Long-Term Moderate Increase in Medium-Chain Fatty Acids Intake Enhances Muscle Metabolism and Function in Mice
Abstract
1. Introduction
2. Results
2.1. Systemic Metabolic and Physiological Responses to MCFA Intake
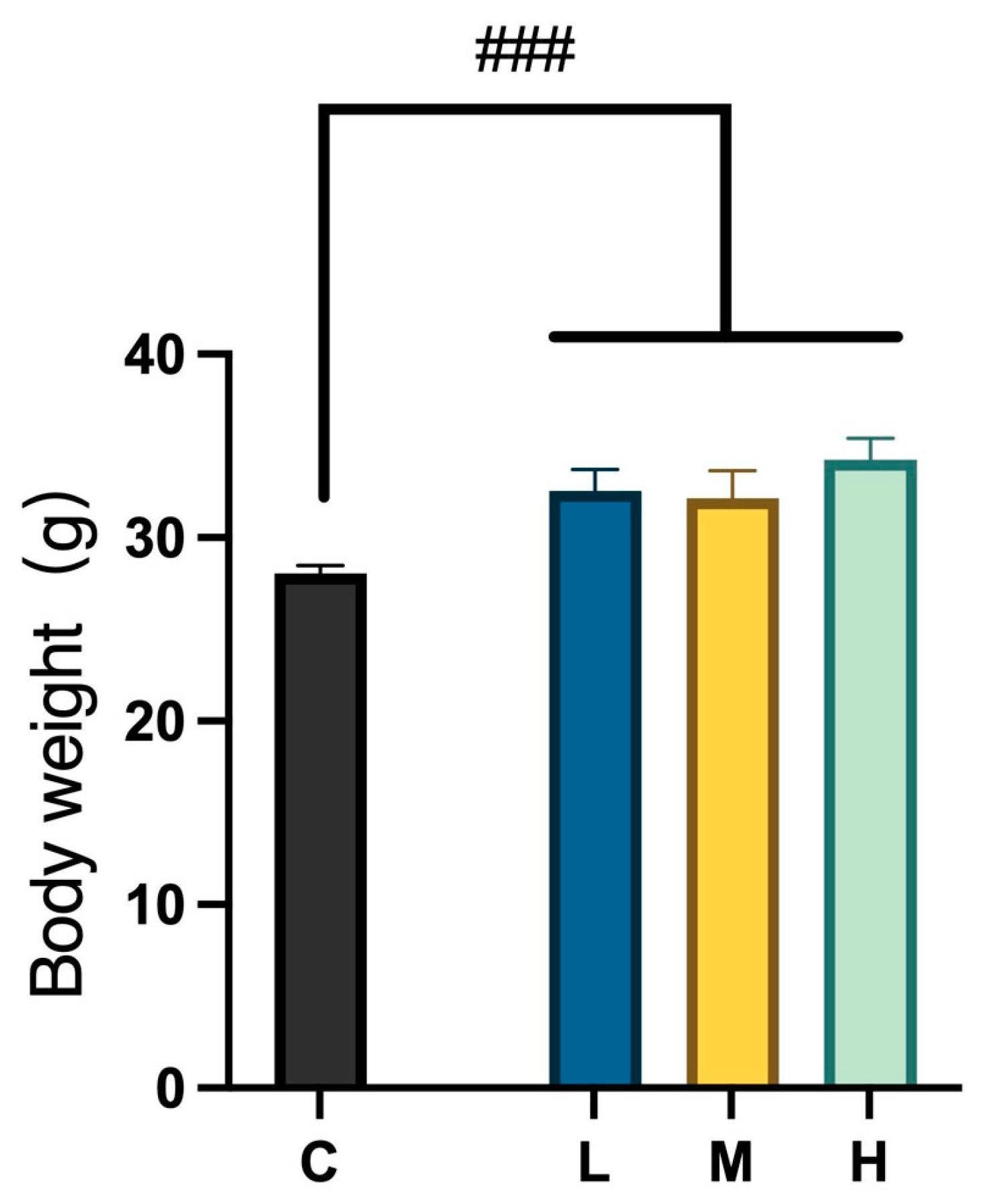
2.1.1. Food and Water Intake and Body Weight Changes During MCFA Administration
2.1.2. Changes in Organ and Tissue Mass Following MCFA Intake
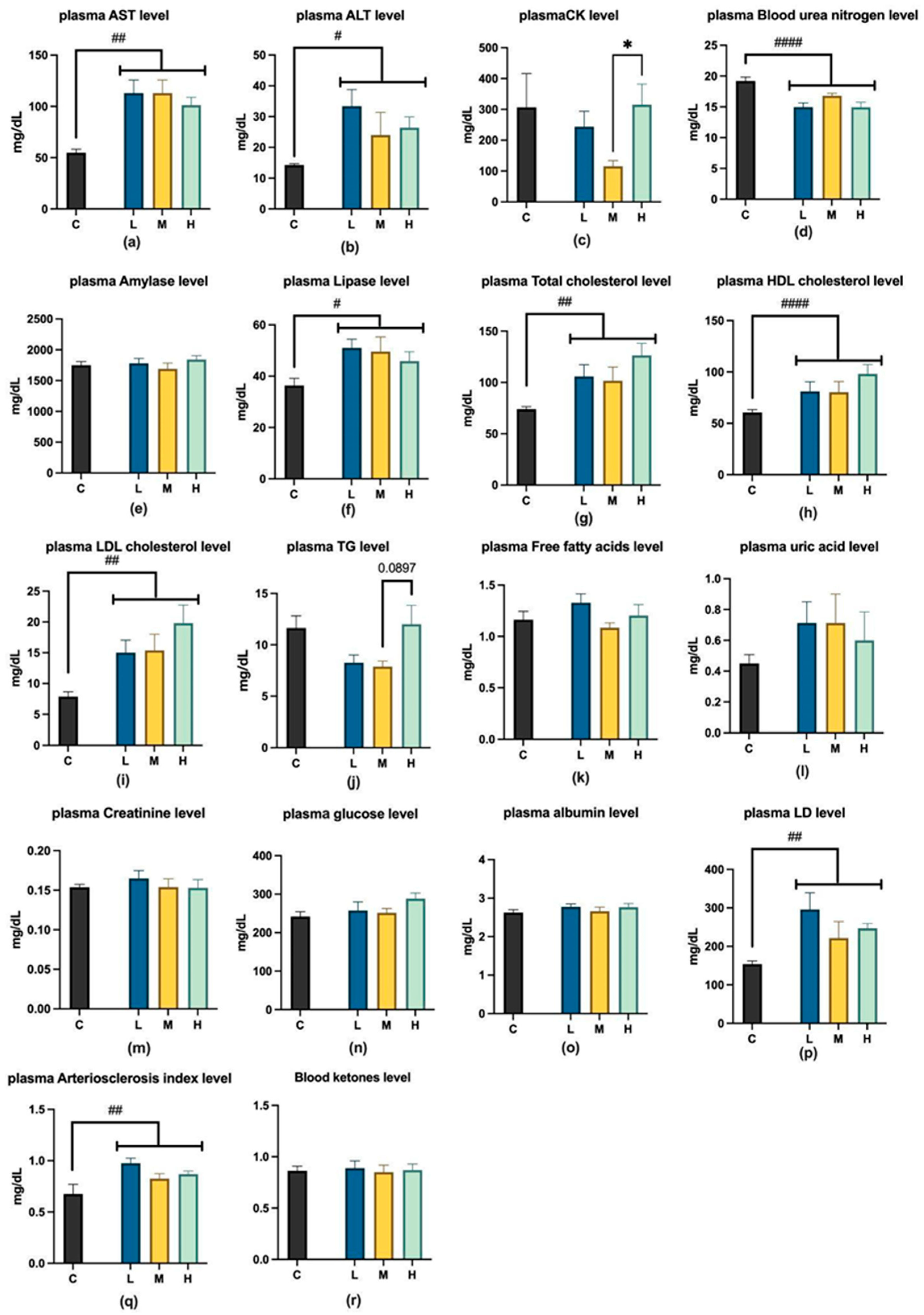
2.1.3. Analysis of Visceral Function and Energy Metabolites
2.2. MCFA Effects on Skeletal Muscle Function
2.2.1. Assessment of Forelimb Grip Strength
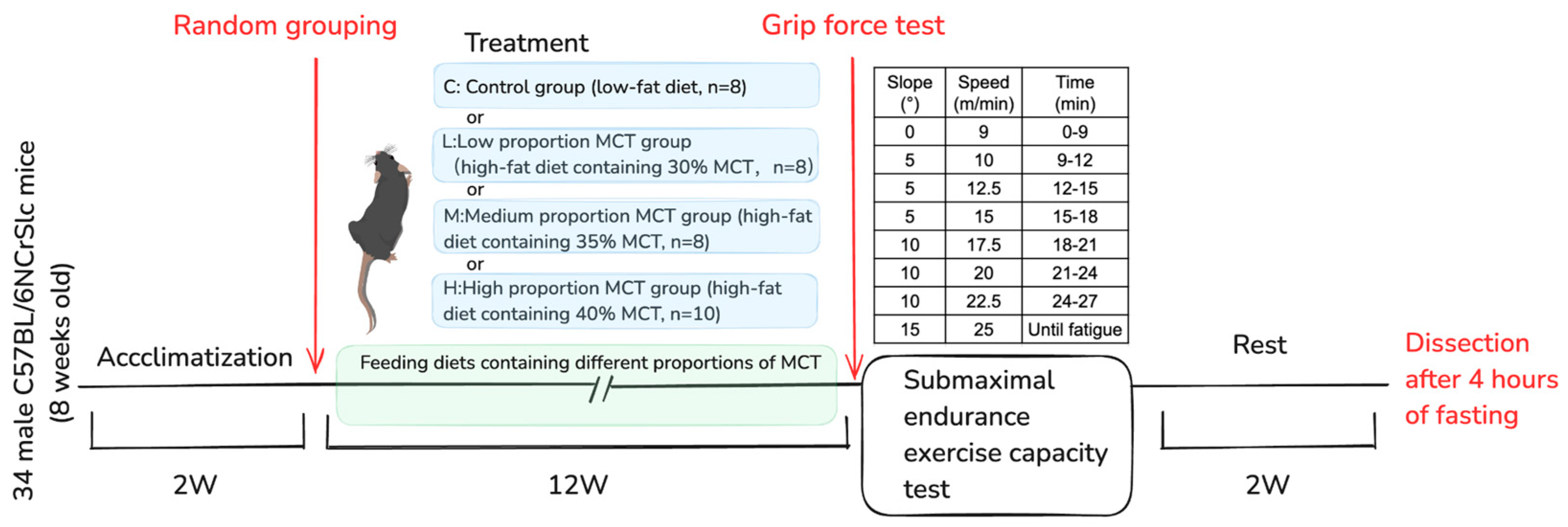
2.2.2. Evaluation of Endurance Exercise Capacity
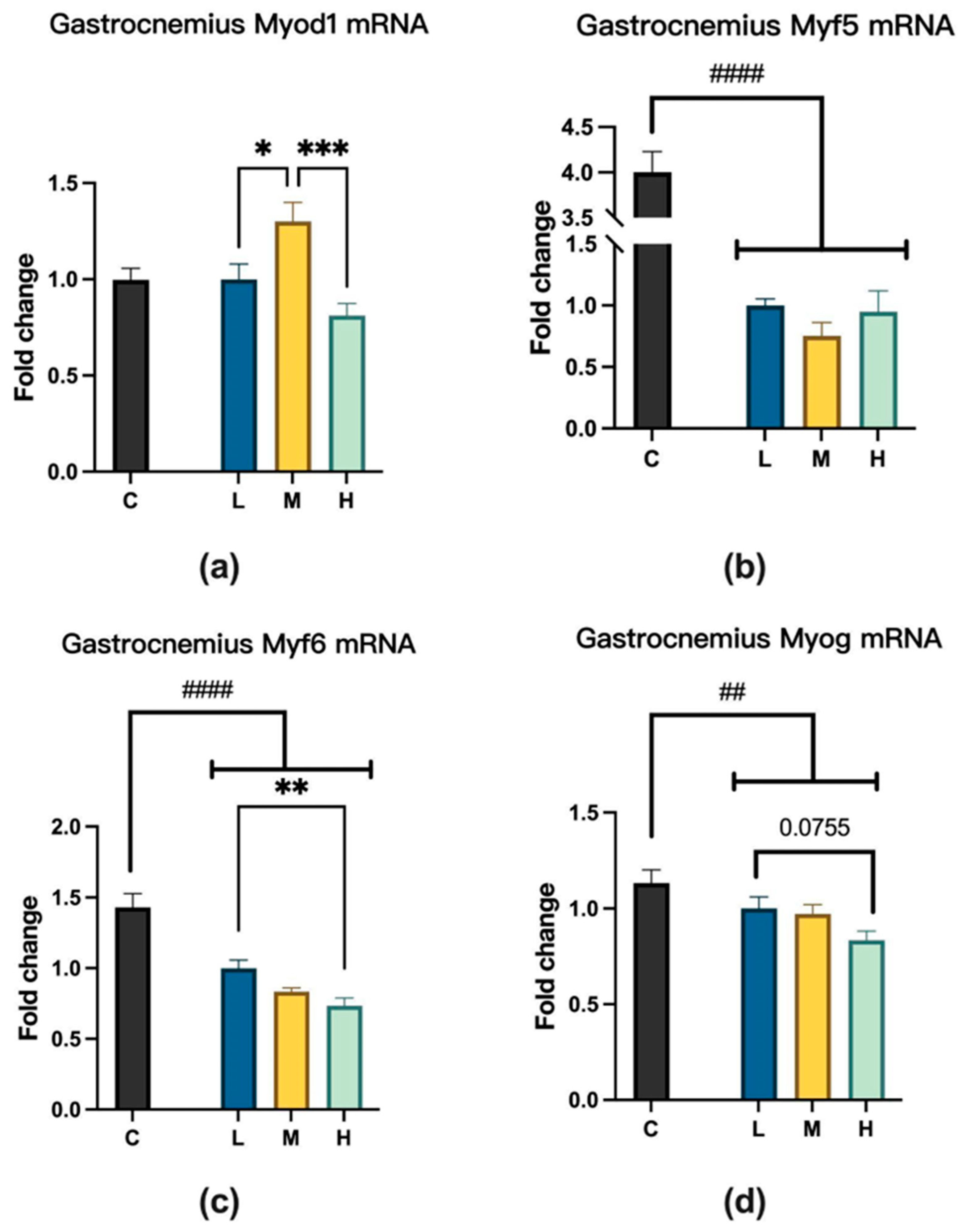
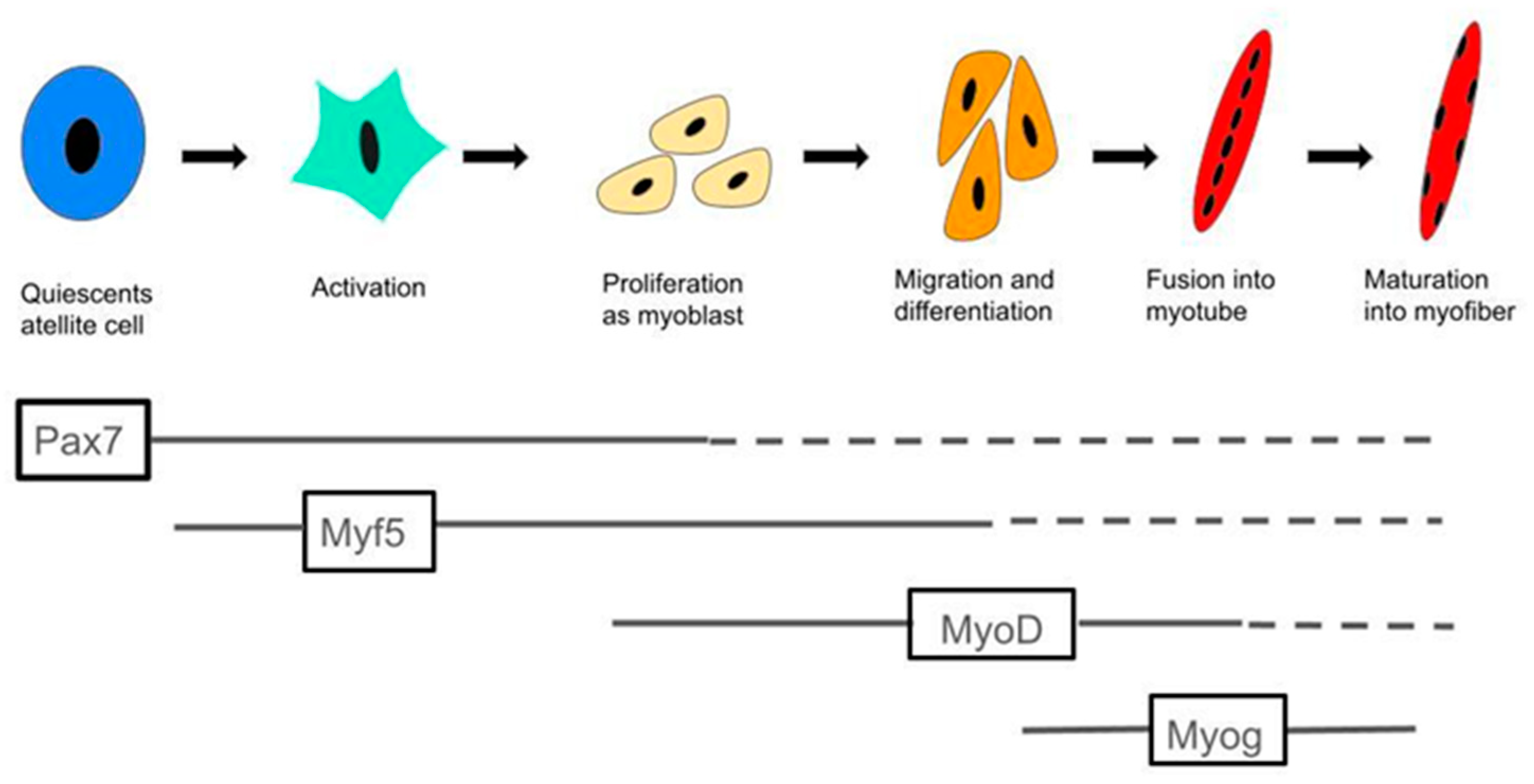
2.3. MCFA Regulation of Muscle Satellite Cell Activity
2.4. Myosin Heavy-Chain Isoform Changes After MCFA Treatment
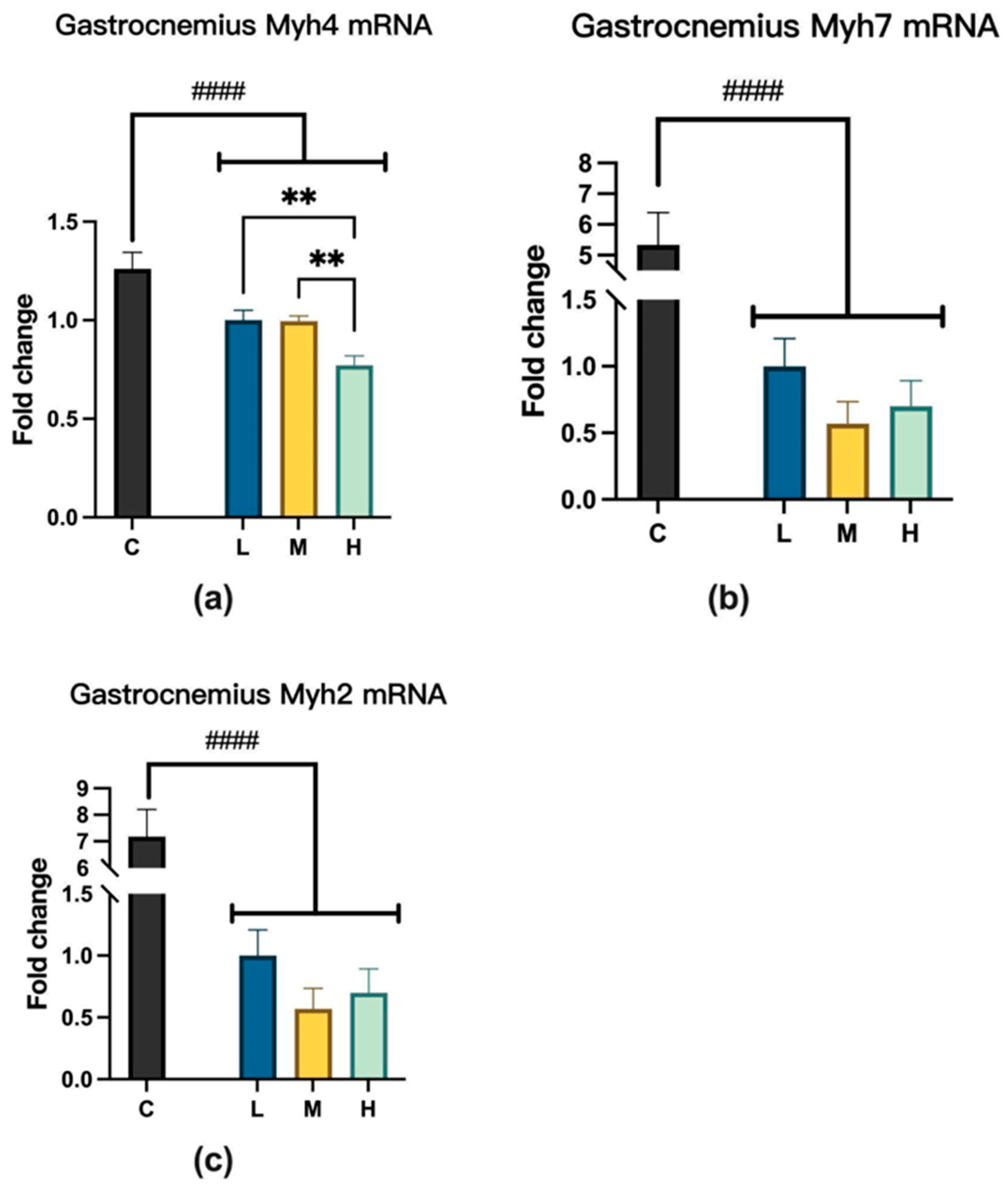
2.4.1. Myosin Heavy Chain mRNA Expression
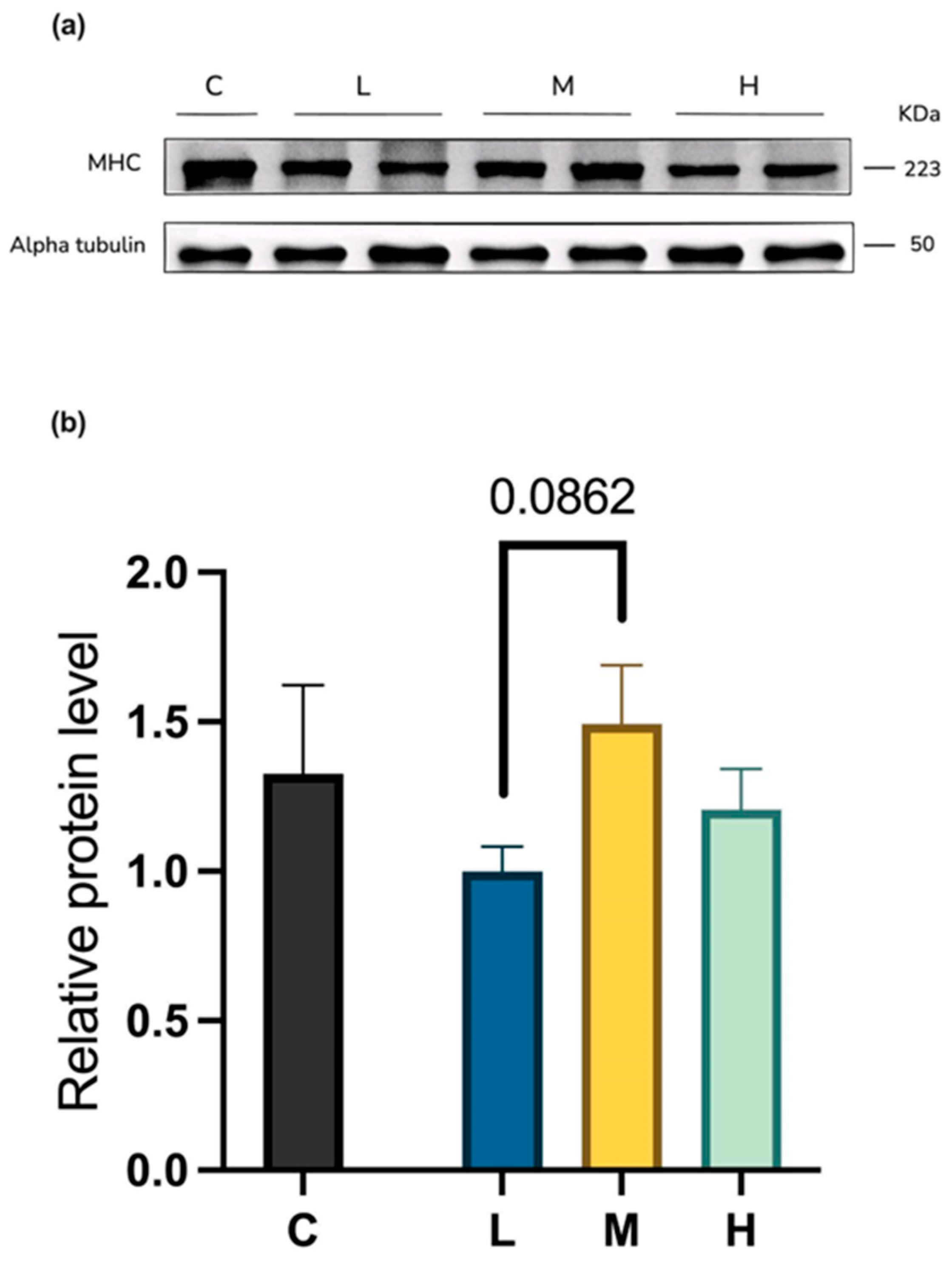
2.4.2. Myosin Heavy-Chain Protein Levels
2.5. MCFA Effects on the Muscle Protein Degradation Pathway
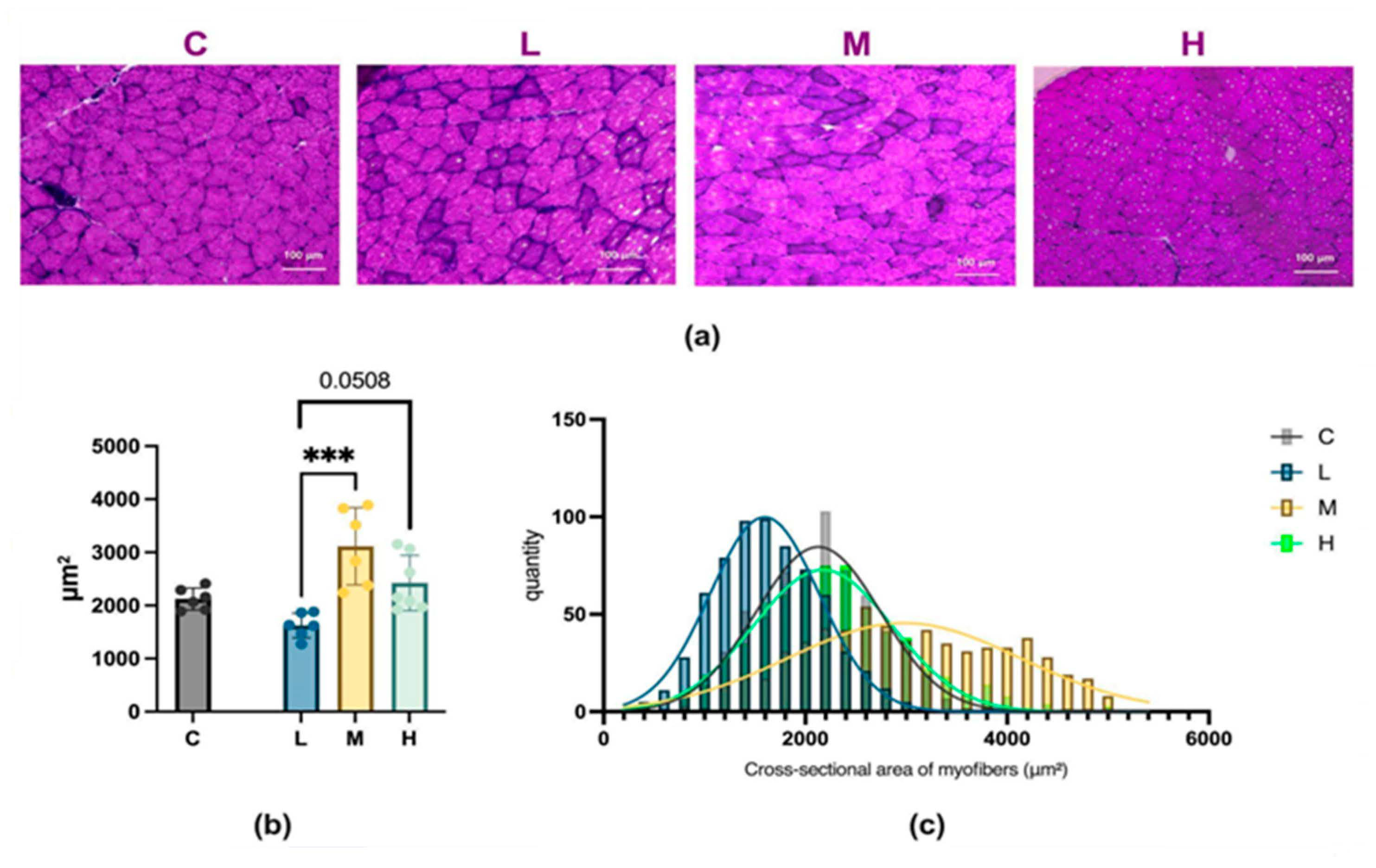
2.6. Analysis of Muscle Fiber Size Following MCFA Administration
3. Discussion
4. Materials and Methods
4.1. Experimental Animals and Ethical Approval
4.2. Diet Composition and Experimental Groups
4.3. Assessment of Skeletal Muscle Function
4.4. Real-Time Quantitative Polymerase Chain Reaction (RT-qPCR) for Gene Expression Analysis
4.5. Muscle Morphology and Immunohistochemistry
4.6. Protein Expression Analysis
4.7. Data Analysis and Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| MCFAs | medium-chain fatty acids |
| MCT | medium-chain triglycerides |
| LCFA | long-chain fatty acids |
| MRFs | muscle regulatory factors |
| Myod1 | myogenic differentiation 1 |
| Myf6 | myogenic factor 6 |
| Myog | myogenin |
| Myf5 | myogenic factor 5 |
| MYH | myosin heavy chain |
| Myh4 | myosin heavy chain 4 |
| Myh7 | myosin heavy chain 7 |
| Myh2 | myosin heavy chain 2 |
| MHC | myosin heavy chain |
| Fbxo32 | F-box protein 32 |
| Trim63 | tripartite motif-containing 63 |
References
- Massaro, E.J. The molecular basis of embryonic development. Investig. Ophthalmol. 1969, 8, 3–16. [Google Scholar]
- Hashim, S.A.; Tantibhedyangkul, P. Medium chain triglyceride in early life: Effects on growth of adipose tissue. Lipids 1987, 22, 429–434. [Google Scholar] [CrossRef] [PubMed]
- Schönfeld, P.; Wojtczak, L. Short- and medium-chain fatty acids in energy metabolism: The cellular perspective. J. Lipid Res. 2016, 57, 943–954. [Google Scholar] [CrossRef] [PubMed]
- Ashbrook, J.D.; Spectro, A.A.; Fletcher, J.E. Medium chain fatty acid binding to human plasma albumin. J. Biol. Chem. 1972, 247, 7038–7042. [Google Scholar] [CrossRef]
- Greenberger, N.J.; Rodgers, J.B.; Isselbacher, K.J. Absorption of medium and long chain triglycerides: Factors influencing their hydrolysis and transport. J. Clin. Investig. 1966, 45, 217–227. [Google Scholar] [CrossRef] [PubMed]
- Papamandjaris, A.A.; MacDougall, D.E.; Jones, P.J. Medium chain fatty acid metabolism and energy expenditure: Obesity treatment implications. Life Sci. 1998, 62, 1203–1215. [Google Scholar] [CrossRef]
- Longo, N.; Frigeni, M.; Pasquali, M. Carnitine transport and fatty acid oxidation. Biochim. Biophys. Acta 2016, 1863, 2422–2435. [Google Scholar] [CrossRef]
- Huang, L.; Gao, L.; Chen, C. Role of Medium-Chain Fatty Acids in Healthy Metabolism: A Clinical Perspective. Trends Endocrinol. Metab. 2021, 32, 351–366. [Google Scholar] [CrossRef]
- Merritt JL2nd Norris, M.; Kanungo, S. Fatty acid oxidation disorders. Ann. Transl. Med. 2018, 6, 473. [Google Scholar] [CrossRef]
- Guo, J.; Yan, E.; He, L.; Wang, Y.; Xiang, Y.; Zhang, P.; Liu, X.; Yin, J. Dietary Supplementation with Lauric Acid Improves Aerobic Endurance in Sedentary Mice via Enhancing Fat Mobilization and Glyconeogenesis. J. Nutr. 2023, 153, 3207–3219. [Google Scholar] [CrossRef]
- Fushiki, T.; Matsumoto, K.; Inoue, K.; Kawada, T.; Sugimoto, E. Swimming endurance capacity of mice is increased by chronic consumption of medium-chain triglycerides. J. Nutr. 1995, 125, 531–539. [Google Scholar] [CrossRef]
- Nosaka, N.; Suzuki, Y.; Nagatoishi, A.; Kasai, M.; Wu, J.; Taguchi, M. Effect of ingestion of medium-chain triacylglycerols on moderate- and high-intensity exercise in recreational athletes. J. Nutr. Sci. Vitaminol. 2009, 55, 120–125. [Google Scholar] [CrossRef] [PubMed]
- Fukazawa, A.; Koike, A.; Karasawa, T.; Tsutsui, M.; Kondo, S.; Terada, S. Effects of a Ketogenic Diet Containing Medium-Chain Triglycerides and Endurance Training on Metabolic Enzyme Adaptations in Rat Skeletal Muscle. Nutrients 2020, 12, 1269. [Google Scholar] [CrossRef] [PubMed]
- Abe, S.; Ezaki, O.; Suzuki, M. Medium-chain triglycerides (8:0 and 10:0) are promising nutrients for sarcopenia: A randomized controlled trial. Am. J. Clin. Nutr. 2019, 110, 652–665. [Google Scholar] [CrossRef]
- Abe, S.; Ezaki, O.; Suzuki, M. Medium-Chain Triglycerides in Combination with Leucine and Vitamin D Increase Muscle Strength and Function in Frail Elderly Adults in a Randomized Controlled Trial. J. Nutr. 2016, 146, 1017–1026. [Google Scholar] [CrossRef] [PubMed]
- Kojima, K.; Ishikawa, H.; Watanabe, S.; Nosaka, N.; Mutoh, T. A Randomized, Double-Blind, Controlled Trial Assessing If Medium-Chain Triglycerides in Combination with Moderate-Intensity Exercise Increase Muscle Strength in Healthy Middle-Aged and Older Adults. Nutrients. 2023, 15, 3275. [Google Scholar] [CrossRef]
- Zhou, S.; Wang, Y.; Jiang, Y.; Zhang, Z.; Sun, X.; Yu, L.L. Dietary Intake of Structured Lipids with Different Contents of Medium-Chain Fatty Acids on Obesity Prevention in C57BL/6J Mice. J. Food Sci. 2017, 82, 1968–1977. [Google Scholar] [CrossRef]
- Montgomery, M.K.; Osborne, B.; Brown, S.H.J.; Small, L.; Mitchell, T.W.; Cooney, G.J.; Turner, N. Contrasting metabolic effects of medium- versus long-chain fatty acids in skeletal muscle. J. Lipid Res. 2013, 54, 3322–3333. [Google Scholar] [CrossRef]
- Turner, N.; Hariharan, K.; TidAng, J.; Frangioudakis, G.; Beale, S.M.; Wright, L.E.; Zeng, X.Y.; Leslie, S.J.; Li, J.-Y.; Kraegen, E.W.; et al. Enhancement of muscle mitochondrial oxidative capacity and alterations in insulin action are lipid species dependent: Potent tissue-specific effects of medium-chain fatty acids. Diabetes 2009, 58, 2547–2554, Erratum in Diabetes 2010, 59, 1283. [Google Scholar] [CrossRef]
- Zhou, S.; Wang, Y.; Jiang, Y.; Yu, L.L. Safety assessment of medium- and long-chain triacylglycerols containing 30% (w/w) medium-chain fatty acids in mice and rats. Regul. Toxicol. Pharmacol. 2017, 86, 42–48. [Google Scholar] [CrossRef]
- Traul, K.A.; Driedger, A.; Ingle, D.L.; Nakhasi, D. Review of the toxicologic properties of medium-chain triglycerides. Food Chem. Toxicol. 2000, 38, 79–98. [Google Scholar] [CrossRef] [PubMed]
- Mänttäri, S.; Järvilehto, M. Comparative analysis of mouse skeletal muscle fibre type composition and contractile responses to calcium channel blocker. BMC Physiol. 2005, 5, 4. [Google Scholar] [CrossRef]
- Weintraub, H.; Davis, R.; Tapscott, S.; Thayer, M.; Krause, M.; Benezra, R.; Blackwell, T.K.; Turner, D.; Rupp, R.; Hollenberg, S.; et al. The myoD gene family: Nodal point during specification of the muscle cell lineage. Science. 1991, 251, 761–766. [Google Scholar] [CrossRef] [PubMed]
- Sassoon, D.; Lyons, G.E.; Wright, W.E.; Lin, V.; Lassar, A.B.; Weintraub, H.; Buckingham, M. Expression of two myogenic regulatory factors myogenin and MyoD1 during mouse embryogenesis. Nature. 1989, 341, 303–307. [Google Scholar] [CrossRef]
- Wood, W.M.; Etemad, S.; Yamamoto, M.; Goldhamer, D.J. MyoD-expressing progenitors are essential for skeletal myogenesis and satellite cell development. Dev. Biol. 2013, 384, 114–127. [Google Scholar] [CrossRef]
- Shirakawa, T.; Toyono, T.; Inoue, A.; Matsubara, T.; Kawamoto, T.; Kokabu, S. Factors Regulating or Regulated by Myogenic Regulatory Factors in Skeletal Muscle Stem Cells. Cells. 2022, 11, 1493. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, M.; Legendre, N.P.; Biswas, A.A.; Lawton, A.; Yamamoto, S.; Tajbakhsh, S.; Kardon, G.; Goldhamer, D.J. Loss of MyoD and Myf5 in Skeletal Muscle Stem Cells Results in Altered Myogenic Programming and Failed Regeneration. Stem Cell Rep. 2018, 10, 956–969. [Google Scholar] [CrossRef]
- Ganassi, M.; Badodi, S.; Wanders, K.; Zammit, P.S.; Hughes, S.M. Myogenin is an essential regulator of adult myofibre growth and muscle stem cell homeostasis. Elife 2020, 9, e60445. [Google Scholar] [CrossRef]
- Lyudinina, A.Y.; Ivankova, G.E.; Bojko, E.R. Priority use of medium-chain fatty acids during high-intensity exercise in cross-country skiers. J. Int. Soc. Sports Nutr. 2018, 15, 57. [Google Scholar] [CrossRef]
- Chargé, S.B.; Rudnicki, M.A. Cellular and molecular regulation of muscle regeneration. Physiol. Rev. 2004, 84, 209–238. [Google Scholar] [CrossRef]
- Sabourin, L.A.; Girgis-Gabardo, A.; Seale, P.; Asakura, A.; Rudnicki, M.A. Reduced differentiation potential of primary MyoD-/- myogenic cells derived from adult skeletal muscle. J. Cell Biol. 1999, 144, 631–643. [Google Scholar] [CrossRef] [PubMed]
- Le Grand, F.; Rudnicki, M.A. Skeletal muscle satellite cells and adult myogenesis. Curr. Opin. Cell Biol. 2007, 19, 628–633. [Google Scholar] [CrossRef] [PubMed]
- Beylkin, D.H.; Allen, D.L.; Leinwand, L.A. MyoD, Myf5, and the calcineurin pathway activate the developmental myosin heavy chain genes. Dev. Biol. 2006, 294, 541–553. [Google Scholar] [CrossRef]
- Bergstrom, D.A.; Penn, B.H.; Strand, A.; Perry, R.L.; Rudnicki, M.A.; Tapscott, S.J. Promoter-specific regulation of MyoD binding and signal transduction cooperate to pattern gene expression. Mol. Cell. 2002, 9, 587–600. [Google Scholar] [CrossRef]
- Astrup, A.; Magkos, F.; Bier, D.M.; Brenna, J.T.; De Oliveira Otto, M.C.; Hill, J.O.; King, J.C.; Mente, A.; Ordovas, J.M.; Volek, J.S.; et al. Saturated Fats and Health: A Reassessment and Proposal for Food-Based Recommendations: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2020, 76, 844–857. [Google Scholar] [CrossRef]
- Nukaga, S.; Fujiwara-Tani, R.; Nishida, R.; Miyagawa, Y.; Goto, K.; Kawahara, I.; Nakashima, C.; Fujii, K.; Ogata, R.; Ohmori, H.; et al. Caprylic Acid Inhibits High Mobility Group Box-1-Induced Mitochondrial Damage in Myocardial Tubes. Int. J. Mol. Sci. 2024, 25, 8081. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pang, X.; Zhang, P.; Chen, X.; Liu, W. Ubiquitin-proteasome pathway in skeletal muscle atrophy. Front. Physiol. 2023, 14, 1289537. [Google Scholar] [CrossRef]
- Mori, T.; Ohmori, H.; Luo, Y.; Mori, S.; Miyagawa, Y.; Nukaga, S.; Goto, K.; Fujiwara-Tani, R.; Kishi, S.; Sasaki, T.; et al. Giving combined medium-chain fatty acids and glucose protects against cancer-associated skeletal muscle atrophy. Cancer Sci. 2019, 110, 3391–3399. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bodine, S.C.; Latres, E.; Baumhueter, S.; Lai, V.K.-M.; Nunez, L.; Clarke, B.A.; Poueymirou, W.T.; Panaro, F.J.; Na, E.; Dharmarajan, K.; et al. Identification of ubiquitin ligases required for skeletal muscle atrophy. Science. 2001, 294, 1704–1708. [Google Scholar] [CrossRef]
- Tong, Y.; Huang, J.; Wang, S.; Awa, R.; Tagawa, T.; Zhang, Z.; Cao, T.; Kobori, H.; Suzuki, K. Effects of 3-(4-Hydroxy-3-methoxyphenyl)propionic Acid on Enhancing Grip Strength and Inhibiting Protein Catabolism Induced by Exhaustive Exercise. Int. J. Mol. Sci. 2024, 25, 6627. [Google Scholar] [CrossRef]
- Akhmedov, D.; Berdeaux, R. The effects of obesity on skeletal muscle regeneration. Front. Physiol. 2013, 4, 371. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Tong, Y.; Wang, S.; Tagawa, T.; Seki, Y.; Ma, S.; Zhang, Z.; Cao, T.; Kobori, H.; Suzuki, K. 8-Week Kaempferia parviflora Extract Administration Improves Submaximal Exercise Capacity in Mice by Enhancing Skeletal Muscle Antioxidant Gene Expression and Plasma Antioxidant Capacity. Antioxidants 2024, 13, 1147. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Yang, J.; Tominaga, T.; Liu, C.; Suzuki, K. A Low-Carbohydrate Ketogenic Diet and Treadmill Training Enhanced Fatty Acid Oxidation Capacity but Did Not Enhance Maximal Exercise Capacity in Mice. Nutrients 2021, 13, 611. [Google Scholar] [CrossRef] [PubMed]
- Avila, J.J.; Kim, S.K.; Massett, M.P. Differences in Exercise Capacity and Responses to Training in 24 Inbred Mouse Strains. Front. Physiol. 2017, 8, 974. [Google Scholar] [CrossRef]



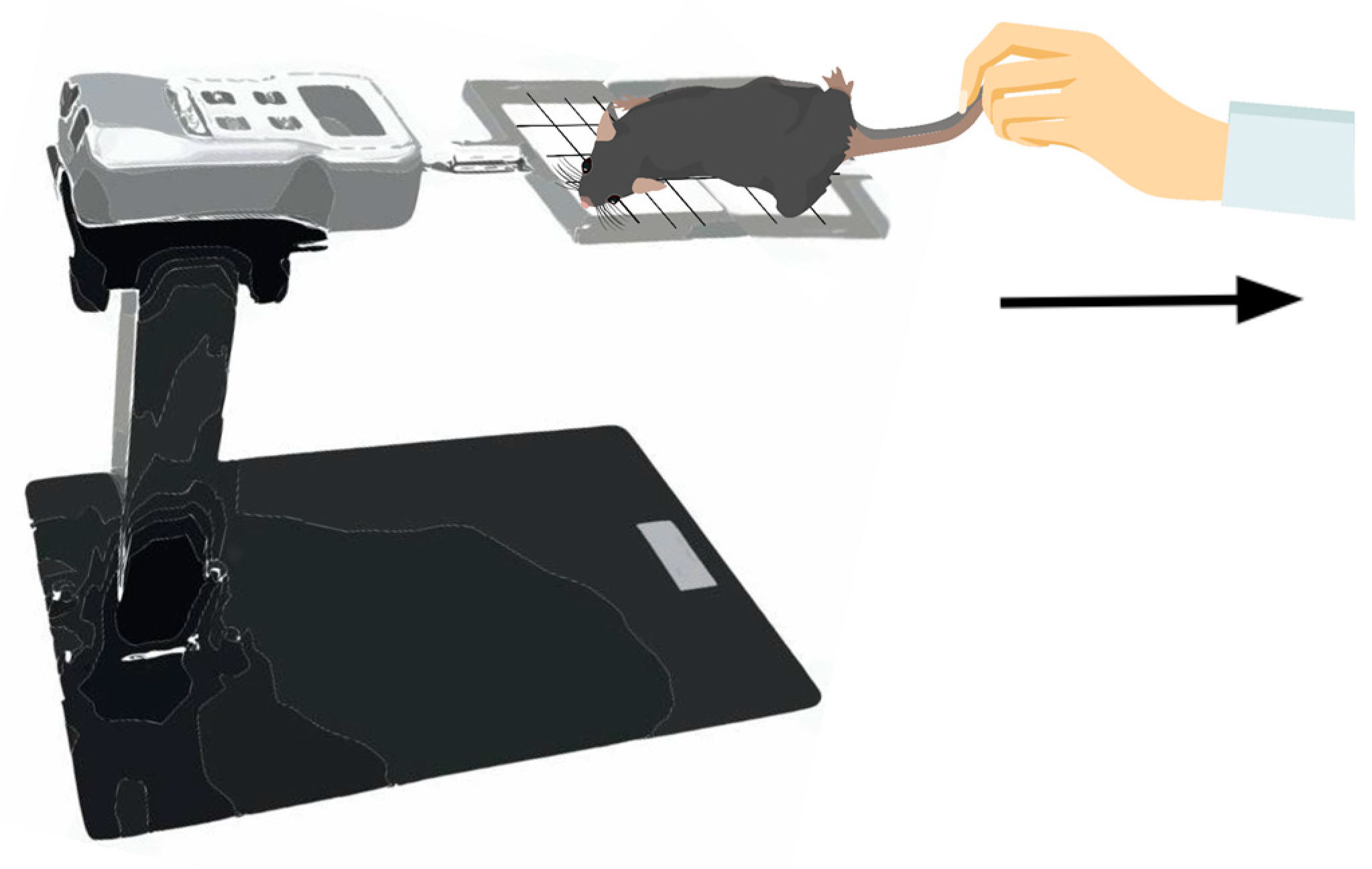
| C | L | M | |||
|---|---|---|---|---|---|
| W1 | Water | 60.20 ± 5.56 | 44.18 ± 8.58 | 41.55 ± 3.78 | 41.04 ± 4.44 |
| Feed | 49.62 ± 1.16 | 50.23 ± 0.68 | 49.81 ± 1.80 | 51.88 ± 1.49 | |
| W2 | Water | 54.45 ± 3.38 | 45.98 ± 6.34 | 40.45 ± 1.89 | 43.64 ± 2.75 |
| Feed | 48.80 ± 2.71 | 47.86 ± 2.12 | 48.46 ± 2.33 | 51.33 ± 2.24 | |
| W3 | Water | 53.50 ± 1.73 | 43.35 ± 8.83 | 41.33 ± 3.02 | 41.48 ± 11.32 |
| Feed | 49.98 ± 2.33 | 48.08 ± 1.05 | 49.00 ± 3.03 | 48.88 ± 3.34 | |
| W4 | Water | 53.63 ± 4.51 | 47.18 ± 8.45 | 46.28 ± 4.36 | 40.98 ± 5.70 |
| Feed | 47.53 ± 2.32 | 50.20 ± 1.63 | 49.45 ± 2.33 | 47.76 ± 4.38 | |
| W5 | Water | 52.98 ± 6.41 | 42.6 ± 10.15 | 41.33 ± 5.87 | 42.28 ± 7.66 |
| Feed | 47.78 ± 0.85 | 45.98 ± 0.39 | 46.78 ± 3.26 | 46.02 ± 4.85 | |
| W6 | Water | 53.58 ± 3.79 | 42.35 ± 10.58 | 42.53 ± 5.36 | 43.42 ± 7.56 |
| Feed | 47.58 ± 1.64 | 46.68 ± 1.67 | 46.20 ± 2.59 | 46.44 ± 3.90 | |
| W7 | Water | 51.30 ± 4.89 | 40.73 ± 13.20 | 36.05 ± 4.25 | 37.80 ± 6.55 |
| Feed | 46.40 ± 5.12 | 45.28 ± 6.37 | 43.23 ± 2.38 | 45.26 ± 2.78 | |
| W8 | Water | 51.28 ± 4.28 | 37.65 ± 12.00 | 35.65 ± 3.84 | 37.62 ± 5.07 |
| Feed | 47.25 ± 1.86 | 43.25 ± 1.90 | 43.35 ± 1.36 | 43.46 ± 1.72 | |
| W9 | Water | 52.33 ± 1.89 | 39.80 ± 11.30 | 36.43 ± 5.51 | 39.62 ± 3.85 |
| Feed | 48.93 ± 3.29 | 41.95 ± 3.01 | 44.10 ± 2.02 | 44.44 ± 4.90 | |
| W10 | Water | 48.90 ± 4.75 | 35.53 ± 9.09 | 35.48 ± 4.08 | 37.34 ± 9.58 |
| Feed | 47.65 ± 3.90 | 43.18 ± 4.21 | 42.25 ± 1.27 | 44.52 ± 2.94 | |
| W11 | Water | 54.8 ± 3.78 | 43.23 ± 15.36 | 38.20 ± 4.28 | 41.76 ± 6.53 |
| Feed | 47.9 ± 2.91 | 44.18 ± 3.29 | 42.85 ± 1.98 | 44.42 ± 3.80 | |
| W12 | Water | 45.60 ± 4.18 | 36.10 ± 15.20 | 31.93 ± 3.01 | 35.9 ± 4.95 |
| Feed | 46.78 ± 2.77 | 41.65 ± 3.94 | 41.90 ± 3.36 | 41.94 ± 3.44 |
| Name | Unit | C | L | M | H |
|---|---|---|---|---|---|
| Heart | mg | 136.01 ± 26.34 | 131.26 ± 17.98 | 139.63 ± 16.77 | 143.85 ± 24.72 |
| % | 0.48 ± 0.1 | 0.41 ± 0.05 | 0.44 ± 0.08 | 0.41 ± 0.07 | |
| Liver | mg | 1159.64 ± 140 | 1180.29 ± 298.39 | 1201.26 ± 265.54 | 1437.27 ± 456.25 |
| % | 4.07 ± 0.48 | 3.61 ± 0.74 | 3.72 ± 0.73 | 3.98 ± 1.04 | |
| Kidney | mg | 342.51 ± 58.44 | 341.13 ± 46.96 | 345.59 ± 48.83 | 354.51 ± 47.48 |
| % | 1.2 ± 0.2 | 1.07 ± 0.2 | 1.08 ± 0.2 | 1 ± 0.16 | |
| Brown fat | mg | 111.08 ± 49 | 192.15 ± 88.08 | 188.46 ± 60.3 | 231.31 ± 66.1 |
| % | 0.39 ± 0.17 | 0.58 ± 0.23 | 0.57 ± 0.14 | 0.65 ± 0.17 | |
| Epididymal white fat | mg | 464.68 ± 150.16 | 986.6 ± 346.51 | 919.03 ± 452.01 | 1217.53 ± 525.89 |
| % | 1.62 ± 0.47 | 2.98 ± 0.87 | 2.73 ± 1.04 | 3.32 ± 1.22 | |
| Retroperitoneal fat | mg | 133.56 ± 74.17 | 314.09 ± 178.73 | 245.04 ± 157.84 | 355.22 ± 123.55 |
| % | 0.46 ± 0.23 | 0.94 ± 0.48 | 0.64 ± 0.44 | 0.98 ± 0.28 | |
| Mesenteric fat | mg | 158.27 ± 71.54 | 317.39 ± 164.01 | 376.93 ± 207.44 | 488.72 ± 212.05 |
| % | 0.56 ± 0.25 | 0.97 ± 0.47 | 1.12 ± 0.55 | 1.38 ± 0.63 | |
| Gastrocnemius muscle, plantaris muscle, soleus muscle | mg | 316.49 ± 20.18 | 314.46 ± 18.75 | 322.9 ± 12.42 | 329.42 ± 16.58 |
| % | 1.11 ± 0.08 | 0.98 ± 0.09 | 1.01 ± 0.11 | 0.93 ± 0.09 | |
| Quadriceps muscle | mg | 244.68 ± 43.77 | 215.65 ± 23.94 | 234.98 ± 20.41 | 231.58 ± 26.44 |
| % | 0.86 ± 0.15 | 0.67 ± 0.09 | 0.74 ± 0.13 | 0.66 ± 0.09 |
| Ingredients (g/100 g) | CON | L, M, and H |
|---|---|---|
| Casein | 17.2 | 14.4 |
| L-Cystine | 0.26 | 0.22 |
| Corn starch | 30.2 | 25.2 |
| Maltodextrin | 3 | 2.5 |
| Sucrose | 35.8 | 29.9 |
| Cellulose | 4.3 | 3.6 |
| Test oils | 4.3 | 20 |
| Vitamin mixture | 0.86 | 0.72 |
| Mineral mixture | 0.86 | 0.72 |
| CaHPO4 | 1.1 | 0.94 |
| CaCO3 | 0.47 | 0.4 |
| K3C6H5O7·H2O | 1.4 | 1.2 |
| Choline bitartrate | 0.25 | 0.2 |
| Gene | Forward | Reverse |
|---|---|---|
| rps18 | TTCTGGCCAACGGTCTAGACAAC | CCAGTGGTCTTGGTGTGCTGA |
| Myod1 | CGCAACGCCATCCGCTACAT | GCATCTGAGTCGCCACTGTAGT |
| Myog | GCAGGCTCAAGAAAGTGAATGA | TAGGCGCTCAATGTACTGGAT |
| Myf6 | CCTCAGCCTCCAGCAGTCTTCA | TTACTTCTCCACCACCTCCTCCAC |
| Myf5 | CTCAGGAATGCCATCCGCTACA | CCGTCAGAGCAGTTGGAGGT |
| Myh4 | GTTCATGCTGACGGATCGTGAGA | AGTCGCCTCCTCCTTCTTCTTGT |
| Myh7 | AGATGAATGCCGAGCTCACT | CACATCCAAACCAGCCATCT |
| Myh2 | GAGCAAAGATGCAGGGAAAG | TAAGGGTTGACGGTGACACA |
| Fbxo32 (ATROGIN1) | GCTGGATTGGAAGAAGATG | AGAGAATGTGGCAGTGTT |
| Trim63 (MuRF1) | CGACATCTTCCAGGCTGCGAAT | ATCACTTCATGGCGGCACGAG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Z.; Wu, C.; Wang, S.; Tong, Y.; Huang, J.; Xue, C.; Cao, T.; Suzuki, K. Long-Term Moderate Increase in Medium-Chain Fatty Acids Intake Enhances Muscle Metabolism and Function in Mice. Int. J. Mol. Sci. 2025, 26, 4126. https://doi.org/10.3390/ijms26094126
Zhang Z, Wu C, Wang S, Tong Y, Huang J, Xue C, Cao T, Suzuki K. Long-Term Moderate Increase in Medium-Chain Fatty Acids Intake Enhances Muscle Metabolism and Function in Mice. International Journal of Molecular Sciences. 2025; 26(9):4126. https://doi.org/10.3390/ijms26094126
Chicago/Turabian StyleZhang, Ziwei, Cong Wu, Shuo Wang, Yishan Tong, Jiapeng Huang, Chuwen Xue, Tiehan Cao, and Katsuhiko Suzuki. 2025. "Long-Term Moderate Increase in Medium-Chain Fatty Acids Intake Enhances Muscle Metabolism and Function in Mice" International Journal of Molecular Sciences 26, no. 9: 4126. https://doi.org/10.3390/ijms26094126
APA StyleZhang, Z., Wu, C., Wang, S., Tong, Y., Huang, J., Xue, C., Cao, T., & Suzuki, K. (2025). Long-Term Moderate Increase in Medium-Chain Fatty Acids Intake Enhances Muscle Metabolism and Function in Mice. International Journal of Molecular Sciences, 26(9), 4126. https://doi.org/10.3390/ijms26094126









