Dysregulated miR-21/SOD3, but Not miR-30b/CAT, Profile in Elderly Patients with Carbohydrate Metabolism Disorders: A Link to Oxidative Stress and Metabolic Dysfunction
Abstract
:1. Introduction
2. Results
2.1. Characteristics of the Participants
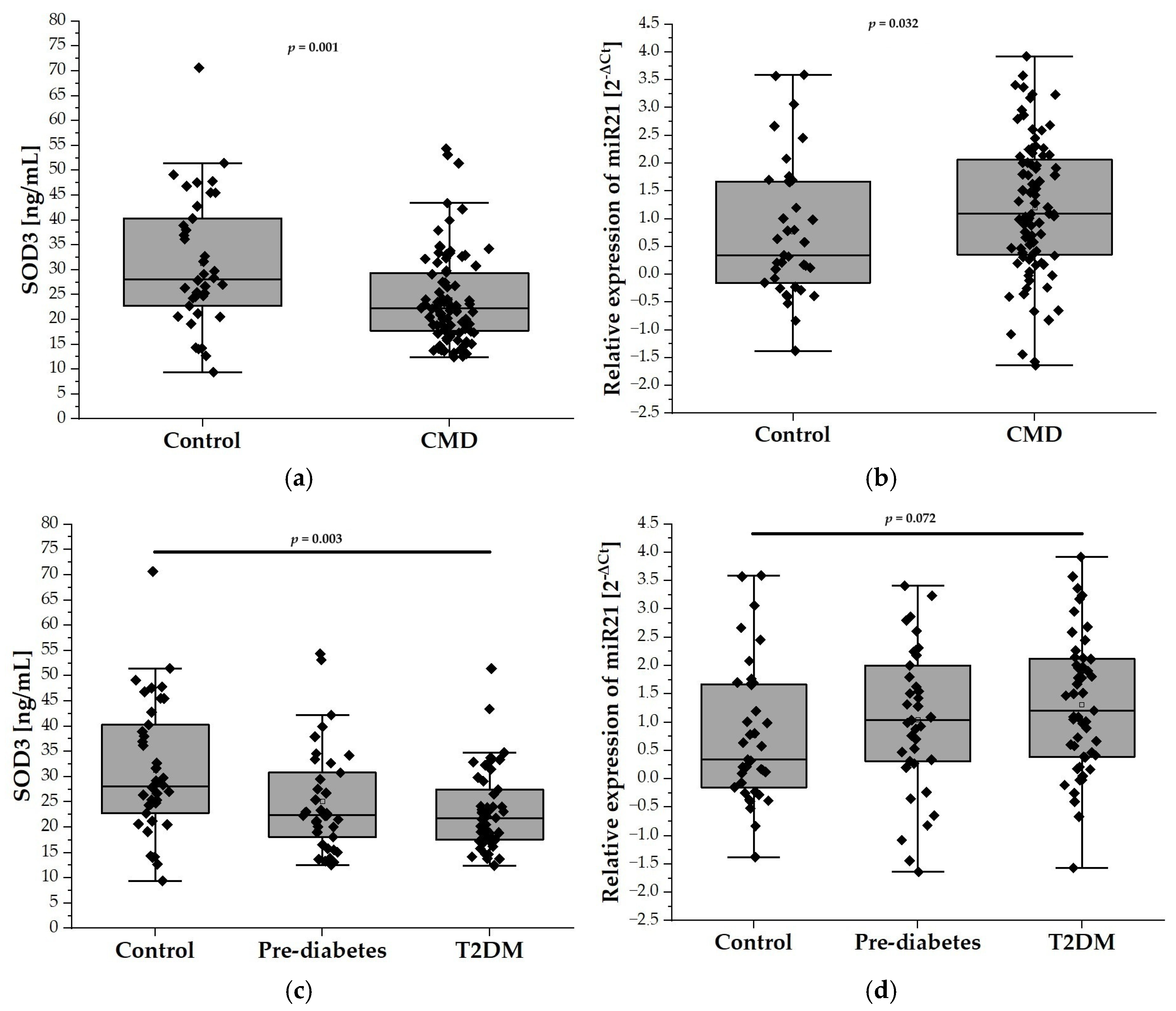
2.2. Relative Expression of miR-21 and SOD3 Levels
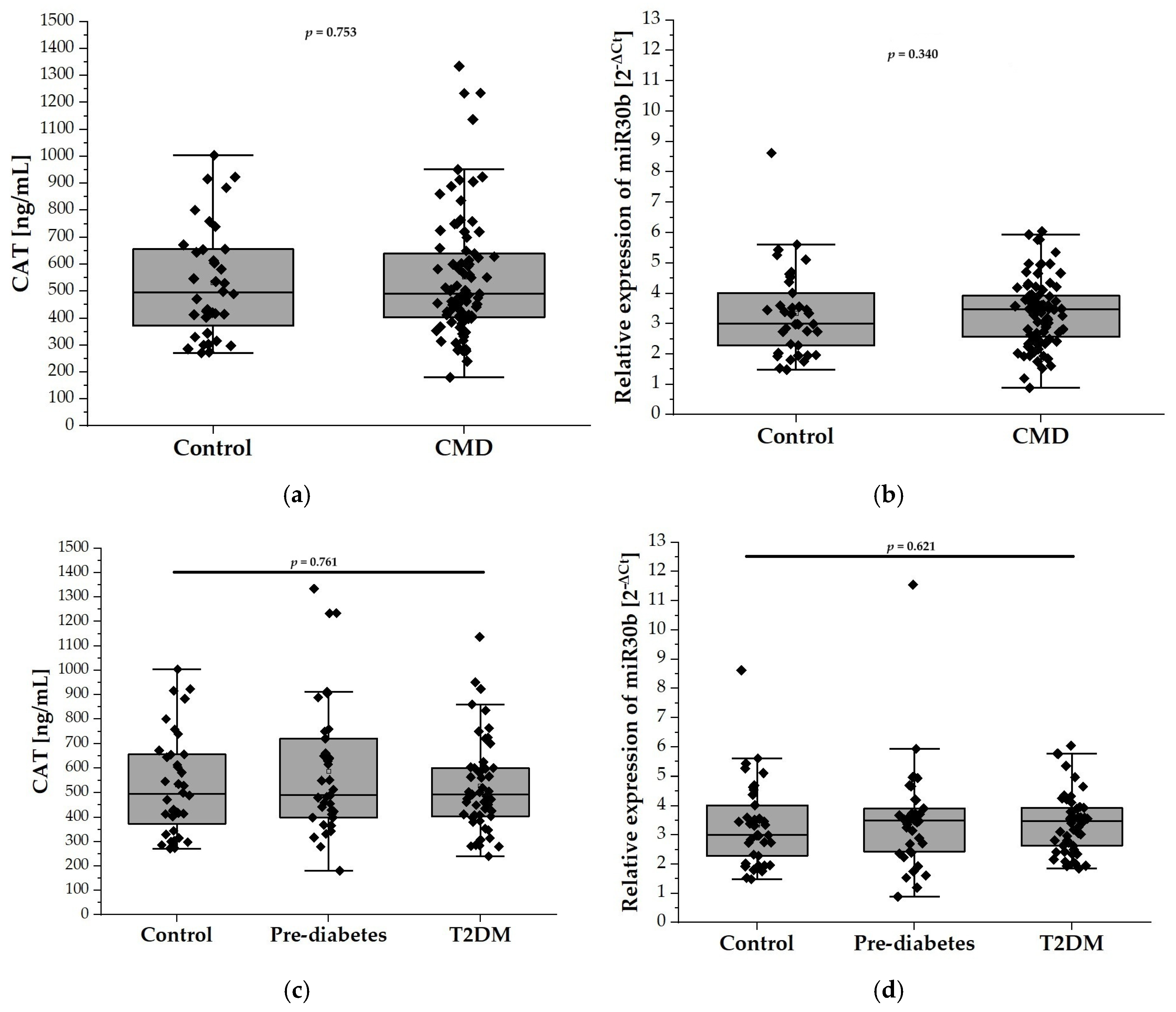
2.3. Relative Expression of miR-30b and CAT Levels
2.4. Correlation Between SOD3 and miR-21 Levels and Anthropometric and Metabolic Parameters
2.5. Correlation Between CAT and miR30b Levels and Anthropometric and Metabolic Parameters
2.6. Diagnostic Utility of Plasma SOD3 Concentration and miR-21 Expression in Assessing CMD Risk
2.7. Evaluation of Plasma CAT Levels and miR-30b Expression as Predictive Biomarkers of CMD in the Elderly
3. Discussion
4. Materials and Methods
4.1. Measurement of SOD3 and CAT Levels—ELISA
4.2. Analysis of microRNA Expression—Quantitative Real-Time PCR Assay (qRT-PCR)
4.3. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hossain, M.J.; Al-Mamun, M.; Islam, M.R. Diabetes Mellitus, the Fastest Growing Global Public Health Concern: Early Detection Should Be Focused. Health Sci. Rep. 2024, 7, e2004. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, Z.; Zhang, K.; Ge, X.; Sun, R.; Zhai, X. Early detection of type 2 diabetes risk: Limitations of current diagnostic criteria. Front. Endocrinol. 2023, 14, 1260623. [Google Scholar] [CrossRef] [PubMed]
- Masenga, S.K.; Kabwe, L.S.; Chakulya, M.; Kirabo, A. Mechanisms of Oxidative Stress in Metabolic Syndrome. Int. J. Mol. Sci. 2023, 24, 7898. [Google Scholar] [CrossRef]
- Busik, J.V.; Mohr, S.; Grant, M.B. Hyperglycemia-Induced Reactive Oxygen Species Toxicity to Endothelial Cells Is Dependent on Paracrine Mediators. Diabetes 2008, 57, 1952–1965. [Google Scholar] [CrossRef] [PubMed]
- Kuilman, T.; Michaloglou, C.; Mooi, W.J.; Peeper, D.S. The Essence of Senescence. Genes Dev. 2010, 24, 2463–2479. [Google Scholar] [CrossRef]
- Kang, C.; Xu, Q.; Martin, T.D.; Li, M.Z.; Demaria, M.; Aron, L.; Lu, T.; Yankner, B.A.; Campisi, J.; Elledge, S.J. The DNA Damage Response Induces Inflammation and Senescence by Inhibiting Autophagy of GATA4. Science 2015, 349, aaa5612. [Google Scholar] [CrossRef]
- Chaudhuri, J.; Bains, Y.; Guha, S.; Kahn, A.; Hall, D.; Bose, N.; Gugliucci, A.; Kapahi, P. The Role of Advanced Glycation End Products in Aging and Metabolic Diseases: Bridging Association and Causality. Cell Metab. 2018, 28, 337–352. [Google Scholar] [CrossRef]
- Caturano, A.; D’Angelo, M.; Mormone, A.; Russo, V.; Mollica, M.P.; Salvatore, T.; Galiero, R.; Rinaldi, L.; Vetrano, E.; Marfella, R.; et al. Oxidative Stress in Type 2 Diabetes: Impacts from Pathogenesis to Lifestyle Modifications. Curr. Issues Mol. Biol. 2023, 45, 6651–6666. [Google Scholar] [CrossRef]
- Manna, P.; Jain, S.K. Obesity, Oxidative Stress, Adipose Tissue Dysfunction, and the Associated Health Risks: Causes and Therapeutic Strategies. Metab. Syndr. Relat. Disord. 2015, 13, 423–444. [Google Scholar] [CrossRef]
- Scioli, M.G.; Storti, G.; D’Amico, F.; Rodríguez Guzmán, R.; Centofanti, F.; Doldo, E.; Céspedes Miranda, E.M.; Orlandi, A. Oxidative Stress and New Pathogenetic Mechanisms in Endothelial Dysfunction: Potential Diagnostic Biomarkers and Therapeutic Targets. J. Clin. Med. 2020, 9, 1995. [Google Scholar] [CrossRef]
- Laurent, G.; Solari, F.; Mateescu, B.; Karaca, M.; Castel, J.; Bourachot, B.; Magnan, C.; Billaud, M.; Mechta-Grigoriou, F. Oxidative Stress Contributes to Aging by Enhancing Pancreatic Angiogenesis and Insulin Signaling. Cell Metab. 2008, 7, 113–124. [Google Scholar] [CrossRef]
- Marklund, S.L. Extracellular Superoxide Dismutase in Human Tissues and Human Cell Lines. J. Clin. Investig. 1984, 74, 1398–1403. [Google Scholar] [CrossRef] [PubMed]
- Fukai, T.; Siegfried, M.R.; Ushio-Fukai, M.; Cheng, Y.; Kojda, G.; Harrison, D.G. Regulation of the Vascular Extracellular Superoxide Dismutase by Nitric Oxide and Exercise Training. J. Clin. Investig. 2000, 105, 1631–1639. [Google Scholar] [CrossRef]
- Walton, P.A.; Pizzitelli, M. Effects of Peroxisomal Catalase Inhibition on Mitochondrial Function. Front. Physiol. 2012, 3, 108. [Google Scholar] [CrossRef] [PubMed]
- Putnam, C.D.; Arvai, A.S.; Bourne, Y.; Tainer, J.A. Active and Inhibited Human Catalase Structures: Ligand and NADPH Binding and Catalytic Mechanism. J. Mol. Biol. 2000, 296, 295–309. [Google Scholar] [CrossRef] [PubMed]
- Dröse, S.; Brandt, U. Molecular Mechanisms of Superoxide Production by the Mitochondrial Respiratory Chain. In Mitochondrial Oxidative Phosphorylation: Nuclear-Encoded Genes, Enzyme Regulation, and Pathophysiology; Kadenbach, B., Ed.; Springer: New York, NY, USA, 2012; pp. 145–169. ISBN 978-1-4614-3573-0. [Google Scholar]
- Msolly, A.A.; Miled, A.; Kassab, A. Hydrogen Peroxide: An Oxidant Stress Indicator in Type 2 Diabetes. Biochem. Indian J. 2013, 7, 166–172. [Google Scholar]
- Nandi, A.; Yan, L.-J.; Jana, C.K.; Das, N. Role of Catalase in Oxidative Stress- and Age-Associated Degenerative Diseases. Oxid. Med. Cell. Longev. 2019, 2019, 9613090. [Google Scholar] [CrossRef]
- Włodarski, A.; Szymczak-Pajor, I.; Kasznicki, J.; Antanaviciute, E.M.; Szymańska, B.; Śliwińska, A. Association of Glutathione Peroxidase 3 (GPx3) and miR-196a with Carbohydrate Metabolism Disorders in the Elderly. Int. J. Mol. Sci. 2024, 25, 5409. [Google Scholar] [CrossRef]
- Włodarski, A.; Strycharz, J.; Wróblewski, A.; Kasznicki, J.; Drzewoski, J.; Śliwińska, A. The Role of microRNAs in Metabolic Syndrome-Related Oxidative Stress. Int. J. Mol. Sci. 2020, 21, 6902. [Google Scholar] [CrossRef]
- Kalinina, E.V.; Ivanova-Radkevich, V.I.; Chernov, N.N. Role of MicroRNAs in the Regulation of Redox-Dependent Processes. Biochemistry 2019, 84, 1233–1246. [Google Scholar] [CrossRef]
- Saha, S. Role of microRNA in Oxidative Stress. Stresses 2024, 4, 269–281. [Google Scholar] [CrossRef]
- Qadir, M.M.F.; Klein, D.; Álvarez-Cubela, S.; Domínguez-Bendala, J.; Pastori, R.L. The Role of MicroRNAs in Diabetes-Related Oxidative Stress. Int. J. Mol. Sci. 2019, 20, 5423. [Google Scholar] [CrossRef]
- Bartel, D.P. MicroRNAs: Target Recognition and Regulatory Functions. Cell 2009, 136, 215–233. [Google Scholar] [CrossRef]
- Carbonell, T.; Gomes, A.V. MicroRNAs in the Regulation of Cellular Redox Status and Its Implications in Myocardial Ischemia-Reperfusion Injury. Redox Biol. 2020, 36, 101607. [Google Scholar] [CrossRef] [PubMed]
- Olejniczak, M.; Kotowska-Zimmer, A.; Krzyzosiak, W. Stress-Induced Changes in miRNA Biogenesis and Functioning. Cell. Mol. Life Sci. 2018, 75, 177–191. [Google Scholar] [CrossRef] [PubMed]
- Rhim, J.; Baek, W.; Seo, Y.; Kim, J.H. From Molecular Mechanisms to Therapeutics: Understanding MicroRNA-21 in Cancer. Cells 2022, 11, 2791. [Google Scholar] [CrossRef]
- Zhang, K.-L.; Han, L.; Chen, L.-Y.; Shi, Z.-D.; Yang, M.; Ren, Y.; Chen, L.-C.; Zhang, J.-X.; Pu, P.-Y.; Kang, C.-S. Blockage of a miR-21/EGFR Regulatory Feedback Loop Augments Anti-EGFR Therapy in Glioblastomas. Cancer Lett. 2014, 342, 139–149. [Google Scholar] [CrossRef] [PubMed]
- Kwak, H.-J.; Kim, Y.-J.; Chun, K.-R.; Woo, Y.M.; Park, S.-J.; Jeong, J.-A.; Jo, S.H.; Kim, T.H.; Min, H.S.; Chae, J.S.; et al. Downregulation of Spry2 by miR-21 Triggers Malignancy in Human Gliomas. Oncogene 2011, 30, 2433–2442. [Google Scholar] [CrossRef]
- Zhou, B.; Wang, D.; Sun, G.; Mei, F.; Cui, Y.; Xu, H. Effect of miR-21 on Apoptosis in Lung Cancer Cell Through Inhibiting the PI3K/ Akt/NF-κB Signaling Pathway in Vitro and in Vivo. Cell. Physiol. Biochem. 2018, 46, 999–1008. [Google Scholar] [CrossRef]
- Zhang, X.; Ng, W.-L.; Wang, P.; Tian, L.; Werner, E.; Wang, H.; Doetsch, P.; Wang, Y. MicroRNA-21 Modulates the Levels of Reactive Oxygen Species Levels by Targeting SOD3 and TNFα. Cancer Res. 2012, 72, 4707–4713. [Google Scholar] [CrossRef]
- Zhang, Q.; Ma, X.-F.; Dong, M.-Z.; Tan, J.; Zhang, J.; Zhuang, L.-K.; Liu, S.-S.; Xin, Y.-N. MiR-30b-5p Regulates the Lipid Metabolism by Targeting PPARGC1A in Huh-7 Cell Line. Lipids Health Dis. 2020, 19, 76. [Google Scholar] [CrossRef] [PubMed]
- Dai, L.-L.; Li, S.-D.; Ma, Y.-C.; Tang, J.-R.; Lv, J.-Y.; Zhang, Y.-Q.; Miao, Y.-L.; Ma, Y.-Q.; Li, C.-M.; Chu, Y.-Y.; et al. MicroRNA-30b Regulates Insulin Sensitivity by Targeting SERCA2b in Non-Alcoholic Fatty Liver Disease. Liver Int. 2019, 39, 1504–1513. [Google Scholar] [CrossRef] [PubMed]
- Haque, R.; Chun, E.; Howell, J.C.; Sengupta, T.; Chen, D.; Kim, H. MicroRNA-30b-Mediated Regulation of Catalase Expression in Human ARPE-19 Cells. PLoS ONE 2012, 7, e42542. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes—2019. Diabetes Care 2019, 42, S13–S28. [Google Scholar] [CrossRef]
- Branco, M.G.; Mateus, C.; Capelas, M.L.; Pimenta, N.; Santos, T.; Mäkitie, A.; Ganhão-Arranhado, S.; Trabulo, C.; Ravasco, P. Bioelectrical Impedance Analysis (BIA) for the Assessment of Body Composition in Oncology: A Scoping Review. Nutrients 2023, 15, 4792. [Google Scholar] [CrossRef]
- Aikaeli, F.; Njim, T.; Gissing, S.; Moyo, F.; Alam, U.; Mfinanga, S.G.; Okebe, J.; Ramaiya, K.; Webb, E.L.; Jaffar, S.; et al. Prevalence of Microvascular and Macrovascular Complications of Diabetes in Newly Diagnosed Type 2 Diabetes in Low-and-Middle-Income Countries: A Systematic Review and Meta-Analysis. PLoS Glob. Public Health 2022, 2, e0000599. [Google Scholar] [CrossRef]
- Franceschi, C.; Capri, M.; Monti, D.; Giunta, S.; Olivieri, F.; Sevini, F.; Panourgia, M.P.; Invidia, L.; Celani, L.; Scurti, M.; et al. Inflammaging and Anti-Inflammaging: A Systemic Perspective on Aging and Longevity Emerged from Studies in Humans. Mech. Ageing Dev. 2007, 128, 92–105. [Google Scholar] [CrossRef]
- Harman, D. The Free Radical Theory of Aging. Antioxid. Redox Signal. 2003, 5, 557–561. [Google Scholar] [CrossRef]
- Fukai, T.; Ushio-Fukai, M. Superoxide Dismutases: Role in Redox Signaling, Vascular Function, and Diseases. Antioxid. Redox Signal. 2011, 15, 1583–1606. [Google Scholar] [CrossRef]
- Strom, A.; Kaul, K.; Brüggemann, J.; Ziegler, I.; Rokitta, I.; Püttgen, S.; Szendroedi, J.; Müssig, K.; Roden, M.; Ziegler, D. Lower Serum Extracellular Superoxide Dismutase Levels Are Associated with Polyneuropathy in Recent-Onset Diabetes. Exp. Mol. Med. 2017, 49, e394. [Google Scholar] [CrossRef]
- Andrés-Blasco, I.; Gallego-Martínez, A.; Machado, X.; Cruz-Espinosa, J.; Di Lauro, S.; Casaroli-Marano, R.; Alegre-Ituarte, V.; Arévalo, J.F.; Pinazo-Durán, M.D. Oxidative Stress, Inflammatory, Angiogenic, and Apoptotic Molecules in Proliferative Diabetic Retinopathy and Diabetic Macular Edema Patients. Int. J. Mol. Sci. 2023, 24, 8227. [Google Scholar] [CrossRef]
- Lewandowski, Ł.; Urbanowicz, I.; Kepinska, M.; Milnerowicz, H. Concentration/Activity of Superoxide Dismutase Isozymes and the pro-/Antioxidative Status, in Context of Type 2 Diabetes and Selected Single Nucleotide Polymorphisms (Genes: INS, SOD1, SOD2, SOD3)—Preliminary Findings. Biomed. Pharmacother. 2021, 137, 111396. [Google Scholar] [CrossRef] [PubMed]
- Sandström, J.; Nilsson, P.; Karlsson, K.; Marklund, S.L. 10-Fold Increase in Human Plasma Extracellular Superoxide Dismutase Content Caused by a Mutation in Heparin-Binding Domain. J. Biol. Chem. 1994, 269, 19163–19166. [Google Scholar] [CrossRef] [PubMed]
- Roy, D.; Modi, A.; Khokhar, M.; Sankanagoudar, S.; Yadav, D.; Sharma, S.; Purohit, P.; Sharma, P. MicroRNA 21 Emerging Role in Diabetic Complications: A Critical Update. Curr. Diabetes Rev. 2021, 17, 122–135. [Google Scholar] [PubMed]
- Chen, Y.; Ye, X.; Zhang, X.; Guo, Z.; Chen, W.; Pan, Z.; Zhang, Z.; Li, B.; Wang, H.; Yao, J. Combination of Evidence from Bibliometrics and Bioinformatics Analysis Identifies miR-21 as a Potential Therapeutical Target for Diabetes. Metabolites 2024, 14, 403. [Google Scholar] [CrossRef]
- La Sala, L.; Mrakic-Sposta, S.; Tagliabue, E.; Prattichizzo, F.; Micheloni, S.; Sangalli, E.; Specchia, C.; Uccellatore, A.C.; Lupini, S.; Spinetti, G.; et al. Circulating microRNA-21 Is an Early Predictor of ROS-Mediated Damage in Subjects with High Risk of Developing Diabetes and in Drug-Naïve T2D. Cardiovasc. Diabetol. 2019, 18, 18. [Google Scholar] [CrossRef]
- López-Armas, G.C.; Yessenbekova, A.; González-Castañeda, R.E.; Arellano-Arteaga, K.J.; Guerra-Librero, A.; Ablaikhanova, N.; Florido, J.; Escames, G.; Acuña-Castroviejo, D.; Rusanova, I. Role of C-miR-21, c-miR-126, Redox Status, and Inflammatory Conditions as Potential Predictors of Vascular Damage in T2DM Patients. Antioxidants 2022, 11, 1675. [Google Scholar] [CrossRef]
- Olivieri, F.; Spazzafumo, L.; Bonafè, M.; Recchioni, R.; Prattichizzo, F.; Marcheselli, F.; Micolucci, L.; Mensà, E.; Giuliani, A.; Santini, G.; et al. MiR-21-5p and miR-126a-3p Levels in Plasma and Circulating Angiogenic Cells: Relationship with Type 2 Diabetes Complications. Oncotarget 2015, 6, 35372–35382. [Google Scholar] [CrossRef]
- Antognelli, C.; Trapani, E.; Delle Monache, S.; Perrelli, A.; Daga, M.; Pizzimenti, S.; Barrera, G.; Cassoni, P.; Angelucci, A.; Trabalzini, L.; et al. KRIT1 Loss-of-Function Induces a Chronic Nrf2-Mediated Adaptive Homeostasis That Sensitizes Cells to Oxidative Stress: Implication for Cerebral Cavernous Malformation Disease. Free Radic. Biol. Med. 2018, 115, 202–218. [Google Scholar] [CrossRef]
- Shi, C.; Liang, Y.; Yang, J.; Xia, Y.; Chen, H.; Han, H.; Yang, Y.; Wu, W.; Gao, R.; Qin, H. MicroRNA-21 Knockout Improve the Survival Rate in DSS Induced Fatal Colitis Through Protecting Against Inflammation and Tissue Injury. PLoS ONE 2013, 8, e66814. [Google Scholar] [CrossRef]
- Ding, X.; Jing, N.; Shen, A.; Guo, F.; Song, Y.; Pan, M.; Ma, X.; Zhao, L.; Zhang, H.; Wu, L.; et al. MiR-21-5p in Macrophage-Derived Extracellular Vesicles Affects Podocyte Pyroptosis in Diabetic Nephropathy by Regulating A20. J. Endocrinol. Investig. 2021, 44, 1175–1184. [Google Scholar] [CrossRef] [PubMed]
- Ghorbani, S.; Mahdavi, R.; Alipoor, B.; Panahi, G.; Nasli Esfahani, E.; Razi, F.; Taghikhani, M.; Meshkani, R. Decreased Serum microRNA-21 Level Is Associated with Obesity in Healthy and Type 2 Diabetic Subjects. Arch. Physiol. Biochem. 2018, 124, 300–305. [Google Scholar] [CrossRef] [PubMed]
- Kuryłowicz, A.; Wicik, Z.; Owczarz, M.; Jonas, M.I.; Kotlarek, M.; Świerniak, M.; Lisik, W.; Jonas, M.; Noszczyk, B.; Puzianowska-Kuźnicka, M. NGS Reveals Molecular Pathways Affected by Obesity and Weight Loss-Related Changes in miRNA Levels in Adipose Tissue. Int. J. Mol. Sci. 2017, 19, 66. [Google Scholar] [CrossRef] [PubMed]
- Nardelli, C.; Iaffaldano, L.; Pilone, V.; Labruna, G.; Ferrigno, M.; Carlomagno, N.; Dodaro, C.A.; Forestieri, P.; Buono, P.; Salvatore, F.; et al. Changes in the MicroRNA Profile Observed in the Subcutaneous Adipose Tissue of Obese Patients after Laparoscopic Adjustable Gastric Banding. J. Obes. 2017, 2017, 6754734. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Sidorkiewicz, I.; Niemira, M.; Maliszewska, K.; Erol, A.; Bielska, A.; Szalkowska, A.; Adamska-Patruno, E.; Szczerbinski, L.; Gorska, M.; Kretowski, A. Circulating miRNAs as a Predictive Biomarker of the Progression from Prediabetes to Diabetes: Outcomes of a 5-Year Prospective Observational Study. J. Clin. Med. 2020, 9, 2184. [Google Scholar] [CrossRef]

| Parameters | Control (n = 38) | Prediabetes (n = 37) | T2DM (n = 51) |
|---|---|---|---|
| Anthropometric | |||
| F/M | 18/20 | 22/15 | 34/17 |
| Age [years] | 73.00(67.75; 85.00) | 76.00(69.5; 85.5) | 75.00(70.00; 82.00) |
| BMI [kg/m2] | 26.26(23.68; 31.14) | 26.50(24.40; 29.25) | 27.25(24.93; 30.93) |
| ST triceps (mm) | 15.60(10.80; 20.20) | 17.40(11.10; 24.50) | 21.80(17.80; 27.60) bb.c |
| ST abdominal (mm) | 26.00(18.70; 33.55) | 31.00(19.60; 37.90) | 34.00(26.80; 41.40) b |
| ST thigh (mm) | 18.40(14.35; 25.20) | 23.40(11.80; 36.20) | 31.20(17.80; 38.80) b |
| BIA-BF [%] | 25.00(20.70; 31.10) | 25.00(20.70; 32.50) c | 30.60(24.10; 37.80) b.c |
| BIA-FFM [%] | 71.00(64.65; 74.65) | 70.20(62.88; 75.28) | 65.90(59.50; 72.00) b |
| BIA-TBW [%] | 52.30(48.60; 56.70) | 52.20(46.90; 55.70) | 48.70(44.50; 51.80) b |
| Metabolic | |||
| HbA1c [%] | 5.50(5.20; 5.60) | 6.00(5.80; 6.20) aaa | 6.50(5.80; 7.60) bbb |
| FPG [mmol/L] | 5.16(4.67; 5.37) | 5.37(4.94; 5.90) | 6.71(5.78; 9.55) bbb. ccc |
| HOMA-IR | 1.88(1.15; 2.83) | 2.41(1.38; 3.80) | 2.68(1.86; 4.86) b |
| TG/HDL ratio | 1.09(0.67; 1.51) | 0.99(0.62; 1.38) | 1.16(0.75; 1.75) |
| Creatinine [μmol/L] | 87.52(71.90; 110.60) | 89.00(74.15; 109.80) | 95.60(69.55; 113.50) |
| Urea [mmol/L] | 5.85(4.50; 7.64) | 7.08(5.31; 9.38) | 7.67(6.02; 9.54) b |
| eGFR [mL/min/1.73 m2] | 70.69(44.40; 90.40) | 61.60(44.00; 85.60) | 55.20(44.30; 83.40) |
| LDL [mmol/L] | 2.53(1.79; 3.42) | 2.15(1.75; 2.85) | 2.13(1.48; 3.14) |
| HDL [mmol/L] | 1.29(1.01; 1.49) | 1.19(0.95; 1.55) | 1.12(0.87; 1.29) b |
| TG [mmol/L] | 1.32(0.82; 1.72) | 1.08(0.86; 1.54) | 1.25(0.91; 1.70) |
| TC [mmol/L] | 4.47(3.74; 5.11) | 4.04(3.32; 4.70) | 3.64(2.95; 5.21) |
| Variable | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|
| Wald’s p | OR | 95% CI | Wald’s p | OR | 95% CI | |
| SOD3 | 0.001 | 0.941 | 0.906–0.976 | 0.012 | 0.948 | 0.909–0.988 |
| CAT | 0.636 | 1.00 | 0.999–1.002 | |||
| miR-21 | 0.180 | 0.648 | 0.344–1.222 | |||
| Age | 0.407 | 1.020 | 0.974–1.067 | |||
| Sex (male) | 0.091 | 0.514 | 0.238–1.112 | |||
| BMI | 0.295 | 1.041 | 0.966–1.122 | |||
| WHR | 0.699 | 2.222 | 0.039–127.410 | |||
| ST triceps | 0.018 | 1.062 | 1.010–1.116 | 0.184 | 1.052 | 0.976–1.134 |
| ST abdominal | 0.043 | 1.034 | 1.011–1.069 | 0.769 | 1.007 | 0.958–1.059 |
| ST thigh | 0.070 | 1.026 | 0.998–1.054 | |||
| BIA-BF [%] | 0.117 | 1.036 | 0.991–1.083 | |||
| BIA-FFM [%] | 0.088 | 0.016 | 0.001–1.856 | |||
| BIA-TBW [%] | 0.104 | 0.005 | 0.001–1.960 | |||
| FPG | <0.001 | 2.585 | 1.500–4.456 | 0.017 | 2.710 | 1.197–6.134 |
| HOMA-IR | 0.045 | 1.794 | 1.012–3.179 | 0.802 | 0.903 | 0.407–2.00 |
| Creatinine | 0.683 | 1.003 | 0.990–1.016 | |||
| eGFR | 0.271 | 0.991 | 0.975–1.007 | |||
| LDL | 0.123 | 0.737 | 0.500–1.086 | |||
| TG/HDL | 0.370 | 1.264 | 0.758–2.108 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Włodarski, A.; Szymczak-Pajor, I.; Kasznicki, J.; Antanaviciute, E.M.; Szymańska, B.; Śliwińska, A. Dysregulated miR-21/SOD3, but Not miR-30b/CAT, Profile in Elderly Patients with Carbohydrate Metabolism Disorders: A Link to Oxidative Stress and Metabolic Dysfunction. Int. J. Mol. Sci. 2025, 26, 4127. https://doi.org/10.3390/ijms26094127
Włodarski A, Szymczak-Pajor I, Kasznicki J, Antanaviciute EM, Szymańska B, Śliwińska A. Dysregulated miR-21/SOD3, but Not miR-30b/CAT, Profile in Elderly Patients with Carbohydrate Metabolism Disorders: A Link to Oxidative Stress and Metabolic Dysfunction. International Journal of Molecular Sciences. 2025; 26(9):4127. https://doi.org/10.3390/ijms26094127
Chicago/Turabian StyleWłodarski, Adam, Izabela Szymczak-Pajor, Jacek Kasznicki, Egle Morta Antanaviciute, Bożena Szymańska, and Agnieszka Śliwińska. 2025. "Dysregulated miR-21/SOD3, but Not miR-30b/CAT, Profile in Elderly Patients with Carbohydrate Metabolism Disorders: A Link to Oxidative Stress and Metabolic Dysfunction" International Journal of Molecular Sciences 26, no. 9: 4127. https://doi.org/10.3390/ijms26094127
APA StyleWłodarski, A., Szymczak-Pajor, I., Kasznicki, J., Antanaviciute, E. M., Szymańska, B., & Śliwińska, A. (2025). Dysregulated miR-21/SOD3, but Not miR-30b/CAT, Profile in Elderly Patients with Carbohydrate Metabolism Disorders: A Link to Oxidative Stress and Metabolic Dysfunction. International Journal of Molecular Sciences, 26(9), 4127. https://doi.org/10.3390/ijms26094127







