Effects of Acrylamide on Mouse Implantation and Decidualization
Abstract
:1. Introduction
2. Results
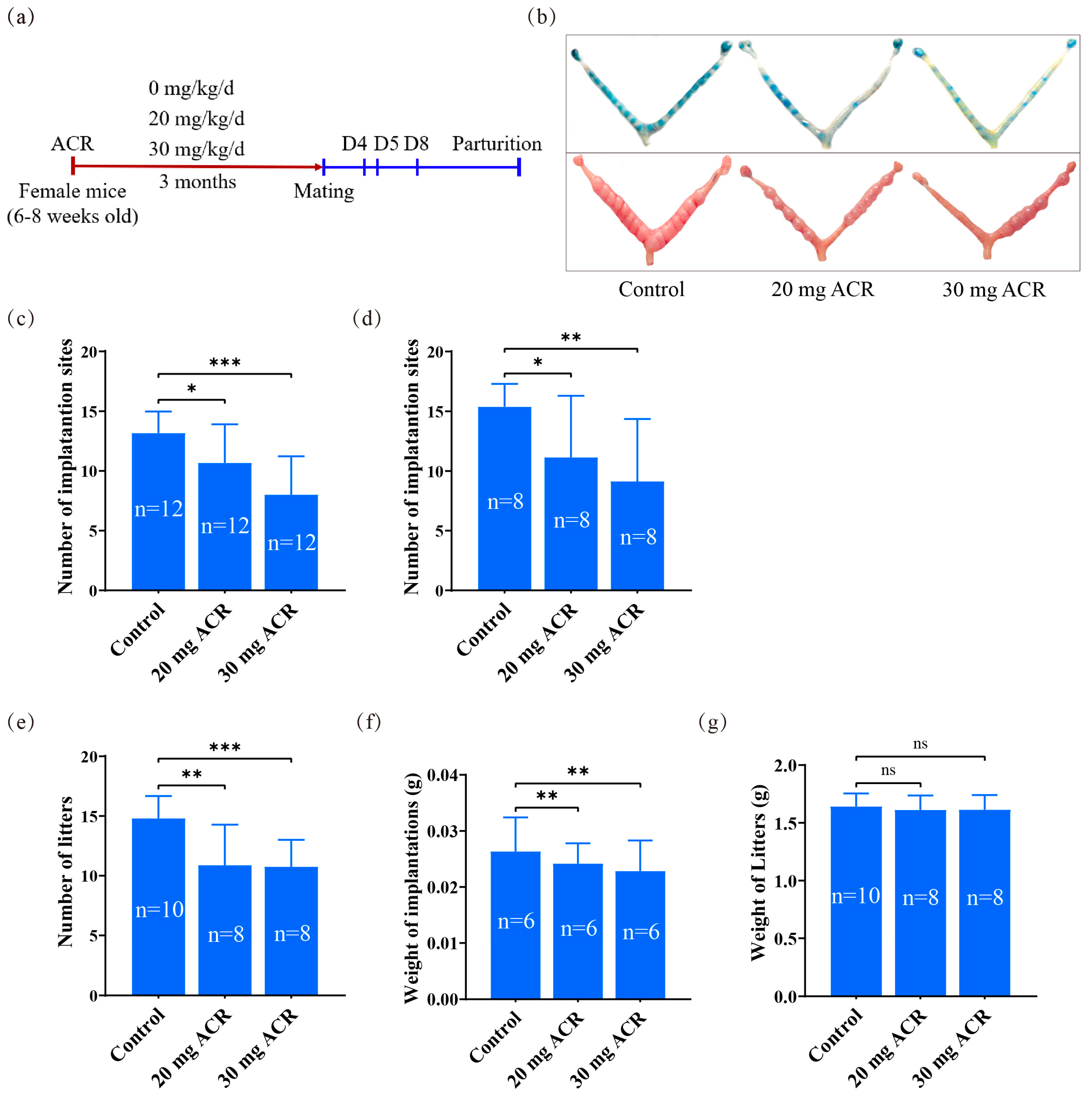
2.1. The Deleterious Effects of Acrylamide on Implantation and Pregnancy Outcomes
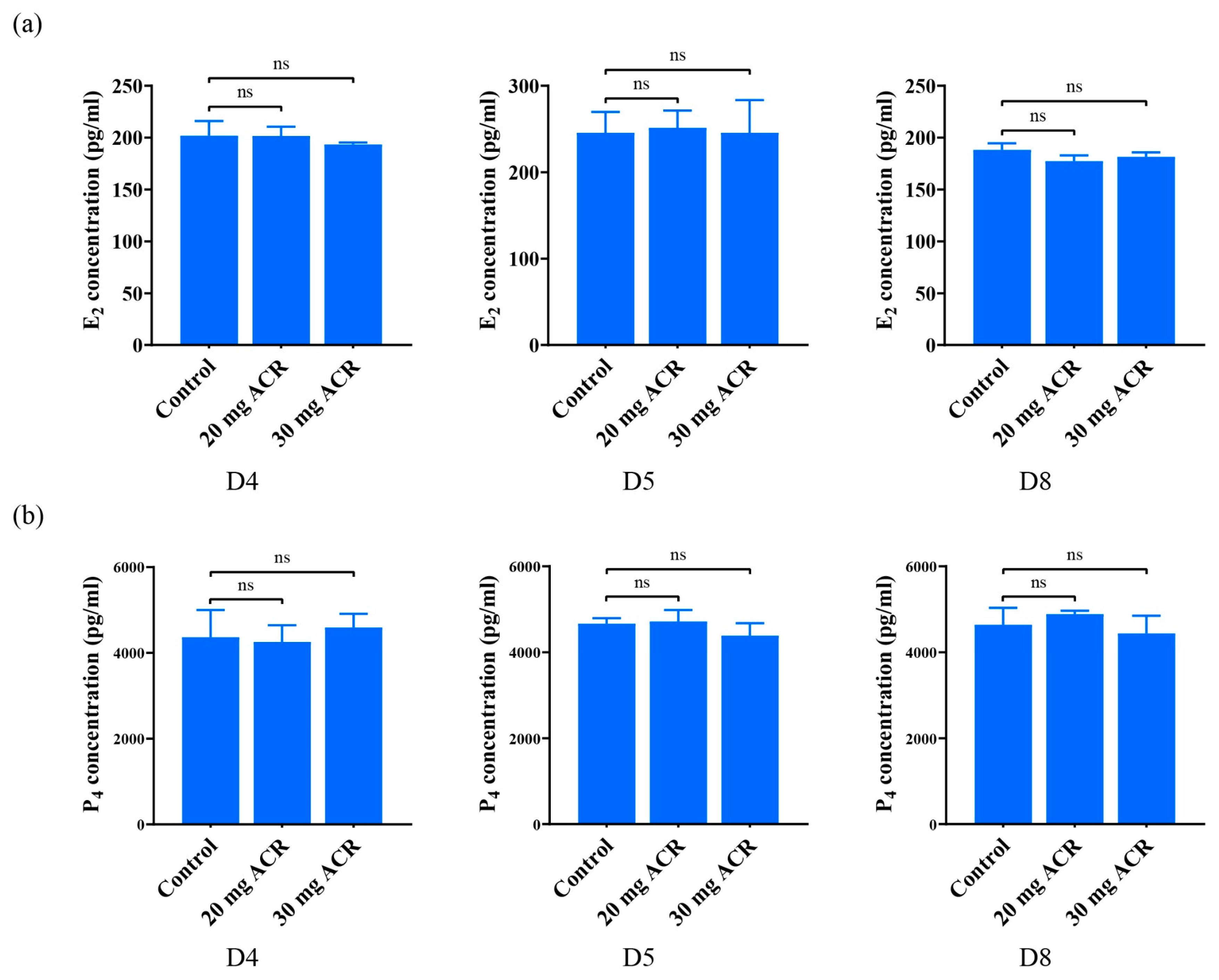
2.2. The Effects of Acrylamide on Ovarian Hormones
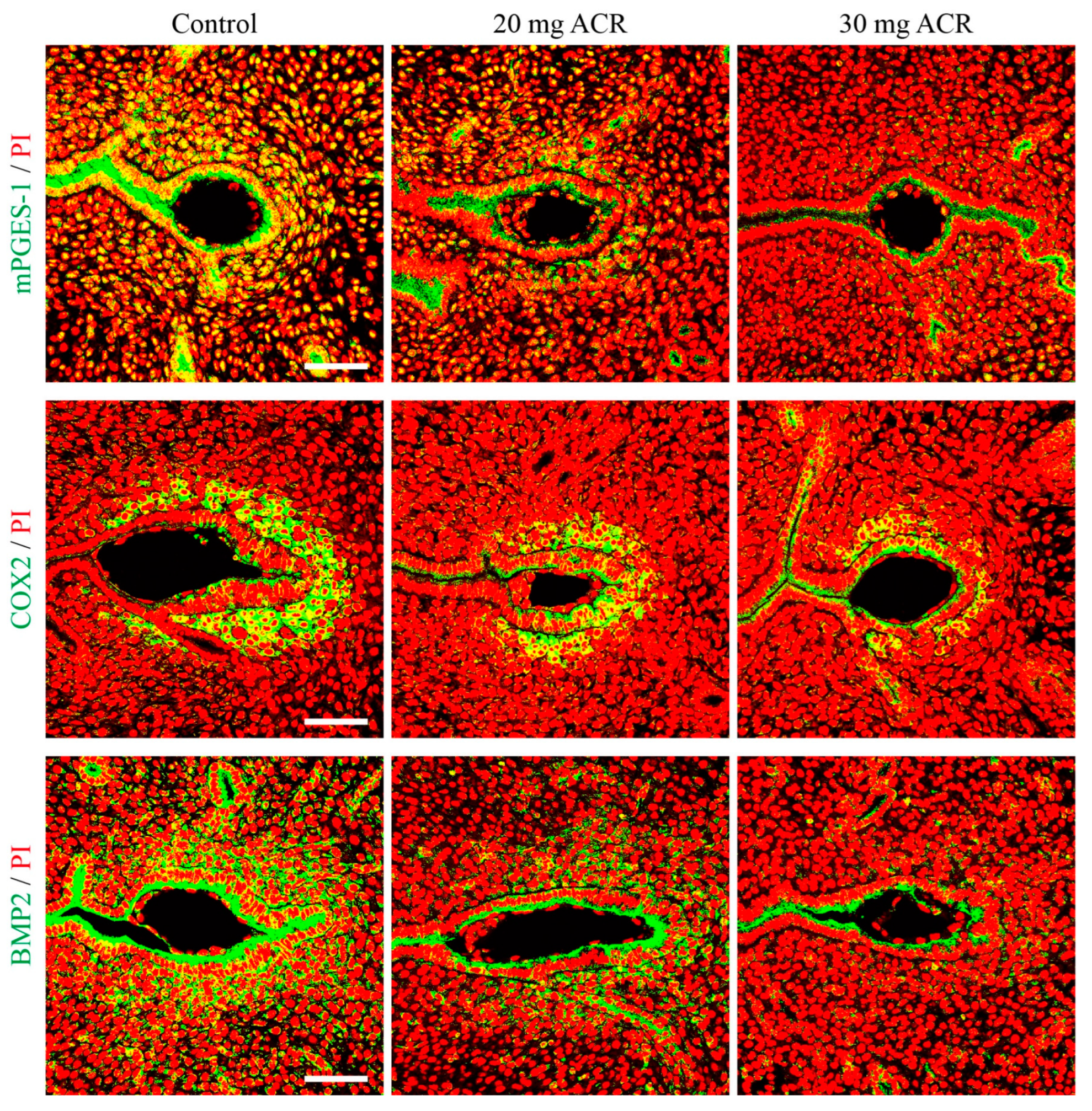
2.3. Acrylamide Treatments Affected Uterine Receptivity
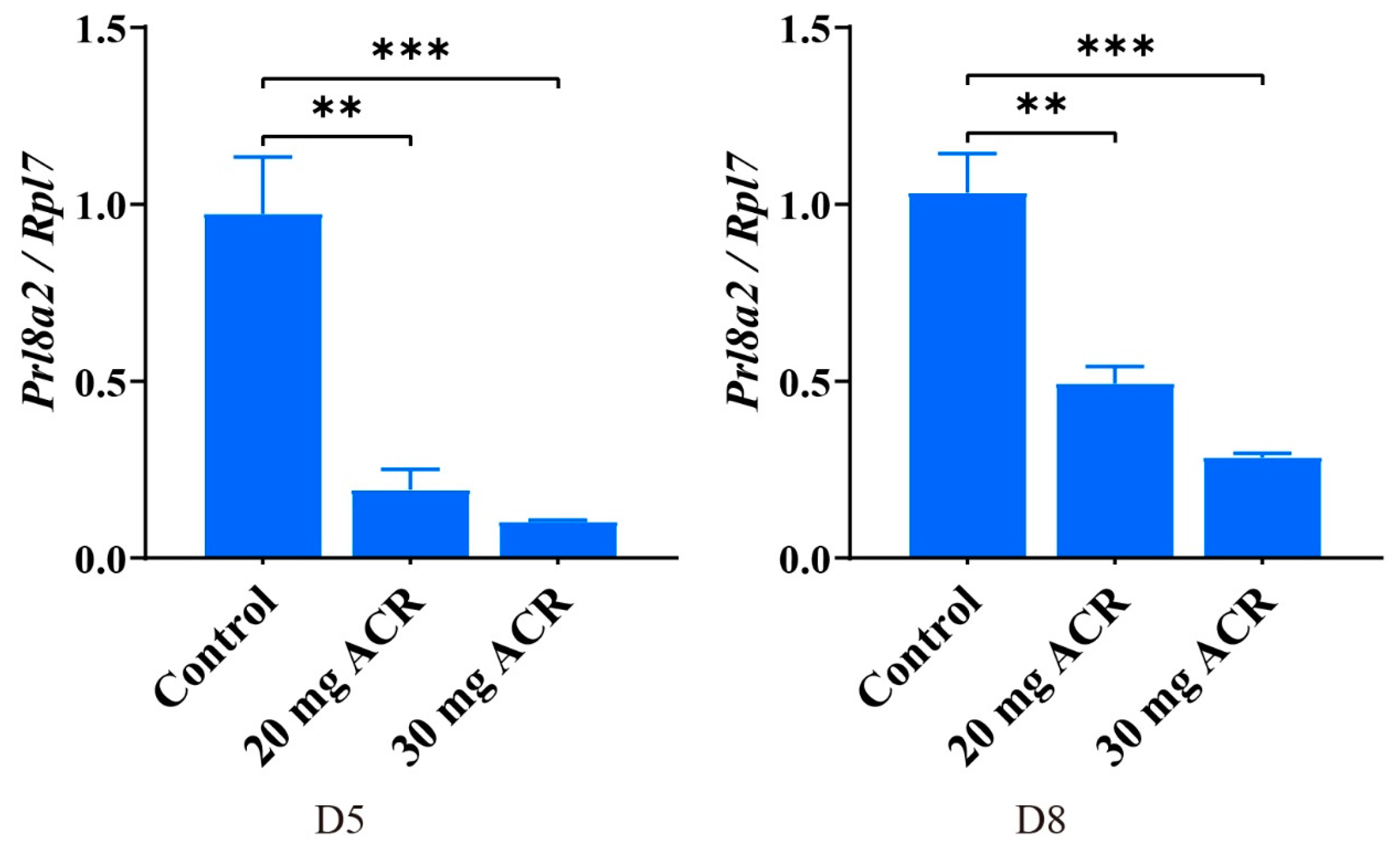
2.4. Acrylamide Suppressed Decidualization-Related Molecules
2.5. Acrylamide Inhibited Decidualization
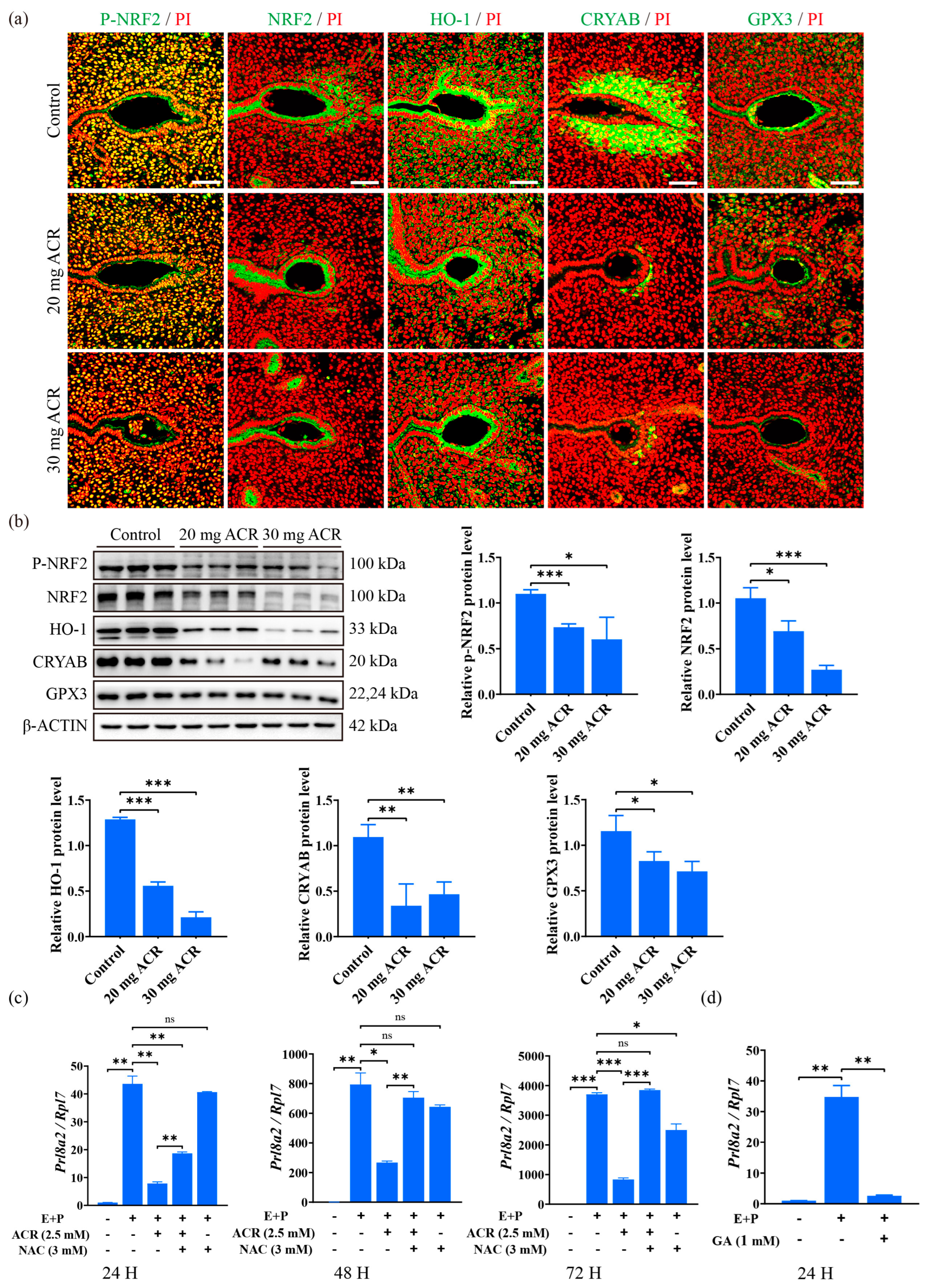
2.6. Acrylamide Caused Abnormal Expression of Oxidative Stress Molecules at Implantation Sites
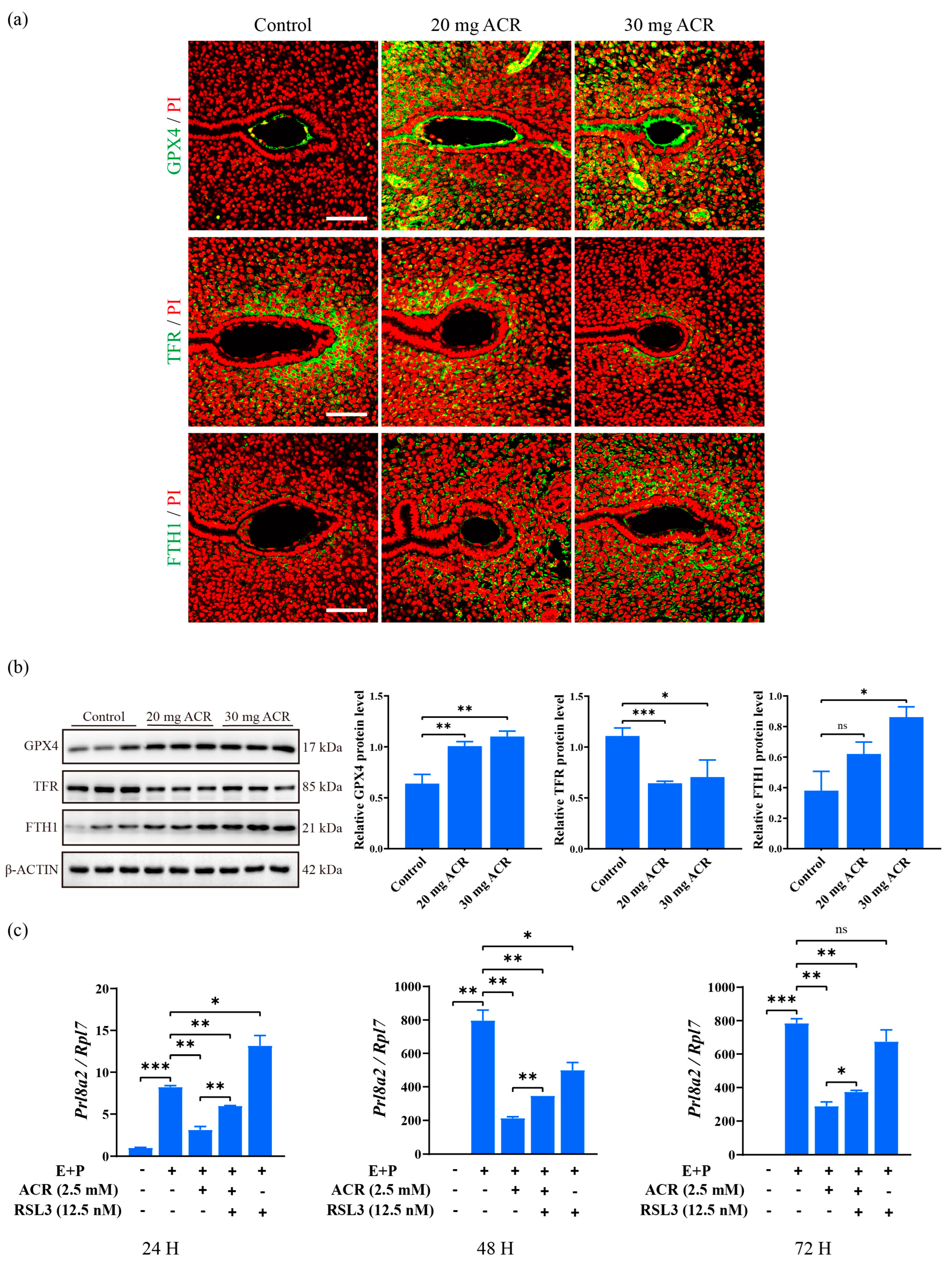
2.7. Acrylamide Caused Abnormal Expression of Ferroptosis-Related Proteins at Implantation Sites
3. Discussion
4. Materials and Methods
4.1. Animals and Treatments
4.2. Immunofluorescence
4.3. Western Blot
4.4. Isolation and Treatment of Mouse Endometrial Stromal Cells
4.5. Real Time RT-PCR
4.6. Measurement of E2 and P4 Concentration
4.7. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Aikawa, S.; Hiraoka, T.; Matsuo, M.; Fukui, Y.; Fujita, H.; Saito-Fujita, T.; Shimizu-Hirota, R.; Takeda, N.; Hiratsuka, D.; He, X.; et al. Spatiotemporal functions of leukemia inhibitory factor in embryo attachment and implantation chamber formation. Cell Death Discov. 2024, 10, 481. [Google Scholar] [CrossRef] [PubMed]
- Conrad, K.P.; Rabaglino, M.B.; Post Uiterweer, E.D. Emerging role for dysregulated decidualization in the genesis of preeclampsia. Placenta 2017, 60, 119–129. [Google Scholar] [CrossRef]
- Koszucka, A.; Nowak, A.; Nowak, I.; Motyl, I. Acrylamide in human diet, its metabolism, toxicity, inactivation and the associated European Union legal regulations in food industry. Crit. Rev. Food Sci. Nutr. 2020, 60, 1677–1692. [Google Scholar] [CrossRef]
- Hogervorst, J.G.; Schouten, L.J.; Konings, E.J.; Goldbohm, R.A.; van den Brandt, P.A. A prospective study of dietary acrylamide intake and the risk of endometrial, ovarian, and breast cancer. Cancer Epidemiol. Biomark. Prev. 2007, 16, 2304–2313. [Google Scholar] [CrossRef]
- Svensson, K.; Abramsson, L.; Becker, W.; Glynn, A.; Hellenas, K.E.; Lind, Y.; Rosen, J. Dietary intake of acrylamide in Sweden. Food Chem. Toxicol. 2003, 41, 1581–1586. [Google Scholar] [CrossRef]
- Exon, J.H. A review of the toxicology of acrylamide. J. Toxicol. Environ. Health Part B Crit. Rev. 2006, 9, 397–412. [Google Scholar] [CrossRef] [PubMed]
- Duan, X.; Wang, Q.C.; Chen, K.L.; Zhu, C.C.; Liu, J.; Sun, S.C. Acrylamide toxic effects on mouse oocyte quality and fertility in vivo. Sci. Rep. 2015, 5, 11562. [Google Scholar] [CrossRef] [PubMed]
- Aras, D.; Cakar, Z.; Ozkavukcu, S.; Can, A.; Cinar, O. In Vivo acrylamide exposure may cause severe toxicity to mouse oocytes through its metabolite glycidamide. PLoS ONE 2017, 12, e0172026. [Google Scholar] [CrossRef]
- Wu, S.L.; Ju, J.Q.; Ji, Y.M.; Zhang, H.L.; Zou, Y.J.; Sun, S.C. Exposure to acrylamide induces zygotic genome activation defects of mouse embryos. Food Chem. Toxicol. 2023, 175, 113753. [Google Scholar] [CrossRef]
- Liu, S.; Jiang, L.; Zhong, T.; Kong, S.; Zheng, R.; Kong, F.; Zhang, C.; Zhang, L.; An, L. Effect of Acrylamide on Oocyte Nuclear Maturation and Cumulus Cells Apoptosis in Mouse In Vitro. PLoS ONE 2015, 10, e0135818. [Google Scholar] [CrossRef]
- Yu, D.; Xie, X.; Qiao, B.; Ge, W.; Gong, L.; Luo, D.; Zhang, D.; Li, Y.; Yang, B.; Kuang, H. Gestational exposure to acrylamide inhibits mouse placental development in vivo. J. Hazard. Mater. 2019, 367, 160–170. [Google Scholar] [CrossRef]
- Aldawood, N.; Alrezaki, A.; Alanazi, S.; Amor, N.; Alwasel, S.; Sirotkin, A.; Harrath, A.H. Acrylamide impairs ovarian function by promoting apoptosis and affecting reproductive hormone release, steroidogenesis and autophagy-related genes: An in vivo study. Ecotoxicol. Environ. Saf. 2020, 197, 110595. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.; Liu, Q.; Qiao, B.; Jiang, W.; Zhang, L.; Shen, X.; Xie, L.; Liu, H.; Zhang, D.; Yang, B.; et al. Exposure to acrylamide inhibits uterine decidualization via suppression of cyclin D3/p21 and apoptosis in mice. J. Hazard. Mater. 2020, 388, 121785. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Wang, Z.; Cao, J.; Chen, Y.; Dong, Y. A novel and compact review on the role of oxidative stress in female reproduction. Reprod. Biol. Endocrinol. 2018, 16, 80. [Google Scholar] [CrossRef]
- Yan, F.; Zhao, Q.; Li, Y.; Zheng, Z.; Kong, X.; Shu, C.; Liu, Y.; Shi, Y. The role of oxidative stress in ovarian aging: A review. J. Ovarian Res. 2022, 15, 100. [Google Scholar] [CrossRef]
- Agarwal, A.; Gupta, S.; Sekhon, L.; Shah, R. Redox considerations in female reproductive function and assisted reproduction: From molecular mechanisms to health implications. Antioxid. Redox Signal. 2008, 10, 1375–1403. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Wang, L.; Zhang, G.; Li, N.; Zhao, Y.; Liu, J.; Jiang, M.; Du, X.; Zeng, Q.; Xiong, D.; et al. Oxidative stress and energy metabolism abnormalities in polycystic ovary syndrome: From mechanisms to therapeutic strategies. Reprod. Biol. Endocrinol. 2024, 22, 159. [Google Scholar] [CrossRef]
- Reiter, R.J.; Sharma, R.; LG, D.A.C.; Zuccari, D.A.; Amaral, F.G.; Cipolla-Neto, J. Melatonin-mediated actions and circadian functions that improve implantation, fetal health and pregnancy outcome. Reprod. Toxicol. 2024, 124, 108534. [Google Scholar] [CrossRef]
- Zhang, Z.; Yang, J.; Zhou, Q.; Zhong, S.; Liu, J.; Zhang, X.; Chang, X.; Wang, H. The cGAS-STING-mediated ROS and ferroptosis are involved in manganese neurotoxicity. J. Environ. Sci. 2025, 152, 71–86. [Google Scholar] [CrossRef]
- You, Y.; Qian, Z.; Jiang, Y.; Chen, L.; Wu, D.; Liu, L.; Zhang, F.; Ning, X.; Zhang, Y.; Xiao, J. Insights into the pathogenesis of gestational and hepatic diseases: The impact of ferroptosis. Front. Cell Dev. Biol. 2024, 12, 1482838. [Google Scholar] [CrossRef]
- Dong, S.; Zhang, C.; Wang, Y.; Liu, S.; Yang, J.; Li, L.; Ma, Y.; Liu, J. The protective effect of rutin on sciatic nerve injury in acrylamide-exposed rats and its mechanisms. Food Chem. Toxicol. 2025, 195, 115106. [Google Scholar] [CrossRef] [PubMed]
- Kachranlouei, L.; Hosseinzadeh, H.; Karimi, G.; Rajabian, F.; Mehri, S. Ameliorative effects of osthole on acrylamide-induced neurotoxicity in PC12 cells: Role of oxidative stress, apoptosis and ERK pathways. Naunyn-Schmiedebergs Arch. Pharmacol. 2024, 398, 4361–4372. [Google Scholar] [CrossRef] [PubMed]
- Johansson, Y.; Awoga, R.A.; Forsby, A. Developmental neurotoxicity evaluation of acrylamide based on in vitro to in vivo extrapolation by pregnancy PBTK modelling. Toxicology 2024, 509, 153950. [Google Scholar] [CrossRef]
- Gur, F.; Cengiz, M.; Gur, B.; Cengiz, O.; Saricicek, O.; Ayhanci, A. Therapeutic role of boron on acrylamide-induced nephrotoxicity, cardiotoxicity, neurotoxicity, and testicular toxicity in rats: Effects on Nrf2/Keap-1 signaling pathway and oxidative stress. J. Trace Elem. Med. Biol. 2023, 80, 127274. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.; Zhang, K.; Wang, J.; He, K.; Zhou, X.; Nie, S. Quercetin Alleviates Acrylamide-Induced Liver Injury by Inhibiting Autophagy-Dependent Ferroptosis. J. Agric. Food Chem. 2023, 71, 7427–7439. [Google Scholar] [CrossRef]
- Yuan, Y.; Yucai, L.; Lu, L.; Hui, L.; Yong, P.; Haiyang, Y. Acrylamide induces ferroptosis in HSC-T6 cells by causing antioxidant imbalance of the XCT-GSH-GPX4 signaling and mitochondrial dysfunction. Toxicol. Lett. 2022, 368, 24–32. [Google Scholar] [CrossRef]
- Dey, S.K.; Lim, H.; Das, S.K.; Reese, J.; Paria, B.C.; Daikoku, T.; Wang, H. Molecular cues to implantation. Endocr. Rev. 2004, 25, 341–373. [Google Scholar] [CrossRef]
- Daikoku, T.; Cha, J.; Sun, X.; Tranguch, S.; Xie, H.; Fujita, T.; Hirota, Y.; Lydon, J.; DeMayo, F.; Maxson, R.; et al. Conditional deletion of Msx homeobox genes in the uterus inhibits blastocyst implantation by altering uterine receptivity. Dev. Cell 2011, 21, 1014–1025. [Google Scholar] [CrossRef]
- Wang, P.; Du, S.; Guo, C.; Ni, Z.; Huang, Z.; Deng, N.; Bao, H.; Deng, W.; Lu, J.; Kong, S.; et al. The presence of blastocyst within the uteri facilitates lumenal epithelium transformation for implantation via upregulating lysosome proteostasis activity. Autophagy 2024, 20, 58–75. [Google Scholar] [CrossRef]
- Xu, Y.; Wu, F.; Qin, C.; Lin, Y. Paradoxical role of phosphorylated STAT3 in normal fertility and the pathogenesis of adenomyosis and endometriosisdagger. Biol. Reprod. 2024, 110, 5–13. [Google Scholar] [CrossRef]
- Matsumoto, H.; Daikoku, T.; Wang, H.; Sato, E.; Dey, S.K. Differential expression of ezrin/radixin/moesin (ERM) and ERM-associated adhesion molecules in the blastocyst and uterus suggests their functions during implantation. Biol. Reprod. 2004, 70, 729–736. [Google Scholar] [CrossRef]
- Heng, S.; Cervero, A.; Simon, C.; Stephens, A.N.; Li, Y.; Zhang, J.; Paule, S.; Rainczuk, A.; Singh, H.; Quinonero, A.; et al. Proprotein convertase 5/6 is critical for embryo implantation in women: Regulating receptivity by cleaving EBP50, modulating ezrin binding, and membrane-cytoskeletal interactions. Endocrinology 2011, 152, 5041–5052. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.; Paria, B.C.; Das, S.K.; Dinchuk, J.E.; Langenbach, R.; Trzaskos, J.M.; Dey, S.K. Multiple female reproductive failures in cyclooxygenase 2-deficient mice. Cell 1997, 91, 197–208. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.Y.; Jeong, J.W.; Wang, J.; Ma, L.; Martin, J.F.; Tsai, S.Y.; Lydon, J.P.; DeMayo, F.J. Bmp2 is critical for the murine uterine decidual response. Mol. Cell. Biol. 2007, 27, 5468–5478. [Google Scholar] [CrossRef]
- Murakami, M.; Naraba, H.; Tanioka, T.; Semmyo, N.; Nakatani, Y.; Kojima, F.; Ikeda, T.; Fueki, M.; Ueno, A.; Oh, S.; et al. Regulation of prostaglandin E2 biosynthesis by inducible membrane-associated prostaglandin E2 synthase that acts in concert with cyclooxygenase-2. J. Biol. Chem. 2000, 275, 32783–32792. [Google Scholar] [CrossRef]
- Ni, H.; Sun, T.; Ding, N.Z.; Ma, X.H.; Yang, Z.M. Differential expression of microsomal prostaglandin e synthase at implantation sites and in decidual cells of mouse uterus. Biol. Reprod. 2002, 67, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Kimura, F.; Takakura, K.; Takebayashi, K.; Ishikawa, H.; Kasahara, K.; Goto, S.; Noda, Y. Messenger ribonucleic acid for the mouse decidual prolactin is present and induced during in vitro decidualization of endometrial stromal cells. Gynecol. Endocrinol. 2001, 15, 426–432. [Google Scholar] [CrossRef]
- Li, N.; Hao, L.; Li, S.; Deng, J.; Yu, F.; Zhang, J.; Nie, A.; Hu, X. The NRF-2/HO-1 Signaling Pathway: A Promising Therapeutic Target for Metabolic Dysfunction-Associated Steatotic Liver Disease. J. Inflamm. Res. 2024, 17, 8061–8083. [Google Scholar] [CrossRef]
- Zhang, N.; Liao, H.; Lin, Z.; Tang, Q. Insights into the Role of Glutathione Peroxidase 3 in Non-Neoplastic Diseases. Biomolecules 2024, 14, 689. [Google Scholar] [CrossRef]
- Zuo, R.J.; Zhao, Y.C.; Lei, W.; Wang, T.S.; Wang, B.C.; Yang, Z.M. Crystallin alphaB acts as a molecular guard in mouse decidualization: Regulation and function during early pregnancy. FEBS Lett. 2014, 588, 2944–2951. [Google Scholar] [CrossRef]
- Mi, Y.; Wei, C.; Sun, L.; Liu, H.; Zhang, J.; Luo, J.; Yu, X.; He, J.; Ge, H.; Liu, P. Melatonin inhibits ferroptosis and delays age-related cataract by regulating SIRT6/p-Nrf2/GPX4 and SIRT6/NCOA4/FTH1 pathways. Biomed. Pharmacother. 2023, 157, 114048. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.C.; Nguyen, T.; Pickett, C.B. Phosphorylation of Nrf2 at Ser-40 by protein kinase C regulates antioxidant response element-mediated transcription. J. Biol. Chem. 2002, 277, 42769–42774. [Google Scholar] [CrossRef] [PubMed]
- Halasi, M.; Wang, M.; Chavan, T.S.; Gaponenko, V.; Hay, N.; Gartel, A.L. ROS inhibitor N-acetyl-L-cysteine antagonizes the activity of proteasome inhibitors. Biochem. J. 2013, 454, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Koszucka, A.; Nowak, A. Thermal processing food-related toxicants: A review. Crit. Rev. Food Sci. Nutr. 2019, 59, 3579–3596. [Google Scholar] [CrossRef]
- Wang, X.; Gao, M.; Lu, X.; Lei, Y.; Sun, J.; Ren, M.; Xu, T.; Lin, H. Resveratrol alleviates Mono-2-ethylhexyl phthalate-induced mitophagy, ferroptosis, and immunological dysfunction in grass carp hepatocytes by regulating the Nrf2 pathway. J. Environ. Manag. 2024, 371, 123235. [Google Scholar] [CrossRef]
- Yan, X.; Xu, L.; Qi, C.; Chang, Y.; Zhang, J.; Li, N.; Shi, B.; Guan, B.; Hu, S.; Huang, C.; et al. Brazilin alleviates acute lung injury via inhibition of ferroptosis through the SIRT3/GPX4 pathway. Apoptosis 2024, 30, 768–783. [Google Scholar] [CrossRef]
- Shin, D.; Kim, E.H.; Lee, J.; Roh, J.L. Nrf2 inhibition reverses resistance to GPX4 inhibitor-induced ferroptosis in head and neck cancer. Free Radic. Biol. Med. 2018, 129, 454–462. [Google Scholar] [CrossRef]
- Kwolek-Mirek, M.; Zadrag-Tecza, R.; Bednarska, S.; Bartosz, G. Yeast Saccharomyces cerevisiae devoid of Cu,Zn-superoxide dismutase as a cellular model to study acrylamide toxicity. Toxicol. In Vitro 2011, 25, 573–579. [Google Scholar] [CrossRef]
- Hogervorst, J.G.; Schouten, L.J.; Konings, E.J.; Goldbohm, R.A.; van den Brandt, P.A. Dietary acrylamide intake and the risk of renal cell, bladder, and prostate cancer. Am. J. Clin. Nutr. 2008, 87, 1428–1438. [Google Scholar] [CrossRef]
- Kacar, S.; Vejselova, D.; Kutlu, H.M.; Sahinturk, V. Acrylamide-derived cytotoxic, anti-proliferative, and apoptotic effects on A549 cells. Hum. Exp. Toxicol. 2018, 37, 468–474. [Google Scholar] [CrossRef]
- Sun, X.; Park, C.B.; Deng, W.; Potter, S.S.; Dey, S.K. Uterine inactivation of muscle segment homeobox (Msx) genes alters epithelial cell junction proteins during embryo implantation. FASEB J. 2016, 30, 1425–1435. [Google Scholar] [CrossRef]
- Lim, H.J.; Wang, H. Uterine disorders and pregnancy complications: Insights from mouse models. J. Clin. Investig. 2010, 120, 1004–1015. [Google Scholar] [CrossRef] [PubMed]
- Andrianifahanana, M.; Moniaux, N.; Batra, S.K. Regulation of mucin expression: Mechanistic aspects and implications for cancer and inflammatory diseases. Biochim. Biophys. Acta 2006, 1765, 189–222. [Google Scholar] [CrossRef]
- Hantak, A.M.; Bagchi, I.C.; Bagchi, M.K. Role of uterine stromal-epithelial crosstalk in embryo implantation. Int. J. Dev. Biol. 2014, 58, 139–146. [Google Scholar] [CrossRef]
- Nallasamy, S.; Kaya Okur, H.S.; Bhurke, A.; Davila, J.; Li, Q.; Young, S.L.; Taylor, R.N.; Bagchi, M.K.; Bagchi, I.C. Msx Homeobox Genes Act Downstream of BMP2 to Regulate Endometrial Decidualization in Mice and in Humans. Endocrinology 2019, 160, 1631–1644. [Google Scholar] [CrossRef] [PubMed]
- Hiraoka, T.; Hirota, Y.; Fukui, Y.; Gebril, M.; Kaku, T.; Aikawa, S.; Hirata, T.; Akaeda, S.; Matsuo, M.; Haraguchi, H.; et al. Differential roles of uterine epithelial and stromal STAT3 coordinate uterine receptivity and embryo attachment. Sci. Rep. 2020, 10, 15523. [Google Scholar] [CrossRef]
- Peloggia, A.; Andres, M.P.; Abrao, M.S. Expression of ezrin protein and phosphorylated ezrin in pelvic endometriotic lesions. Clinics 2022, 77, 100074. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Li, G.; Du, M.; Bao, S. Effect of Ezrin on regulating trophoblast cell invasion via PKC signaling pathway in unexplained recurrent spontaneous abortion. Reprod. Biol. 2022, 22, 100634. [Google Scholar] [CrossRef]
- Morales, F.C.; Takahashi, Y.; Kreimann, E.L.; Georgescu, M.M. Ezrin-radixin-moesin (ERM)-binding phosphoprotein 50 organizes ERM proteins at the apical membrane of polarized epithelia. Proc. Natl. Acad. Sci. USA 2004, 101, 17705–17710. [Google Scholar] [CrossRef]
- Zhou, Y.J.; Geng, Y.Q.; Gao, R.F.; Liu, X.Q.; Chen, X.M.; He, J.L. Early pregnancy exposure to beta-cypermethrin compromises endometrial decidualisation in mice via downregulation of cyclin D3, CDK4/6, and p21. Food Chem. Toxicol. 2022, 169, 113382. [Google Scholar] [CrossRef]
- Cong, J.; Diao, H.L.; Zhao, Y.C.; Ni, H.; Yan, Y.Q.; Yang, Z.M. Differential expression and regulation of cylooxygenases, prostaglandin E synthases and prostacyclin synthase in rat uterus during the peri-implantation period. Reproduction 2006, 131, 139–151. [Google Scholar] [CrossRef] [PubMed]
- Pakrasi, P.L.; Jain, A.K. Cyclooxygenase-2 derived PGE2 and PGI2 play an important role via EP2 and PPARdelta receptors in early steps of oil induced decidualization in mice. Placenta 2008, 29, 523–530. [Google Scholar] [CrossRef] [PubMed]
- Han, H.; Zhang, G.; Zhang, X.; Zhao, Q. Nrf2-mediated ferroptosis inhibition: A novel approach for managing inflammatory diseases. Inflammopharmacology 2024, 32, 2961–2986. [Google Scholar] [CrossRef] [PubMed]
- von Mengden, L.; Klamt, F.; Smitz, J. Redox Biology of Human Cumulus Cells: Basic Concepts, Impact on Oocyte Quality, and Potential Clinical Use. Antioxid. Redox Signal. 2020, 32, 522–535. [Google Scholar] [CrossRef]
- Frohlich, D.A.; McCabe, M.T.; Arnold, R.S.; Day, M.L. The role of Nrf2 in increased reactive oxygen species and DNA damage in prostate tumorigenesis. Oncogene 2008, 27, 4353–4362. [Google Scholar] [CrossRef]
- Padron, J.G.; Norman Ing, N.D.; Ng, P.K.; Kendal-Wright, C.E. Stretch Causes Cell Stress and the Downregulation of Nrf2 in Primary Amnion Cells. Biomolecules 2022, 12, 766. [Google Scholar] [CrossRef]
- Guo, F.; Zhuang, X.; Han, M.; Lin, W. Polysaccharides from Enteromorpha prolifera protect against carbon tetrachloride-induced acute liver injury in mice via activation of Nrf2/HO-1 signaling, and suppression of oxidative stress, inflammation and apoptosis. Food Funct. 2020, 11, 4485–4498. [Google Scholar] [CrossRef]
- Ekuban, F.A.; Zong, C.; Takikawa, M.; Morikawa, K.; Sakurai, T.; Ichihara, S.; Itoh, K.; Yamamoto, M.; Ohsako, S.; Ichihara, G. Genetic ablation of Nrf2 exacerbates neurotoxic effects of acrylamide in mice. Toxicology 2021, 456, 152785. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, X.; Xiong, X.; Zhu, H.; Chen, R.; Zhang, S.; Chen, G.; Jian, Z. Nrf2 Regulates Oxidative Stress and Its Role in Cerebral Ischemic Stroke. Antioxidants 2022, 11, 2377. [Google Scholar] [CrossRef]
- Jansen, T.; Hortmann, M.; Oelze, M.; Opitz, B.; Steven, S.; Schell, R.; Knorr, M.; Karbach, S.; Schuhmacher, S.; Wenzel, P.; et al. Conversion of biliverdin to bilirubin by biliverdin reductase contributes to endothelial cell protection by heme oxygenase-1-evidence for direct and indirect antioxidant actions of bilirubin. J. Mol. Cell. Cardiol. 2010, 49, 186–195. [Google Scholar] [CrossRef]
- Cakmak, F.; Kucukler, S.; Gur, C.; Ileriturk, M.; Gul, M.; Varisli, B. Cardiotoxicity caused by acrylamide in rats can be alleviated as a result of suppression of oxidative stress, endoplasmic reticulum stress, inflammation, and apoptosis by morin treatment. Iran. J. Basic Med. Sci. 2025, 28, 376–384. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Leng, J.Y.; Gao, F.; Zhao, Z.A.; Deng, W.B.; Liang, X.H.; Zhang, Y.J.; Zhang, Z.R.; Li, M.; Sha, A.G.; et al. Differential expression and anti-oxidant function of glutathione peroxidase 3 in mouse uterus during decidualization. FEBS Lett. 2014, 588, 1580–1589. [Google Scholar] [CrossRef]
- Yang, W.S.; SriRamaratnam, R.; Welsch, M.E.; Shimada, K.; Skouta, R.; Viswanathan, V.S.; Cheah, J.H.; Clemons, P.A.; Shamji, A.F.; Clish, C.B.; et al. Regulation of ferroptotic cancer cell death by GPX4. Cell 2014, 156, 317–331. [Google Scholar] [CrossRef] [PubMed]
- Orino, K.; Lehman, L.; Tsuji, Y.; Ayaki, H.; Torti, S.V.; Torti, F.M. Ferritin and the response to oxidative stress. Biochem. J. 2001, 357, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Arosio, P.; Ingrassia, R.; Cavadini, P. Ferritins: A family of molecules for iron storage, antioxidation and more. Biochim. Biophys. Acta 2009, 1790, 589–599. [Google Scholar] [CrossRef]
- Nadadur, S.S.; Srirama, K.; Mudipalli, A. Iron transport & homeostasis mechanisms: Their role in health & disease. Indian J. Med. Res. 2008, 128, 533–544. [Google Scholar]
- Kawabata, H. Transferrin and transferrin receptors update. Free Radic. Biol. Med. 2019, 133, 46–54. [Google Scholar] [CrossRef]
- Levy, J.E.; Jin, O.; Fujiwara, Y.; Kuo, F.; Andrews, N.C. Transferrin receptor is necessary for development of erythrocytes and the nervous system. Nat. Genet. 1999, 21, 396–399. [Google Scholar] [CrossRef]
- Pourentezari, M.; Talebi, A.; Abbasi, A.; Khalili, M.A.; Mangoli, E.; Anvari, M. Effects of acrylamide on sperm parameters, chromatin quality, and the level of blood testosterone in mice. Iran. J. Reprod. Med. 2014, 12, 335–342. [Google Scholar]
- Li, M.Y.; Wu, Y.; Tang, H.L.; Wang, Y.; Li, B.; He, Y.Y.; Yan, G.J.; Yang, Z.M. Embryo-Derived Cathepsin B Promotes Implantation and Decidualization by Activating Pyroptosis. Adv. Sci. 2024, 11, e2402299. [Google Scholar] [CrossRef]
- Chen, S.T.; Shi, W.W.; Ran, F.; Liu, C.K.; Luo, H.N.; Wu, L.J.; Wu, Y.; Zhang, T.T.; Yang, Z.M. The activation of cGAS-STING pathway causes abnormal uterine receptivity in aged mice. Aging Cell 2024, 23, e14303. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Chen, S.T.; He, Y.Y.; Li, B.; Yang, C.; Yang, Z.S.; Yang, Z.M. The regulation and function of acetylated high-mobility group box 1 during implantation and decidualization. Front. Immunol. 2023, 14, 1024706. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; Li, B.; Li, M.; Luo, J.; Hong, Y.; He, Y.; Chen, S.; Yang, Z.; Liang, C.; Yang, Z. Caveolin-1 Regulation and Function in Mouse Uterus During Early Pregnancy and Under Human In Vitro Decidualization. Int. J. Mol. Sci. 2022, 23, 3699. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, H.-Y.; Luo, H.-N.; Wang, Z.-M.; Jin, D.-D.; Yang, Z.-M. Effects of Acrylamide on Mouse Implantation and Decidualization. Int. J. Mol. Sci. 2025, 26, 4129. https://doi.org/10.3390/ijms26094129
Yang H-Y, Luo H-N, Wang Z-M, Jin D-D, Yang Z-M. Effects of Acrylamide on Mouse Implantation and Decidualization. International Journal of Molecular Sciences. 2025; 26(9):4129. https://doi.org/10.3390/ijms26094129
Chicago/Turabian StyleYang, Hong-Yuan, Hui-Na Luo, Zai-Mei Wang, Dan-Dan Jin, and Zeng-Ming Yang. 2025. "Effects of Acrylamide on Mouse Implantation and Decidualization" International Journal of Molecular Sciences 26, no. 9: 4129. https://doi.org/10.3390/ijms26094129
APA StyleYang, H.-Y., Luo, H.-N., Wang, Z.-M., Jin, D.-D., & Yang, Z.-M. (2025). Effects of Acrylamide on Mouse Implantation and Decidualization. International Journal of Molecular Sciences, 26(9), 4129. https://doi.org/10.3390/ijms26094129





