y.J.; Lee, C.S. Protocatechuic acid inhibits Toll-like receptor-4-dependent activation of NF-κB by suppressing activation of the Akt, mTOR, JNK and p38-MAPK. Int. Immunopharmacol. 2018, 55, 272–281. [Google Scholar] [CrossRef] [PubMed]
- Hanigan, M.H.; Devarajan, P. Cisplatin nephrotoxicity: Molecular mechanisms. Cancer Ther. 2003, 1, 47–61. [Google Scholar] [PubMed]
- Fu, S.; Hu, X.; Ma, Z.; Wei, Q.; Xiang, X.; Li, S.; Wen, L.; Liang, Y.; Dong, Z. p53 in Proximal Tubules Mediates Chronic Kidney Problems after Cisplatin Treatment. Cells 2022, 11, 712. [Google Scholar] [CrossRef]
- Li, F.; Sun, A.; Cheng, G.; Liu, D.; Xiao, J.; Zhao, Z.; Dong, Z. Compound C Protects Against Cisplatin-Induced Nephrotoxicity Through Pleiotropic Effects. Front. Physiol. 2020, 11, 614244. [Google Scholar] [CrossRef]
- Tang, X.L.; Liu, J.X.; Dong, W.; Li, P.; Li, L.; Lin, C.R.; Zheng, Y.Q.; Cong, W.H.; Hou, J.C. Cardioprotective effect of protocatechuic acid on myocardial ischemia/reperfusion injury. J. Pharmacol. Sci. 2014, 125, 176–183. [Google Scholar] [CrossRef] [PubMed]
- Owumi, S.E.; Ochaoga, S.E.; Odunola, O.A.; Farombi, E.O. Protocatechuic acid inhibits testicular and epididymal toxicity associated with methotrexate in rats. Andrologia 2019, 51, e13350. [Google Scholar] [CrossRef]
- Deng, J.-S.; Lee, S.-D.; Kuo, W.-W.; Fan, M.-J.; Lin, Y.-M.; Hu, W.-S.; Huang, Y.-C.; Velmurugan, B.K.; Tsai, F.-J.; Tsai, C.-H.; et al. Anti-apoptotic and pro-survival effect of protocatechuic acid on hypertensive hearts. Chem.-Biol. Interact. 2014, 209, 77–84. [Google Scholar] [CrossRef]
- Förster, C. Tight junctions and the modulation of barrier function in disease. Histochem. Cell Biol. 2008, 130, 55–70. [Google Scholar] [CrossRef]
- Monaco, A.; Ovryn, B.; Axis, J.; Amsler, K. The Epithelial Cell Leak Pathway. Int. J. Mol. Sci. 2021, 22, 7677. [Google Scholar] [CrossRef]
- Vermette, D.; Hu, P.; Canarie, M.F.; Funaro, M.; Glover, J.; Pierce, R.W. Tight junction structure, function, and assessment in the critically ill: A systematic review. Intensive Care Med. Exp. 2018, 6, 37. [Google Scholar] [CrossRef]
- Fromm, M.; Piontek, J.; Rosenthal, R.; Günzel, D.; Krug, S.M. Tight junctions of the proximal tubule and their channel proteins. Pflug. Arch. 2017, 469, 877–887. [Google Scholar] [CrossRef] [PubMed]
- Kuo, W.; Odenwald, M.A.; Turner, J.R.; Zuo, L. Tight junction proteins occludin and ZO-1 as regulators of epithelial proliferation and survival. Ann. N. Y. Acad. Sci. 2022, 1514, 21–33. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.; Moon, K.M.; Kim, C.Y. Tight Junction in the Intestinal Epithelium: Its Association with Diseases and Regulation by Phytochemicals. J. Immunol. Res. 2018, 2018, 2645465. [Google Scholar] [CrossRef] [PubMed]
- Ikari, A.; Nagatani, Y.; Tsukimoto, M.; Harada, H.; Miwa, M.; Takagi, K. Sodium-dependent glucose transporter reduces peroxynitrite and cell injury caused by cisplatin in renal tubular epithelial cells. Biochim. Biophys. Acta-Biomembr. 2005, 1717, 109–117. [Google Scholar] [CrossRef]
- Trujillo, J.; Molina-Jijón, E.; Medina-Campos, O.N.; Rodríguez-Muñoz, R.; Reyes, J.L.; Loredo, M.L.; Tapia, E.; Sánchez-Lozada, L.G.; Barrera-Oviedo, D.; Pedraza-Chaverri, J. Renal tight junction proteins are decreased in cisplatin-induced nephrotoxicity in rats. Toxicol. Mech. Methods 2014, 24, 520–528. [Google Scholar] [CrossRef]
- Rao, R. Oxidative stress-induced disruption of epithelial and endothelial tight junctions. Front. Biosci. 2008, 13, 7210–7226. [Google Scholar] [CrossRef]
- Bailey, T.A.; Kanuga, N.; Romero, I.A.; Greenwood, J.; Luthert, P.J.; Cheetham, M.E. Oxidative Stress Affects the Junctional Integrity of Retinal Pigment Epithelial Cells. Investig. Ophthalmol. Vis. Sci. 2004, 45, 675–684. [Google Scholar] [CrossRef]
- Hirata, J.; Ko, J.-A.; Mochizuki, H.; Funaishi, K.; Yamane, K.; Sonoda, K.-H.; Kiuchi, Y. Oxidative stress regulates expression of claudin-1 in human RPE cells. Cent. Eur. J. Biol. 2014, 9, 461–468. [Google Scholar] [CrossRef]
- Li, J.; Zhou, J.; Tian, B.; Chu, Y.; Zhang, N.; Hu, X.; Wan, X.; Ye, Y. Activation of HO-1 protects placental cells function in oxidative stress via regulating ZO-1/occludin. Biochem. Biophys. Res. Commun. 2019, 511, 903–909. [Google Scholar] [CrossRef]
- Qin, Y.; Stokman, G.; Yan, K.; Ramaiahgari, S.; Verbeek, F.; de Graauw, M.; van de Water, B.; Price, L.S. cAMP signalling protects proximal tubular epithelial cells from cisplatin-induced apoptosis via activation of Epac. Br. J. Pharmacol. 2012, 165, 1137–1150. [Google Scholar] [CrossRef]
- Yang, X.; Sun, X.; Zhou, F.; Xiao, S.; Zhong, L.; Hu, S.; Zhou, Z.; Li, L.; Tan, Y. Protocatechuic Acid Alleviates Dextran-Sulfate-Sodium-Induced Ulcerative Colitis in Mice via the Regulation of Intestinal Flora and Ferroptosis. Molecules 2023, 28, 3775. [Google Scholar] [CrossRef] [PubMed]
- Xi, Z.; Chen, X.; Xu, C.; Wang, B.; Zhong, Z.; Sun, Q.; Sun, Y.; Bian, L. Protocatechuic acid attenuates brain edema and blood-brain barrier disruption after intracerebral hemorrhage in mice by promoting Nrf2/HO-1 pathway. NeuroReport 2020, 31, 1274–1282. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Jin, C.; Cui, L.; Xing, H.; Liu, J.; Liao, W.; Liao, H.; Yu, Y. HMGB1 contributes to the irradiation-induced endothelial barrier injury through receptor for advanced glycation endproducts (RAGE). J. Cell. Physiol. 2018, 233, 6714–6721. [Google Scholar] [CrossRef]
- Sinha, D.; Wang, Z.; Price, V.R.; Schwartz, J.H.; Lieberthal, W. Chemical anoxia of tubular cells induces activation of c-Src and its translocation to the zonula adherens. Am. J. Physiol.-Ren. Physiol. 2003, 284, F488–F497. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Liu, Z.; Tang, C.; Cai, J.; Dong, Z. FGF21 is induced in cisplatin nephrotoxicity to protect against kidney tubular cell injury. FASEB J. 2018, 32, 3423–3433. [Google Scholar] [CrossRef]
- Othman, A.; Ahmad, S.; Megyerdi, S.; Mussell, R.; Choksi, K.; Maddipati, K.R.; Elmarakby, A.; Rizk, N.; Al-Shabrawey, M. 12/15-Lipoxygenase-derived lipid metabolites induce retinal endothelial cell barrier dysfunction: Contribution of NADPH oxidase. PLoS ONE 2013, 8, e57254. [Google Scholar] [CrossRef]
- Kim, H.; Xue, X. Detection of Total Reactive Oxygen Species in Adherent Cells by 2′,7′-Dichlorodihydrofluorescein Diacetate Staining. J. Vis. Exp. 2020, 160, e60682. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saad, K.M.; Elmasry, K.; Baban, B.; Livingston, M.J.; Dong, Z.; Abdelmageed, M.E.; Abdelaziz, R.R.; Suddek, G.M.; Elmarakby, A.A. Protocatechuic Acid Ameliorates Cisplatin-Induced Inflammation and Apoptosis in Mouse Proximal Tubular Cells. Int. J. Mol. Sci. 2025, 26, 4115. https://doi.org/10.3390/ijms26094115
Saad KM, Elmasry K, Baban B, Livingston MJ, Dong Z, Abdelmageed ME, Abdelaziz RR, Suddek GM, Elmarakby AA. Protocatechuic Acid Ameliorates Cisplatin-Induced Inflammation and Apoptosis in Mouse Proximal Tubular Cells. International Journal of Molecular Sciences. 2025; 26(9):4115. https://doi.org/10.3390/ijms26094115
Chicago/Turabian StyleSaad, Karim M., Khaled Elmasry, Babak Baban, Man J. Livingston, Zheng Dong, Marwa E. Abdelmageed, Rania R. Abdelaziz, Ghada M. Suddek, and Ahmed A. Elmarakby. 2025. "Protocatechuic Acid Ameliorates Cisplatin-Induced Inflammation and Apoptosis in Mouse Proximal Tubular Cells" International Journal of Molecular Sciences 26, no. 9: 4115. https://doi.org/10.3390/ijms26094115
APA StyleSaad, K. M., Elmasry, K., Baban, B., Livingston, M. J., Dong, Z., Abdelmageed, M. E., Abdelaziz, R. R., Suddek, G. M., & Elmarakby, A. A. (2025). Protocatechuic Acid Ameliorates Cisplatin-Induced Inflammation and Apoptosis in Mouse Proximal Tubular Cells. International Journal of Molecular Sciences, 26(9), 4115. https://doi.org/10.3390/ijms26094115






