Photoreactive Properties of Melanin Obtained from Human Induced Pluripotent Stem Cell-Derived Melanocytes
Abstract
1. Introduction
2. Results and Discussion
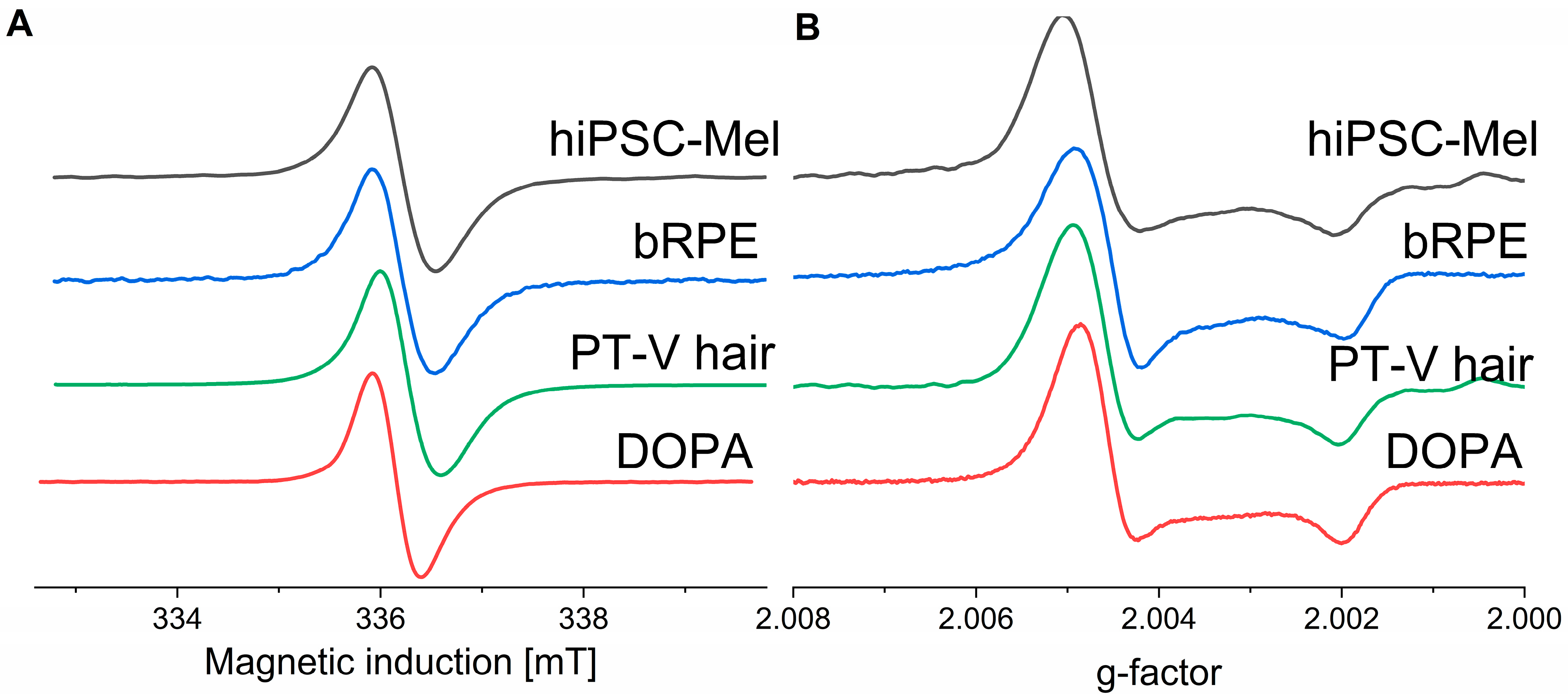
2.1. Paramagnetic Properties of the Examined Melanins
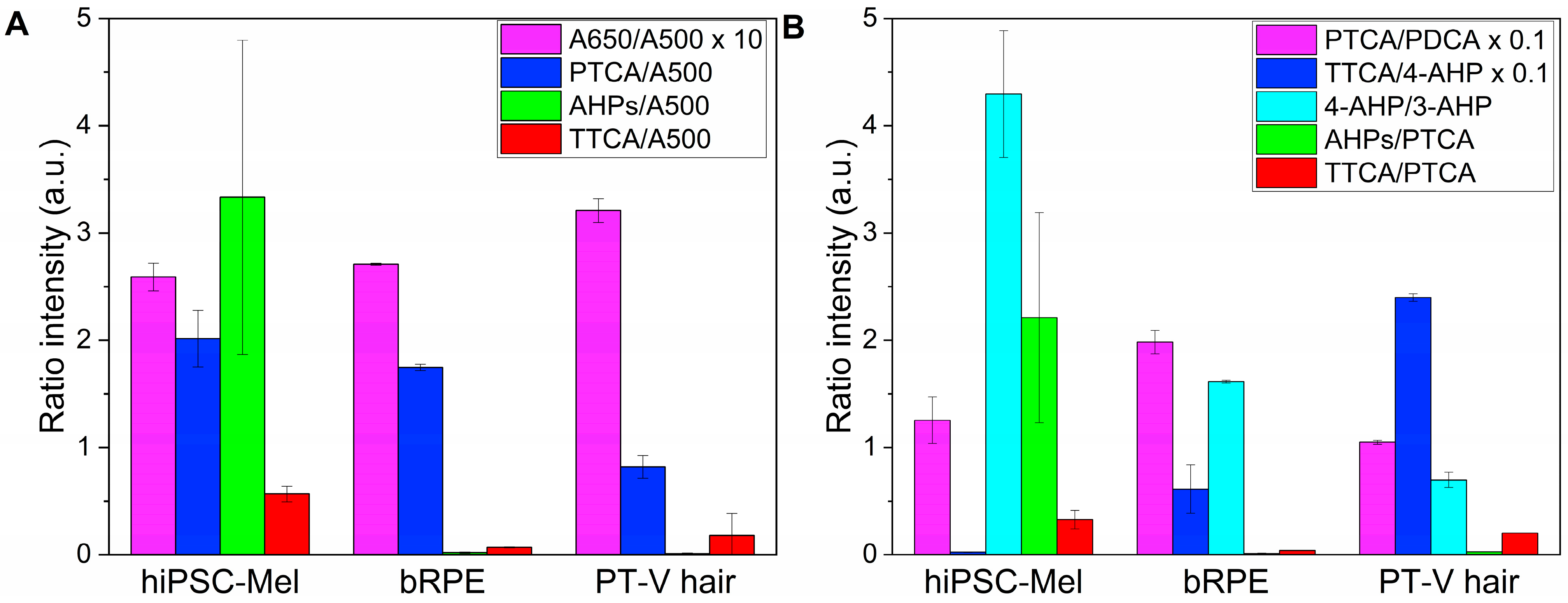
2.2. Chemical Analysis of Melanin Degradation Products
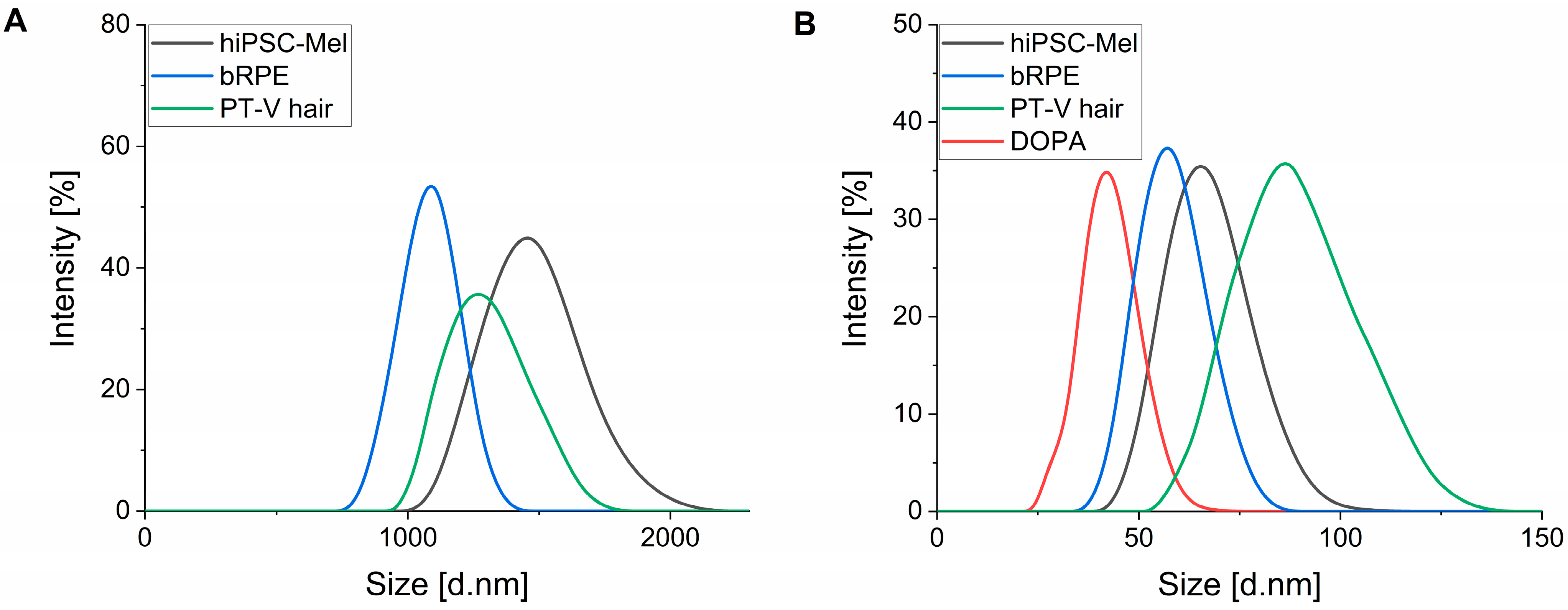
2.3. Size of Melanosomes and Nanoaggregates
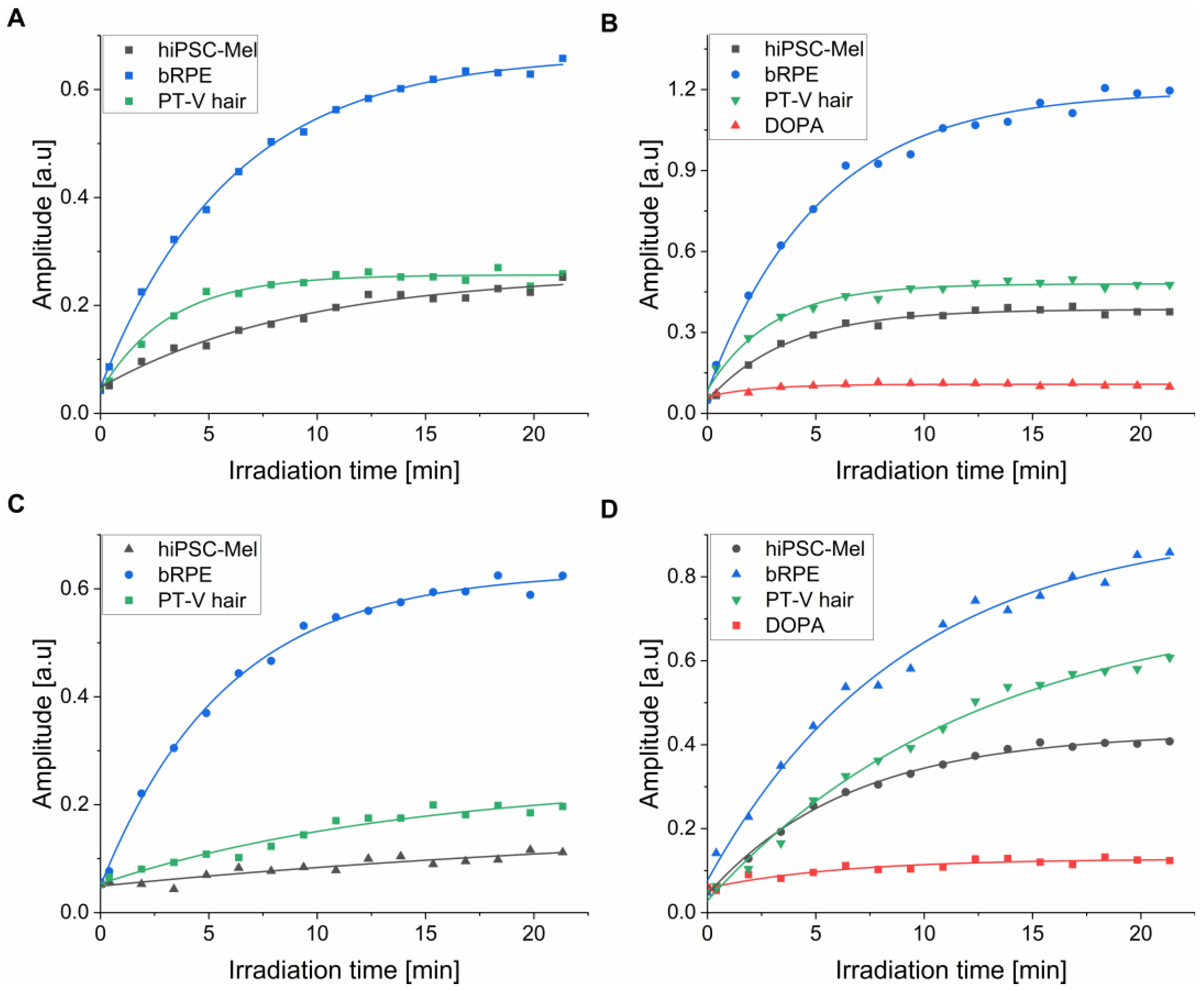
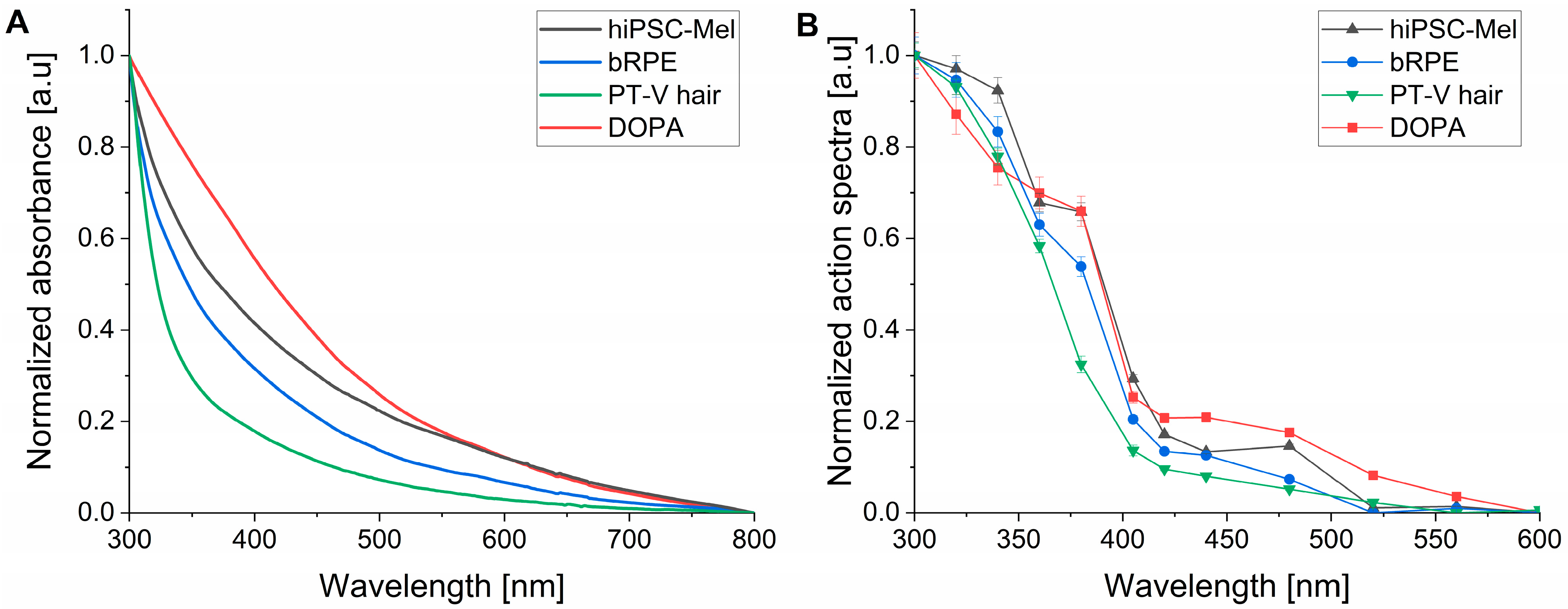
2.4. Photoconsumption of Oxygen by the Examined Melanins
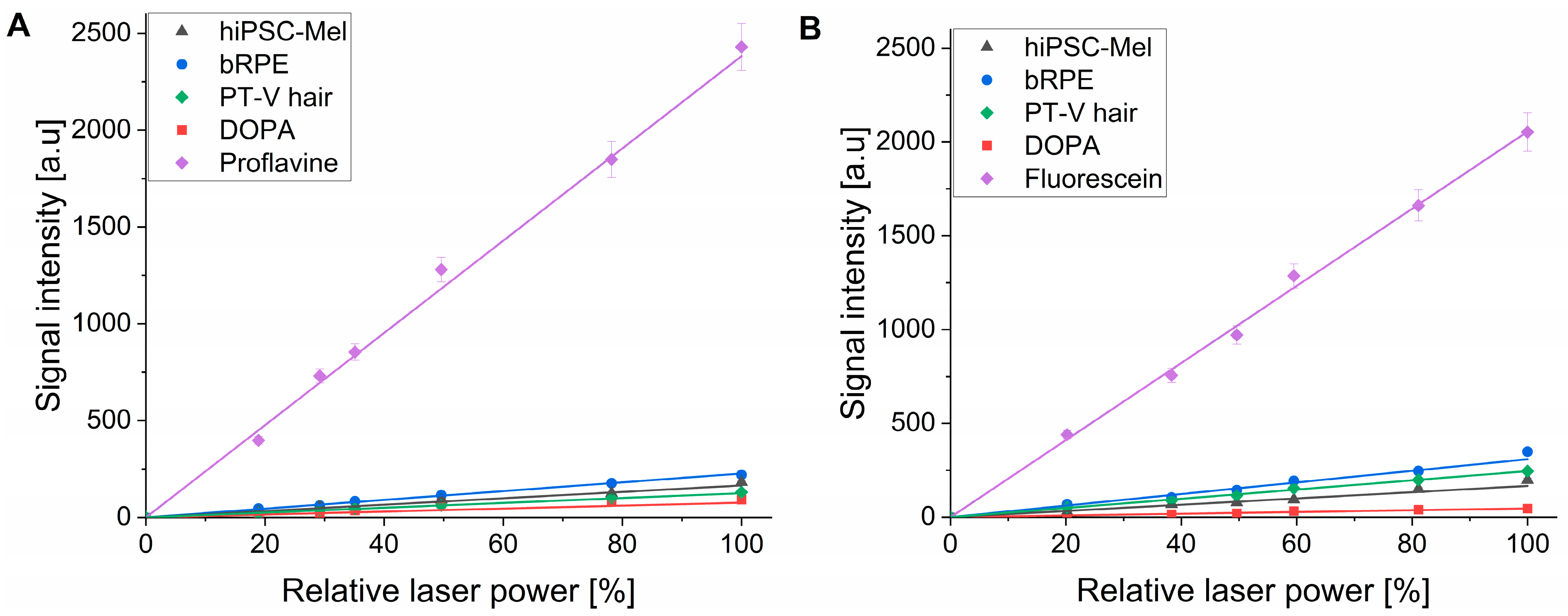
2.5. Photoproduction of Superoxide Anion by the Examined Melanins
2.6. Generation of Singlet Oxygen by the Examined Melanins
3. Materials and Methods
3.1. Generation of Melanocytes from hiPSCs
3.2. Isolation of Melanin from hiPSC-Mel
3.3. Isolation of Melanin from Human Hair
3.4. Isolation of Melanin from Bovine Retinal Pigment Epithelium
3.5. Preparation of Synthetic Melanin
3.6. X-Band Electron Paramagnetic Resonance Spectroscopy Measurements
3.7. W-Band Electron Paramagnetic Resonance Spectroscopy Measurements
3.8. Chemical Analysis of Melanin Subunits
3.9. UV–Vis Absorption Spectroscopy
3.10. Size Analysis
3.11. Oxygen Consumption Measurements
3.12. Electron Paramagnetic Resonance Spin Trapping Studies
3.13. Time-Resolved Singlet Oxygen Phosphorescence
3.14. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Guo, L.; Li, W.; Gu, Z.; Wang, L.; Guo, L.; Ma, S.; Li, C.; Sun, J.; Han, B.; Chang, J. Recent Advances and Progress on Melanin: From Source to Application. Int. J. Mol. Sci. 2023, 24, 4360. [Google Scholar] [CrossRef] [PubMed]
- Zecca, L.; Zucca, F.A.; Wilms, H.; Sulzer, D. Neuromelanin of the Substantia Nigra: A Neuronal Black Hole with Protective and Toxic Characteristics. Trends Neurosci. 2003, 26, 578–580. [Google Scholar] [CrossRef]
- Boulton, M.E. Studying Melanin and Lipofuscin in RPE Cell Culture Models. Exp. Eye Res. 2014, 126, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Borovanský, J.; Hach, P. Isolation of Melanosomes from Keratinous Structures: Current State of the Art. Arch. Dermatol. Res. 1986, 279, 54–58. [Google Scholar] [CrossRef]
- Joly-Tonetti, N.; Wibawa, J.I.D.; Bell, M.; Tobin, D.J. Basis of Melanin Distribution in the Human Skin Epidermis. Brit. J. Dermatol. 2018, 179, 1115–1126. [Google Scholar] [CrossRef] [PubMed]
- Zamudio Díaz, D.F.; Busch, L.; Kröger, M.; Klein, A.L.; Lohan, S.B.; Mewes, K.R.; Vierkotten, L.; Witzel, C.; Rohn, S.; Meinke, M.C. Significance of Melanin Distribution in the Epidermis for the Protective Effect against UV Light. Sci. Rep. 2024, 14, 3488. [Google Scholar] [CrossRef]
- Yakimov, B.P.; Shirshin, E.A.; Schleusener, J.; Allenova, A.S.; Fadeev, V.V.; Darvin, M.E. Melanin Distribution from the Dermal–Epidermal Junction to the Stratum Corneum: Non-Invasive in Vivo Assessment by Fluorescence and Raman Microspectroscopy. Sci. Rep. 2020, 10, 14374. [Google Scholar] [CrossRef]
- D’Alba, L.; Shawkey, M.D. Melanosomes: Biogenesis, Properties, and Evolution of an Ancient Organelle. Physiol. Rev. 2019, 99, 1–19. [Google Scholar] [CrossRef]
- Ando, H.; Niki, Y.; Ito, M.; Akiyama, K.; Matsui, M.S.; Yarosh, D.B.; Ichihashi, M. Melanosomes Are Transferred from Melanocytes to Keratinocytes through the Processes of Packaging, Release, Uptake, and Dispersion. J. Investig. Dermatol. 2012, 132, 1222–1229. [Google Scholar] [CrossRef]
- Moreiras, H.; Seabra, M.C.; Barral, D.C. Melanin Transfer in the Epidermis: The Pursuit of Skin Pigmentation Control Mechanisms. Int. J. Mol. Sci. 2021, 22, 4466. [Google Scholar] [CrossRef]
- Brenner, M.; Hearing, V.J. The Protective Role of Melanin against UV Damage in Human Skin. Photochem. Photobiol. 2008, 84, 539–549. [Google Scholar] [CrossRef] [PubMed]
- Solano, F. Photoprotection and Skin Pigmentation: Melanin-Related Molecules and Some Other New Agents Obtained from Natural Sources. Molecules 2020, 25, 1537. [Google Scholar] [CrossRef]
- Mokrzyński, K.; Ito, S.; Wakamatsu, K.; Camenish, T.G.; Sarna, T.; Sarna, M. Photoreactivity of Hair Melanin from Different Skin Phototypes—Contribution of Melanin Subunits to the Pigments Photoreactive Properties. Int. J. Mol. Sci. 2021, 22, 4465. [Google Scholar] [CrossRef] [PubMed]
- Mokrzyński, K.; Sarna, M.; Sarna, T. Photoreactivity and Phototoxicity of Experimentally Photodegraded Hair Melanosomes from Individuals of Different Skin Phototypes. J. Photochem. Photobiol. B 2023, 243, 112704. [Google Scholar] [CrossRef] [PubMed]
- Chiarelli-Neto, O.; Ferreira, A.S.; Martins, W.K.; Pavani, C.; Severino, D.; Faião-Flores, F.; Maria-Engler, S.S.; Aliprandini, E.; Martinez, G.R.; Di Mascio, P.; et al. Melanin Photosensitization and the Effect of Visible Light on Epithelial Cells. PLoS ONE 2014, 9, e113266. [Google Scholar] [CrossRef]
- Mostert, A.B.; Rienecker, S.B.; Noble, C.; Hanson, G.R.; Meredith, P. The Photoreactive Free Radical in Eumelanin. Sci. Adv. 2018, 4, eaaq1293. [Google Scholar] [CrossRef]
- Mokrzyński, K.; Żądło, A.; Szewczyk, G.; Sarna, M.; Camenisch, T.G.; Ito, S.; Wakamatsu, K.; Sarna, T. The Effect of Oxidative Degradation of Dopa-Melanin on Its Basic Physicochemical Properties and Photoreactivity. Pigment Cell Melanoma Res. 2024, 37, 769–782. [Google Scholar] [CrossRef]
- Cabaço, L.C.; Tomás, A.; Pojo, M.; Barral, D.C. The Dark Side of Melanin Secretion in Cutaneous Melanoma Aggressiveness. Front. Oncol. 2022, 12, 887366. [Google Scholar] [CrossRef]
- Slominski, R.M.; Zmijewski, M.A.; Slominski, A.T. The Role of Melanin Pigment in Melanoma. Exp. Dermatol. 2015, 24, 258–259. [Google Scholar] [CrossRef]
- Snyman, M.; Walsdorf, R.E.; Wix, S.N.; Gill, J.G. The Metabolism of Melanin Synthesis-From Melanocytes to Melanoma. Pigment. Cell Melanoma Res. 2024, 37, 438–452. [Google Scholar] [CrossRef]
- Noonan, F.P.; Zaidi, M.R.; Wolnicka-Glubisz, A.; Anver, M.R.; Bahn, J.; Wielgus, A.; Cadet, J.; Douki, T.; Mouret, S.; Tucker, M.A.; et al. Melanoma Induction by Ultraviolet A but Not Ultraviolet B Radiation Requires Melanin Pigment. Nat. Commun. 2012, 3, 884. [Google Scholar] [CrossRef]
- Khan, A.Q.; Travers, J.B.; Kemp, M.G. Roles of UVA Radiation and DNA Damage Responses in Melanoma Pathogenesis. Environ. Mol. Mutagen. 2018, 59, 438–460. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, D.; Fernandez, A. The Photobiology of Melanocytes Modulates the Impact of UVA on Sunlight-Induced Melanoma. Photochem. Photobiol. Sci. 2012, 11, 69–73. [Google Scholar] [CrossRef] [PubMed]
- Pudroma, X.; Duoji, G.; Grigalavicius, M.; Jie, D.; Juzeniene, A. Molecular Mechanisms of UVA-Induced Melanoma. J. Environ. Pathol. Toxicol. Oncol. 2017, 36, 217–228. [Google Scholar] [CrossRef]
- Cadet, J.; Douki, T. Formation of UV-Induced DNA Damage Contributing to Skin Cancer Development. Photochem. Photobiol. Sci. 2018, 17, 1816–1841. [Google Scholar] [CrossRef] [PubMed]
- Sample, A.; He, Y.-Y. Mechanisms and Prevention of UV-Induced Melanoma. Photodermatol. Photoimmunol. Photomed. 2018, 34, 13–24. [Google Scholar] [CrossRef]
- Zhao, B.; Shah, P.; Qiang, L.; He, T.-C.; Budanov, A.; He, Y.-Y. Distinct Role of Sesn2 in Response to UVB-Induced DNA Damage and UVA-Induced Oxidative Stress in Melanocytes. Photochem. Photobiol. 2017, 93, 375–381. [Google Scholar] [CrossRef]
- Cadet, J.; Davies, K.J.A.; Medeiros, M.H.; Di Mascio, P.; Wagner, J.R. Formation and Repair of Oxidatively Generated Damage in Cellular DNA. Free Radic. Biol. Med. 2017, 107, 13–34. [Google Scholar] [CrossRef]
- Calkins, M.J.; Vartanian, V.; Owen, N.; Kirkali, G.; Jaruga, P.; Dizdaroglu, M.; McCullough, A.K.; Lloyd, R.S. Enhanced Sensitivity of Neil1−/− Mice to Chronic UVB Exposure. DNA Repair 2016, 48, 43–50. [Google Scholar] [CrossRef]
- Sage, E.; Girard, P.-M.; Francesconi, S. Unravelling UVA-Induced Mutagenesis. Photochem. Photobiol. Sci. 2012, 11, 74–80. [Google Scholar] [CrossRef]
- Żądło, A.; Szewczyk, G.; Sarna, M.; Kozinska, A.; Pilat, A.; Kaczara, P.; Sarna, T. Photoaging of Retinal Pigment Epithelial Melanosomes: The Effect of Photobleaching on Morphology and Reactivity of the Pigment Granules. Free Radic. Biol. Med. 2016, 97, 320–329. [Google Scholar] [CrossRef] [PubMed]
- Panzella, L.; Leone, L.; Greco, G.; Vitiello, G.; D’Errico, G.; Napolitano, A.; d’Ischia, M. Red Human Hair Pheomelanin Is a Potent Pro-Oxidant Mediating UV-Independent Contributory Mechanisms of Melanomagenesis. Pigment Cell Melanoma Res. 2014, 27, 244–252. [Google Scholar] [CrossRef] [PubMed]
- Nissan, X.; Larribere, L.; Saidani, M.; Hurbain, I.; Delevoye, C.; Feteira, J.; Lemaitre, G.; Peschanski, M.; Baldeschi, C. Functional Melanocytes Derived from Human Pluripotent Stem Cells Engraft into Pluristratified Epidermis. Proc. Natl. Acad. Sci. USA 2011, 108, 14861–14866. [Google Scholar] [CrossRef]
- Ohta, S.; Imaizumi, Y.; Akamatsu, W.; Okano, H.; Kawakami, Y. Generation of Human Melanocytes from Induced Pluripotent Stem Cells. Methods Mol. Biol. 2013, 989, 193–215. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Jiang, M.; Kumar, S.M.; Xu, T.; Wang, F.; Xiang, L.; Xu, X. Generation of Melanocytes from Induced Pluripotent Stem Cells. J. Investig. Dermatol. 2011, 131, 2458–2466. [Google Scholar] [CrossRef]
- Kobori, C.; Takagi, R.; Yokomizo, R.; Yoshihara, S.; Mori, M.; Takahashi, H.; Javaregowda, P.K.; Akiyama, T.; Ko, M.S.H.; Kishi, K.; et al. Functional and Long-Lived Melanocytes from Human Pluripotent Stem Cells with Transient Ectopic Expression of JMJD3. Stem Cell Res. Ther. 2023, 14, 242. [Google Scholar] [CrossRef]
- Parat, V.; Onteniente, B.; Maruotti, J. A High-Throughput Screening Platform for Pigment Regulating Agents Using Pluripotent Stem Cell-Derived Melanocytes. Exp. Dermatol. 2021, 30, 691–697. [Google Scholar] [CrossRef]
- Sułkowski, M.; Kot, M.; Badyra, B.; Paluszkiewicz, A.; Płonka, P.M.; Sarna, M.; Michalczyk-Wetula, D.; Zucca, F.A.; Zecca, L.; Majka, M. Highly Effective Protocol for Differentiation of Induced Pluripotent Stem Cells (iPS) into Melanin-Producing Cells. Int. J. Mol. Sci. 2021, 22, 12787. [Google Scholar] [CrossRef]
- Hosaka, C.; Kunisada, M.; Koyanagi-Aoi, M.; Masaki, T.; Takemori, C.; Taniguchi-Ikeda, M.; Aoi, T.; Nishigori, C. Induced Pluripotent Stem Cell-Derived Melanocyte Precursor Cells Undergoing Differentiation into Melanocytes. Pigment Cell Melanoma Res. 2019, 32, 623–633. [Google Scholar] [CrossRef]
- Najder-Kozdrowska, L.; Pilawa, B.; Buszman, E.; Wrześniok, D.; Więckowski, A.B. Electron Paramagnetic Resonance (EPR) Study of DOPA–Melanin Complexes with Kanamycin and Copper(II) Ions. Spectrosc. 2011, 25, 197–205. [Google Scholar] [CrossRef]
- Pasenkiewicz-Gierula, M.; Sealy, R.C. Analysis of the ESR Spectrum of Synthetic Dopa Melanin. Biochim. Biophys. Acta-Gen. Subj. 1986, 884, 510–516. [Google Scholar] [CrossRef] [PubMed]
- Ito, S.; Nakanishi, Y.; Valenzuela, R.K.; Brilliant, M.H.; Kolbe, L.; Wakamatsu, K. Usefulness of Alkaline Hydrogen Peroxide Oxidation to Analyze Eumelanin and Pheomelanin in Various Tissue Samples: Application to Chemical Analysis of Human Hair Melanins. Pigment. Cell Melanoma Res. 2011, 24, 605–613. [Google Scholar] [CrossRef] [PubMed]
- Wakamatsu, K.; Ito, S.; Rees, J.L. The Usefulness of 4-Amino-3-Hydroxyphenylalanine as a Specific Marker of Pheomelanin. Pigment Cell Res. 2002, 15, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Ozeki, H.; Ito, S.; Wakamatsu, K. Chemical Characterization of Melanins in Sheep Wool and Human Hair. Pigment. Cell Res. 1996, 9, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Ito, S.; Wakamatsu, K.; Sarna, T. Photodegradation of Eumelanin and Pheomelanin and Its Pathophysiological Implications. Photochem. Photobiol. 2018, 74, 409–420. [Google Scholar] [CrossRef]
- Del Bino, S.; Ito, S.; Sok, J.; Nakanishi, Y.; Bastien, P.; Wakamatsu, K.; Bernerd, F. Chemical Analysis of Constitutive Pigmentation of Human Epidermis Reveals Constant Eumelanin to Pheomelanin Ratio. Pigment Cell Melanoma Res. 2015, 28, 707–717. [Google Scholar] [CrossRef]
- Żądło, A.; Ito, S.; Sarna, M.; Wakamatsu, K.; Mokrzyński, K.; Sarna, T. The Role of Hydrogen Peroxide and Singlet Oxygen in the Photodegradation of Melanin. Photochem. Photobiol. Sci. 2020, 19, 654–667. [Google Scholar] [CrossRef]
- Thong, H.-Y.; Jee, S.-H.; Sun, C.-C.; Boissy, R.E. The Patterns of Melanosome Distribution in Keratinocytes of Human Skin as One Determining Factor of Skin Colour. Brit. J. Dermatol. 2003, 149, 498–505. [Google Scholar] [CrossRef]
- Pelkonen, L.; Reinisalo, M.; Morin-Picardat, E.; Kidron, H.; Urtti, A. Isolation of Intact and Functional Melanosomes from the Retinal Pigment Epithelium. PLoS ONE 2016, 11, e0160352. [Google Scholar] [CrossRef]
- Reszka, K.; Chignell, C.F. Spin-Trapping of the Superoxide Radical in Aprotic Solvents. Free Radic. Res. Commun. 1991, 14, 97–106. [Google Scholar] [CrossRef]
- Wilkinson, F.; Abdel-Shafi, A.A. Mechanism of Quenching of Triplet States by Molecular Oxygen: Biphenyl Derivatives in Different Solvents. J. Phys. Chem. A 1999, 103, 5425–5435. [Google Scholar] [CrossRef]
- Nofsinger, J.B.; Ye, T.; Simon, J.D. Ultrafast Nonradiative Relaxation Dynamics of Eumelanin. J. Phys. Chem. B 2001, 105, 2864–2866. [Google Scholar] [CrossRef]
- Wang, A.; Marino, A.R.; Gasyna, E.M.; Sarna, T.; Norris, J.R., Jr. Investigation of Photoexcited States in Porcine Eumelanin through Their Transient Radical Products. J. Phys. Chem. B 2009, 113, 10480–10482. [Google Scholar] [CrossRef] [PubMed]
- Premi, S.; Wallisch, S.; Mano, C.M.; Weiner, A.B.; Bacchiocchi, A.; Wakamatsu, K.; Bechara, E.J.H.; Halaban, R.; Douki, T.; Brash, D.E. Chemiexcitation of Melanin Derivatives Induces DNA Photoproducts Long after UV Exposure. Science 2015, 347, 842–847. [Google Scholar] [CrossRef]
- Chiarelli-Neto, O.; Pavani, C.S.; Ferreira, A.; Uchoa, A.F.; Severino, D.; Baptista, M.S. Generation and Suppression of Singlet Oxygen in Hair by Photosensitization of Melanin. Free Radic. Biol. Med. 2011, 51, 1195–1202. [Google Scholar] [CrossRef] [PubMed]
- Felix, C.C.; Hyde, J.S.; Sealy, R.C. Photoreactions of Melanin: A New Transient Species and Evidence for Triplet State Involvement. Biochem. Biophys. Res. Commun. 1979, 88, 456–461. [Google Scholar] [CrossRef]
- Conti, F.; Panzella, L.; Napolitano, A.; d’Ischia, M.; Toffoletti, A. Time-Resolved EPR Investigation of Oxygen and Temperature Effects on Synthetic Eumelanin. J. Spectrosc. 2010, 24, 289–295. [Google Scholar] [CrossRef]
- Wiktor, A.; Sarna, M.; Wnuk, D.; Sarna, T. Lipofuscin-Mediated Photodynamic Stress Induces Adverse Changes in Nanomechanical Properties of Retinal Pigment Epithelium Cells. Sci. Rep. 2018, 8, 17929. [Google Scholar] [CrossRef]
- Sidabras, J.W.; Mett, R.R.; Froncisz, W.; Camenisch, T.G.; Anderson, J.R.; Hyde, J.S. Multipurpose EPR Loop-Gap Resonator and Cylindrical TE011 Cavity for Aqueous Samples at 94 GHz. Rev. Sci. Instrum. 2007, 78, 034701. [Google Scholar] [CrossRef]
- Hyde, J.S.; Froncisz, W.; Sidabras, J.W.; Camenisch, T.G.; Anderson, J.R.; Strangeway, R.A. Microwave Frequency Modulation in CW EPR at W-Band Using a Loop-Gap Resonator. J. Magn. Reson. 2007, 185, 259–263. [Google Scholar] [CrossRef]
- Ito, S.; Del Bino, S.; Hirobe, T.; Wakamatsu, K. Improved HPLC Conditions to Determine Eumelanin and Pheomelanin Contents in Biological Samples Using an Ion Pair Reagent. Int. J. Mol. Sci. 2020, 21, 5134. [Google Scholar] [CrossRef] [PubMed]

| Origin of the Pigment | A650/ A500 1 | PTCA (µg)/ A500 1 | AHPs (µg)/ A500 1 | TTCA (µg)/ A500 1 |
|---|---|---|---|---|
| hiPSC-Mel | 0.259 ± 0.013 | 2.015± 0.263 | 3.333 ± 1.465 | 0.566 ± 0.072 |
| bRPE | 0.271 ± 0.001 | 1.747 ± 0.030 | 0.021 ± 0.006 | 0.069 ± 0.005 |
| PT-V hair | 0.321 ± 0.001 | 0.819 ± 0.106 | 0.010 ± 0.008 | 0.178 ± 0.205 |
| DOPA-mel 2 | 0.321 ± 0.002 | 0.340 ± 0.007 | 0.000 ± 0.000 | 0.000 ± 0.000 |
| Origin of the Pigment | PTCA/ PDCA 1 | TTCA/ 4-AHP 1 | 4-AHP/ 3-AHP 1 | AHPs/ PTCA 1 | TTCA/ PTCA 1 |
|---|---|---|---|---|---|
| hiPSC-Mel | 12.539 ± 2.179 | 0.252 ± 0.033 | 4.295 ± 0.59 | 2.210 ± 0.981 | 0.327 ± 0.087 |
| bRPE | 19.821 ± 1.089 | 6.111 ± 2.265 | 1.613 ± 0.013 | 0.012 ± 0.004 | 0.040 ± 0.002 |
| PT-V hair | 10.486 ± 0.020 | 23.980 ± 0.357 | 0.697 ± 0.07 | 0.028 ± 0.001 | 0.202 ± 0.001 |
| DOPA-mel 2 | 2.104 ± 1.234 | 0.000 ± 0.000 | 0.000 ± 0.000 | 0.000 ± 0.000 | 0.000 ± 0.000 |
| Origin of the Pigment | Oxygen Consumption at 365 nm [nM (O2)/s] 1 | Oxygen Consumption at 445 nm [nM (O2)/s] 1 | |
|---|---|---|---|
| hiPSC-Mel | Melanosomes | 60.47 ± 3.20 | 19.00 ± 1.26 |
| bRPE | Melanosomes | 67.71 ± 4.52 | 23.67 ± 2.87 |
| PT-V hair | Melanosomes | 64.09 ± 2.29 | 21.67 ± 2.32 |
| hiPSC-Mel | Nanoaggregates | 142.65 ± 8.92 | 38.83 ± 4.55 |
| bRPE | Nanoaggregates | 297.71 ± 19.33 | 139.33 ± 11.79 |
| PT-V hair | Nanoaggregates | 160.74 ± 5.35 | 40.17 ± 3.79 |
| DOPA-melanin | 77.53 ± 8.92 | 20.16 ± 2.64 | |
| Origin of the Pigment | Rate of DMPO-OOH Generation at 365 nm [a.u × 10−4/s] 1 | Rate of DMPO-OOH Generation at 445 nm [a.u × 10−4/s] 1 | |
|---|---|---|---|
| hiPSC-Mel | Melanosomes | 11.87 ± 1.21 | 0.67 ± 0.13 |
| bRPE | Melanosomes | 52.56 ± 4.62 | 15.60 ± 1.22 |
| PT-V hair | Melanosomes | 34.91 ± 3.39 | 2.24 ± 0.11 |
| hiPSC-Mel | Nanoaggregates | 47.24 ± 4.20 | 9.59 ± 1.23 |
| bRPE | Nanoaggregates | 110.22 ± 8.97 | 16.65 ± 2.22 |
| PT-V hair | Nanoaggregates | 67.98 ± 5.24 | 9.75 ± 1.05 |
| DOPA-melanin | 9.85 ± 1.11 | 1.91 ± 0.21 | |
| Origin of the Pigment | Quantum Yield at 365 nm [%] 1 | Quantum Yield at 445 nm [%] 1 |
|---|---|---|
| hiPSC-Mel | 0.53 ± 0.05 | 0.28 ± 0.02 |
| bRPE | 0.95 ± 0.13 | 0.53 ± 0.03 |
| PT-V hair | 0.69 ± 0.09 | 0.42 ± 0.07 |
| DOPA-melanin | 0.16 ± 0.03 | 0.08 ± 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mokrzynski, K.; Wojtala, M.; Sulkowski, M.; Ito, S.; Wakamatsu, K.; Zadlo, A.; Majka, M.; Sarna, T.; Sarna, M. Photoreactive Properties of Melanin Obtained from Human Induced Pluripotent Stem Cell-Derived Melanocytes. Int. J. Mol. Sci. 2025, 26, 4119. https://doi.org/10.3390/ijms26094119
Mokrzynski K, Wojtala M, Sulkowski M, Ito S, Wakamatsu K, Zadlo A, Majka M, Sarna T, Sarna M. Photoreactive Properties of Melanin Obtained from Human Induced Pluripotent Stem Cell-Derived Melanocytes. International Journal of Molecular Sciences. 2025; 26(9):4119. https://doi.org/10.3390/ijms26094119
Chicago/Turabian StyleMokrzynski, Krystian, Mateusz Wojtala, Maciej Sulkowski, Shosuke Ito, Kazumasa Wakamatsu, Andrzej Zadlo, Marcin Majka, Tadeusz Sarna, and Michal Sarna. 2025. "Photoreactive Properties of Melanin Obtained from Human Induced Pluripotent Stem Cell-Derived Melanocytes" International Journal of Molecular Sciences 26, no. 9: 4119. https://doi.org/10.3390/ijms26094119
APA StyleMokrzynski, K., Wojtala, M., Sulkowski, M., Ito, S., Wakamatsu, K., Zadlo, A., Majka, M., Sarna, T., & Sarna, M. (2025). Photoreactive Properties of Melanin Obtained from Human Induced Pluripotent Stem Cell-Derived Melanocytes. International Journal of Molecular Sciences, 26(9), 4119. https://doi.org/10.3390/ijms26094119












