Azurin: A Model to Study a Metal Coordination Sphere or Electron Transfer in Metalloproteins
Abstract
:1. Introduction
1.1. Redox Reactions Are One of the Cornerstones of Life
1.2. Blue Copper Proteins—Structure and Properties
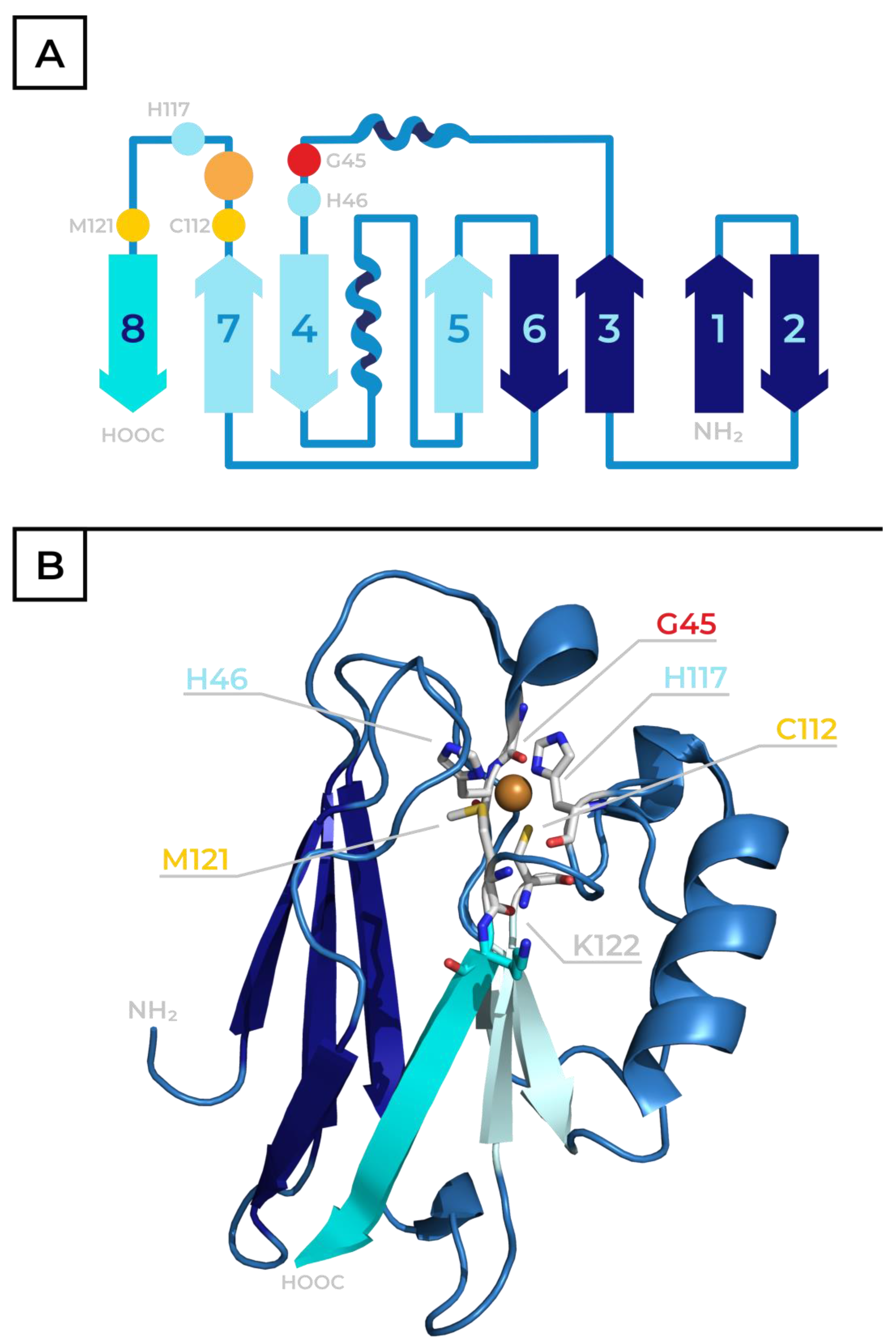
2. Azurin—Physiological Role, Structure and Properties
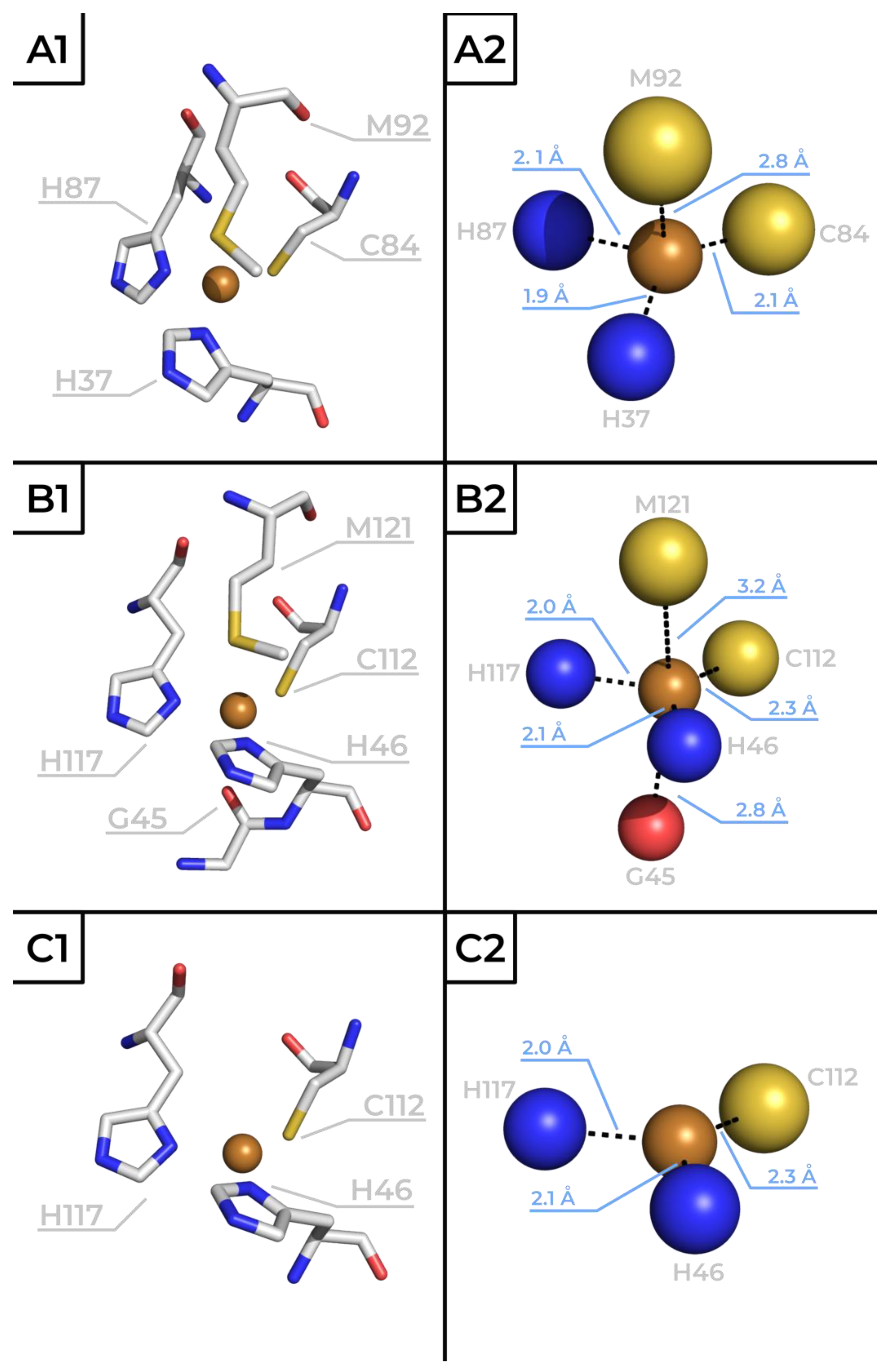
3. The Role of Metal and Amino Acid Residues in the Active Site of Azurin
4. Azurin—ET Studies
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gray, H.B.; Winkler, J.R. Electron Transfer in Proteins. Annu. Rev. Biochem. 1996, 65, 537–561. [Google Scholar] [CrossRef] [PubMed]
- Fereiro, J.A.; Yu, X.; Pecht, I.; Sheves, M.; Cuevas, J.C.; Cahen, D. Tunneling Explains Efficient Electron Transport via Protein Junctions. Proc. Natl. Acad. Sci. USA 2018, 115, E4577–E4583. [Google Scholar] [CrossRef]
- Winkler, J.R.; Gray, H.B. Long-Range Electron Tunneling. J. Am. Chem. Soc. 2014, 136, 2930–2939. [Google Scholar] [CrossRef]
- Warren, J.J.; Ener, M.E.; Vlček, A., Jr.; Winkler, J.R.; Gray, H.B. Electron Hopping through Proteins. Coord. Chem. Rev. 2012, 256, 2478–2487. [Google Scholar] [CrossRef] [PubMed]
- Hosseinzadeh, P.; Marshall, N.M.; Chacón, K.N.; Yu, Y.; Nilges, M.J.; New, S.Y.; Tashkov, S.A.; Blackburn, N.J.; Lu, Y. Design of a Single Protein That Spans the Entire 2-V Range of Physiological Redox Potentials. Proc. Natl. Acad. Sci. USA 2016, 113, 262–267. [Google Scholar] [CrossRef]
- Liu, J.; Chakraborty, S.; Hosseinzadeh, P.; Yu, Y.; Tian, S.; Petrik, I.; Bhagi, A.; Lu, Y. Metalloproteins Containing Cytochrome, Iron–Sulfur, or Copper Redox Centers. Chem. Rev. 2014, 114, 4366–4469. [Google Scholar] [CrossRef] [PubMed]
- Nersissian, A.M.; Shipp, E.L. Blue Copper-Binding Domains. Adv. Protein Chem. 2002, 60, 271–340. [Google Scholar] [CrossRef]
- Savelieff, M.G.; Wilson, T.D.; Elias, Y.; Nilges, M.J.; Garner, D.K.; Lu, Y. Experimental Evidence for a Link among Cupredoxins: Red, Blue, and Purple Copper Transformations in Nitrous Oxide Reductase. Proc. Natl. Acad. Sci. USA 2008, 105, 7919–7924. [Google Scholar] [CrossRef]
- Harris, R.L.; Eady, R.R.; Samar Hasnain, S.; Gary Sawers, R. Coordinate Synthesis of Azurin I and Copper Nitrite Reductase in Alcaligenes Xylosoxidans during Denitrification. Arch. Microbiol. 2006, 186, 241–249. [Google Scholar] [CrossRef]
- Pérez-Henarejos, S.A.; Alcaraz, L.A.; Donaire, A. Blue Copper Proteins: A Rigid Machine for Efficient Electron Transfer, a Flexible Device for Metal Uptake. Arch. Biochem. Biophys. 2015, 584, 134–148. [Google Scholar] [CrossRef]
- Colman, P.M.; Freeman, H.C.; Guss, J.M.; Murata, M.; Norris, V.A.; Ramshaw, J.A.M.; Venkatappa, M.P. X-Ray Crystal Structure Analysis of Plastocyanin at 2.7 Å Resolution. Nature 1978, 272, 319–324. [Google Scholar] [CrossRef]
- Guss, J.M.; Bartunik, H.D.; Freeman, H.C. Accuracy and Precision in Protein Structure Analysis: Restrained Least-Squares Refinement of the Structure of Poplar Plastocyanin at 1.33 Å Resolution. Acta Crystallogr. B 1992, 48 Pt 6, 790–811. [Google Scholar] [CrossRef] [PubMed]
- Nar, H.; Messerschmidt, A.; Huber, R.; van de Kamp, M.; Canters, G.W. Crystal Structure Analysis of Oxidized Pseudomonas Aeruginosa Azurin at pH 5·5 and pH 9·0. J. Mol. Biol. 1991, 221, 765–772. [Google Scholar] [CrossRef] [PubMed]
- Bank, R.P.D. RCSB PDB—1E5Y: Azurin from Pseudomonas Aeruginosa, Reduced Form, pH 5.5. Available online: https://www.rcsb.org/structure/1e5y (accessed on 3 February 2025).
- Estrada, E.; Uriarte, E. Folding Degrees of Azurins and Pseudoazurins: Implications for Structure and Function. Comput. Biol. Chem. 2005, 29, 345–353. [Google Scholar] [CrossRef]
- De Rienzo, F.; Gabdoulline, R.R.; Menziani, M.C.; Wade, R.C. Blue Copper Proteins: A Comparative Analysis of Their Molecular Interaction Properties. Protein Sci. Publ. Protein Soc. 2000, 9, 1439–1454. [Google Scholar] [CrossRef]
- Tsai, L.-C.; Sjölin, L.; Langer, V.; Bonander, N.; Karlsson, B.G.; Vänngård, T.; Hammann, C.; Nar, H. Structure of the Azurin Mutant Nickel–Trp48Met from Pseudomonas Aeruginosa at 2.2 Å Resolution. Acta Crystallogr. D Biol. Crystallogr. 1995, 51, 711–717. [Google Scholar] [CrossRef]
- Silvestrini, M.C.; Falcinelli, S.; Ciabatti, I.; Cutruzzolà, F.; Brunori, M. Pseudomonas Aeruginosa Nitrite Reductase (or Cytochrome Oxidase): An Overview. Biochimie 1994, 76, 641–654. [Google Scholar] [CrossRef] [PubMed]
- van Gastel, M.; Coremans, J.W.A.; Mol, J.; Jeuken, L.J.C.; Canters, G.W.; Groenen, E.J.J. The Binding of Imidazole in an Azurin-like Blue-Copper Site. JBIC J. Biol. Inorg. Chem. 1999, 4, 257–265. [Google Scholar] [CrossRef]
- Leckner, J.; Bonander, N.; Wittung-Stafshede, P.; Malmström, B.G.; Karlsson, B.G. The Effect of the Metal Ion on the Folding Energetics of Azurin: A Comparison of the Native, Zinc and Apoprotein. Biochim. Biophys. Acta BBA-Protein Struct. Mol. Enzymol. 1997, 1342, 19–27. [Google Scholar] [CrossRef]
- Rosen, P.; Pecht, I. Conformational Equilibria Accompanying the Electron Transfer between Cytochrome c (P551) and Azurin from Pseudomonas Aeruginosa. Biochemistry 1976, 15, 775–786. [Google Scholar] [CrossRef]
- Dennison, C. Investigating the Structure and Function of Cupredoxins. Coord. Chem. Rev. 2005, 249, 3025–3054. [Google Scholar] [CrossRef]
- Gewirth, A.A.; Solomon, E.I. Electronic Structure of Plastocyanin: Excited State Spectral Features. J. Am. Chem. Soc. 1988, 110, 3811–3819. [Google Scholar] [CrossRef]
- van de Kamp, M.; Hali, F.C.; Rosato, N.; Agro, A.F.; Canters, G.W. Purification and Characterization of a Non-Reconstitutable Azurin, Obtained by Heterologous Expression of the Pseudomonas Aeruginosa Azu Gene in Escherichia Coli. Biochim. Biophys. Acta 1990, 1019, 283–292. [Google Scholar] [CrossRef]
- Margoliash, E.; Schejter, A. Cytochrome c. In Advances in Protein Chemistry; Anfinsen, C.B., Anson, M.L., Edsall, J.T., Richards, F.M., Eds.; Academic Press: Cambridge, MA, USA, 1966; Volume 21, pp. 113–286. [Google Scholar]
- Augustin, M.A.; Chapman, S.K.; Davies, D.M.; Sykes, A.G.; Speck, S.H.; Margoliash, E. Interaction of Cytochrome c with the Blue Copper Proteins, Plastocyanin and Azurin. J. Biol. Chem. 1983, 258, 6405–6409. [Google Scholar] [CrossRef] [PubMed]
- Bunkute, E.; Cummins, C.; Crofts, F.J.; Bunce, G.; Nabney, I.T.; Flower, D.R. PIP-DB: The Protein Isoelectric Point Database. Bioinformatics 2015, 31, 295–296. [Google Scholar] [CrossRef]
- Slater, J.C. Atomic Radii in Crystals. J. Chem. Phys. 1964, 41, 3199–3204. [Google Scholar] [CrossRef]
- Bonander, N.; Vänngård, T.; Tsai, L.-C.; Langer, V.; Nar, H.; Sjölin, L. The Metal Site of Pseudomonas Aeruginosa Azurin, Revealed by a Crystal Structure Determination of the Co(II) Derivative and Co-EPR Spectroscopy. Proteins Struct. Funct. Bioinform. 1997, 27, 385–394. [Google Scholar] [CrossRef]
- McLaughlin, M.P.; Retegan, M.; Bill, E.; Payne, T.M.; Shafaat, H.S.; Peña, S.; Sudhamsu, J.; Ensign, A.A.; Crane, B.R.; Neese, F.; et al. Azurin as a Protein Scaffold for a Low-Coordinate Nonheme Iron Site with a Small-Molecule Binding Pocket. J. Am. Chem. Soc. 2012, 134, 19746–19757. [Google Scholar] [CrossRef]
- Zampino, A.P.; Masters, F.M.; Bladholm, E.L.; Panzner, M.J.; Berry, S.M.; Leeper, T.C.; Ziegler, C.J. Mercury Metallation of the Copper Protein Azurin and Structural Insight into Possible Heavy Metal Reactivity. J. Inorg. Biochem. 2014, 141, 152–160. [Google Scholar] [CrossRef]
- Nar, H.; Huber, R.; Messerschmidt, A.; Filippou, A.C.; Barth, M.; Jaquinod, M.; van de Kamp, M.; Canters, G.W. Characterization and Crystal Structure of Zinc Azurin, a by-Product of Heterologous Expression in Escherichia Coli of Pseudomonas Aeruginosa Copper Azurin. Eur. J. Biochem. 1992, 205, 1123–1129. [Google Scholar] [CrossRef]
- Bhagi-Damodaran, A.; Lu, Y. The Periodic Table’s Impact on Bioinorganic Chemistry and Biology’s Selective Use of Metal Ions. In The Periodic Table II: Catalytic, Materials, Biological and Medical Applications; Mingos, D.M.P., Ed.; Structure and Bonding; Springer International Publishing: Cham, Switzerland, 2019; pp. 153–173. ISBN 978-3-030-40010-1. [Google Scholar]
- Amdursky, N.; Sepunaru, L.; Raichlin, S.; Pecht, I.; Sheves, M.; Cahen, D. Electron Transfer Proteins as Electronic Conductors: Significance of the Metal and Its Binding Site in the Blue Cu Protein, Azurin. Adv. Sci. 2015, 2, 1400026. [Google Scholar] [CrossRef] [PubMed]
- Lawrance, G.A. Introduction to Coordination Chemistry; John Wiley & Sons: Hoboken, NJ, USA, 2013; ISBN 978-1-118-68140-4. [Google Scholar]
- Maret, W. Metalloproteomics, Metalloproteomes, and the Annotation of Metalloproteins. Met. Integr. Biometal Sci. 2010, 2, 117–125. [Google Scholar] [CrossRef]
- Clementi, E.; Raimondi, D.L.; Reinhardt, W.P. Atomic Screening Constants from SCF Functions. II. Atoms with 37 to 86 Electrons. J. Chem. Phys. 1967, 47, 1300–1307. [Google Scholar] [CrossRef]
- Pascher, T.; Karlsson, B.G.; Nordling, M.; Malmström, B.G.; Vänngård, T. Reduction Potentials and Their pH Dependence in Site-Directed-Mutant Forms of Azurin from Pseudomonas Aeruginosa. Eur. J. Biochem. 1993, 212, 289–296. [Google Scholar] [CrossRef]
- Di Bilio, A.J.; Chang, T.K.; Malmström, B.G.; Gray, H.B.; Göran Karlsson, B.; Nordling, M.; Pascher, T.; Lundberg, L.G. Electronic Absorption Spectra of M(II)(Met121X) Azurins (MCo, Ni, Cu; XLeu, Gly, Asp, Glu): Charge-Transfer Energies and Reduction Potentials. Inorganica Chim. Acta 1992, 198–200, 145–148. [Google Scholar] [CrossRef]
- Garner, D.K.; Vaughan, M.D.; Hwang, H.J.; Savelieff, M.G.; Berry, S.M.; Honek, J.F.; Lu, Y. Reduction Potential Tuning of the Blue Copper Center in Pseudomonas Aeruginosa Azurin by the Axial Methionine as Probed by Unnatural Amino Acids. J. Am. Chem. Soc. 2006, 128, 15608–15617. [Google Scholar] [CrossRef]
- Lowery, M.D.; Solomon, E.I. Axial Ligand Bonding in Blue Copper Proteins. Inorganica Chim. Acta 1992, 198–200, 233–243. [Google Scholar] [CrossRef]
- Marshall, N.M.; Garner, D.K.; Wilson, T.D.; Gao, Y.-G.; Robinson, H.; Nilges, M.J.; Lu, Y. Rationally Tuning the Reduction Potential of a Single Cupredoxin beyond the Natural Range. Nature 2009, 462, 113–116. [Google Scholar] [CrossRef]
- Winkler, J.R.; Gray, H.B. Electron Flow through Metalloproteins. Chem. Rev. 2014, 114, 3369–3380. [Google Scholar] [CrossRef]
- Voityuk, A.A. Long-Range Electron Transfer in Biomolecules. Tunneling or Hopping? J. Phys. Chem. B 2011, 115, 12202–12207. [Google Scholar] [CrossRef]
- Gray, H.B.; Winkler, J.R. Long-Range Electron Transfer. Proc. Natl. Acad. Sci. USA 2005, 102, 3534–3539. [Google Scholar] [CrossRef] [PubMed]
- Prytkova, T.R.; Kurnikov, I.V.; Beratan, D.N. Coupling Coherence Distinguishes Structure Sensitivity in Protein Electron Transfer. Science 2007, 315, 622–625. [Google Scholar] [CrossRef] [PubMed]
- Takematsu, K.; Williamson, H.R.; Nikolovski, P.; Kaiser, J.T.; Sheng, Y.; Pospíšil, P.; Towrie, M.; Heyda, J.; Hollas, D.; Záliš, S.; et al. Two Tryptophans Are Better Than One in Accelerating Electron Flow through a Protein. ACS Cent. Sci. 2019, 5, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Walden, S.E.; Wheeler, R.A. Distinguishing Features of Indolyl Radical and Radical Cation: Implications for Tryptophan Radical Studies. J. Phys.Chem. 1996, 100, 1530–1535. [Google Scholar] [CrossRef]
| Azurin (Metal) | pI | Calculated M Atomic Radius | M-Nε (His46) | M-Sγ (Cys112) | M-Nε (His117) | M-Sδ (Met121) | M-O (Gly45) | PDB |
|---|---|---|---|---|---|---|---|---|
| Cu(II) | 5.5 | 1.45 | 2.11 | 2.27 | 1.99 | 3.18 | 2.84 | 4AZU |
| Cu(I) | 4.6 | - | 2.05 | 2.30 | 1.98 | 3.16 | 3.11 | 1E5Y |
| Ni(II) | 5.7 | 1.49 | 2.15 | 2.49 | 2.07 | 3.34 | 3.35 | 1NZR |
| Co(II) | 5.7 | 1.52 | 2.39 | 2.34 | 2.27 | 3.56 | 2.23 | 1VLX |
| Zn(II) | 5.7 | 1.42 | 2.07 | 2.30 | 2.01 | 3.38 | 2.32 | 1E67 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tuzhilkin, R.; Ondruška, V.; Šulc, M. Azurin: A Model to Study a Metal Coordination Sphere or Electron Transfer in Metalloproteins. Int. J. Mol. Sci. 2025, 26, 4125. https://doi.org/10.3390/ijms26094125
Tuzhilkin R, Ondruška V, Šulc M. Azurin: A Model to Study a Metal Coordination Sphere or Electron Transfer in Metalloproteins. International Journal of Molecular Sciences. 2025; 26(9):4125. https://doi.org/10.3390/ijms26094125
Chicago/Turabian StyleTuzhilkin, Roman, Vladimír Ondruška, and Miroslav Šulc. 2025. "Azurin: A Model to Study a Metal Coordination Sphere or Electron Transfer in Metalloproteins" International Journal of Molecular Sciences 26, no. 9: 4125. https://doi.org/10.3390/ijms26094125
APA StyleTuzhilkin, R., Ondruška, V., & Šulc, M. (2025). Azurin: A Model to Study a Metal Coordination Sphere or Electron Transfer in Metalloproteins. International Journal of Molecular Sciences, 26(9), 4125. https://doi.org/10.3390/ijms26094125






