Intrinsic Antibacterial Urushiol-Based Benzoxazine Polymer Coating for Marine Antifouling Applications
Abstract
1. Introduction
2. Results and Discussion
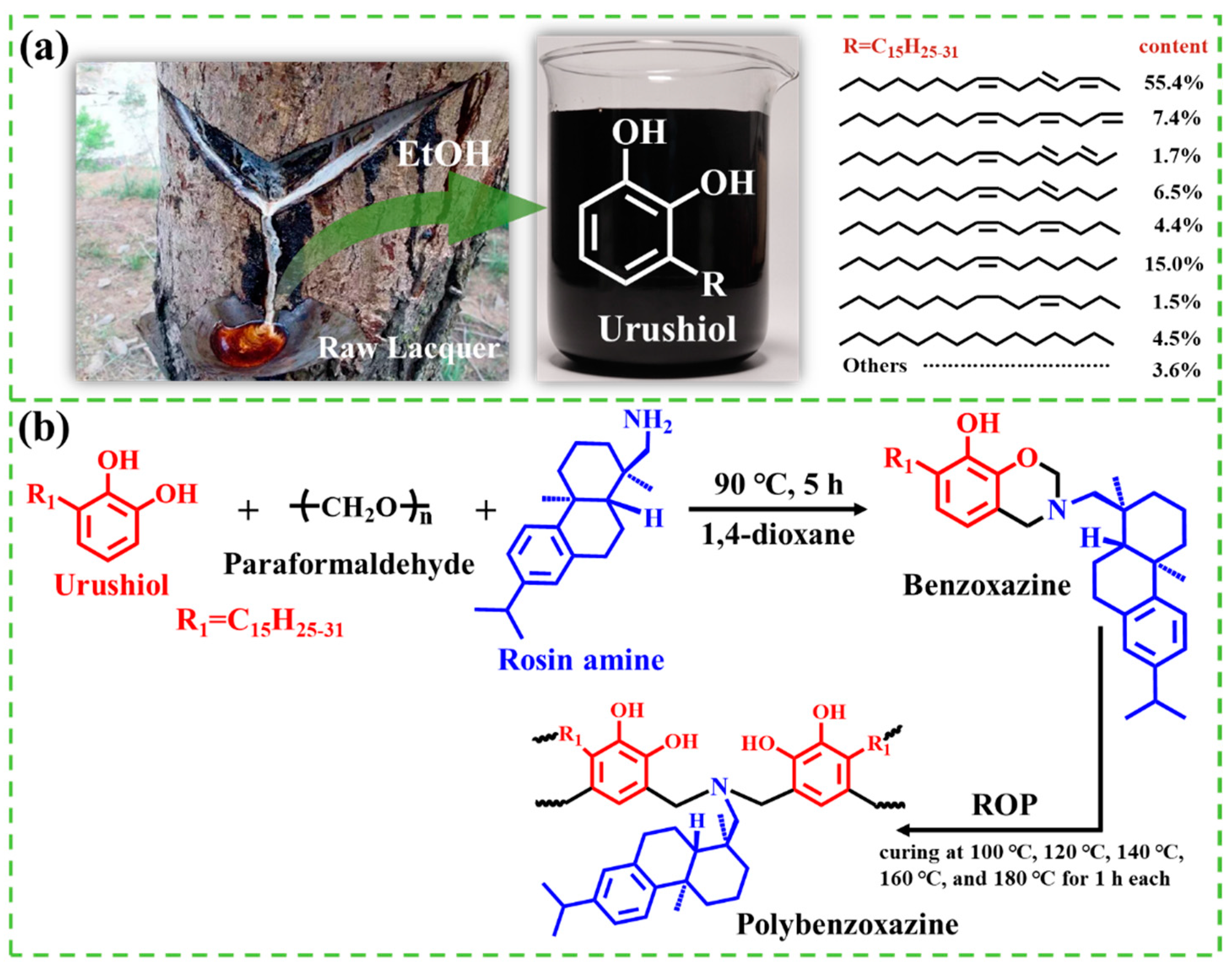
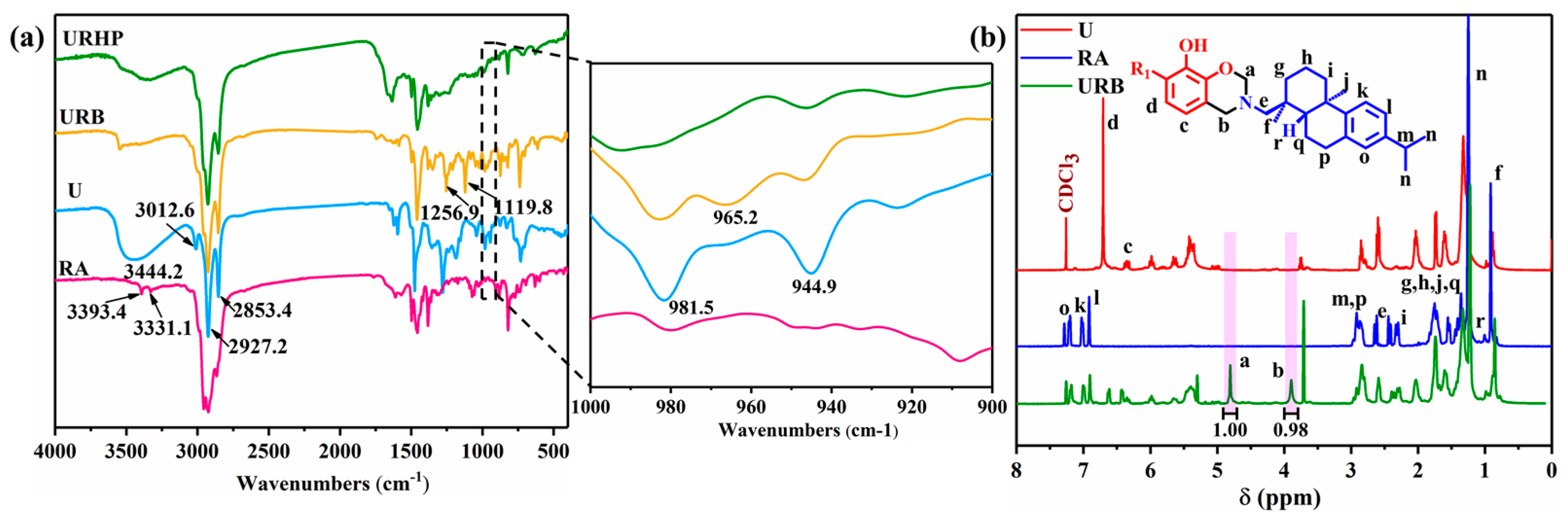
2.1. Synthesis and Characterization of URB Monomer
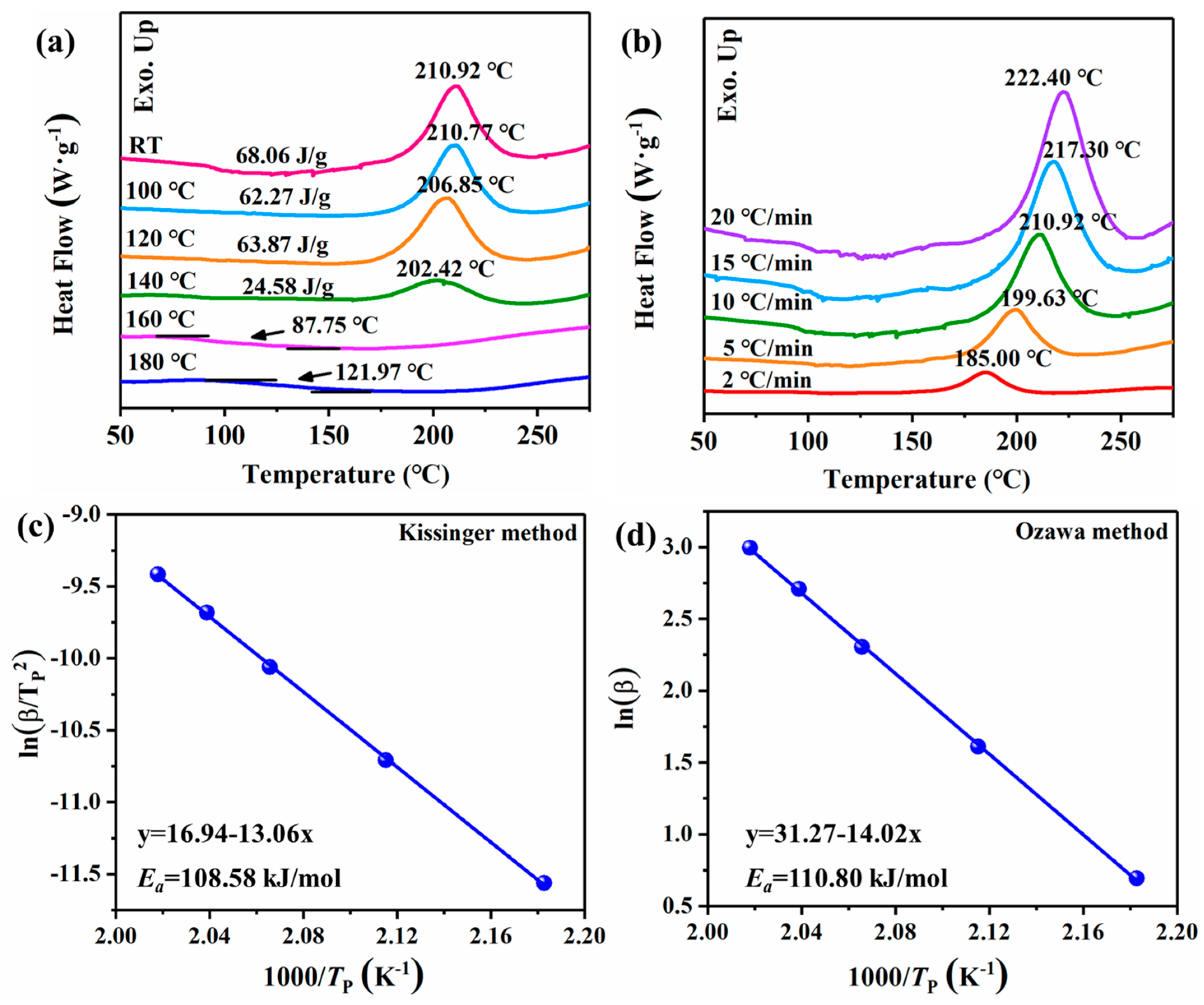
2.2. Curing Process of URB Monomers
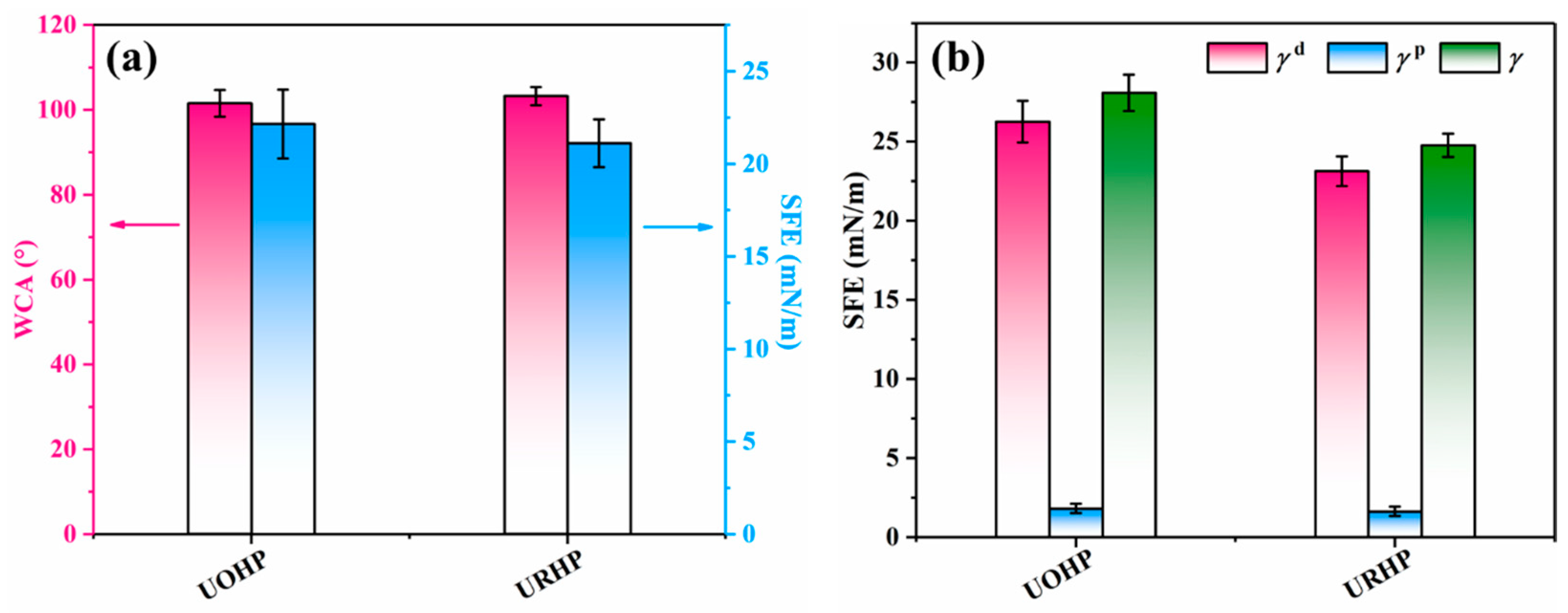
2.3. Surface Analysis of URHP Coatings
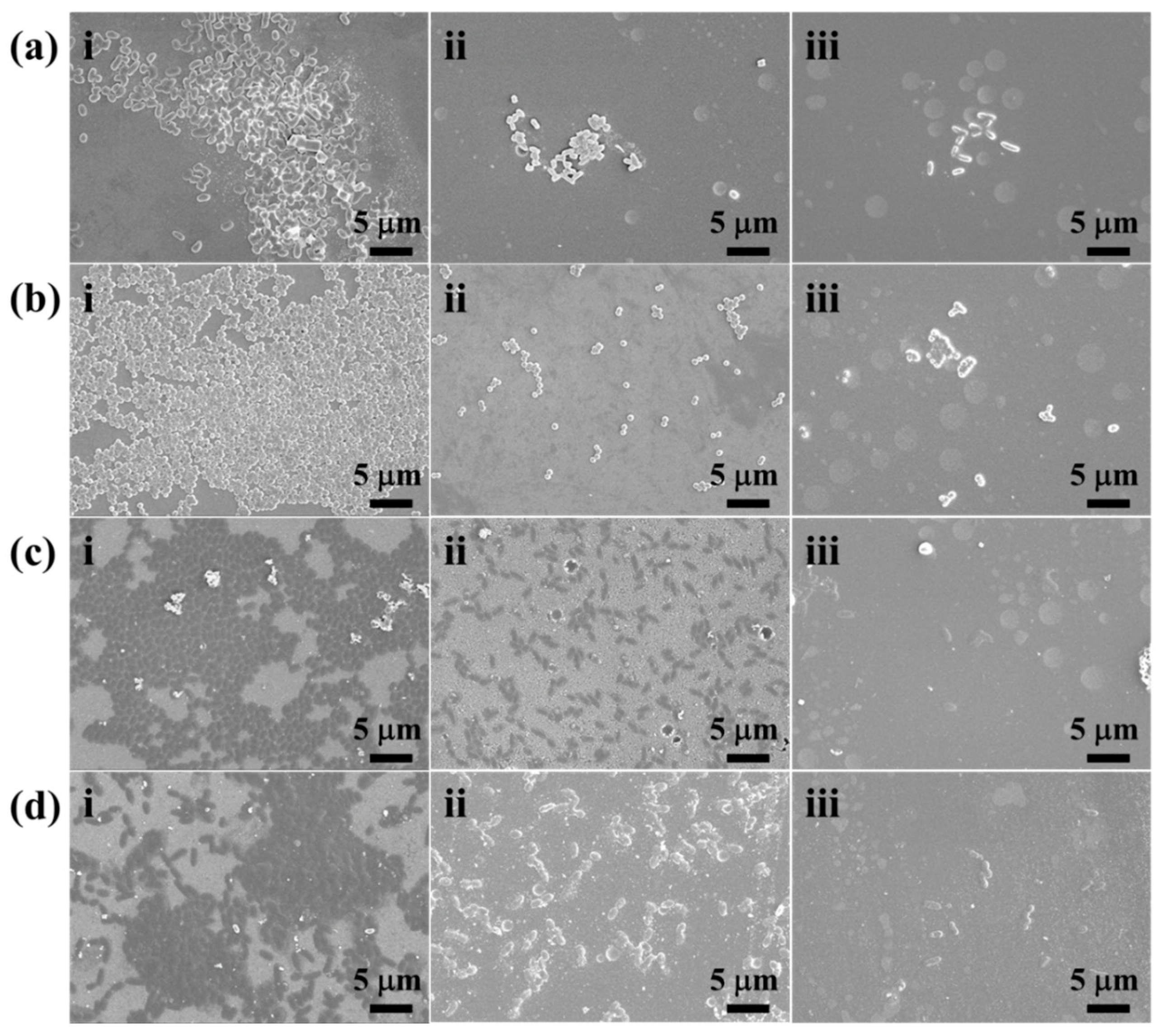
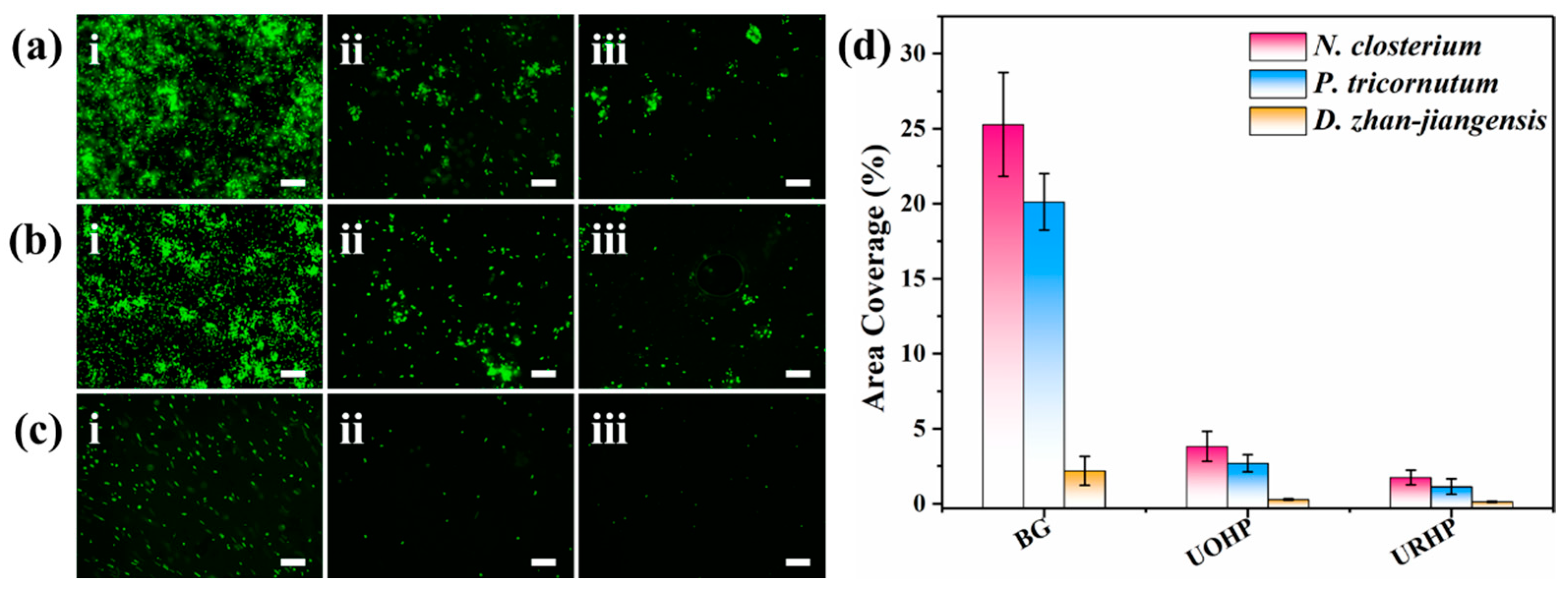
2.4. Fouling-Resistant Assays of URHP Coating
3. Materials and Methods
3.1. Materials
3.2. Synthesis of Urushiol–Rosin Amine–Benzoxazine Monomer (URB)
3.3. Urushiol–Rosin Amine–Benzoxazine Polymer (URHP) Coatings
3.4. Characterizations
3.5. Antibacterial Assessments
3.6. Algal Biofouling Assessments
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| URB | Urushiol-based benzoxazine |
| URHP | Urushiol-based benzoxazine polymer |
| U | Urushiol |
| RA | Rosin amine |
| DI | Deionized |
| ASW | Artificial seawater |
| E. coli | Escherichia coli |
| S. aureus | Staphylococcus aureus |
| V. alginolyticus | Vibrio alginolyticus |
| N. closterium | Nitzschiaclosterium |
| P. tricornutum | Phaeodactylumtricornutum |
| D. zhan-jiangensis | Dicrateria zhanjiangensis |
| LB | Luria-Bertani |
| PBS | Phosphate-buffered saline |
| A.R. | Antibacterial rate |
| BG | Bare glass slide |
| ROP | Ring-opening polymerization |
| PDMS | Polydimethylsiloxane |
| UOHP | Urushiol-based benzoxazine polymer |
| ATR-FTIR | Attenuated Total Reflection Flourier Transformed Infrared |
| NMR | Nuclear Magnetic Resonance |
| FE-SEM | Field emission scanning electron microscope |
| EDS | Energy-Dispersive Spectroscopy |
| XPS | X-ray Photoelectron Spectroscopy |
| AFM | Atomic Force Microscopy |
| DSC | Differential Scanning Calorimeter |
| EOS | Equation of State |
| OWK | Owens–Wendte–Kaelble |
| TGA | Thermal Gravimetric Analyzer |
| DTG | Derivative Thermogravimetry |
References
- Qiu, H.; Feng, K.; Gapeeva, A.; Meurisch, K.; Kaps, S.; Li, X.; Yu, L.; Mishra, Y.K.; Adelung, R.; Baum, M. Functional polymer materials for modern marine biofouling control. Prog. Polym. Sci. 2022, 127, 101516. [Google Scholar] [CrossRef]
- Jin, H.; Wang, J.; Tian, L.; Gao, M.; Zhao, J.; Ren, L. Recent advances in emerging integrated antifouling and anticorrosion coatings. Mater. Design 2022, 213, 110307. [Google Scholar] [CrossRef]
- Liu, M.; Jing, L.; Zhang, Z.; Li, X.; Yuan, H.; Cui, J.; Li, M.; Zhang, Y. Superior mechanical performance polyurethane-silicone coatings for synergistic antimicrobial and antifouling coatings. J. Macromol. Sci. Part A 2024, 61, 241–251. [Google Scholar] [CrossRef]
- Chen, J.; Zhao, J.; Lin, F.; Zheng, X.; Jian, R.; Lin, Y.; Wei, F.; Lin, Q.; Bai, W.; Xu, Y. Polymerized tung oil toughened urushiol-based benzoxazine copper polymer coatings with excellent antifouling performances. Prog. Org. Coat. 2023, 177, 107411. [Google Scholar] [CrossRef]
- Dai, G.; Ai, X.; Mei, L.; Ma, C.; Zhang, G. Kill-Resist-Renew trinity: Hyperbranched polymer with self-regenerating attack and defense for antifouling coatings. ACS Appl. Mater. Interfaces 2021, 13, 13735–13743. [Google Scholar] [CrossRef]
- Wang, R.; Zhou, T.; Liu, J.; Zhang, X.; Yang, J.; Hu, W.; Liu, L. Designing novel anti-biofouling coatings on titanium based on the ferroelectric-induced strategy. Mater. Design 2021, 203, 109584. [Google Scholar] [CrossRef]
- Callow, J.A.; Callow, M.E. Trends in the development of environmentally friendly fouling-resistant marine coatings. Nat. Commun. 2011, 2, 244. [Google Scholar] [CrossRef] [PubMed]
- Hu, P.; Xie, Q.; Ma, C.; Zhang, G. Silicone-based fouling-release coatings for marine antifouling. Langmuir 2020, 36, 2170–2183. [Google Scholar] [CrossRef]
- Xie, Q.; Pan, J.; Ma, C.; Zhang, G. Dynamic surface antifouling: Mechanism and systems. Soft Matter 2019, 15, 1087–1107. [Google Scholar] [CrossRef]
- Chen, J.; Zheng, X.; Jian, R.; Bai, W.; Zheng, G.; Xie, Z.; Lin, Q.; Lin, F.; Xu, Y. In Situ Reduction of Silver Nanoparticles/Urushiol-Based Polybenzoxazine Composite Coatings with Enhanced Antimicrobial and Antifouling Performances. Polymers 2024, 16, 1167. [Google Scholar] [CrossRef]
- Li, P.; Cai, C.; Long, Y.; Zhu, T.; Dong, H.; Zhu, C.; Zhao, N.; Xu, J. Cu2O-clay composites with sub-micrometer-sized Cu2O particles for marine antifouling paints. J. Coat. Technol. Res. 2018, 16, 25–30. [Google Scholar] [CrossRef]
- Turner, A. Paint particles in the marine environment: An overlooked component of microplastics. Water Res. X 2021, 12, 100110. [Google Scholar] [CrossRef] [PubMed]
- Zou, W.; Cheng, J.; Ou, Y.; Deng, Y.; Zhao, Q. Copper I-II-containing composites and coatings with high and broad-spectrum antimicrobial activity. Chem. Eng. J. 2024, 498, 155193. [Google Scholar] [CrossRef]
- Yang, Z.; He, X.; Lou, T.; Su, D.; Bai, X.; Yuan, C. Fabrication of dual functional marine antifouling coatings by infusing epoxy silicone oil modification of quaternary ammonium. Eur. Polym. J. 2024, 210, 112947. [Google Scholar] [CrossRef]
- Marzullo, P.; Gruttadauria, M.; D’Anna, F. Quaternary Ammonium Salts-Based Materials: A Review on Environmental Toxicity, Anti-Fouling Mechanisms and Applications in Marine and Water Treatment Industries. Biomolecules 2024, 14, 957. [Google Scholar] [CrossRef]
- Song, Y.; Wang, Q.; Ning, Y.; Tian, H.; Liu, B. Developing a novel antibacterial coating system for marine corrosion and fouling control. Mar. Pollut. Bull. 2025, 210, 117347. [Google Scholar] [CrossRef]
- Xie, Q.; Zeng, H.; Peng, Q.; Bressy, C.; Ma, C.; Zhang, G. Self-stratifying silicone coating with nonleaching antifoulant for marine anti-biofouling. Adv. Mater. Interfaces 2019, 6, 1900535. [Google Scholar] [CrossRef]
- Wu, H.X.; Tan, L.; Tang, Z.W.; Yang, M.Y.; Xiao, J.Y.; Liu, C.J.; Zhuo, R.X. Highly efficient antibacterial surface grafted with a triclosan-decorated poly(N-hydroxyethylacrylamide) brush. ACS Appl. Mater. Interfaces 2015, 7, 7008–7015. [Google Scholar] [CrossRef]
- Tan, J.; Liang, X.; Yang, J.; Zhou, S. Sol-gel-derived hard coatings from tetraethoxysilane and organoalkoxysilanes bearing zwitterionic and isothiazolinone groups and their antifouling behaviors. J. Mater. Chem. B 2022, 10, 406–417. [Google Scholar] [CrossRef]
- Peng, K.; Dai, X.; Mao, H.; Zou, H.; Yang, Z.; Tu, W.; Hu, J. Development of direct contact-killing non-leaching antimicrobial polyurethanes through click chemistry. J. Coat. Technol. Res. 2018, 15, 1239–1250. [Google Scholar] [CrossRef]
- Gao, Z.; Zhang, S.; Duan, Y.; Chang, H.; Cui, M.; Huang, R.; Su, R. Photoinitiated Thiol–Ene Click Reaction for Preparation of Highly Adhesive and Mechanically Stable Silicone Coatings for Marine Antifouling and Anticorrosion. ACS Appl. Mater. Interfaces 2025, 17, 8299–8311. [Google Scholar] [CrossRef] [PubMed]
- Tonelli, M.; Perini, I.; Ridi, F.; Baglioni, P. Improving the properties of antifouling hybrid composites: The use of Halloysites as nano-containers in epoxy coatings. Colloids Surf. A 2021, 623, 126779. [Google Scholar] [CrossRef]
- Zhao, W.; Liu, J.; Wang, S.; Dai, J.; Liu, X. Bio-Based Thermosetting Resins: From Molecular Engineering to Intrinsically Multifunctional Customization. Adv. Mater. 2024, 36, 2311242. [Google Scholar] [CrossRef]
- Chen, Q.; Zhang, L.; Zhang, J.; Habib, S.; Lu, G.; Dai, J.; Liu, X. Bio-based polybenzoxazines coatings for efficient marine antifouling. Prog. Org. Coat. 2023, 174, 107298. [Google Scholar] [CrossRef]
- Asrafali, S.P.; Periyasamy, T.; Lee, J. A Comprehensive Review on Bio-Based Polybenzoxazines Emphasizing Their Antimicrobial Property. Microorganisms 2025, 13, 164. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Li, K.; Fang, J. Research Progress on Modification and Application of Raw Lacquer. ChemistrySelect 2022, 7, e202200943. [Google Scholar] [CrossRef]
- Xia, J.; Lin, J.; Xu, Y.; Chen, Q. On the UV-Induced Polymeric Behavior of Chinese Lacquer. ACS Appl. Mater. Interfaces 2010, 3, 482–489. [Google Scholar] [CrossRef]
- Chen, J.; Jian, R.; Yang, K.; Bai, W.; Huang, C.; Lin, Y.; Zheng, B.; Wei, F.; Lin, Q.; Xu, Y. Urushiol-based benzoxazine copper polymer with low surface energy, strong substrate adhesion and antibacterial for marine antifouling application. J. Clean. Prod. 2021, 318, 128527. [Google Scholar] [CrossRef]
- Chen, J.; Bai, W.; Jian, R.; Lin, Y.; Zheng, X.; Wei, F.; Lin, Q.; Lin, F.; Xu, Y. Molecular structure design of polybenzoxazines with low surface energy and low modulus for marine antifouling application. Prog. Org. Coat. 2024, 187, 108165. [Google Scholar] [CrossRef]
- Ribeiro, F.W.M.; Omari, I.; Thomas, G.T.; Paul, M.; Williams, P.J.H.; McIndoe, J.S.; Correra, T.C. Microstructural Analysis of Benzoxazine Cationic Ring-Opening Polymerization Pathways. Macromol. Rapid Commun. 2024, 45, e2300470. [Google Scholar] [CrossRef]
- Zhao, W.; Yang, R.; Yang, S.; Zhang, K. Experimental and computational investigations on ring-opening polymerization mechanisms of amide-functional benzoxazines. Macromol. Res. 2023, 31, 45–52. [Google Scholar] [CrossRef]
- Qi, L.; Hu, L.-X.; Wang, Z.-C.; Yuan, Z.-G.; Wen, H.-L.; Liu, W.-B.; Wang, J.; Derradji, M. Bio-based benzoxazine-terminated hyperbranched polyesters. React. Funct. Polym. 2024, 199, 105888. [Google Scholar] [CrossRef]
- Zhang, K.; Liu, Y.; Han, M.; Froimowicz, P. Smart and sustainable design of latent catalyst-containing benzoxazine-bio-resins and application studies. Green Chem. 2020, 22, 1209–1219. [Google Scholar] [CrossRef]
- Zeng, M.; Wang, J.; Li, R.; Liu, J.; Chen, W.; Xu, Q.; Gu, Y. The curing behavior and thermal property of graphene oxide/benzoxazine nanocomposites. Polymer 2013, 54, 3107–3116. [Google Scholar] [CrossRef]
- Ye, Q.; He, B.; Zhang, Y.; Zhang, J.; Liu, S.; Zhou, F. Grafting Robust Thick Zwitterionic Polymer Brushes via Subsurface-Initiated Ring-Opening Metathesis Polymerization for Antimicrobial and Anti-Biofouling. ACS Appl. Mater. Interfaces 2019, 11, 39171–39178. [Google Scholar] [CrossRef] [PubMed]
- Lejars, M.; Margaillan, A.; Bressy, C. Fouling release coatings: A nontoxic alternative to biocidal antifouling coatings. Chem. Rev. 2012, 112, 4347–4390. [Google Scholar] [CrossRef]
- Baier, R.E. Surface behaviour of biomaterials: The theta surface for biocompatibility. J. Mater. Sci. Mater. Med. 2006, 17, 1057–1062. [Google Scholar] [CrossRef]
- Xu, H.; Lu, Z.; Zhang, G. Synthesis and properties of thermosetting resin based on urushiol. RSC Adv. 2012, 2, 2768–2772. [Google Scholar] [CrossRef]
- ASTM D1141-1998; Standard Practice for the Preparation of Substitute Ocean Water. ASTM International: West Conshohocken, PA, USA, 2013.
- ASTM B499-2009; Standard Test Method for Measurement of Coating Thicknesses by the Magnetic Method: Nonmagnetic Coatings on Magnetic Basis Metals. ASTM International: West Conshohocken, PA, USA, 2014.
- Zheng, L.; Zhu, J.; Chen, J.; Xu, Y.; Jiang, L. Fabrication of Highly Stable Polyurushiol-Decorated Silver Nanoparticles and Evaluation of Their Antibacterial and Anti-Microalgae Activities. J. Inorg. Organomet. Polym. Mater. 2024, 30, 570–582. [Google Scholar] [CrossRef]
- Xu, K.; Xie, H.; Sun, C.; Lin, W.; You, Z.; Zheng, G.; Zheng, X.; Xu, Y.; Chen, J.; Lin, F. Sustainable Coating Based on Zwitterionic Functionalized Polyurushiol with Antifouling and Antibacterial Properties. Molecules 2023, 28, 8040. [Google Scholar] [CrossRef]
- Zhao, J.; Chen, J.; Zheng, X.; Lin, Q.; Zheng, G.; Xu, Y.; Lin, F. Urushiol-Based Benzoxazine Containing Sulfobetaine Groups for Sustainable Marine Antifouling Applications. Polymers 2023, 15, 2383. [Google Scholar] [CrossRef] [PubMed]




| Samples | T5% (°C) | Tmax (°C) | Residue at 600 °C (wt%) |
|---|---|---|---|
| URB | 205.9 | 438.3 | 13.9 |
| URHP | 269.5 | 432.6 | 24.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, N.; Zhu, J.; Chen, X.; Lin, F.; Zheng, X.; Zheng, G.; Lin, Q.; Chen, J.; Xu, Y. Intrinsic Antibacterial Urushiol-Based Benzoxazine Polymer Coating for Marine Antifouling Applications. Int. J. Mol. Sci. 2025, 26, 4118. https://doi.org/10.3390/ijms26094118
Chen N, Zhu J, Chen X, Lin F, Zheng X, Zheng G, Lin Q, Chen J, Xu Y. Intrinsic Antibacterial Urushiol-Based Benzoxazine Polymer Coating for Marine Antifouling Applications. International Journal of Molecular Sciences. 2025; 26(9):4118. https://doi.org/10.3390/ijms26094118
Chicago/Turabian StyleChen, Nuo, Jide Zhu, Xinrong Chen, Fengcai Lin, Xiaoxiao Zheng, Guocai Zheng, Qi Lin, Jipeng Chen, and Yanlian Xu. 2025. "Intrinsic Antibacterial Urushiol-Based Benzoxazine Polymer Coating for Marine Antifouling Applications" International Journal of Molecular Sciences 26, no. 9: 4118. https://doi.org/10.3390/ijms26094118
APA StyleChen, N., Zhu, J., Chen, X., Lin, F., Zheng, X., Zheng, G., Lin, Q., Chen, J., & Xu, Y. (2025). Intrinsic Antibacterial Urushiol-Based Benzoxazine Polymer Coating for Marine Antifouling Applications. International Journal of Molecular Sciences, 26(9), 4118. https://doi.org/10.3390/ijms26094118







