Molecular Hydrogen in the Treatment of Respiratory Diseases
Abstract
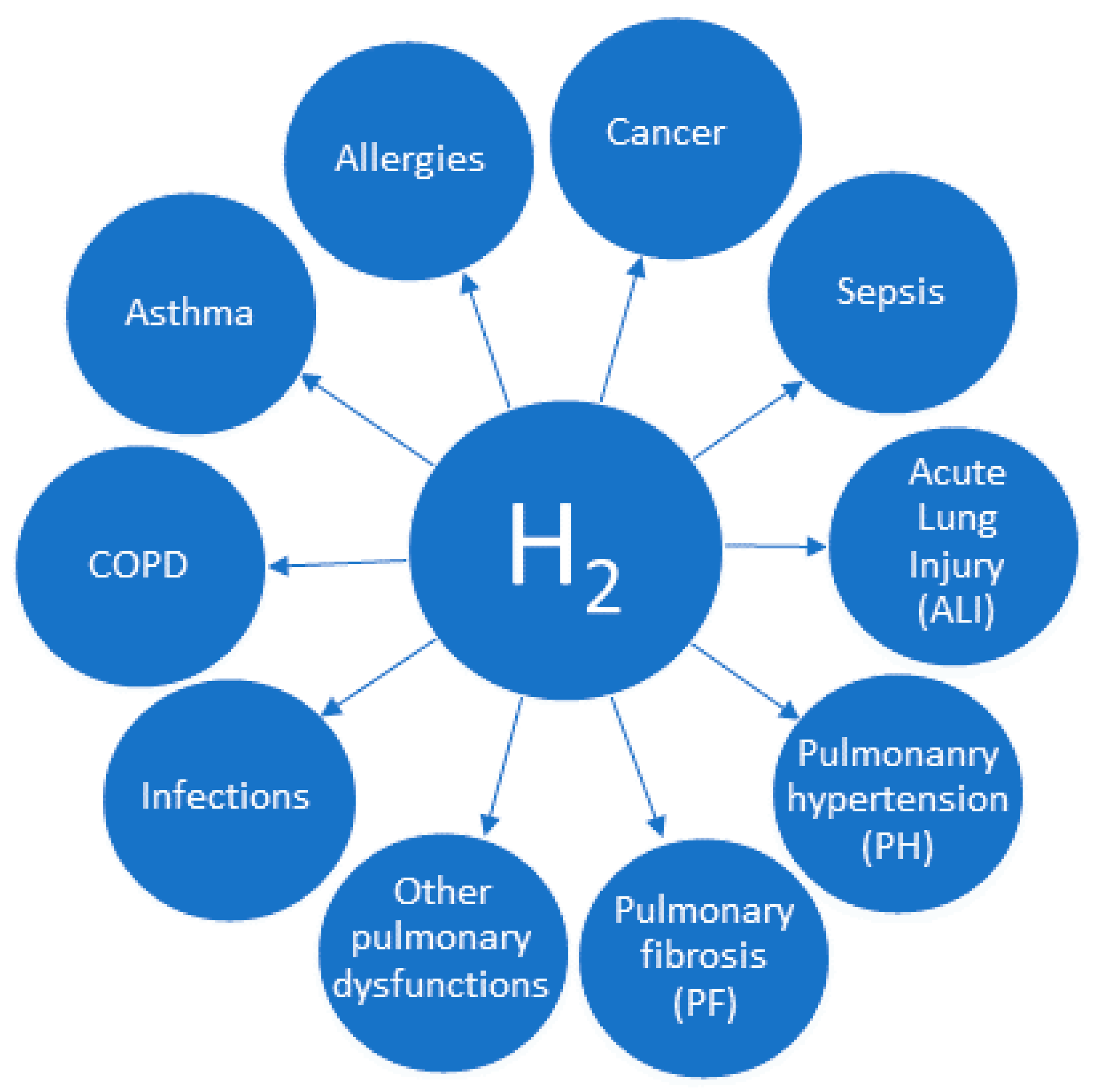
:1. Introduction
2. Mechanisms of Action of Molecular Hydrogen
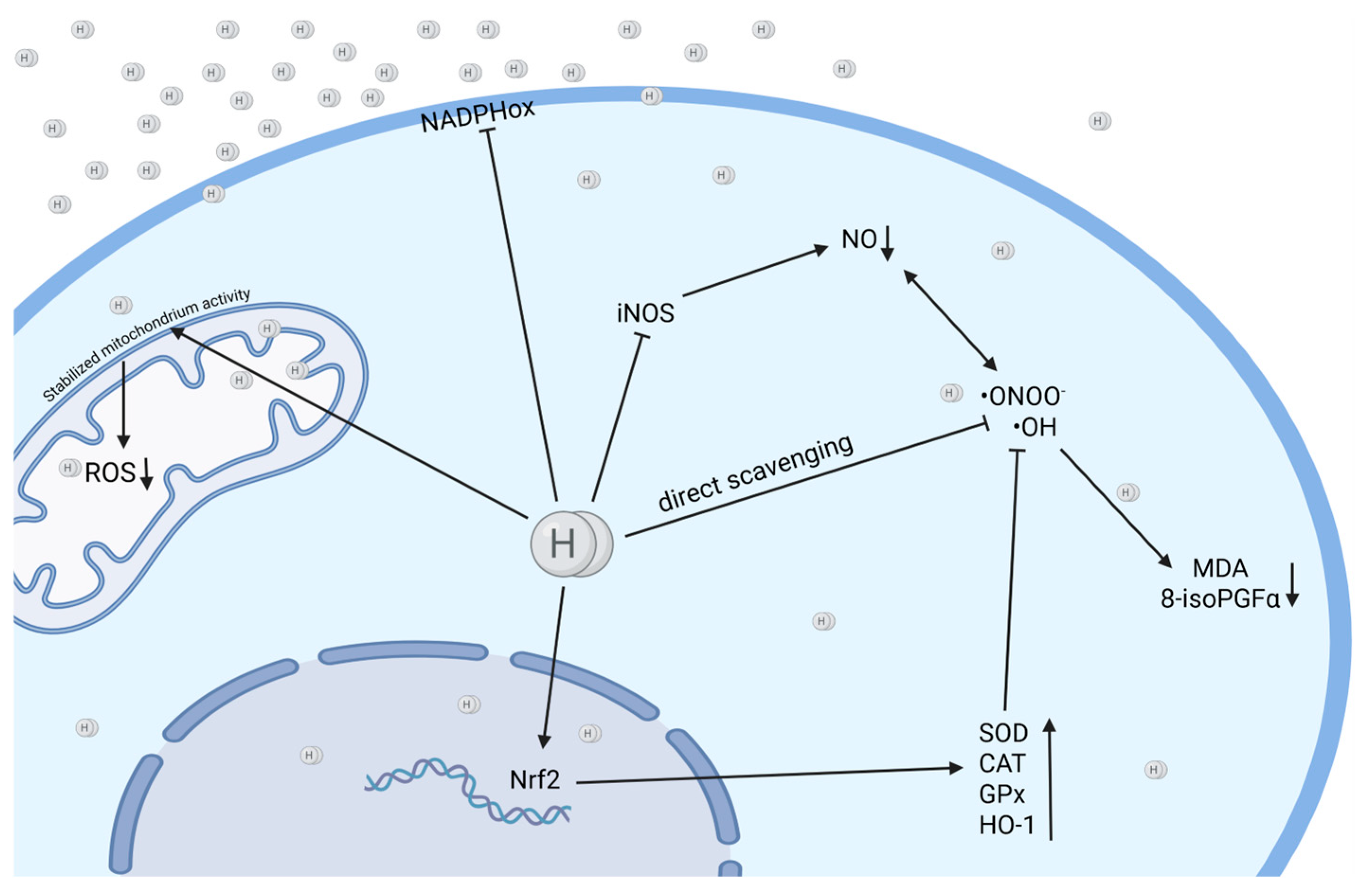
2.1. Anti-Oxidant Activity of Molecular Hydrogen
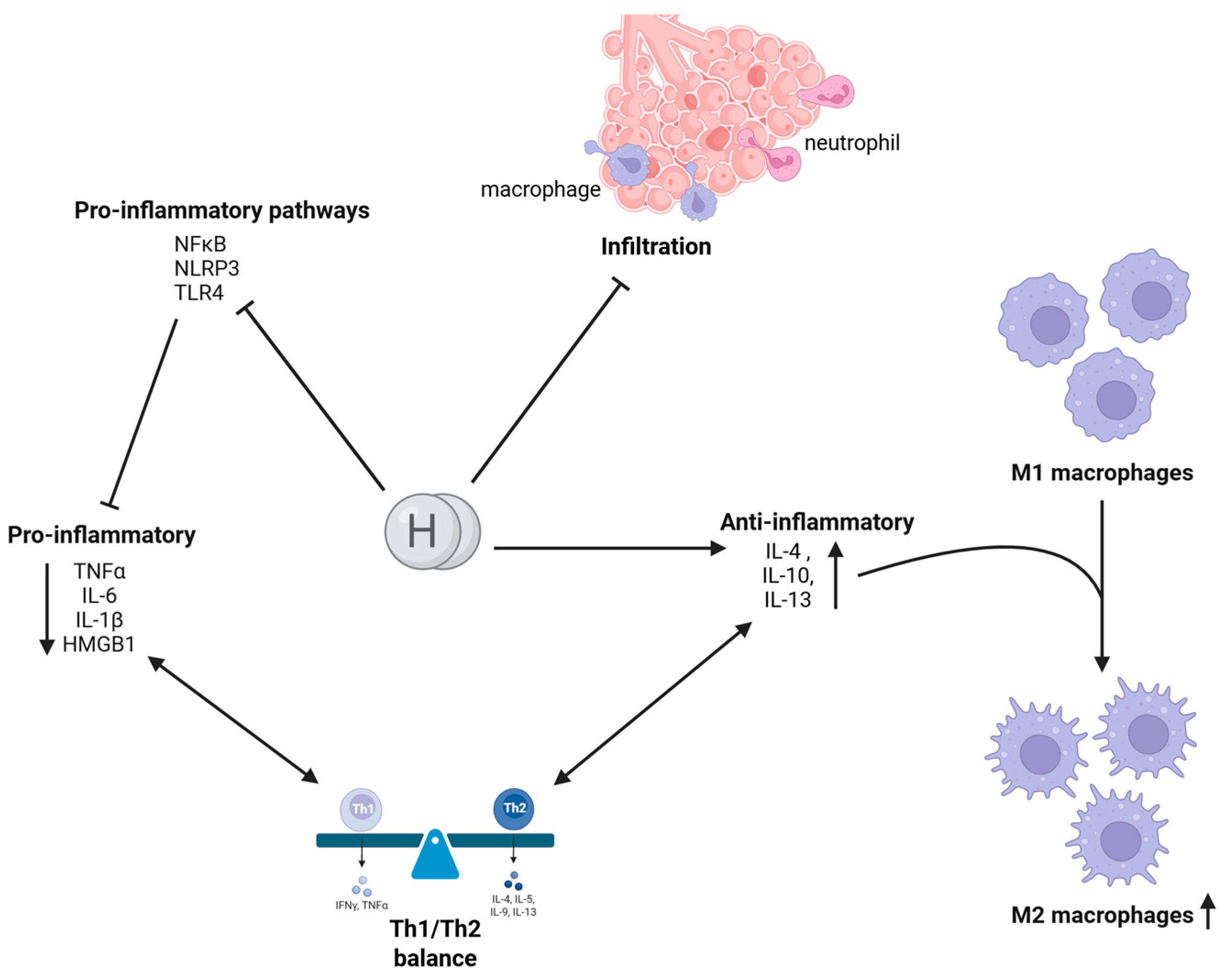
2.2. Anti-Inflammatory Activity of Molecular Hydrogen
2.3. Antiapoptotic Activity of Molecular Hydrogen
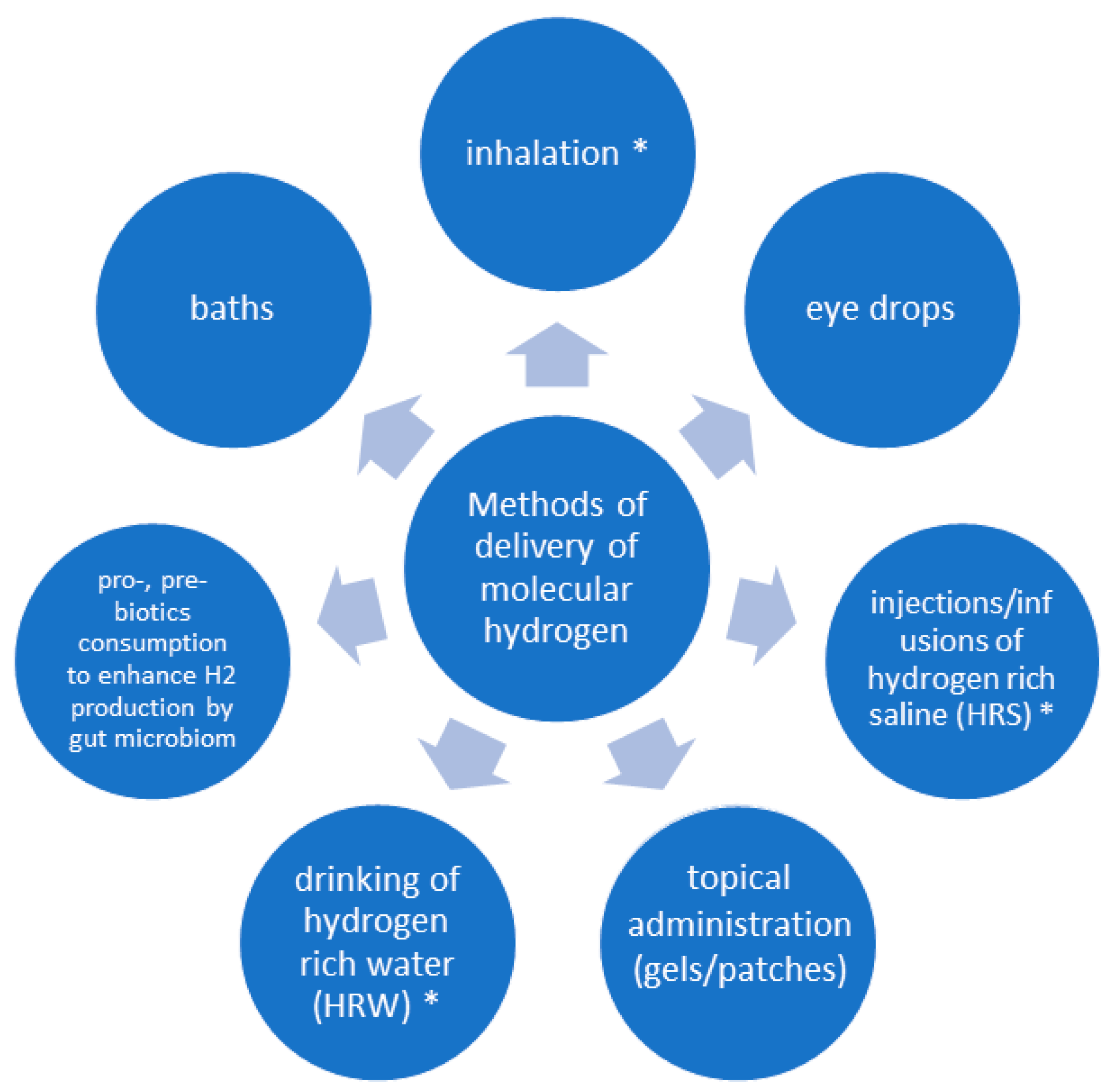
3. Methods of Administration
4. Molecular Hydrogen and Allergies
5. Molecular Hydrogen and Asthma
6. Molecular Hydrogen and COPD
7. Molecular Hydrogen and Pulmonary Fibrosis
8. Molecular Hydrogen and Other Pulmonary Diseases
9. Molecular Hydrogen and Lung Injuries
9.1. Sepsis-Related Lung Injury
9.2. General Lung Injury
9.3. Hypoxia-Reoxygenation Lung Injury
9.4. Hyperoxia-Related Lung Injury
9.5. Ventilator-Induced Lung Injury
9.6. Seawater-Induced Lung Injury
9.7. Other Forms of Lung Injury
10. Molecular Hydrogen and Infections
11. Molecular Hydrogen and Cancer
12. Future Perspectives and Limitations
13. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| •OH | hydroxyl radical |
| 4HNE | 4-hydroxyl-2-nonenal |
| 6-MWT | 6-min walking test |
| 8-OHdG | 8-hydroxydeoxyguanosine |
| A549 cells | adenocarcinomic human alveolar basal epithelial cells |
| ALI | acute lung injury |
| AR | allergic rhinitis |
| ARDS | acute respiratory distress syndrome |
| BALF | bronchoalveolar lavage fluid |
| BCSS | breathless-cough-sputum scale |
| BMSC | bone marrow-derived mesenchymal stem cells |
| CAT | catalase |
| CLP | cecal-ligation-and-puncture |
| COPD | chronic obstructive pulmonary disease |
| COVID-19 | coronavirus disease 2019 |
| CS | cigarette smoke |
| Drp1 | dynamin-related protein 1 |
| EBC | exhaled breath condensate |
| EGFR | epidermal growth factor receptor |
| EMT | epithelial-to-mesenchymal transition |
| ER | endoplasmic reticulum |
| FAS | fatigue assessment scale |
| FcεRI | high-affinity IgE receptor |
| FEV1 | forced expiratory volume in 1 s |
| FGFR4 | fibroblast growth factor receptor 4 |
| FVC | forced vital capacity |
| GPx | glutathione peroxidase |
| H/R | hypoxia-reoxygenation |
| H1975 | human non-small cell lung cancer cells |
| H2O2 | hydrogen peroxide |
| HaCaT | aneuploid immortal keratinocyte cell line from adult human skin |
| HIEC | human intestinal epithelial cells |
| HIF-1α | hypoxia-inducible factor-1 α |
| HMGB1 | high mobility group box 1 protein |
| HO-1 | hemoxygenase-1 |
| HRS | hydrogen-enriched saline |
| HRW | hydrogen-enriched water |
| ICAM-1 | intercellular adhesion molecule 1 |
| IgE | immunoglobulin E |
| IL | interleukin |
| INF-γ | interferon gamma |
| ip. | intraperitoneal (injection) |
| it. | intratracheal (administration) |
| iv. | intravenous (administration) |
| LI | lung injury |
| LPS | lipopolysaccharide |
| MAPK | mitogen-activated protein kinase |
| MCP-1 | monocyte chemoattractant protein-1 |
| MDA | malondialdehyde |
| MMP-12 | matrix metalloproteinase-12 |
| MP1α | macrophage protein 1α |
| MPO | myeloperoxidase |
| MUC5AC | mucin-5AC |
| NFκB | nuclear factor kappa-light-chain-enhancer of activated B cells |
| NLRP3 | NLR family pyrin domain containing 3 |
| Nrf2 | nuclear factor erythroid 2-related factor-2 |
| O2- | superoxide |
| ONOO⁻ | peroxynitrite |
| OVA | ovalbumin |
| pCO2 | partial pressure of carbon dioxide |
| PF | pulmonary fibrosis |
| PGE2 | prostaglandin E2 |
| PM2.5 | particulate matter of a diameter of less than 2.5 µm |
| pO2 | partial pressure of oxygen |
| ROS/RNS | reactive oxygen and nitrogen species |
| SARS-CoV-2 | severe acute respiratory syndrome coronavirus-2 |
| SIRT-1 | Sirtuin-1 |
| SMA | smooth muscle actin |
| SMC | structural maintenance of chromosomes complex |
| SOD | superoxide dismutase |
| TBARS | thiobarbituric acid reactive substances |
| TGF1β | transforming growth factor |
| TIMP-1 | tissue inhibitor of metalloproteinase-1 |
| TLR4 | toll-like receptor 4 |
| TNF-α | tumor necrosis factor-α |
| TUNEL | terminal deoxynucleotidyl transferase dUTP nick end labeling |
| VCAM-1 | vascular cell adhesion molecule-1 |
| VE | minute ventilation |
| VEGF | vascular endothelial growth factor |
| VEGFR2 | vascular endothelial growth factor receptor 2 |
| VILI | ventilatory-induced lung injury |
| XEN gas | 2.4% H2 in air |
| ZO-1 | tight junction protein ZO-1/zonula occludens-1 |
References
- Ohta, S. Molecular Hydrogen as a Novel Antioxidant: Overview of the Advantages of Hydrogen for Medical Applications. Methods Enzymol. 2015, 555, 289–317. [Google Scholar] [CrossRef]
- Hong, Y.; Chen, S.; Zhang, J.-M. Hydrogen as a Selective Antioxidant: A Review of Clinical and Experimental Studies. J. Int. Med. Res. 2010, 38, 1893–1903. [Google Scholar] [CrossRef]
- Ohsawa, I.; Ishikawa, M.; Takahashi, K.; Watanabe, M.; Nishimaki, K.; Yamagata, K.; Katsura, K.-I.; Katayama, Y.; Asoh, S.; Ohta, S. Hydrogen Acts as a Therapeutic Antioxidant by Selectively Reducing Cytotoxic Oxygen Radicals. Nat. Med. 2007, 13, 688–694. [Google Scholar] [CrossRef]
- Hirano, S.; Ichikawa, Y.; Sato, B.; Yamamoto, H.; Takefuji, Y.; Satoh, F. Potential Therapeutic Applications of Hydrogen in Chronic Inflammatory Diseases: Possible Inhibiting Role on Mitochondrial Stress. Int. J. Mol. Sci. 2021, 22, 2549. [Google Scholar] [CrossRef] [PubMed]
- Tao, G.; Song, G.; Qin, S. Molecular Hydrogen: Current Knowledge on Mechanism in Alleviating Free Radical Damage and Diseases. Acta Biochim. Biophys. Sin. 2019, 51, 1189–1197. [Google Scholar] [CrossRef]
- Tian, Y.; Zhang, Y.; Wang, Y.; Chen, Y.; Fan, W.; Zhou, J.; Qiao, J.; Wei, Y. Hydrogen, a Novel Therapeutic Molecule, Regulates Oxidative Stress, Inflammation, and Apoptosis. Front. Physiol. 2021, 12, 789507. [Google Scholar] [CrossRef] [PubMed]
- Shimouchi, A.; Nose, K.; Shirai, M.; Kondo, T. Estimation of Molecular Hydrogen Consumption in the Human Whole Body after the Ingestion of Hydrogen-Rich Water. Adv. Exp. Med. Biol. 2012, 737, 245–250. [Google Scholar] [CrossRef] [PubMed]
- Alwazeer, D.; Liu, F.F.-C.; Wu, X.Y.; LeBaron, T.W. Combating Oxidative Stress and Inflammation in COVID-19 by Molecular Hydrogen Therapy: Mechanisms and Perspectives. Oxid. Med. Cell. Longev. 2021, 2021, 5513868. [Google Scholar] [CrossRef]
- Hylemon, P.B.; Harris, S.C.; Ridlon, J.M. Metabolism of Hydrogen Gases and Bile Acids in the Gut Microbiome. FEBS Lett. 2018, 592, 2070–2082. [Google Scholar] [CrossRef]
- Smith, N.W.; Shorten, P.R.; Altermann, E.H.; Roy, N.C.; McNabb, W.C. Hydrogen Cross-Feeders of the Human Gastrointestinal Tract. Gut Microbes 2019, 10, 270–288. [Google Scholar] [CrossRef]
- Wang, J.; Yin, Y. Clostridium Species for Fermentative Hydrogen Production: An Overview. Int. J. Hydrogen Energy 2021, 46, 34599–34625. [Google Scholar] [CrossRef]
- Wolf, P.G.; Biswas, A.; Morales, S.E.; Greening, C.; Gaskins, H.R. H2 Metabolism Is Widespread and Diverse among Human Colonic Microbes. Gut Microbes 2016, 7, 235–245. [Google Scholar] [CrossRef] [PubMed]
- Pilcher, J.E. Senn on the diagnosis of gastro-intestinal perforation by the rectal insufflation of hydrogen gas. Ann. Surg. 1888, 8, 190–204. [Google Scholar] [CrossRef] [PubMed]
- Zetterstrom, A. Deep-Sea Diving with Synthetic Gas Mixtures. Mil. Surg. 1948, 103, 104–106. [Google Scholar] [CrossRef]
- Dole, M.; Wilson, F.R.; Fife, W.P. Hyperbaric Hydrogen Therapy: A Possible Treatment for Cancer. Science 1975, 190, 152–154. [Google Scholar] [CrossRef]
- Gharib, B.; Hanna, S.; Abdallahi, O.M.; Lepidi, H.; Gardette, B.; De Reggi, M. Anti-Inflammatory Properties of Molecular Hydrogen: Investigation on Parasite-Induced Liver Inflammation. C. R. Acad. Sci. III 2001, 324, 719–724. [Google Scholar] [CrossRef]
- Fu, Z.; Zhang, Z.; Wu, X.; Zhang, J. Hydrogen-Rich Saline Inhibits Lipopolysaccharide-Induced Acute Lung Injury and Endothelial Dysfunction by Regulating Autophagy through mTOR/TFEB Signaling Pathway. BioMed Res. Int. 2020, 2020, 9121894. [Google Scholar] [CrossRef]
- Johnsen, H.M.; Hiorth, M.; Klaveness, J. Molecular Hydrogen Therapy—A Review on Clinical Studies and Outcomes. Molecules 2023, 28, 7785. [Google Scholar] [CrossRef]
- Ohta, S. Molecular Hydrogen as a Preventive and Therapeutic Medical Gas: Initiation, Development and Potential of Hydrogen Medicine. Pharmacol. Ther. 2014, 144, 1–11. [Google Scholar] [CrossRef]
- Russell, G.; Nenov, A.; Hancock, J.T. Oxy-Hydrogen Gas: The Rationale Behind Its Use as a Novel and Sustainable Treatment for COVID-19 and Other Respiratory Diseases. Eur. Med. J. 2021, 10, 21-00027. [Google Scholar] [CrossRef]
- Yang, M.; Dong, Y.; He, Q.; Zhu, P.; Zhuang, Q.; Shen, J.; Zhang, X.; Zhao, M. Hydrogen: A Novel Option in Human Disease Treatment. Oxid. Med. Cell. Longev. 2020, 2020, e8384742. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.-B.; Kong, X.-F.; Qian, W.; Mu, F.; Lu, T.-Y.; Lu, Y.-Y.; Xu, K.-C. Two Weeks of Hydrogen Inhalation Can Significantly Reverse Adaptive and Innate Immune System Senescence Patients with Advanced Non-Small Cell Lung Cancer: A Self-Controlled Study. Med. Gas Res. 2020, 10, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.-B.; Kong, X.-F.; Mu, F.; Lu, T.-Y.; Lu, Y.-Y.; Xu, K.-C. Hydrogen Therapy Can Be Used to Control Tumor Progression and Alleviate the Adverse Events of Medications in Patients with Advanced Non-Small Cell Lung Cancer. Med. Gas Res. 2020, 10, 75–80. [Google Scholar] [CrossRef]
- Hirano, S.; Ichikawa, Y.; Sato, B.; Yamamoto, H.; Takefuji, Y.; Satoh, F. Molecular Hydrogen as a Potential Clinically Applicable Radioprotective Agent. Int. J. Mol. Sci. 2021, 22, 4566. [Google Scholar] [CrossRef] [PubMed]
- Hirano, S.; Yamamoto, H.; Ichikawa, Y.; Sato, B.; Takefuji, Y.; Satoh, F. Molecular Hydrogen as a Novel Antitumor Agent: Possible Mechanisms Underlying Gene Expression. Int. J. Mol. Sci. 2021, 22, 8724. [Google Scholar] [CrossRef]
- Hu, Q.; Zhou, Y.; Wu, S.; Wu, W.; Deng, Y.; Shao, A. Molecular Hydrogen: A Potential Radioprotective Agent. Biomed. Pharmacother. 2020, 130, 110589. [Google Scholar] [CrossRef]
- Qian, L.; Shen, J.; Chuai, Y.; Cai, J. Hydrogen as a New Class of Radioprotective Agent. Int. J. Biol. Sci. 2013, 9, 887–894. [Google Scholar] [CrossRef]
- Zhou, W.; Zhang, J.; Chen, W.; Miao, C. Prospects of Molecular Hydrogen in Cancer Prevention and Treatment. J. Cancer Res. Clin. Oncol. 2024, 150, 170. [Google Scholar] [CrossRef]
- Qiu, P.; Liu, Y.; Zhang, J. Recent Advances in Studies of Molecular Hydrogen against Sepsis. Int. J. Biol. Sci. 2019, 15, 1261–1275. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, J.; Fu, Z. Molecular Hydrogen Is a Potential Protective Agent in the Management of Acute Lung Injury. Mol. Med. 2022, 28, 27. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Z.; Lian, N.; Wang, Y.; Zheng, W.; Xie, K. Molecular Hydrogen: A Promising Adjunctive Strategy for the Treatment of the COVID-19. Front. Med. 2021, 8, 671215. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Yue, R.; Luo, X.; Liu, R.; Huang, X. Hydrogen: A Potential New Adjuvant Therapy for COVID-19 Patients. Front. Pharmacol. 2020, 11, 543718. [Google Scholar] [CrossRef]
- Fu, Z.; Zhang, J. Molecular Hydrogen Is a Promising Therapeutic Agent for Pulmonary Disease. J. Zhejiang Univ.-Sci. B 2022, 23, 102–122. [Google Scholar] [CrossRef] [PubMed]
- Niki, E. Biomarkers of Lipid Peroxidation in Clinical Material. Biochim. Biophys. Acta BBA-Gen. Subj. 2014, 1840, 809–817. [Google Scholar] [CrossRef]
- Valavanidis, A.; Vlachogianni, T.; Fiotakis, C. 8-Hydroxy-2′ -Deoxyguanosine (8-OHdG): A Critical Biomarker of Oxidative Stress and Carcinogenesis. J. Environ. Sci. Health Part C 2009, 27, 120–139. [Google Scholar] [CrossRef]
- Radi, R. Oxygen Radicals, Nitric Oxide, and Peroxynitrite: Redox Pathways in Molecular Medicine. Proc. Natl. Acad. Sci. USA 2018, 115, 5839–5848. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Zhang, H.; Ji, M.; Jia, M.; Chen, H.; Yang, J.; Duan, M. Hydrogen-Rich Saline Attenuates Neuronal Ischemia–Reperfusion Injury by Protecting Mitochondrial Function in Rats. J. Surg. Res. 2014, 192, 564–572. [Google Scholar] [CrossRef]
- Gvozdjáková, A.; Kucharská, J.; Kura, B.; Vančová, O.; Rausová, Z.; Sumbalová, Z.; Uličná, O.; Slezák, J. A New Insight into the Molecular Hydrogen Effect on Coenzyme Q and Mitochondrial Function of Rats. Can. J. Physiol. Pharmacol. 2020, 98, 29–34. [Google Scholar] [CrossRef]
- Ishihara, G.; Kawamoto, K.; Komori, N.; Ishibashi, T. Molecular Hydrogen Suppresses Superoxide Generation in the Mitochondrial Complex I and Reduced Mitochondrial Membrane Potential. Biochem. Biophys. Res. Commun. 2020, 522, 965–970. [Google Scholar] [CrossRef]
- Chandhok, G.; Lazarou, M.; Neumann, B. Structure, Function, and Regulation of Mitofusin-2 in Health and Disease. Biol. Rev. 2018, 93, 933–949. [Google Scholar] [CrossRef]
- Dong, A.; Yu, Y.; Wang, Y.; Li, C.; Chen, H.; Bian, Y.; Zhang, P.; Zhao, Y.; Yu, Y.; Xie, K. Protective Effects of Hydrogen Gas against Sepsis-Induced Acute Lung Injury via Regulation of Mitochondrial Function and Dynamics. Int. Immunopharmacol. 2018, 65, 366–372. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Li, Y.; Pan, W.; Yang, B.; Fu, C. Role of Dynamin-Related Protein 1-Dependent Mitochondrial Fission in Drug-Induced Toxicity. Pharmacol. Res. 2024, 206, 107250. [Google Scholar] [CrossRef] [PubMed]
- Fu, Z.; Zhang, J.; Zhang, Y. Role of Molecular Hydrogen in Ageing and Ageing-Related Diseases. Oxid. Med. Cell. Longev. 2022, 2022, 2249749. [Google Scholar] [CrossRef]
- Chen, H.; Mao, X.; Meng, X.; Li, Y.; Feng, J.; Zhang, L.; Zhang, Y.; Wang, Y.; Yu, Y.; Xie, K. Hydrogen Alleviates Mitochondrial Dysfunction and Organ Damage via Autophagy-mediated NLRP3 Inflammasome Inactivation in Sepsis. Int. J. Mol. Med. 2019, 44, 1309–1324. [Google Scholar] [CrossRef] [PubMed]
- Chelikani, P.; Fita, I.; Loewen, P.C. Diversity of Structures and Properties among Catalases. Cell. Mol. Life Sci. CMLS 2004, 61, 192–208. [Google Scholar] [CrossRef]
- Idriss, N.K.; Blann, A.D.; Lip, G.Y.H. Hemoxygenase-1 in Cardiovascular Disease. J. Am. Coll. Cardiol. 2008, 52, 971–978. [Google Scholar] [CrossRef]
- Liu, M.; Sun, X.; Chen, B.; Dai, R.; Xi, Z.; Xu, H. Insights into Manganese Superoxide Dismutase and Human Diseases. Int. J. Mol. Sci. 2022, 23, 15893. [Google Scholar] [CrossRef]
- Nguyen, N.H.; Tran, G.-B.; Nguyen, C.T. Anti-Oxidative Effects of Superoxide Dismutase 3 on Inflammatory Diseases. J. Mol. Med. 2020, 98, 59–69. [Google Scholar] [CrossRef]
- Zhai, X.; Chen, X.; Shi, J.; Shi, D.; Ye, Z.; Liu, W.; Li, M.; Wang, Q.; Kang, Z.; Bi, H.; et al. Lactulose Ameliorates Cerebral Ischemia–Reperfusion Injury in Ratsby Inducing Hydrogen by Activating Nrf2 Expression. Free Radic. Biol. Med. 2013, 65, 731–741. [Google Scholar] [CrossRef]
- He, F.; Ru, X.; Wen, T. NRF2, a Transcription Factor for Stress Response and Beyond. Int. J. Mol. Sci. 2020, 21, 4777. [Google Scholar] [CrossRef]
- Rangasamy, T.; Guo, J.; Mitzner, W.A.; Roman, J.; Singh, A.; Fryer, A.D.; Yamamoto, M.; Kensler, T.W.; Tuder, R.M.; Georas, S.N.; et al. Disruption of Nrf2 Enhances Susceptibility to Severe Airway Inflammation and Asthma in Mice. J. Exp. Med. 2005, 202, 47–59. [Google Scholar] [CrossRef] [PubMed]
- Kawamura, T.; Wakabayashi, N.; Shigemura, N.; Huang, C.-S.; Masutani, K.; Tanaka, Y.; Noda, K.; Peng, X.; Takahashi, T.; Billiar, T.R.; et al. Hydrogen Gas Reduces Hyperoxic Lung Injury via the Nrf2 Pathway in Vivo. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2013, 304, L646–L656. [Google Scholar] [CrossRef] [PubMed]
- Morse, D.; Lin, L.; Choi, A.M.K.; Ryter, S.W. Heme Oxygenase-1, a Critical Arbitrator of Cell Death Pathways in Lung Injury and Disease. Free Radic. Biol. Med. 2009, 47, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Sies, H. Oxidative Stress: A Concept in Redox Biology and Medicine. Redox Biol. 2015, 4, 180–183. [Google Scholar] [CrossRef]
- Bezerra, F.S.; Lanzetti, M.; Nesi, R.T.; Nagato, A.C.; Silva, C.P.E.; Kennedy-Feitosa, E.; Melo, A.C.; Cattani-Cavalieri, I.; Porto, L.C.; Valenca, S.S. Oxidative Stress and Inflammation in Acute and Chronic Lung Injuries. Antioxidants 2023, 12, 548. [Google Scholar] [CrossRef]
- Fredenburgh, L.E.; Perrella, M.A.; Mitsialis, S.A. The Role of Heme Oxygenase-1 in Pulmonary Disease. Am. J. Respir. Cell Mol. Biol. 2007, 36, 158–165. [Google Scholar] [CrossRef]
- Kelley, N.; Jeltema, D.; Duan, Y.; He, Y. The NLRP3 Inflammasome: An Overview of Mechanisms of Activation and Regulation. Int. J. Mol. Sci. 2019, 20, 3328. [Google Scholar] [CrossRef]
- Itoh, T.; Fujita, Y.; Ito, M.; Masuda, A.; Ohno, K.; Ichihara, M.; Kojima, T.; Nozawa, Y.; Ito, M. Molecular Hydrogen Suppresses FcεRI-Mediated Signal Transduction and Prevents Degranulation of Mast Cells. Biochem. Biophys. Res. Commun. 2009, 389, 651–656. [Google Scholar] [CrossRef]
- Vermot, A.; Petit-Härtlein, I.; Smith, S.M.E.; Fieschi, F. NADPH Oxidases (NOX): An Overview from Discovery, Molecular Mechanisms to Physiology and Pathology. Antioxidants 2021, 10, 890. [Google Scholar] [CrossRef]
- Aratani, Y. Myeloperoxidase: Its Role for Host Defense, Inflammation, and Neutrophil Function. Arch. Biochem. Biophys. 2018, 640, 47–52. [Google Scholar] [CrossRef]
- Diao, M.; Zhang, S.; Wu, L.; Huan, L.; Huang, F.; Cui, Y.; Lin, Z. Hydrogen Gas Inhalation Attenuates Seawater Instillation-Induced Acute Lung Injury via the Nrf2 Pathway in Rabbits. Inflammation 2016, 39, 2029–2039. [Google Scholar] [CrossRef] [PubMed]
- Guan, W.-J.; Wei, C.-H.; Chen, A.-L.; Sun, X.-C.; Guo, G.-Y.; Zou, X.; Shi, J.-D.; Lai, P.-Z.; Zheng, Z.-G.; Zhong, N.-S. Hydrogen/Oxygen Mixed Gas Inhalation Improves Disease Severity and Dyspnea in Patients with Coronavirus Disease 2019 in a Recent Multicenter, Open-Label Clinical Trial. J. Thorac. Dis. 2020, 12, 3448–3452. [Google Scholar] [CrossRef]
- Zou, R.; Wang, M.-H.; Chen, Y.; Fan, X.; Yang, B.; Du, J.; Wang, X.-B.; Liu, K.-X.; Zhou, J. Hydrogen-Rich Saline Attenuates Acute Lung Injury Induced by Limb Ischemia/Reperfusion via Down-Regulating Chemerin and NLRP3 in Rats. Shock 2019, 52, 134–141. [Google Scholar] [CrossRef] [PubMed]
- Qiu, X.; Li, H.; Tang, H.; Jin, Y.; Li, W.; YuSun; PingFeng; Sun, X.; Xia, Z. Hydrogen Inhalation Ameliorates Lipopolysaccharide-Induced Acute Lung Injury in Mice. Int. Immunopharmacol. 2011, 11, 2130–2137. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Y.; Zhang, J. Saturated Hydrogen Saline Attenuates Endotoxin-Induced Lung Dysfunction. J. Surg. Res. 2015, 198, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Xie, K.; Han, H.; Li, Y.; Liu, L.; Yang, T.; Yu, Y. Molecular Hydrogen Protects Mice against Polymicrobial Sepsis by Ameliorating Endothelial Dysfunction via an Nrf2/HO-1 Signaling Pathway. Int. Immunopharmacol. 2015, 28, 643–654. [Google Scholar] [CrossRef]
- Xie, K.; Yu, Y.; Huang, Y.; Zheng, L.; Li, J.; Chen, H.; Han, H.; Hou, L.; Gong, G.; Wang, G. Molecular Hydrogen Ameliorates Lipopolysaccharide-Induced Acute Lung Injury in Mice Through Reducing Inflammation and Apoptosis. Shock 2012, 37, 548–555. [Google Scholar] [CrossRef]
- Chen, M.; Zhang, J.; Chen, Y.; Qiu, Y.; Luo, Z.; Zhao, S.; Du, L.; Tian, D. Hydrogen Protects Lung from Hypoxia/Re-Oxygenation Injury by Reducing Hydroxyl Radical Production and Inhibiting Inflammatory Responses. Sci. Rep. 2018, 8, 8004. [Google Scholar] [CrossRef]
- He, B.; Zhang, Y.; Kang, B.; Xiao, J.; Xie, B.; Wang, Z. Protection of Oral Hydrogen Water as an Antioxidant on Pulmonary Hypertension. Mol. Biol. Rep. 2013, 40, 5513–5521. [Google Scholar] [CrossRef]
- Itoh, T.; Hamada, N.; Terazawa, R.; Ito, M.; Ohno, K.; Ichihara, M.; Nozawa, Y.; Ito, M. Molecular Hydrogen Inhibits Lipopolysaccharide/Interferon γ-Induced Nitric Oxide Production through Modulation of Signal Transduction in Macrophages. Biochem. Biophys. Res. Commun. 2011, 411, 143–149. [Google Scholar] [CrossRef]
- Du, J.; Li, J.; Li, R.; Yan, X. High Concentration of Hydrogen Ameliorates Lipopolysaccharide-Induced Acute Lung Injury in a Sirt1-Dependent Manner. Respir. Physiol. Neurobiol. 2022, 296, 103808. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Han, W.; Hu, H.; Fan, D.; Li, Y.; Zhang, Y.; Lv, Y.; Li, M.; Pan, S. Hydrogen Alleviates Hyperoxic Acute Lung Injury Related Endoplasmic Reticulum Stress in Rats through Upregulation of SIRT1. Free Radic. Res. 2017, 51, 622–632. [Google Scholar] [CrossRef]
- Li, H.; Zhou, R.; Liu, J.; Li, Q.; Zhang, J.; Mu, J.; Sun, X. Hydrogen-Rich Saline Attenuates Lung Ischemia-Reperfusion Injury in Rabbits. J. Surg. Res. 2012, 174, e11–e16. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.; Wei, S.; Huang, W.; Wu, P.; Chen, S.; Tao, A.; Wang, H.; Liang, Z.; Chen, R.; Yan, J.; et al. Hydrogen Gas Inhalation Enhances Alveolar Macrophage Phagocytosis in an Ovalbumin-Induced Asthma Model. Int. Immunopharmacol. 2019, 74, 105646. [Google Scholar] [CrossRef]
- Wu, D.; Liang, M.; Dang, H.; Fang, F.; Xu, F.; Liu, C. Hydrogen Protects against Hyperoxia-Induced Apoptosis in Type II Alveolar Epithelial Cells via Activation of PI3K/Akt/Foxo3a Signaling Pathway. Biochem. Biophys. Res. Commun. 2018, 495, 1620–1627. [Google Scholar] [CrossRef] [PubMed]
- Russell, G.; Nenov, A.; Kisher, H.; Hancock, J.T. Molecular Hydrogen as Medicine: An Assessment of Administration Methods. Hydrogen 2021, 2, 444–460. [Google Scholar] [CrossRef]
- Korovljev, D.; Stajer, V.; Ostojic, J.; LeBaron, T.W.; Ostojic, S.M. Hydrogen-Rich Water Reduces Liver Fat Accumulation and Improves Liver Enzyme Profiles in Patients with Non-Alcoholic Fatty Liver Disease: A Randomized Controlled Pilot Trial. Clin. Res. Hepatol. Gastroenterol. 2019, 43, 688–693. [Google Scholar] [CrossRef]
- LeBaron, T.W.; Laher, I.; Kura, B.; Slezak, J. Hydrogen Gas: From Clinical Medicine to an Emerging Ergogenic Molecule for Sports Athletes. Can. J. Physiol. Pharmacol. 2019, 97, 797–807. [Google Scholar] [CrossRef]
- Lucas, K.; Rosch, M.; Langguth, P. Molecular Hydrogen (H2) as a Potential Treatment for Acute and Chronic Fatigue. Arch. Pharm. 2021, 354, 2000378. [Google Scholar] [CrossRef]
- Alharbi, A.A.D.; Ebine, N.; Nakae, S.; Hojo, T.; Fukuoka, Y. Application of Molecular Hydrogen as an Antioxidant in Responses to Ventilatory and Ergogenic Adjustments during Incremental Exercise in Humans. Nutrients 2021, 13, 459. [Google Scholar] [CrossRef]
- Asada, R.; Kageyama, K.; Tanaka, H.; Matsui, H.; Kimura, M.; Saitoh, Y.; Miwa, N. Antitumor Effects of Nano-Bubble Hydrogen-Dissolved Water Are Enhanced by Coexistent Platinum Colloid and the Combined Hyperthermia with Apoptosis-like Cell Death. Oncol. Rep. 2010, 24, 1463–1470. [Google Scholar] [CrossRef] [PubMed]
- Ito, M.; Hirayama, M.; Yamai, K.; Goto, S.; Ito, M.; Ichihara, M.; Ohno, K. Drinking Hydrogen Water and Intermittent Hydrogen Gas Exposure, but Not Lactulose or Continuous Hydrogen Gas Exposure, Prevent 6-Hydorxydopamine-Induced Parkinson’s Disease in Rats. Med. Gas Res. 2012, 2, 15. [Google Scholar] [CrossRef] [PubMed]
- Yang, N.; Gong, F.; Liu, B.; Hao, Y.; Chao, Y.; Lei, H.; Yang, X.; Gong, Y.; Wang, X.; Liu, Z.; et al. Magnesium Galvanic Cells Produce Hydrogen and Modulate the Tumor Microenvironment to Inhibit Cancer Growth. Nat. Commun. 2022, 13, 2336. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhao, P.; Yue, C.; Jin, Z.; Liu, Q.; Du, X.; He, Q. Sustained Release of Bioactive Hydrogen by Pd Hydride Nanoparticles Overcomes Alzheimer’s Disease. Biomaterials 2019, 197, 393–404. [Google Scholar] [CrossRef]
- Xu, F.; Yu, S.; Qin, M.; Mao, Y.; Jin, L.; Che, N.; Liu, S.; Ge, R. Hydrogen-Rich Saline Ameliorates Allergic Rhinitis by Reversing the Imbalance of Th1/Th2 and Up-Regulation of CD4+CD25+Foxp3+Regulatory T Cells, Interleukin-10, and Membrane-Bound Transforming Growth Factor-β in Guinea Pigs. Inflammation 2018, 41, 81–92. [Google Scholar] [CrossRef]
- Yu, S.; Zhao, C.; Che, N.; Jing, L.; Ge, R. Hydrogen-Rich Saline Attenuates Eosinophil Activation in a Guinea Pig Model of Allergic Rhinitis via Reducing Oxidative Stress. J. Inflamm. 2017, 14, 1. [Google Scholar] [CrossRef]
- Fang, S.; Li, X.; Wei, X.; Zhang, Y.; Ma, Z.; Wei, Y.; Wang, W. Beneficial Effects of Hydrogen Gas Inhalation on a Murine Model of Allergic Rhinitis. Exp. Ther. Med. 2018, 16, 5178–5184. [Google Scholar] [CrossRef]
- Zhao, C.; Yu, S.; Li, J.; Xu, W.; Ge, R. Changes in IL-4 and IL-13 Expression in Allergic-Rhinitis Treated with Hydrogen-Rich Saline in Guinea-Pig Model. Allergol. Immunopathol. 2017, 45, 350–355. [Google Scholar] [CrossRef]
- Jin, L.; Tan, S.; Fan, K.; Wang, Y.; Yu, S. Research Progress of Hydrogen on Chronic Nasal Inflammation. J. Inflamm. Res. 2023, 16, 2149–2157. [Google Scholar] [CrossRef]
- Jin, L.; Fan, K.; Tan, S.; Liu, S.; Ge, Q.; Wang, Y.; Ai, Z.; Yu, S. The Beneficial Effects of Hydrogen-Rich Saline Irrigation on Chronic Rhinitis: A Randomized, Double-Blind Clinical Trial. J. Inflamm. Res. 2022, 15, 3983–3995. [Google Scholar] [CrossRef]
- Jin, L.; Yu, S.Q.; Zhang, X.; Ge, Q.; Zhang, X.L.; Wang, Y.; Qin, M.L. Clinical study of hydrogen-rich saline in the treatment of moderate to severe allergic rhinitis. Lin Chuang Er Bi Yan Hou Tou Jing Wai Ke Za Zhi J. Clin. Otorhinolaryngol. Head Neck Surg. 2018, 32, 493–496. [Google Scholar] [CrossRef]
- Zhao, S.; Yang, Y.; Liu, W.; Xuan, Z.; Wu, S.; Yu, S.; Mei, K.; Huang, Y.; Zhang, P.; Cai, J.; et al. Protective Effect of Hydrogen-Rich Saline against Radiation-Induced Immune Dysfunction. J. Cell. Mol. Med. 2014, 18, 938–946. [Google Scholar] [CrossRef] [PubMed]
- Karlidaǧ, T.; Keles, E.; İlhan, N.; Yakclin, S.; Kaygusuz, İ.; Yildiz, M. Roles of Free Radicals, Nitric Oxide, and Scavenging Enzymes in Nasal Polyp Development. Ann. Otol. Rhinol. Laryngol. 2005, 114, 122–126. [Google Scholar] [CrossRef]
- Uneri, C.; Oztürk, O.; Polat, S.; Yüksel, M.; Haklar, G. Determination of Reactive Oxygen Species in Nasal Polyps. Rhinology 2005, 43, 185–189. [Google Scholar]
- Choi, J.; Suk An, E.; Ban, Y.-H.; Woom Seo, D.; Kim, T.-S.; Lee, S.-P.; Lin, Y.; Choi, E.-K.; Kim, Y.-B. Hydrogen-Enriched Water Eliminates Fine Particles from the Lungs and Blood by Enhancing Phagocytic Activity. J. Biomed. Res. 2017, 31, 503–511. [Google Scholar] [CrossRef]
- GINA 2022, Global Initiative for Asthma. Global Strategy for Asthma Management and Prevention. Available online: https://ginasthma.org/gina-reports/ (accessed on 20 November 2024).
- Zhang, N.; Deng, C.; Zhang, X.; Zhang, J.; Bai, C. Inhalation of Hydrogen Gas Attenuates Airway Inflammation and Oxidative Stress in Allergic Asthmatic Mice. Asthma Res. Pract. 2018, 4, 3. [Google Scholar] [CrossRef] [PubMed]
- Xiao, M.; Zhu, T.; Wang, T.; Wen, F.-Q. Hydrogen-Rich Saline Reduces Airway Remodeling via Inactivation of NF-κB in a Murine Model of Asthma. Eur. Rev. Med. Pharmacol. Sci. 2013, 17, 1033–1043. [Google Scholar]
- Zhang, J.; Feng, X.; Fan, Y.; Zhu, G.; Bai, C. Molecular Hydrogen Alleviates Asthma through Inhibiting IL-33/ILC2 Axis. Inflamm. Res. 2021, 70, 569–579. [Google Scholar] [CrossRef]
- Cho, H.-Y.; Park, S.; Miller, L.; Lee, H.-C.; Langenbach, R.; Kleeberger, S.R. Role for Mucin-5AC in Upper and Lower Airway Pathogenesis in Mice. Toxicol. Pathol. 2021, 49, 1077–1099. [Google Scholar] [CrossRef]
- Kuo, W.; Odenwald, M.A.; Turner, J.R.; Zuo, L. Tight Junction Proteins Occludin and ZO-1 as Regulators of Epithelial Proliferation and Survival. Ann. N. Y. Acad. Sci. 2022, 1514, 21–33. [Google Scholar] [CrossRef]
- Niu, Y.; Nie, Q.; Dong, L.; Zhang, J.; Liu, S.F.; Song, W.; Wang, X.; Wu, G.; Song, D. Hydrogen Attenuates Allergic Inflammation by Reversing Energy Metabolic Pathway Switch. Sci. Rep. 2020, 10, 1962. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.B.; Tarnava, A.; Fatima, G.; Fedacko, J.; Mojto, V.; LeBaron, T.W. Can Hydrogen Water Enhance Oxygen Saturation in Patients with Chronic Lung Disease? A Non-Randomized, Observational Pilot Study. Diseases 2023, 11, 127. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.-T.; Bao, C.; He, Y.; Tian, X.; Yang, Y.; Zhang, T.; Xu, K.-F. Hydrogen Gas (XEN) Inhalation Ameliorates Airway Inflammation in Asthma and COPD Patients. QJM Int. J. Med. 2020, 113, 870–875. [Google Scholar] [CrossRef]
- Feng, S.; Duan, E.; Shi, X.; Zhang, H.; Li, H.; Zhao, Y.; Chao, L.; Zhong, X.; Zhang, W.; Li, R.; et al. Hydrogen Ameliorates Lung Injury in a Rat Model of Subacute Exposure to Concentrated Ambient PM2.5 via Aryl Hydrocarbon Receptor. Int. Immunopharmacol. 2019, 77, 105939. [Google Scholar] [CrossRef]
- 2025 GOLD Report. Available online: https://goldcopd.org/2025-gold-report/ (accessed on 11 February 2025).
- Barnes, P.J. Inflammatory Mechanisms in Patients with Chronic Obstructive Pulmonary Disease. J. Allergy Clin. Immunol. 2016, 138, 16–27. [Google Scholar] [CrossRef]
- Celli, B.; Fabbri, L.; Criner, G.; Martinez, F.J.; Mannino, D.; Vogelmeier, C.; Montes De Oca, M.; Papi, A.; Sin, D.D.; Han, M.K.; et al. Definition and Nomenclature of Chronic Obstructive Pulmonary Disease: Time for Its Revision. Am. J. Respir. Crit. Care Med. 2022, 206, 1317–1325. [Google Scholar] [CrossRef]
- Domej, W.; Oettl, K.; Renner, W. Oxidative Stress and Free Radicals in COPD--Implications and Relevance for Treatment. Int. J. Chron. Obstruct. Pulmon. Dis. 2014, 9, 1207–1224. [Google Scholar] [CrossRef]
- Liu, X.; Ma, C.; Wang, X.; Wang, W.; Li, Z.; Wang, X.; Wang, P.; Sun, W.; Xue, B. Hydrogen Coadministration Slows the Development of COPD-like Lung Disease in a Cigarette Smoke-Induced Rat Model. Int. J. Chron. Obstruct. Pulmon. Dis. 2017, 12, 1309–1324. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Li, D.; Hu, J.; Mei, H.; Shu, J.; Long, Z.; Yuan, L.; Li, D.; Guan, R.; Li, Y.; et al. Hydrogen Gas Inhalation Protects against Cigarette Smoke-Induced COPD Development in Mice. J. Thorac. Dis. 2018, 10, 3232–3243. [Google Scholar] [CrossRef]
- Suzuki, Y.; Sato, T.; Sugimoto, M.; Baskoro, H.; Karasutani, K.; Mitsui, A.; Nurwidya, F.; Arano, N.; Kodama, Y.; Hirano, S.; et al. Hydrogen-Rich Pure Water Prevents Cigarette Smoke-Induced Pulmonary Emphysema in SMP30 Knockout Mice. Biochem. Biophys. Res. Commun. 2017, 492, 74–81. [Google Scholar] [CrossRef]
- Ning, Y.; Shang, Y.; Huang, H.; Zhang, J.; Dong, Y.; Xu, W.; Li, Q. Attenuation of Cigarette Smoke-Induced Airway Mucus Production by Hydrogen-Rich Saline in Rats. PLoS ONE 2013, 8, e83429. [Google Scholar] [CrossRef] [PubMed]
- Grünwald, B.; Schoeps, B.; Krüger, A. Recognizing the Molecular Multifunctionality and Interactome of TIMP-1. Trends Cell Biol. 2019, 29, 6–19. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.-G.; Sun, W.-Z.; Hu, J.-Y.; Jie, Z.-J.; Xu, J.-F.; Cao, J.; Song, Y.-L.; Wang, C.-H.; Wang, J.; Zhao, H.; et al. Hydrogen/Oxygen Therapy for the Treatment of an Acute Exacerbation of Chronic Obstructive Pulmonary Disease: Results of a Multicenter, Randomized, Double-Blind, Parallel-Group Controlled Trial. Respir. Res. 2021, 22, 149. [Google Scholar] [CrossRef]
- Aokage, T.; Seya, M.; Hirayama, T.; Nojima, T.; Iketani, M.; Ishikawa, M.; Terasaki, Y.; Taniguchi, A.; Miyahara, N.; Nakao, A.; et al. The Effects of Inhaling Hydrogen Gas on Macrophage Polarization, Fibrosis, and Lung Function in Mice with Bleomycin-Induced Lung Injury. BMC Pulm. Med. 2021, 21, 339. [Google Scholar] [CrossRef] [PubMed]
- Dong, W.-W.; Zhang, Y.-Q.; Zhu, X.-Y.; Mao, Y.-F.; Sun, X.-J.; Liu, Y.-J.; Jiang, L. Protective Effects of Hydrogen-Rich Saline Against Lipopolysaccharide-Induced Alveolar Epithelial-to-Mesenchymal Transition and Pulmonary Fibrosis. Med. Sci. Monit. 2017, 23, 2357–2364. [Google Scholar] [CrossRef]
- Gao, L.; Jiang, D.; Geng, J.; Dong, R.; Dai, H. Hydrogen Inhalation Attenuated Bleomycin-induced Pulmonary Fibrosis by Inhibiting Transforming Growth Factor-β1 and Relevant Oxidative Stress and Epithelial-to-mesenchymal Transition. Exp. Physiol. 2019, 104, 1942–1951. [Google Scholar] [CrossRef]
- Liu, S.; Liu, K.; Sun, Q.; Liu, W.; Xu, W.; Denoble, P.; Tao, H.; Sun, X. Consumption of Hydrogen Water Reduces Paraquat-Induced Acute Lung Injury in Rats. BioMed Res. Int. 2011, 2011, 305086. [Google Scholar] [CrossRef]
- Zhou, Z.-Q.; Zhong, C.-H.; Su, Z.-Q.; Li, X.-Y.; Chen, Y.; Chen, X.-B.; Tang, C.-L.; Zhou, L.-Q.; Li, S.-Y. Breathing Hydrogen-Oxygen Mixture Decreases Inspiratory Effort in Patients with Tracheal Stenosis. Respiration 2019, 97, 42–51. [Google Scholar] [CrossRef]
- Ozeki, N.; Yamawaki-Ogata, A.; Narita, Y.; Mii, S.; Ushida, K.; Ito, M.; Hirano, S.; Kurokawa, R.; Ohno, K.; Usui, A. Hydrogen Water Alleviates Obliterative Airway Disease in Mice. Gen. Thorac. Cardiovasc. Surg. 2020, 68, 158–163. [Google Scholar] [CrossRef]
- Muramatsu, Y.; Ito, M.; Oshima, T.; Kojima, S.; Ohno, K. Hydrogen-Rich Water Ameliorates Bronchopulmonary Dysplasia (BPD) in Newborn Rats. Pediatr. Pulmonol. 2016, 51, 928–935. [Google Scholar] [CrossRef]
- Dao, D.T.; Nandivada, P.; Vuong, J.T.; Anez-Bustillos, L.; Pan, A.; Kishikawa, H.; Mitchell, P.D.; Baker, M.A.; Fell, G.L.; Martin, T.; et al. Vascular Endothelial Growth Factor Accelerates Compensatory Lung Growth by Increasing the Alveolar Units. Pediatr. Res. 2018, 83, 1182–1189. [Google Scholar] [CrossRef] [PubMed]
- Hattori, Y.; Kotani, T.; Tsuda, H.; Mano, Y.; Tu, L.; Li, H.; Hirako, S.; Ushida, T.; Imai, K.; Nakano, T.; et al. Maternal Molecular Hydrogen Treatment Attenuates Lipopolysaccharide-Induced Rat Fetal Lung Injury. Free Radic. Res. 2015, 49, 1026–1037. [Google Scholar] [CrossRef] [PubMed]
- Kishimoto, Y.; Kato, T.; Ito, M.; Azuma, Y.; Fukasawa, Y.; Ohno, K.; Kojima, S. Hydrogen Ameliorates Pulmonary Hypertension in Rats by Anti-Inflammatory and Antioxidant Effects. J. Thorac. Cardiovasc. Surg. 2015, 150, 645–654.e3. [Google Scholar] [CrossRef] [PubMed]
- Zhai, Y.; Zhou, X.; Dai, Q.; Fan, Y.; Huang, X. Hydrogen-Rich Saline Ameliorates Lung Injury Associated with Cecal Ligation and Puncture-Induced Sepsis in Rats. Exp. Mol. Pathol. 2015, 98, 268–276. [Google Scholar] [CrossRef]
- Ren, J.-D.; Wu, X.-B.; Jiang, R.; Hao, D.-P.; Liu, Y. Molecular Hydrogen Inhibits Lipopolysaccharide-Triggered NLRP3 Inflammasome Activation in Macrophages by Targeting the Mitochondrial Reactive Oxygen Species. Biochim. Biophys. Acta BBA-Mol. Cell Res. 2016, 1863, 50–55. [Google Scholar] [CrossRef]
- Chen, H.; Bai, C.; Wang, X. The Value of the Lipopolysaccharide-Induced Acute Lung Injury Model in Respiratory Medicine. Expert Rev. Respir. Med. 2010, 4, 773–783. [Google Scholar] [CrossRef]
- Tao, B.; Liu, L.; Wang, N.; Wang, W.; Jiang, J.; Zhang, J. Effects of Hydrogen-Rich Saline on Aquaporin 1, 5 in Septic Rat Lungs. J. Surg. Res. 2016, 202, 291–298. [Google Scholar] [CrossRef]
- Liu, H.; Liang, X.; Wang, D.; Zhang, H.; Liu, L.; Chen, H.; Li, Y.; Duan, Q.; Xie, K. Combination Therapy with Nitric Oxide and Molecular Hydrogen in a Murine Model of Acute Lung Injury. Shock 2015, 43, 504. [Google Scholar] [CrossRef]
- Liu, L.-D.; Wu, X.-Y.; Tao, B.-D.; Wang, N.; Zhang, J. Protective Effect and Mechanism of Hydrogen Treatment on Lung Epithelial Barrier Dysfunction in Rats with Sepsis. Genet. Mol. Res. 2016, 15, gmr.15016050. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, J.; Bo, J.; Wang, X.; Zhu, J. Hydrogen-Rich Saline Ameliorated LPS-Induced Acute Lung Injury via Autophagy Inhibition through the ROS/AMPK/mTOR Pathway in Mice. Exp. Biol. Med. 2019, 244, 721–727. [Google Scholar] [CrossRef]
- Saramago, E.A.; Borges, G.S.; Singolani, C.G., Jr.; Nogueira, J.E.; Soriano, R.N.; Cárnio, E.C.; Branco, L.G.S. Molecular Hydrogen Potentiates Hypothermia and Prevents Hypotension and Fever in LPS-Induced Systemic Inflammation. Brain. Behav. Immun. 2019, 75, 119–128. [Google Scholar] [CrossRef]
- Mirzayans, R.; Murray, D. Do TUNEL and Other Apoptosis Assays Detect Cell Death in Preclinical Studies? Int. J. Mol. Sci. 2020, 21, 9090. [Google Scholar] [CrossRef]
- Kawamura, T.; Huang, C.-S.; Peng, X.; Masutani, K.; Shigemura, N.; Billiar, T.R.; Okumura, M.; Toyoda, Y.; Nakao, A. The Effect of Donor Treatment with Hydrogen on Lung Allograft Function in Rats. Surgery 2011, 150, 240–249. [Google Scholar] [CrossRef] [PubMed]
- Kawamura, T.; Huang, C.-S.; Tochigi, N.; Lee, S.; Shigemura, N.; Billiar, T.R.; Okumura, M.; Nakao, A.; Toyoda, Y. Inhaled Hydrogen Gas Therapy for Prevention of Lung Transplant-Induced Ischemia/Reperfusion Injury in Rats. Transplantation 2010, 90, 1344–1351. [Google Scholar] [CrossRef] [PubMed]
- Haam, S.; Lee, J.G.; Paik, H.C.; Park, M.S.; Lim, B.J. Hydrogen Gas Inhalation during Ex Vivo Lung Perfusion of Donor Lungs Recovered after Cardiac Death. J. Heart Lung Transpl. 2018, 37, 1271–1278. [Google Scholar] [CrossRef]
- Amarelle, L.; Quintela, L.; Hurtado, J.; Malacrida, L. Hyperoxia and Lungs: What We Have Learned From Animal Models. Front. Med. 2021, 8, 606678. [Google Scholar] [CrossRef] [PubMed]
- Kallet, R.H.; Matthay, M.A. Hyperoxic Acute Lung Injury. Respir. Care 2013, 58, 123–141. [Google Scholar] [CrossRef]
- Huang, C.-S.; Kawamura, T.; Peng, X.; Tochigi, N.; Shigemura, N.; Billiar, T.R.; Nakao, A.; Toyoda, Y. Hydrogen Inhalation Reduced Epithelial Apoptosis in Ventilator-Induced Lung Injury via a Mechanism Involving Nuclear Factor-Kappa B Activation. Biochem. Biophys. Res. Commun. 2011, 408, 253–258. [Google Scholar] [CrossRef]
- Huang, C.-S.; Kawamura, T.; Lee, S.; Tochigi, N.; Shigemura, N.; Buchholz, B.M.; Kloke, J.D.; Billiar, T.R.; Toyoda, Y.; Nakao, A. Hydrogen Inhalation Ameliorates Ventilator-Induced Lung Injury. Crit. Care 2010, 14, R234. [Google Scholar] [CrossRef]
- Ibsen, L.M.; Koch, T. Submersion and Asphyxial Injury. Crit. Care Med. 2002, 30, S402–S408. [Google Scholar] [CrossRef]
- Chen, X.; Liu, Q.; Wang, D.; Feng, S.; Zhao, Y.; Shi, Y.; Liu, Q. Protective Effects of Hydrogen-Rich Saline on Rats with Smoke Inhalation Injury. Oxid. Med. Cell. Longev. 2015, 2015, 106836. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Zhou, X.; Qiu, Y.; Song, Y.; Feng, F.; Feng, J.; Song, Q.; Jia, Q.; Wang, J. Clinical Characteristics of 82 Cases of Death from COVID-19. PLoS ONE 2020, 15, e0235458. [Google Scholar] [CrossRef]
- Tay, M.Z.; Poh, C.M.; Rénia, L.; MacAry, P.A.; Ng, L.F.P. The Trinity of COVID-19: Immunity, Inflammation and Intervention. Nat. Rev. Immunol. 2020, 20, 363–374. [Google Scholar] [CrossRef]
- Erlich, J.R.; To, E.E.; Liong, S.; Brooks, R.; Vlahos, R.; O’Leary, J.J.; Brooks, D.A.; Selemidis, S. Targeting Evolutionary Conserved Oxidative Stress and Immunometabolic Pathways for the Treatment of Respiratory Infectious Diseases. Antioxid. Redox Signal. 2020, 32, 993–1013. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Liu, L.; Zhang, D.; Xu, J.; Dai, H.; Tang, N.; Su, X.; Cao, B. SARS-CoV-2 and Viral Sepsis: Observations and Hypotheses. Lancet 2020, 395, 1517–1520. [Google Scholar] [CrossRef]
- Zeng, Y.; Guan, W.; Wang, K.; Jie, Z.; Zou, X.; Tan, X.; Li, X.; Chen, X.; Ren, X.; Jiang, J.; et al. Effect of Hydrogen/Oxygen Therapy for Ordinary COVID-19 Patients: A Propensity-Score Matched Case-Control Study. BMC Infect. Dis. 2023, 23, 440. [Google Scholar] [CrossRef]
- Luo, P.; Ding, Y.; He, Y.; Chen, D.; He, Q.; Huang, Z.; Huang, S.; Lei, W. Hydrogen-Oxygen Therapy Alleviates Clinical Symptoms in Twelve Patients Hospitalized with COVID-19: A Retrospective Study of Medical Records. Medicine 2022, 101, e27759. [Google Scholar] [CrossRef]
- Yu, Y.; Yang, Y.; Yang, M.; Wang, C.; Xie, K.; Yu, Y. Hydrogen Gas Reduces HMGB1 Release in Lung Tissues of Septic Mice in an Nrf2/HO-1-Dependent Pathway. Int. Immunopharmacol. 2019, 69, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.-M.; Chen, Y.-T.; Wang, X.-D.; Zhang, Y.-F.; Cheng, T.; Chen, H.; Sun, F.; Bao, H.; Chen, R.; Xiong, W.-N.; et al. The Efficacy of Hydrogen/Oxygen Therapy Favored the Recovery of Omicron SARS-CoV-2 Variant Infection: Results of a Multicenter, Randomized, Controlled Trial. J. Clin. Biochem. Nutr. 2023, 73, 228–233. [Google Scholar] [CrossRef]
- Botek, M.; Krejčí, J.; Valenta, M.; McKune, A.; Sládečková, B.; Konečný, P.; Klimešová, I.; Pastucha, D. Molecular Hydrogen Positively Affects Physical and Respiratory Function in Acute Post-COVID-19 Patients: A New Perspective in Rehabilitation. Int. J. Environ. Res. Public. Health 2022, 19, 1992. [Google Scholar] [CrossRef]
- Pozdnyakova, D.D.; Bakhareva, T.A.; Baranova, I.A.; Selemir, V.D.; Chuchalin, A.G. Rehabilitation Program of Post-COVID-19 Syndrome with the Use of Nitric Oxide and Molecular Hydrogen. Ter. Arkh. 2024, 96, 260–265. [Google Scholar] [CrossRef] [PubMed]
- Saitoh, Y.; Okayasu, H.; Xiao, L.; Harata, Y.; Miwa, N. Neutral pH Hydrogen-Enriched Electrolyzed Water Achieves Tumor-Preferential Clonal Growth Inhibition Over Normal Cells and Tumor Invasion Inhibition Concurrently with Intracellular Oxidant Repression. Oncol. Res. Featur. Preclin. Clin. Cancer Ther. 2008, 17, 247–255. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Wang, L.; Zhang, Y.; Zhao, Y.; Chen, G. Hydrogen Gas Inhibits Lung Cancer Progression through Targeting SMC3. Biomed. Pharmacother. 2018, 104, 788–797. [Google Scholar] [CrossRef]
- Akagi, J.; Baba, H. Hydrogen Gas Restores Exhausted CD8+ T Cells in Patients with Advanced Colorectal Cancer to Improve Prognosis. Oncol. Rep. 2019, 41, 301–311. [Google Scholar] [CrossRef] [PubMed]
- Saitoh, Y.; Yoshimura, Y.; Nakano, K.; Miwa, N. Platinum Nanocolloid-Supplemented Hydrogendissolved Water Inhibits Growth of Human Tongue Carcinoma Cells Preferentially over Normal Cells. Exp. Oncol. 2009, 31, 156–162. [Google Scholar]
- Chen, J.-B.; Kong, X.-F.; Lv, Y.-Y.; Qin, S.-C.; Sun, X.-J.; Mu, F.; Lu, T.-Y.; Xu, K.-C. “Real World Survey” of Hydrogen-Controlled Cancer: A Follow-up Report of 82 Advanced Cancer Patients. Med. Gas Res. 2019, 9, 115–121. [Google Scholar] [CrossRef]
- Akagi, J.; Baba, H. Hydrogen Gas Activates Coenzyme Q10 to Restore Exhausted CD8+ T Cells, Especially PD-1+Tim3+terminal CD8+ T Cells, Leading to Better Nivolumab Outcomes in Patients with Lung Cancer. Oncol. Lett. 2020, 20, 258. [Google Scholar] [CrossRef]
- Kong, X.; Lu, T.; Lu, Y.-Y.; Yin, Z.; Xu, K. Effect of Hydrogen Inhalation Therapy on Hearing Loss of Patients with Nasopharyngeal Carcinoma After Radiotherapy. Front. Med. 2022, 9, 828370. [Google Scholar] [CrossRef]
- Nakashima-Kamimura, N.; Mori, T.; Ohsawa, I.; Asoh, S.; Ohta, S. Molecular Hydrogen Alleviates Nephrotoxicity Induced by an Anti-Cancer Drug Cisplatin without Compromising Anti-Tumor Activity in Mice. Cancer Chemother. Pharmacol. 2009, 64, 753–761. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, P.Y.; Bao, W.; Chen, S.J.; Wu, F.S.; Zhu, P.Y. Hydrogen Inhibits Endometrial Cancer Growth via a ROS/NLRP3/Caspase-1/GSDMD-Mediated Pyroptotic Pathway. BMC Cancer 2020, 20, 28. [Google Scholar] [CrossRef]
- Meng, J.; Liu, L.; Wang, D.; Yan, Z.; Chen, G. Hydrogen Gas Represses the Progression of Lung Cancer via Down-Regulating CD47. Biosci. Rep. 2020, 40, BSR20192761. [Google Scholar] [CrossRef]
- Lin, Y.; Ohkawara, B.; Ito, M.; Misawa, N.; Miyamoto, K.; Takegami, Y.; Masuda, A.; Toyokuni, S.; Ohno, K. Molecular Hydrogen Suppresses Activated Wnt/β-Catenin Signaling. Sci. Rep. 2016, 6, 31986. [Google Scholar] [CrossRef]
- Mishra, K.; Alsbeih, G. Appraisal of Biochemical Classes of Radioprotectors: Evidence, Current Status and Guidelines for Future Development. 3 Biotech 2017, 7, 292. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.; Kinoshita, M.; Yamamoto, T.; Ito, M.; Nishida, T.; Takeuchi, M.; Saitoh, D.; Seki, S.; Mukai, Y. Treatment of Irradiated Mice with High-Dose Ascorbic Acid Reduced Lethality. PLoS ONE 2015, 10, e0117020. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zong, C.; Jia, J.; Liu, Y.; Zhang, Z.; Cai, B.; Tian, L. A Study on the Protective Effect of Molecular Hydrogen on Osteoradionecrosis of the Jaw in Rats. Int. J. Oral Maxillofac. Surg. 2020, 49, 1648–1654. [Google Scholar] [CrossRef] [PubMed]
- Mei, K.; Zhao, S.; Qian, L.; Li, B.; Ni, J.; Cai, J. Hydrogen Protects Rats from Dermatitis Caused by Local Radiation. J. Dermatol. Treat. 2014, 25, 182–188. [Google Scholar] [CrossRef]
- Qian, L.; Li, B.; Cao, F.; Huang, Y.; Liu, S.; Cai, J.; Gao, F. Hydrogen-Rich PBS Protects Cultured Human Cells from Ionizing Radiation-Induced Cellular Damage. Nucl. Technol. Radiat. Prot. 2010, 25, 23–29. [Google Scholar] [CrossRef]
- Terasaki, Y.; Ohsawa, I.; Terasaki, M.; Takahashi, M.; Kunugi, S.; Dedong, K.; Urushiyama, H.; Amenomori, S.; Kaneko-Togashi, M.; Kuwahara, N.; et al. Hydrogen Therapy Attenuates Irradiation-Induced Lung Damage by Reducing Oxidative Stress. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2011, 301, L415–L426. [Google Scholar] [CrossRef]
- Yang, Y.; Li, B.; Liu, C.; Chuai, Y.; Lei, J.; Gao, F.; Cui, J.; Sun, D.; Cheng, Y.; Zhou, C.; et al. Hydrogen-Rich Saline Protects Immunocytes from Radiation-Induced Apoptosis. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2012, 18, BR144–BR148. [Google Scholar] [CrossRef]
- Qian, L.; Cao, F.; Cui, J.; Wang, Y.; Huang, Y.; Chuai, Y.; Zaho, L.; Jiang, H.; Cai, J. The Potential Cardioprotective Effects of Hydrogen in Irradiated Mice. J. Radiat. Res. 2010, 51, 741–747. [Google Scholar] [CrossRef]
- Qian, L.; Cao, F.; Cui, J.; Huang, Y.; Zhou, X.; Liu, S.; Cai, J. Radioprotective Effect of Hydrogen in Cultured Cells and Mice. Free Radic. Res. 2010, 44, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Qiu, X.; Dong, K.; Guan, J.; He, J. Hydrogen Attenuates Radiation-Induced Intestinal Damage by Reducing Oxidative Stress and Inflammatory Response. Int. Immunopharmacol. 2020, 84, 106517. [Google Scholar] [CrossRef] [PubMed]
- Xiao, H.-W.; Li, Y.; Luo, D.; Dong, J.-L.; Zhou, L.-X.; Zhao, S.-Y.; Zheng, Q.-S.; Wang, H.-C.; Cui, M.; Fan, S.-J. Hydrogen-Water Ameliorates Radiation-Induced Gastrointestinal Toxicity via MyD88’s Effects on the Gut Microbiota. Exp. Mol. Med. 2018, 50, e433. [Google Scholar] [CrossRef]
- Zhou, P.; Lin, B.; Wang, P.; Pan, T.; Wang, S.; Chen, W.; Cheng, S.; Liu, S. The Healing Effect of Hydrogen-Rich Water on Acute Radiation-Induced Skin Injury in Rats. J. Radiat. Res. 2019, 60, 17–22. [Google Scholar] [CrossRef]
- Hirano, S.; Aoki, Y.; Li, X.-K.; Ichimaru, N.; Takahara, S.; Takefuji, Y. Protective Effects of Hydrogen Gas Inhalation on Radiation-Induced Bone Marrow Damage in Cancer Patients: A Retrospective Observational Study. Med. Gas Res. 2021, 11, 104–109. [Google Scholar] [CrossRef]
- Kang, K.-M.; Kang, Y.-N.; Choi, I.-B.; Gu, Y.; Kawamura, T.; Toyoda, Y.; Nakao, A. Effects of Drinking Hydrogen-Rich Water on the Quality of Life of Patients Treated with Radiotherapy for Liver Tumors. Med. Gas Res. 2011, 1, 11. [Google Scholar] [CrossRef]
- Alwazeer, D.; Tan, K.; Örs, B. Reducing Atmosphere Packaging as a Novel Alternative Technique for Extending Shelf Life of Fresh Cheese. J. Food Sci. Technol. 2020, 57, 3013–3023. [Google Scholar] [CrossRef] [PubMed]
- Zajac, D. Inhalations with Thermal Waters in Respiratory Diseases. J. Ethnopharmacol. 2021, 281, 114505. [Google Scholar] [CrossRef]
- Köster, S.; Upadhyay, S.; Chandra, P.; Papavinasasundaram, K.; Yang, G.; Hassan, A.; Grigsby, S.J.; Mittal, E.; Park, H.S.; Jones, V.; et al. Mycobacterium tuberculosis Is Protected from NADPH Oxidase and LC3-Associated Phagocytosis by the LCP Protein CpsA. Proc. Natl. Acad. Sci. USA 2017, 114, E8711–E8720. [Google Scholar] [CrossRef]
- Maier, R.J.; Olczak, A.; Maier, S.; Soni, S.; Gunn, J. Respiratory Hydrogen Use by Salmonella enterica Serovar Typhimurium Is Essential for Virulence. Infect. Immun. 2004, 72, 6294–6299. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zajac, D.; Jampolska, M.; Wojciechowski, P. Molecular Hydrogen in the Treatment of Respiratory Diseases. Int. J. Mol. Sci. 2025, 26, 4116. https://doi.org/10.3390/ijms26094116
Zajac D, Jampolska M, Wojciechowski P. Molecular Hydrogen in the Treatment of Respiratory Diseases. International Journal of Molecular Sciences. 2025; 26(9):4116. https://doi.org/10.3390/ijms26094116
Chicago/Turabian StyleZajac, Dominika, Monika Jampolska, and Piotr Wojciechowski. 2025. "Molecular Hydrogen in the Treatment of Respiratory Diseases" International Journal of Molecular Sciences 26, no. 9: 4116. https://doi.org/10.3390/ijms26094116
APA StyleZajac, D., Jampolska, M., & Wojciechowski, P. (2025). Molecular Hydrogen in the Treatment of Respiratory Diseases. International Journal of Molecular Sciences, 26(9), 4116. https://doi.org/10.3390/ijms26094116







