The Regulator of G Protein Signaling 14 Knockout Mouse, a Model of Healthful Longevity Protects Against Obesity and Glucose Intolerance Through a Brown Adipose Tissue Mechanism
Abstract
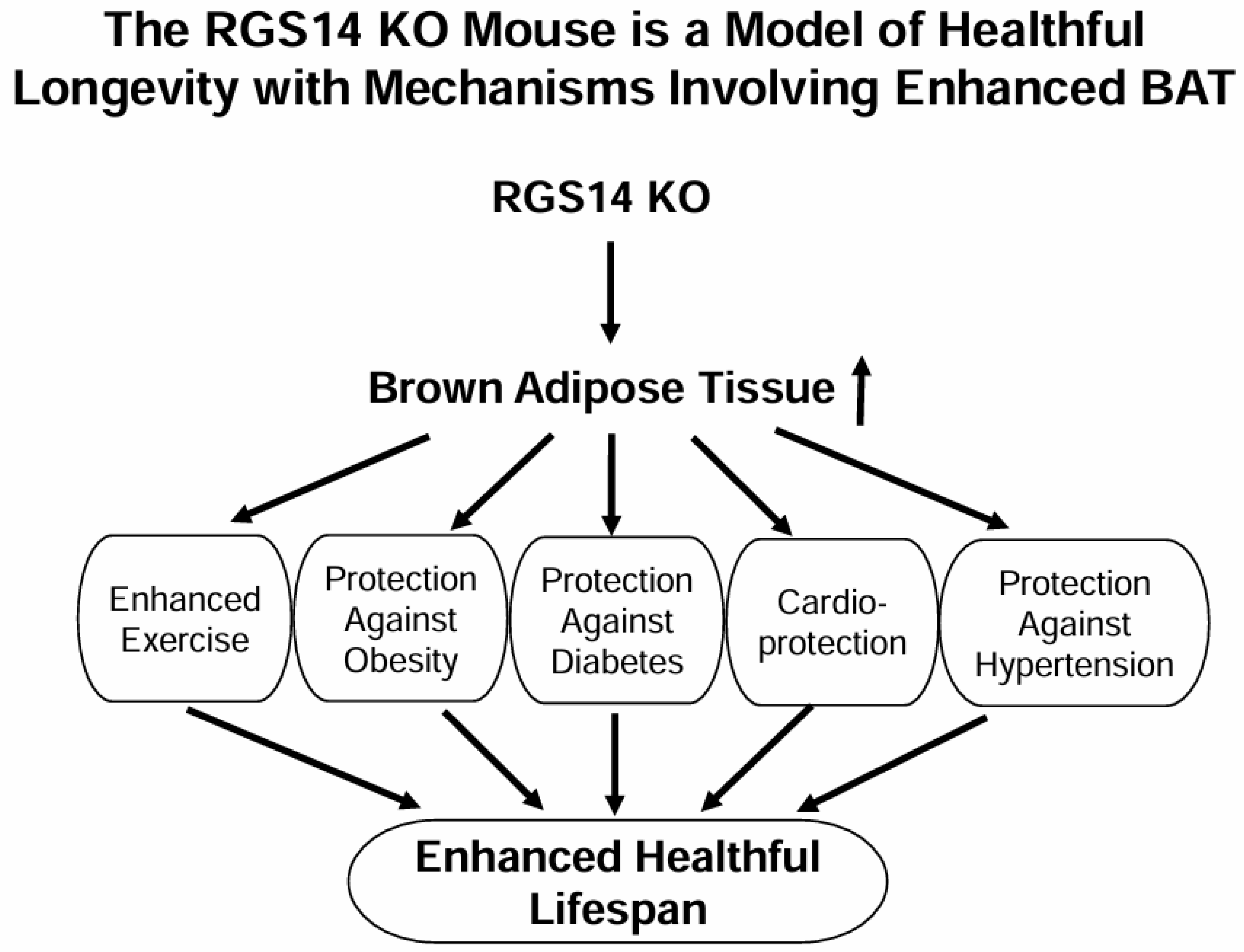
1. Introduction
2. Results
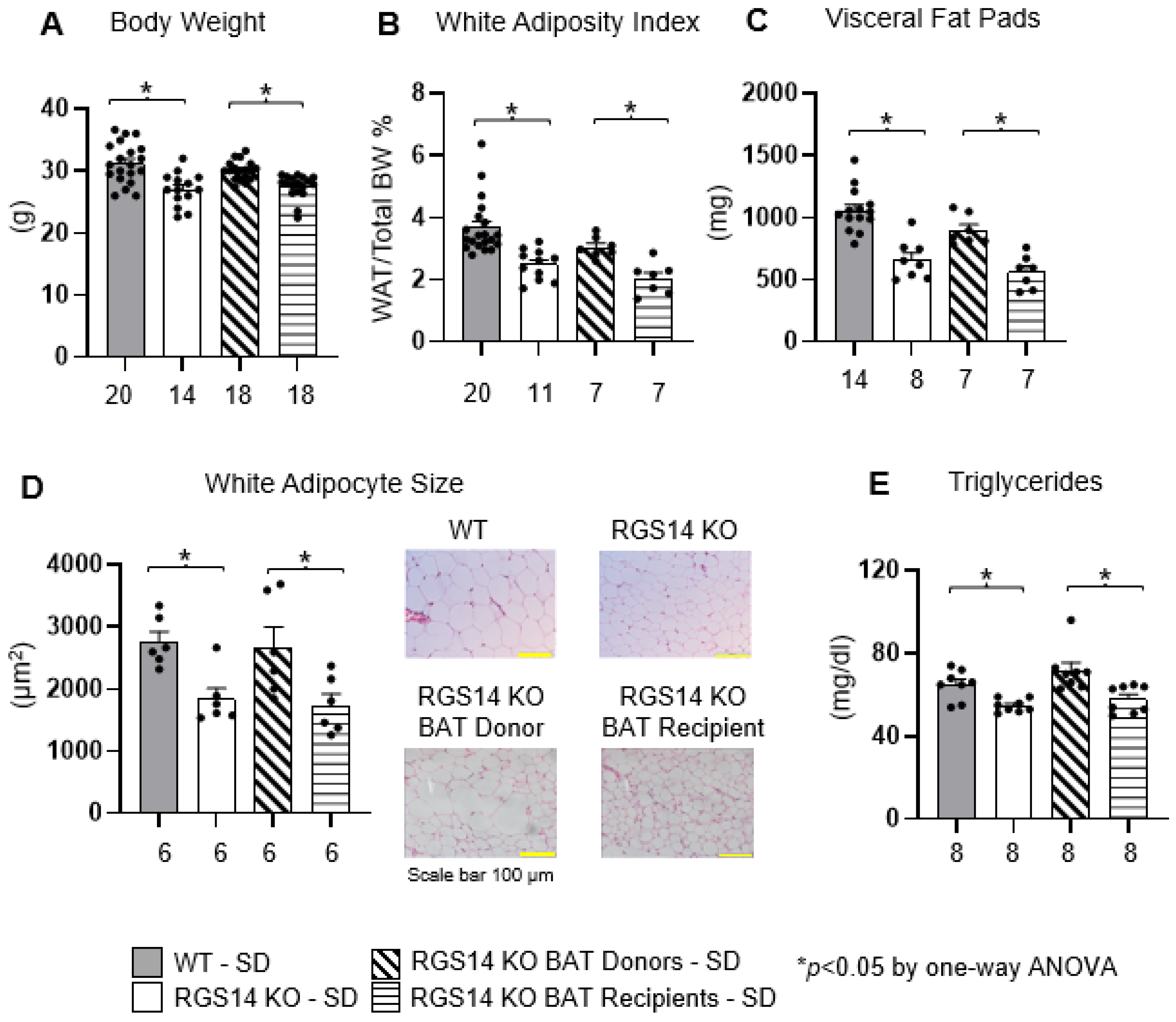
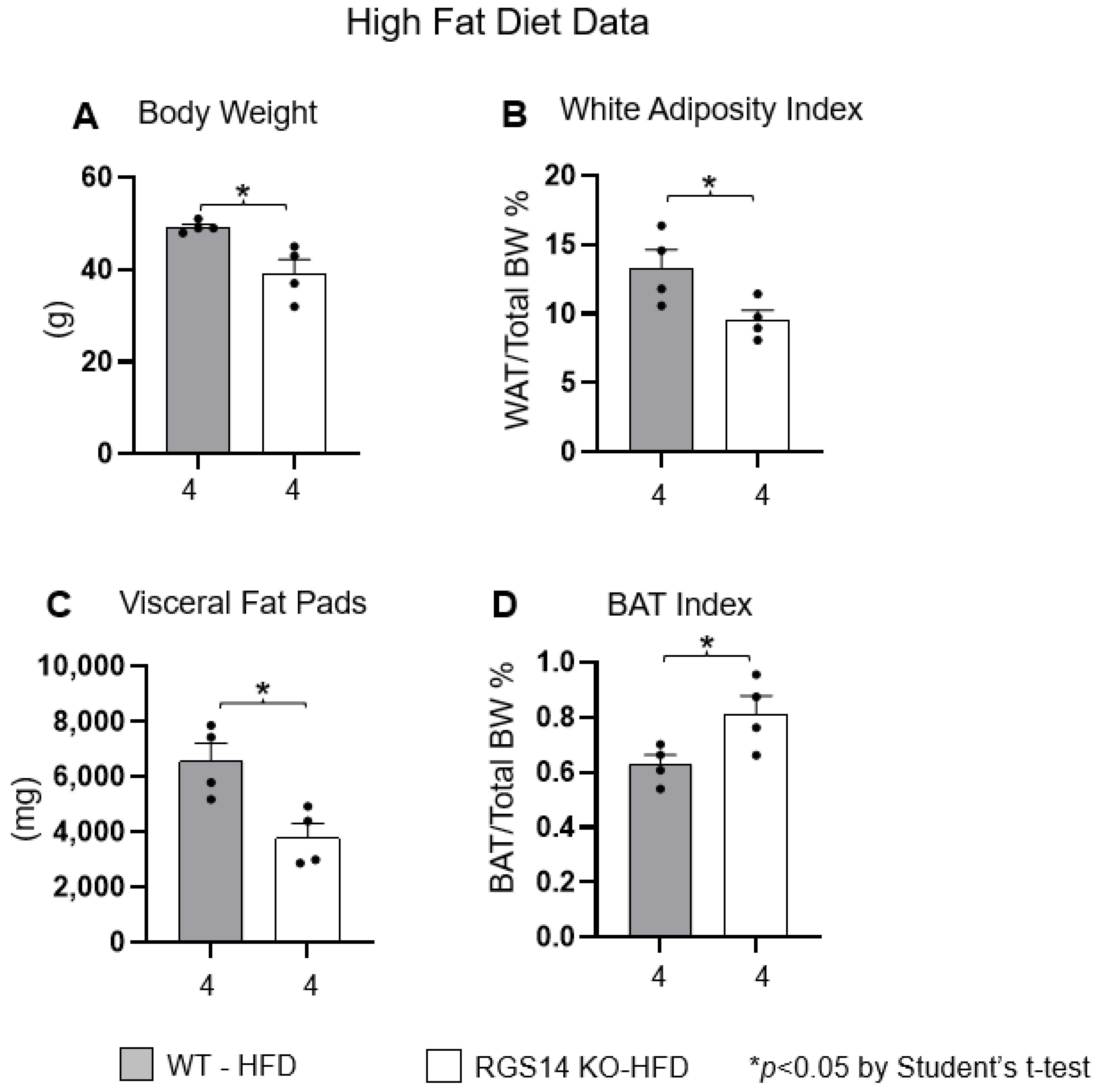
2.1. RGS14 Deficiency Improves Body Composition by Enhanced Brown Adipose Tissue
2.2. RGS14 Deficiency Improves Glucose Tolerance and Insulin Sensitivity by Enhanced Brown Adipose Tissue
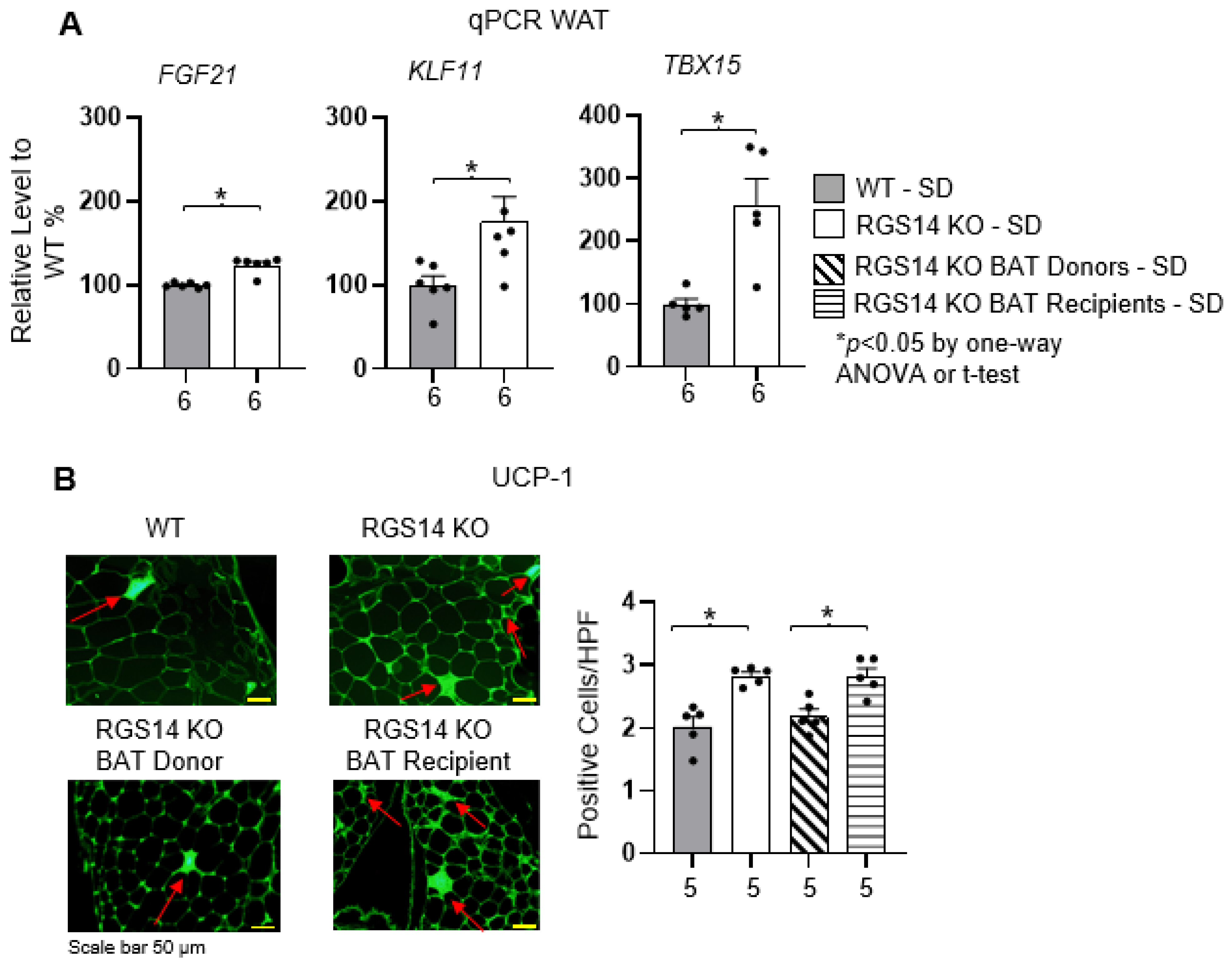
2.3. RGS14 Deficiency Promotes White Adipose Tissue Browning by Enhanced Brown Adipose Tissue
3. Discussion
4. Materials and Methods
4.1. Animal Experimental Procedures
4.2. Brown Adipose Tissue Removal and Transplantation
4.3. Fasting Glucose and Lipid Profile
4.4. Calculation of White Adiposity Index and BAT Index
4.5. Glucose Tolerance Test
4.6. Insulin Tolerance Test
4.7. Quantitative RT-PCR
4.8. Histology and Immunofluorescence Staining
4.9. Statistical Analyses
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vatner, D.E.; Zhang, J.; Oydanich, M.; Guers, J.; Katsyuba, E.; Yan, L.; Sinclair, D.; Auwerx, J.; Vatner, S.F. Enhanced longevity and metabolism by brown adipose tissue with disruption of the regulator of G protein signaling 14. Aging Cell 2018, 17, e12751. [Google Scholar] [CrossRef] [PubMed]
- Vatner, D.E.; Oydanich, M.; Zhang, J.; Campbell, S.C.; Vatner, S.F. Exercise enhancement by RGS14 disruption is mediated by brown adipose tissue. Aging Cell 2023, 22, e13791. [Google Scholar] [CrossRef] [PubMed]
- Dhana, K.; Nano, J.; Ligthart, S.; Peeters, A.; Hofman, A.; Nusselder, W.; Dehghan, A.; Franco, O.H. Obesity and Life Expectancy with and without Diabetes in Adults Aged 55 Years and Older in the Netherlands: A Prospective Cohort Study. PLoS Med. 2016, 13, e1002086. [Google Scholar] [CrossRef]
- Pi-Sunyer, X. The medical risks of obesity. Postgrad. Med. 2009, 121, 21–33. [Google Scholar] [CrossRef]
- Caballero-Franco, C.; Kissler, S. The autoimmunity-associated gene RGS1 affects the frequency of T follicular helper cells. Genes. Immun. 2016, 17, 228–238. [Google Scholar] [CrossRef]
- Ruiz de Azua, I.; Scarselli, M.; Rosemond, E.; Gautam, D.; Jou, W.; Gavrilova, O.; Ebert, P.J.; Levitt, P.; Wess, J. RGS4 is a negative regulator of insulin release from pancreatic beta-cells in vitro and in vivo. Proc. Natl. Acad. Sci. USA 2010, 107, 7999–8004. [Google Scholar] [CrossRef]
- Deng, W.; Wang, X.; Xiao, J.; Chen, K.; Zhou, H.; Shen, D.; Li, H.; Tang, Q. Loss of regulator of G protein signaling 5 exacerbates obesity, hepatic steatosis, inflammation and insulin resistance. PLoS ONE 2012, 7, e30256. [Google Scholar] [CrossRef]
- Walker, P.D.; Jarosz, P.A.; Bouhamdan, M.; MacKenzie, R.G. Effects of gender on locomotor sensitivity to amphetamine, body weight, and fat mass in regulator of G protein signaling 9 (RGS9) knockout mice. Physiol. Behav. 2015, 138, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Vivot, K.; Moulle, V.S.; Zarrouki, B.; Tremblay, C.; Mancini, A.D.; Maachi, H.; Ghislain, J.; Poitout, V. The regulator of G-protein signaling RGS16 promotes insulin secretion and beta-cell proliferation in rodent and human islets. Mol. Metab. 2016, 5, 988–996. [Google Scholar] [CrossRef]
- Smith, R.E.; Roberts, J.C. Thermogenesis of Brown Adipose Tissue in Cold-Acclimated Rats. Am. J. Physiol. 1964, 206, 143–148. [Google Scholar] [CrossRef]
- Nedergaard, J.; Cannon, B. The browning of white adipose tissue: Some burning issues. Cell Metab. 2014, 20, 396–407. [Google Scholar] [CrossRef] [PubMed]
- Feldmann, H.M.; Golozoubova, V.; Cannon, B.; Nedergaard, J. UCP1 ablation induces obesity and abolishes diet-induced thermogenesis in mice exempt from thermal stress by living at thermoneutrality. Cell Metab. 2009, 9, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Stanford, K.I.; Middelbeek, R.J.; Townsend, K.L.; An, D.; Nygaard, E.B.; Hitchcox, K.M.; Markan, K.R.; Nakano, K.; Hirshman, M.F.; Tseng, Y.H.; et al. Brown adipose tissue regulates glucose homeostasis and insulin sensitivity. J. Clin. Investig. 2013, 123, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wang, S.; You, Y.; Meng, M.; Zheng, Z.; Dong, M.; Lin, J.; Zhao, Q.; Zhang, C.; Yuan, X.; et al. Brown Adipose Tissue Transplantation Reverses Obesity in Ob/Ob Mice. Endocrinology 2015, 156, 2461–2469. [Google Scholar] [CrossRef]
- Da Peres Valgas Silva, C.; Shettigar, V.K.; Baer, L.A.; Abay, E.; Madaris, K.L.; Mehling, M.R.; Hernandez-Saavedra, D.; Pinckard, K.M.; Seculov, N.P.; Ziolo, M.T.; et al. Brown adipose tissue prevents glucose intolerance and cardiac remodeling in high-fat-fed mice after a mild myocardial infarction. Int. J. Obes. 2022, 46, 350–358. [Google Scholar] [CrossRef]
- Nedergaard, J.; Bengtsson, T.; Cannon, B. Unexpected evidence for active brown adipose tissue in adult humans. Am. J. Physiol. Endocrinol. Metab. 2007, 293, E444–E452. [Google Scholar] [CrossRef]
- Matsushita, M.; Yoneshiro, T.; Aita, S.; Kameya, T.; Sugie, H.; Saito, M. Impact of brown adipose tissue on body fatness and glucose metabolism in healthy humans. Int. J. Obes. 2014, 38, 812–817. [Google Scholar] [CrossRef]
- Li, Y.; Knapp, J.R.; Kopchick, J.J. Enlargement of interscapular brown adipose tissue in growth hormone antagonist transgenic and in growth hormone receptor gene-disrupted dwarf mice. Exp. Biol. Med. 2003, 228, 207–215. [Google Scholar] [CrossRef]
- Becher, T.; Palanisamy, S.; Kramer, D.J.; Eljalby, M.; Marx, S.J.; Wibmer, A.G.; Butler, S.D.; Jiang, C.S.; Vaughan, R.; Schoder, H.; et al. Brown adipose tissue is associated with cardiometabolic health. Nat. Med. 2021, 27, 58–65. [Google Scholar] [CrossRef]
- White, J.D.; Dewal, R.S.; Stanford, K.I. The beneficial effects of brown adipose tissue transplantation. Mol. Asp. Med. 2019, 68, 74–81. [Google Scholar] [CrossRef]
- Liu, X.; Zheng, Z.; Zhu, X.; Meng, M.; Li, L.; Shen, Y.; Chi, Q.; Wang, D.; Zhang, Z.; Li, C.; et al. Brown adipose tissue transplantation improves whole-body energy metabolism. Cell Res. 2013, 23, 851–854. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Spicer, E.G.; Gavini, C.K.; Goudjo-Ako, A.J.; Novak, C.M.; Shi, H. Enhanced sympathetic activity in mice with brown adipose tissue transplantation (transBATation). Physiol. Behav. 2014, 125, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Gunawardana, S.C.; Piston, D.W. Reversal of type 1 diabetes in mice by brown adipose tissue transplant. Diabetes 2012, 61, 674–682. [Google Scholar] [CrossRef] [PubMed]
- Gunawardana, S.C.; Piston, D.W. Insulin-independent reversal of type 1 diabetes in nonobese diabetic mice with brown adipose tissue transplant. Am. J. Physiol. Endocrinol. Metab. 2015, 308, E1043–E1055. [Google Scholar] [CrossRef]
- Ho, D.; Zhao, X.; Yan, L.; Yuan, C.; Zong, H.; Vatner, D.E.; Pessin, J.E.; Vatner, S.F. Adenylyl Cyclase Type 5 Deficiency Protects Against Diet-Induced Obesity and Insulin Resistance. Diabetes 2015, 64, 2636–2645. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vatner, S.F.; Zhang, J.; Oydanich, M.; Vatner, D.E. The Regulator of G Protein Signaling 14 Knockout Mouse, a Model of Healthful Longevity Protects Against Obesity and Glucose Intolerance Through a Brown Adipose Tissue Mechanism. Int. J. Mol. Sci. 2025, 26, 4113. https://doi.org/10.3390/ijms26094113
Vatner SF, Zhang J, Oydanich M, Vatner DE. The Regulator of G Protein Signaling 14 Knockout Mouse, a Model of Healthful Longevity Protects Against Obesity and Glucose Intolerance Through a Brown Adipose Tissue Mechanism. International Journal of Molecular Sciences. 2025; 26(9):4113. https://doi.org/10.3390/ijms26094113
Chicago/Turabian StyleVatner, Stephen F., Jie Zhang, Marko Oydanich, and Dorothy E. Vatner. 2025. "The Regulator of G Protein Signaling 14 Knockout Mouse, a Model of Healthful Longevity Protects Against Obesity and Glucose Intolerance Through a Brown Adipose Tissue Mechanism" International Journal of Molecular Sciences 26, no. 9: 4113. https://doi.org/10.3390/ijms26094113
APA StyleVatner, S. F., Zhang, J., Oydanich, M., & Vatner, D. E. (2025). The Regulator of G Protein Signaling 14 Knockout Mouse, a Model of Healthful Longevity Protects Against Obesity and Glucose Intolerance Through a Brown Adipose Tissue Mechanism. International Journal of Molecular Sciences, 26(9), 4113. https://doi.org/10.3390/ijms26094113





