Localizations of Laminin Chains Suggest Their Multifaceted Functions in Mouse Tooth Development
Abstract
:1. Introduction
2. Results
2.1. Primary Laminin Isoforms in the Basement Membrane Structures Surrounding Inner and Outer Enamel Epithelium Were Laminins 511 and 111
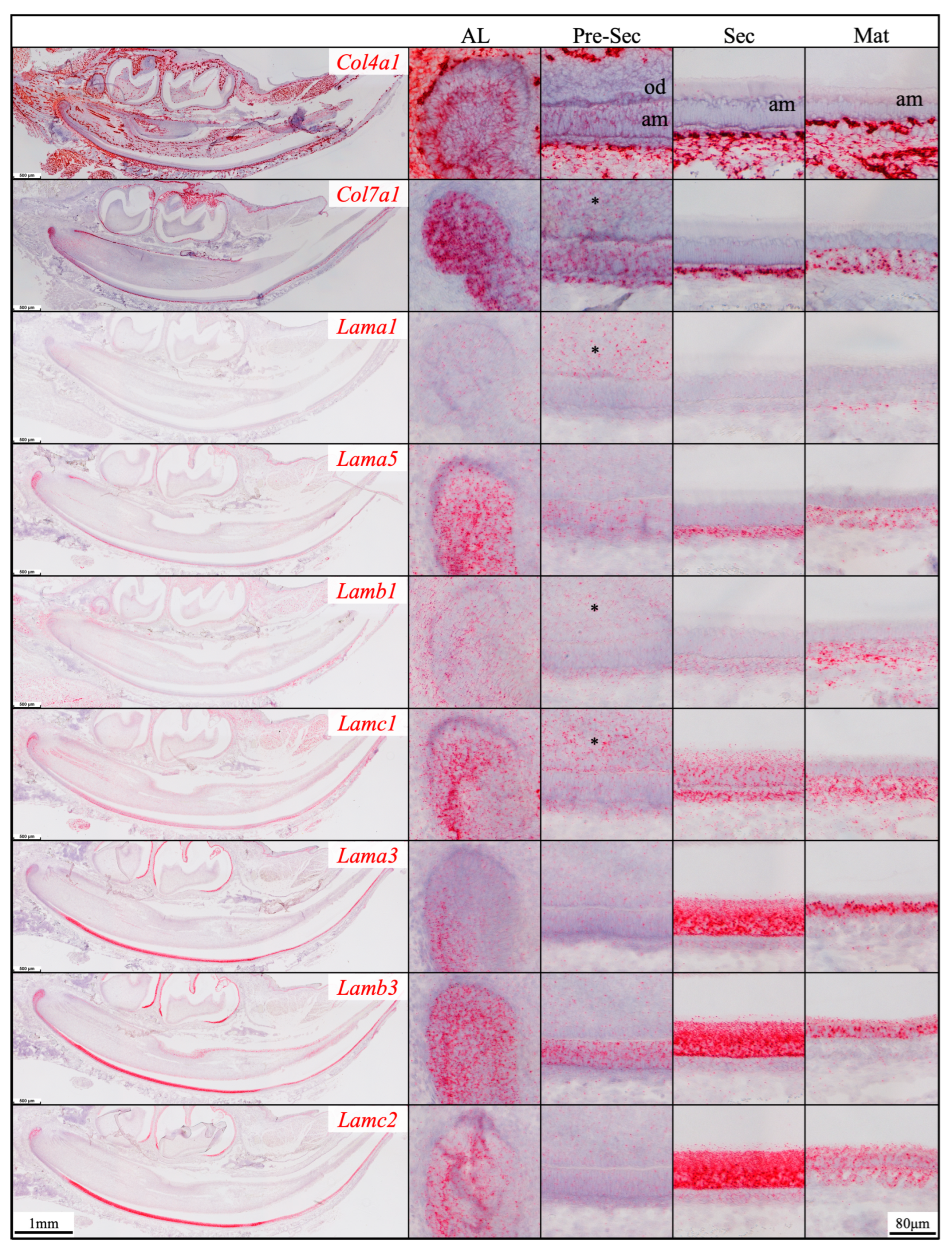
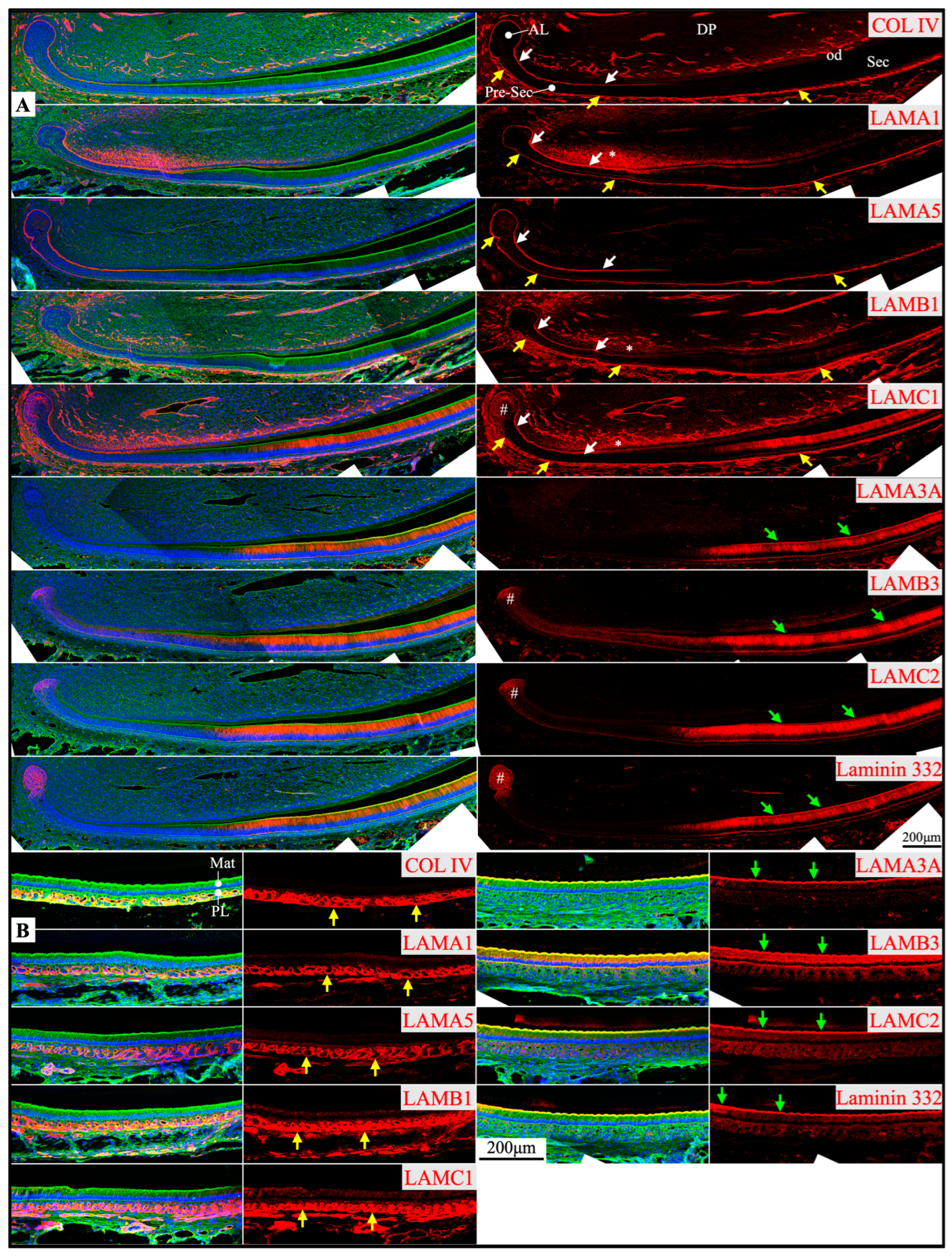
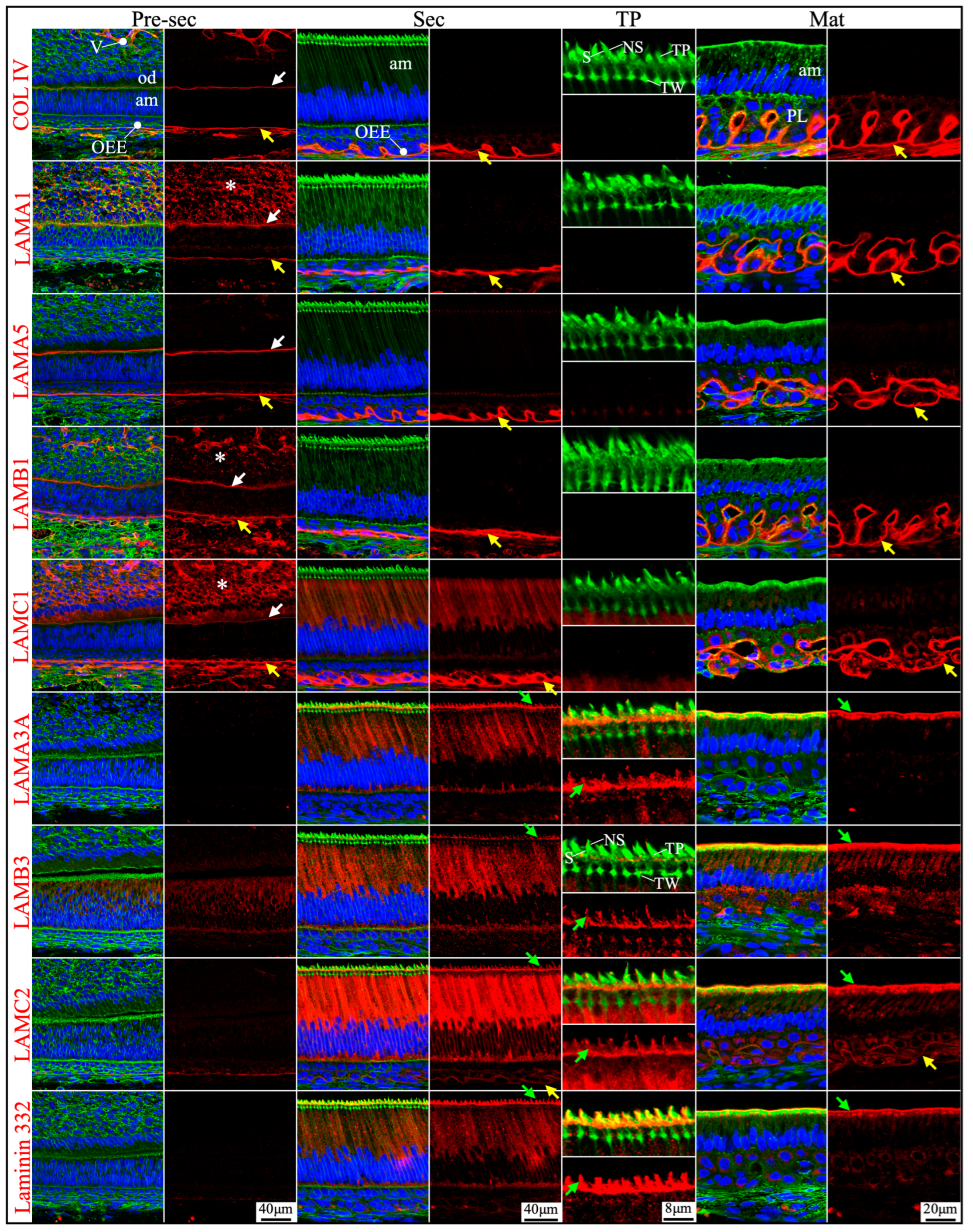
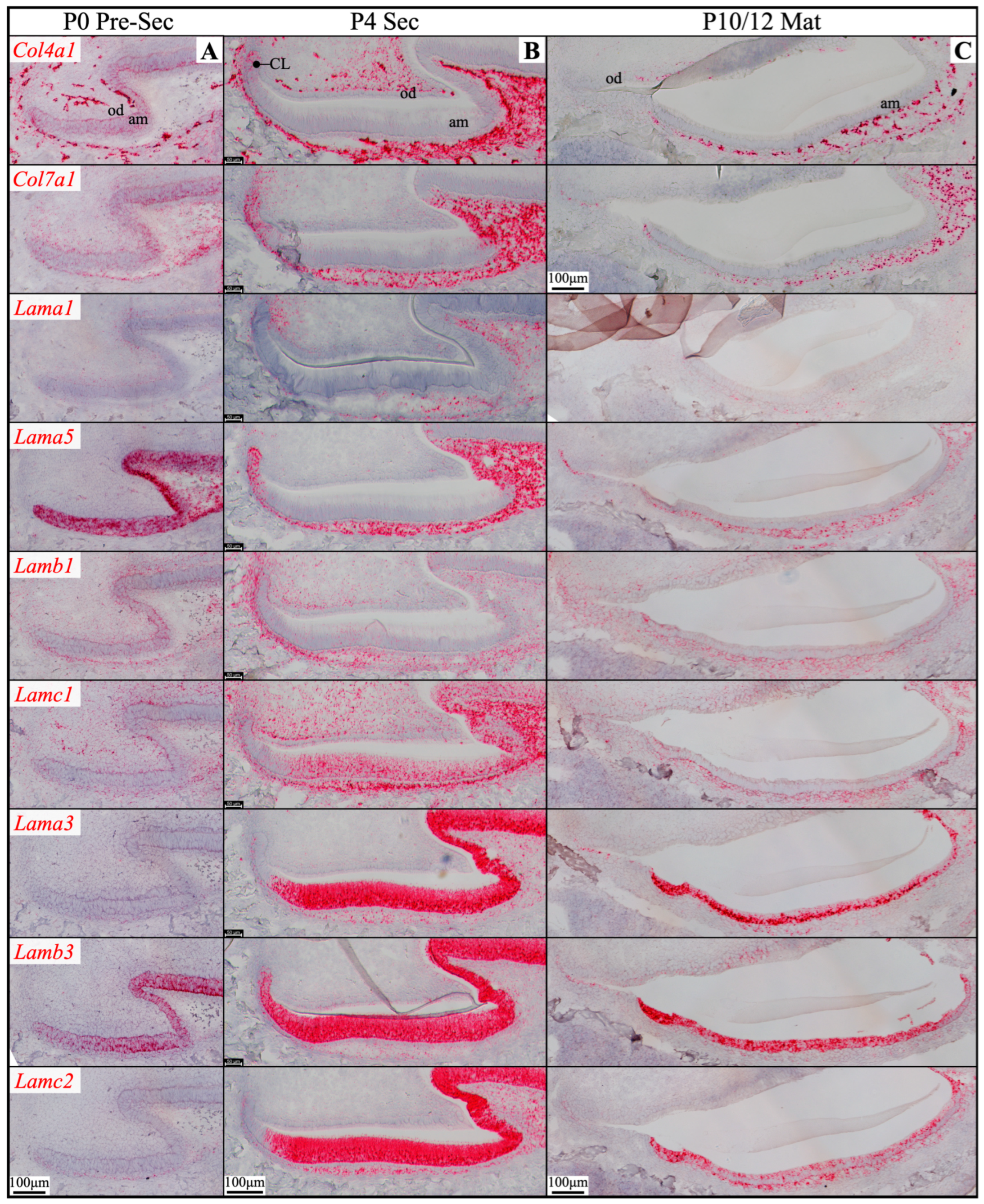
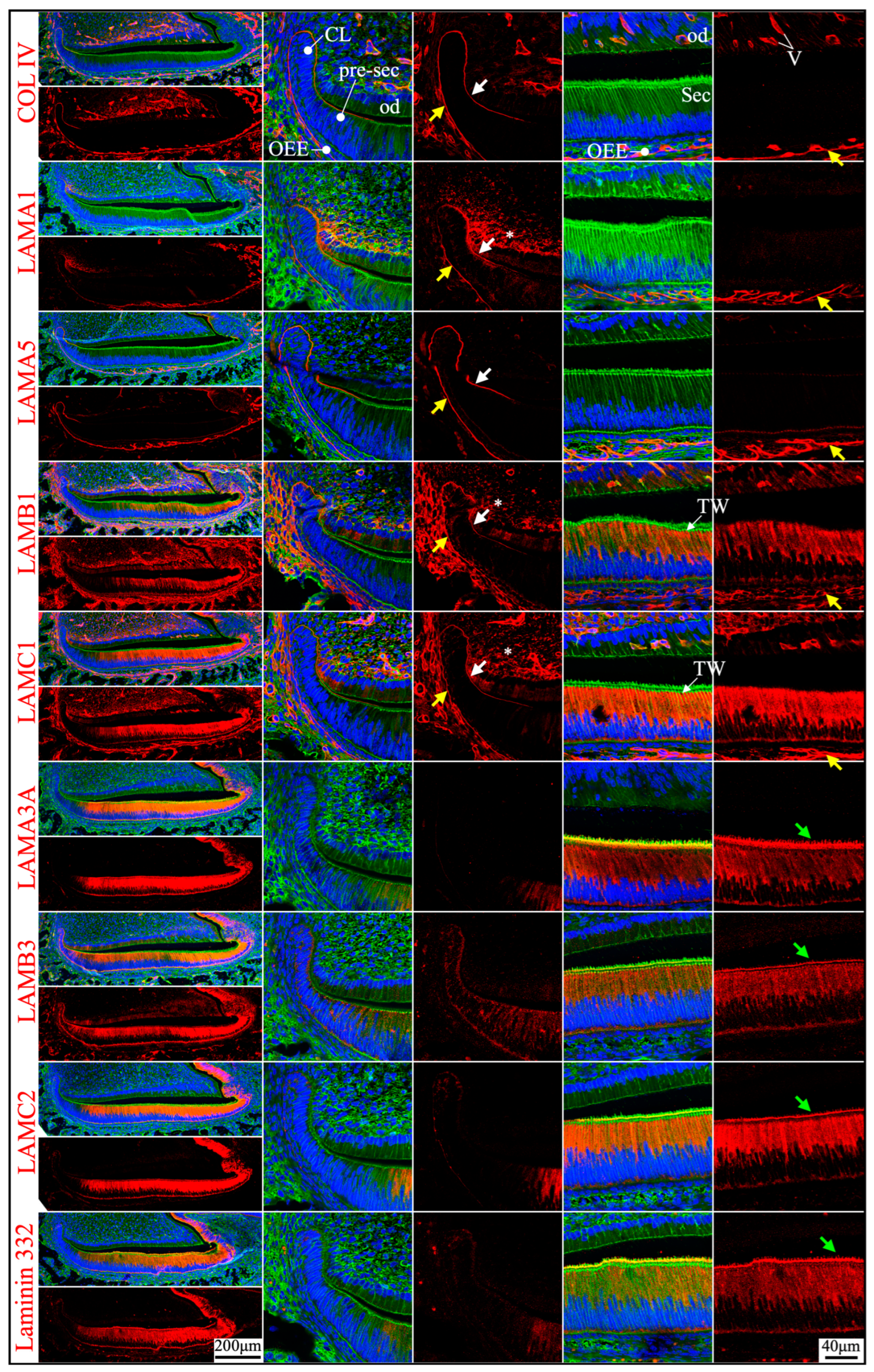
2.2. Laminin 3A32 Were Localized Along the Secretory Face of Tomes’ Process During Secretory Stage Enamel Formation Without Forming a Basement Membrane Structure

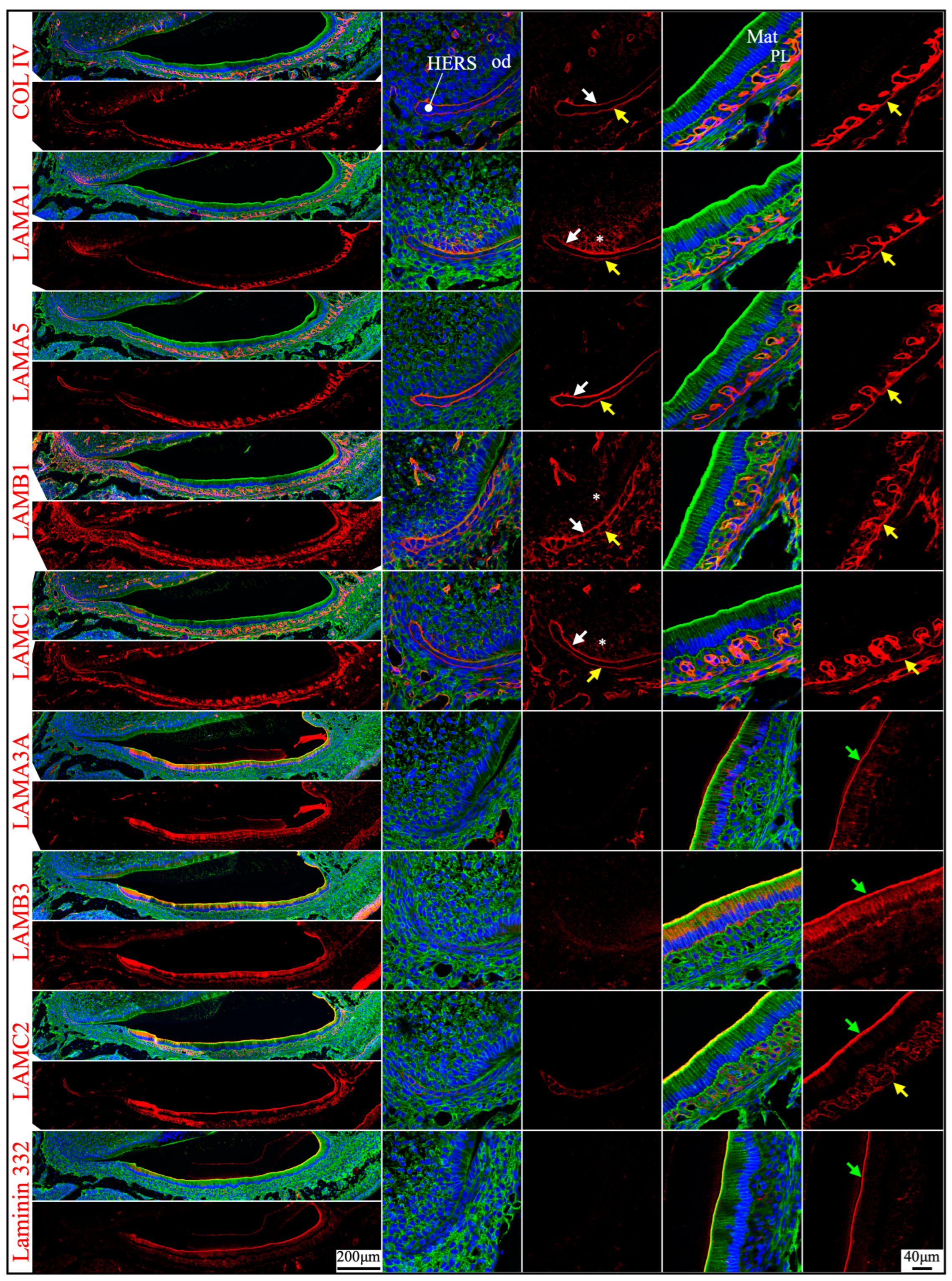
2.3. The Atypical Basement Membrane of Maturation Stage Ameloblasts Contained Laminin 3A32
2.4. Junctional Epithelium Was Connected to the Enamel via Laminin 3A32 and to the Gingival Connective Tissues via Laminins 111 and 511

2.5. Laminin 411 Was in the Endothelial Basement Membrane of Capillaries That Supplied the Dental Papilla and the Enamel Organ
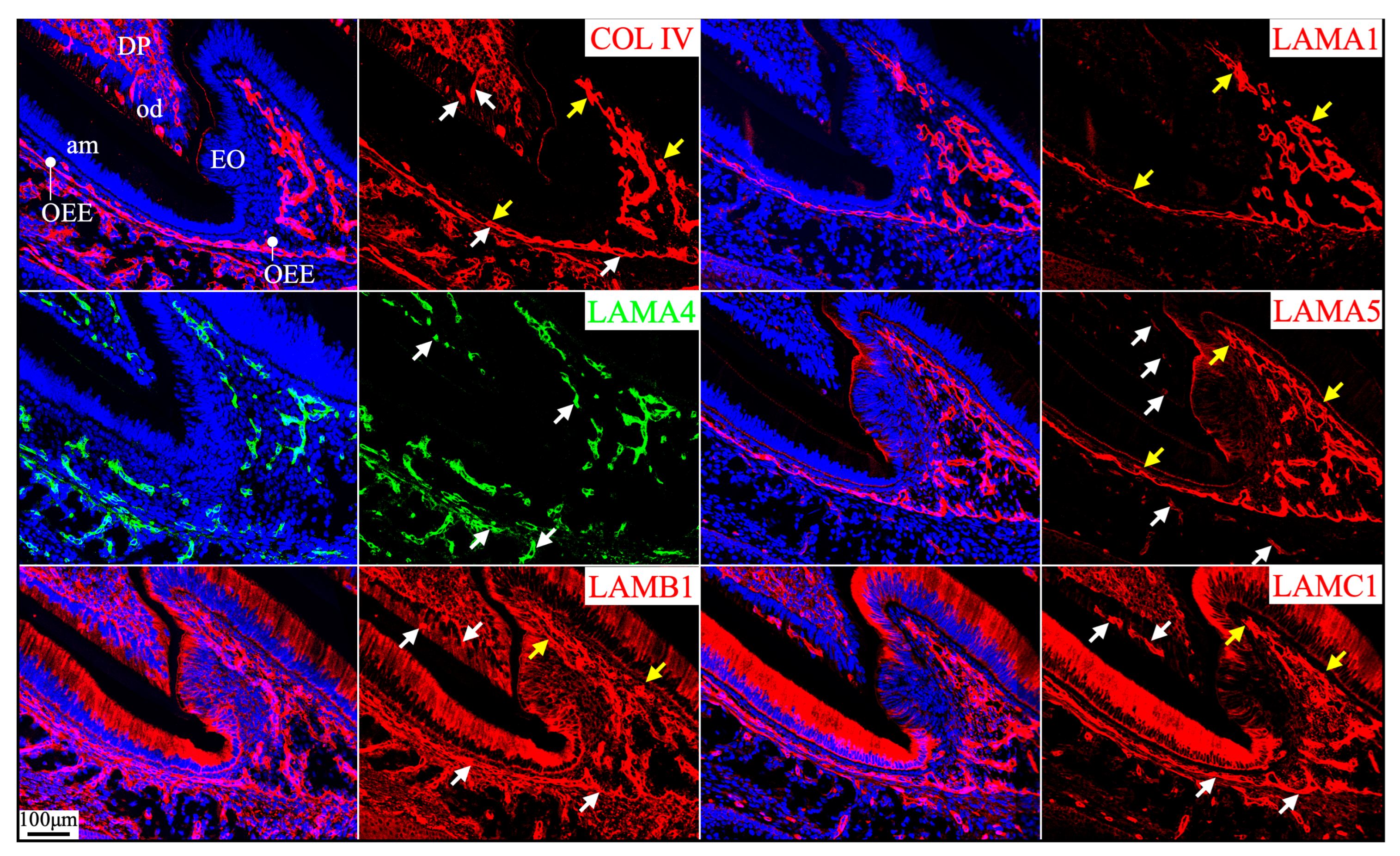
2.6. Laminin 111 Was Found in the Extracellular Matrix of Apical Dental Papilla Cells Without Forming a Basement Membrane Structure
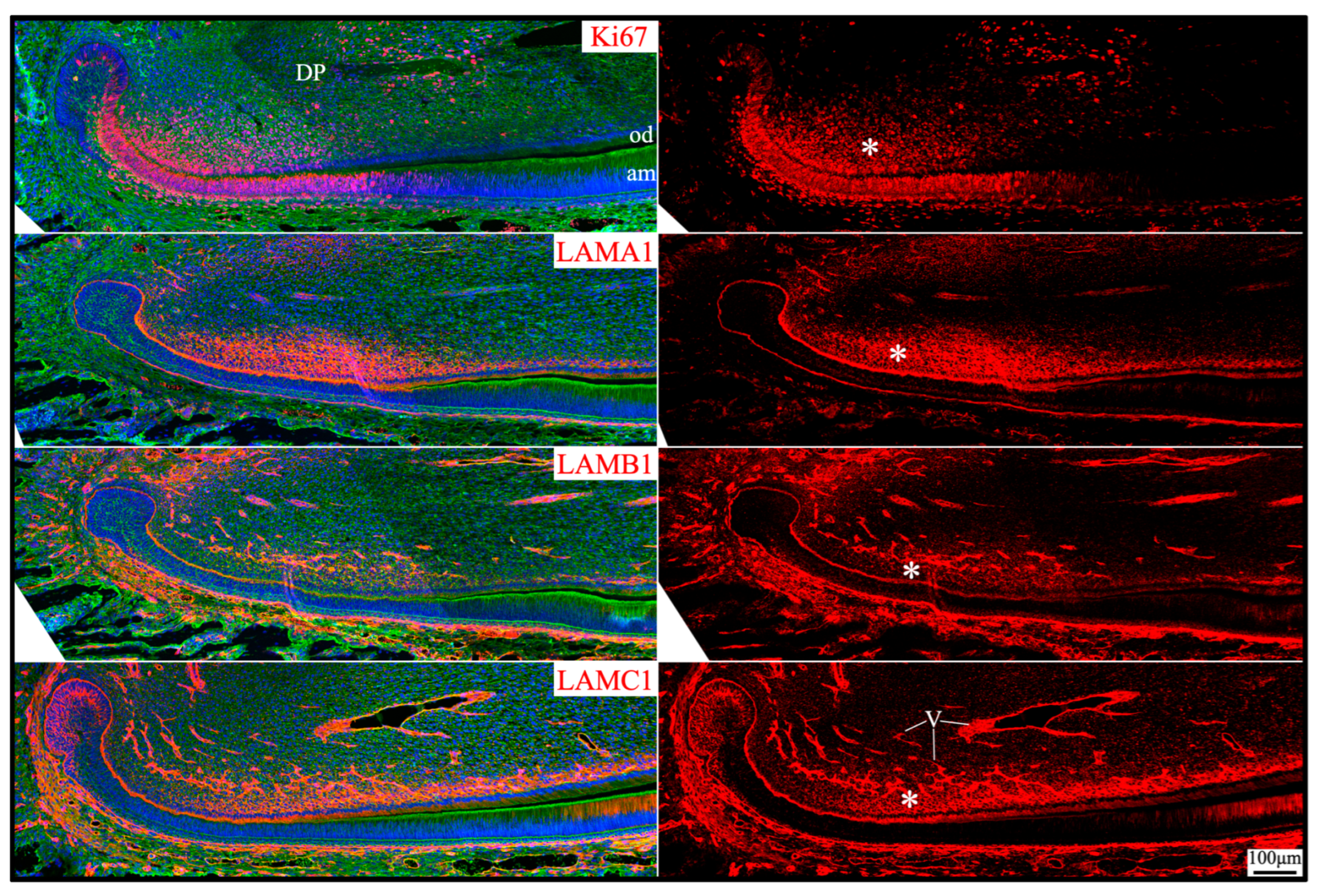
3. Discussion
4. Materials and Methods
4.1. Mice
4.2. Sample Preparation
4.3. RNAscope In Situ Hybridization
4.4. Immunohistochemistry
4.5. Reverse Transcriptase Polymerase Chain Reaction (RT-PCR)
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Keeley, D.P.; Sherwood, D.R. Tissue linkage through adjoining basement membranes: The long and the short term of it. Matrix Biol. J. Int. Soc. Matrix Biol. 2019, 75–76, 58–71. [Google Scholar] [CrossRef] [PubMed]
- Pastor-Pareja, J.C. Atypical basement membranes and basement membrane diversity—What is normal anyway? J. Cell Sci. 2020, 133, jcs241794. [Google Scholar] [CrossRef] [PubMed]
- Yurchenco, P.D. Basement membranes: Cell scaffoldings and signaling platforms. Cold Spring Harb Perspect Biol. 2011, 3, a004911. [Google Scholar] [CrossRef] [PubMed]
- Evans, M.J.; Fanucchi, M.V.; Plopper, C.G.; Hyde, D.M. Postnatal development of the lamina reticularis in primate airways. Anat. Rec. 2010, 293, 947–954. [Google Scholar] [CrossRef]
- Merker, H.J. Morphology of the basement membrane. Microsc. Res. Tech. 1994, 28, 95–124. [Google Scholar] [CrossRef]
- Sekiguchi, R.; Yamada, K.M. Basement Membranes in Development and Disease. Curr. Top Dev. Biol. 2018, 130, 143–191. [Google Scholar] [CrossRef]
- Jayadev, R.; Sherwood, D.R. Basement membranes. Curr. Biol. CB 2017, 27, R207–R211. [Google Scholar] [CrossRef]
- Domogatskaya, A.; Rodin, S.; Tryggvason, K. Functional diversity of laminins. Annu. Rev. Cell Dev. Biol. 2012, 28, 523–553. [Google Scholar] [CrossRef]
- Aumailley, M.; Bruckner-Tuderman, L.; Carter, W.G.; Deutzmann, R.; Edgar, D.; Ekblom, P.; Engel, J.; Engvall, E.; Hohenester, E.; Jones, J.C.; et al. A simplified laminin nomenclature. Matrix Biol. J. Int. Soc. Matrix Biol. 2005, 24, 326–332. [Google Scholar] [CrossRef]
- Balic, A.; Thesleff, I. Tissue Interactions Regulating Tooth Development and Renewal. Curr. Top Dev. Biol. 2015, 115, 157–186. [Google Scholar] [CrossRef]
- Lacruz, R.S.; Habelitz, S.; Wright, J.T.; Paine, M.L. Dental Enamel Formation and Implications for Oral Health and Disease. Physiol. Rev. 2017, 97, 939–993. [Google Scholar] [CrossRef] [PubMed]
- Inai, T.; Kukita, T.; Ohsaki, Y.; Nagata, K.; Kukita, A.; Kurisu, K. Immunohistochemical demonstration of amelogenin penetration toward the dental pulp in the early stages of ameloblast development in rat molar tooth germs. Anat. Rec. 1991, 229, 259–270. [Google Scholar] [CrossRef] [PubMed]
- Simmer, J.P.; Hu, J.C.; Hu, Y.; Zhang, S.; Liang, T.; Wang, S.K.; Kim, J.W.; Yamakoshi, Y.; Chun, Y.H.; Bartlett, J.D.; et al. A genetic model for the secretory stage of dental enamel formation. J. Struct. Biol. 2021, 213, 107805. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.E.; Hu, Y.; Hu, J.C.; Simmer, J.P. Ultrastructure of early amelogenesis in wild-type, Amelx(-/-), and Enam(-/-) mice: Enamel ribbon initiation on dentin mineral and ribbon orientation by ameloblasts. Mol. Genet. Genom. Med. 2016, 4, 662–683. [Google Scholar] [CrossRef]
- Nanci, A.; Warshawsky, H. Characterization of putative secretory sites on ameloblasts of the rat incisor. Am. J. Anat. 1984, 171, 163–189. [Google Scholar] [CrossRef]
- Hu, J.C.; Chun, Y.H.; Al Hazzazzi, T.; Simmer, J.P. Enamel formation and amelogenesis imperfecta. Cells Tissues Organs 2007, 186, 78–85. [Google Scholar] [CrossRef]
- Fukumoto, S.; Miner, J.H.; Ida, H.; Fukumoto, E.; Yuasa, K.; Miyazaki, H.; Hoffman, M.P.; Yamada, Y. Laminin alpha5 is required for dental epithelium growth and polarity and the development of tooth bud and shape. J. Biol. Chem. 2006, 281, 5008–5016. [Google Scholar] [CrossRef]
- Wright, J.T.; Johnson, L.B.; Fine, J.D. Development defesssscts of enamel in humans with hereditary epidermolysis bullosa. Arch. Oral Biol. 1993, 38, 945–955. [Google Scholar] [CrossRef]
- McGrath, J.A.; Kivirikko, S.; Ciatti, S.; Moss, C.; Dunnill, G.S.; Eady, R.A.; Rodeck, C.H.; Christiano, A.M.; Uitto, J. A homozygous nonsense mutation in the alpha 3 chain gene of laminin 5 (LAMA3) in Herlitz junctional epidermolysis bullosa: Prenatal exclusion in a fetus at risk. Genomics 1995, 29, 282–284. [Google Scholar] [CrossRef]
- McLean, W.H.; Irvine, A.D.; Hamill, K.J.; Whittock, N.V.; Coleman-Campbell, C.M.; Mellerio, J.E.; Ashton, G.S.; Dopping-Hepenstal, P.J.; Eady, R.A.; Jamil, T.; et al. An unusual N-terminal deletion of the laminin alpha3a isoform leads to the chronic granulation tissue disorder laryngo-onycho-cutaneous syndrome. Hum. Mol. Genet. 2003, 12, 2395–2409. [Google Scholar] [CrossRef]
- McGrath, J.A.; Pulkkinen, L.; Christiano, A.M.; Leigh, I.M.; Eady, R.A.; Uitto, J. Altered laminin 5 expression due to mutations in the gene encoding the beta 3 chain (LAMB3) in generalized atrophic benign epidermolysis bullosa. J. Investig. Dermatol. 1995, 104, 467–474. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.K.; Zhang, H.; Wang, Y.L.; Seymen, F.; Koruyucu, M.; Simmer, J.P.; Hu, J.C. Phenotypic variability in LAMA3-associated amelogenesis imperfecta. Oral Dis. 2023, 29, 3514–3524. [Google Scholar] [CrossRef] [PubMed]
- Gostyńska, K.B.; Yan Yuen, W.; Pasmooij, A.M.G.; Stellingsma, C.; Pas, H.H.; Lemmink, H.; Jonkman, M.F. Carriers with functional null mutations in LAMA3 have localized enamel abnormalities due to haploinsufficiency. Eur. J. Hum. Genet. EJHG 2016, 25, 94–99. [Google Scholar] [CrossRef] [PubMed]
- Yuen, W.Y.; Pasmooij, A.M.; Stellingsma, C.; Jonkman, M.F. Enamel defects in carriers of a novel LAMA3 mutation underlying epidermolysis bullosa. Acta Derm.-Venereol. 2012, 92, 695–696. [Google Scholar] [CrossRef]
- Kim, Y.J.; Shin, T.J.; Hyun, H.K.; Lee, S.H.; Lee, Z.H.; Kim, J.W. A novel de novo mutation in LAMB3 causes localized hypoplastic enamel in the molar region. Eur. J. Oral Sci. 2016, 124, 403–405. [Google Scholar] [CrossRef]
- Lee, K.E.; Ko, J.; Le, C.G.; Shin, T.J.; Hyun, H.K.; Lee, S.H.; Kim, J.W. Novel LAMB3 mutations cause non-syndromic amelogenesis imperfecta with variable expressivity. Clin. Genet. 2015, 87, 90–92. [Google Scholar] [CrossRef]
- Poulter, J.A.; El-Sayed, W.; Shore, R.C.; Kirkham, J.; Inglehearn, C.F.; Mighell, A.J. Whole-exome sequencing, without prior linkage, identifies a mutation in LAMB3 as a cause of dominant hypoplastic amelogenesis imperfecta. Eur. J. Hum. Genet. EJHG 2014, 22, 132–135. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, Y.; Yang, Y.; Qin, M. Novel ENAM and LAMB3 mutations in Chinese families with hypoplastic amelogenesis imperfecta. PLoS ONE 2015, 10, e0116514. [Google Scholar] [CrossRef]
- Kim, J.W.; Seymen, F.; Lee, K.E.; Ko, J.; Yildirim, M.; Tuna, E.B.; Gencay, K.; Shin, T.J.; Kyun, H.K.; Simmer, J.P.; et al. LAMB3 mutations causing autosomal-dominant amelogenesis imperfecta. J. Dent. Res. 2013, 92, 899–904. [Google Scholar] [CrossRef]
- Ryan, M.C.; Lee, K.; Miyashita, Y.; Carter, W.G. Targeted disruption of the LAMA3 gene in mice reveals abnormalities in survival and late stage differentiation of epithelial cells. J. Cell Biol. 1999, 145, 1309–1323. [Google Scholar] [CrossRef]
- Salmivirta, K.; Sorokin, L.M.; Ekblom, P. Differential expression of laminin alpha chains during murine tooth development. Dev. Dyn. Off. Publ. Am. Assoc. Anat. 1997, 210, 206–215. [Google Scholar] [CrossRef]
- Galliano, M.F.; Aberdam, D.; Aguzzi, A.; Ortonne, J.P.; Meneguzzi, G. Cloning and complete primary structure of the mouse laminin alpha 3 chain. Distinct expression pattern of the laminin alpha 3A and alpha 3B chain isoforms. J. Biol. Chem. 1995, 270, 21820–21826. [Google Scholar] [CrossRef] [PubMed]
- He, P.; Zhang, Y.; Kim, S.O.; Radlanski, R.J.; Butcher, K.; Schneider, R.A.; DenBesten, P.K. Ameloblast differentiation in the human developing tooth: Effects of extracellular matrices. Matrix Biol. J. Int. Soc. Matrix Biol. 2010, 29, 411–419. [Google Scholar] [CrossRef] [PubMed]
- Yoshiba, K.; Yoshiba, N.; Aberdam, D.; Meneguzzi, G.; Perrin-Schmitt, F.; Stoetzel, C.; Ruch, J.V.; Lesot, H. Expression and localization of laminin-5 subunits during mouse tooth development. Dev. Dyn. Off. Publ. Am. Assoc. Anat. 1998, 211, 164–176. [Google Scholar] [CrossRef]
- Yoshiba, N.; Yoshiba, K.; Aberdam, D.; Meneguzzi, G.; Perrin-Schmitt, F.; Stoetzel, C.; Ruch, J.V.; Lesot, H. Expression and localization of laminin-5 subunits in the mouse incisor. Cell Tissue Res. 1998, 292, 143–149. [Google Scholar] [CrossRef]
- Sahlberg, C.; Hormia, M.; Airenne, T.; Thesleff, I. Laminin gamma2 expression is developmentally regulated during murine tooth morphogenesis and is intense in ameloblasts. J. Dent. Res. 1998, 77, 1589–1596. [Google Scholar] [CrossRef]
- Sawada, T. Ultrastructural and immunocytochemical characterization of ameloblast-enamel adhesion at maturation stage in amelogenesis in Macaca fuscata tooth germ. Histochem. Cell Biol. 2015, 144, 587–596. [Google Scholar] [CrossRef]
- Hormia, M.; Sahlberg, C.; Thesleff, I.; Airenne, T. The epithelium-tooth interface--a basal lamina rich in laminin-5 and lacking other known laminin isoforms. J. Dent. Res. 1998, 77, 1479–1485. [Google Scholar] [CrossRef]
- Altera, A.; Tosi, G.M.; Regoli, M.; De Benedetto, E.; Bertelli, E. The extracellular matrix complexity of idiopathic epiretinal membranes and the bilaminar arrangement of the associated internal limiting membrane in the posterior retina. Graefes Arch. Clin. Exp. Ophthalmol. 2021, 259, 2559–2571. [Google Scholar] [CrossRef]
- Sasaki, T.; Göhring, W.; Mann, K.; Brakebusch, C.; Yamada, Y.; Fässler, R.; Timpl, R. Short arm region of laminin-5 gamma2 chain: Structure, mechanism of processing and binding to heparin and proteins. J. Mol. Biol. 2001, 314, 751–763. [Google Scholar] [CrossRef]
- Tunggal, L.; Ravaux, J.; Pesch, M.; Smola, H.; Krieg, T.; Gaill, F.; Sasaki, T.; Timpl, R.; Mauch, C.; Aumailley, M. Defective laminin 5 processing in cylindroma cells. Am. J. Pathol. 2002, 160, 459–468. [Google Scholar] [CrossRef] [PubMed]
- Zeichner-David, M.; Oishi, K.; Su, Z.; Zakartchenko, V.; Chen, L.S.; Arzate, H.; Bringas, P., Jr. Role of Hertwig’s epithelial root sheath cells in tooth root development. Dev. Dyn. Off. Publ. Am. Assoc. Anat. 2003, 228, 651–663. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.C.; Fukae, M.; Uchida, T.; Qian, Q.; Zhang, C.H.; Ryu, O.H.; Tanabe, T.; Yamakoshi, Y.; Murakami, C.; Dohi, N.; et al. Cloning and characterization of porcine enamelin mRNAs. J. Dent. Res. 1997, 76, 1720–1729. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, M. Histological and immunological characteristics of the junctional epithelium. Jpn Dent Sci Rev. 2018, 54, 59–65. [Google Scholar] [CrossRef]
- Bernick, S. Vascular supply to the developing teeth of rats. Anat. Rec. 1960, 137, 141–151. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, Y.; Song, J.; Gerwien, H.; Chuquisana, O.; Chashchina, A.; Denz, C.; Sorokin, L. The endothelial basement membrane acts as a checkpoint for entry of pathogenic T cells into the brain. J. Exp. Med. 2020, 217, e20191339. [Google Scholar] [CrossRef]
- Yoshida, S.; Ohshima, H.; Kobayashi, S. Vascularization of the enamel organ in developing molar teeth of rats--scanning electron microscope study of corrosion casts. Okajimas Folia Anat. Jpn. 1989, 66, 99–111. [Google Scholar] [CrossRef]
- Decker, J.D. The development of a vascular supply to the rat molar enamel organ. An electron microscopic study. Arch. Oral Biol. 1967, 12, 453–458. [Google Scholar] [CrossRef]
- Jing, J.; Feng, J.; Yuan, Y.; Guo, T.; Lei, J.; Pei, F.; Ho, T.V.; Chai, Y. Spatiotemporal single-cell regulatory atlas reveals neural crest lineage diversification and cellular function during tooth morphogenesis. Nat. Commun. 2022, 13, 4803. [Google Scholar] [CrossRef]
- Linde, A.; Goldberg, M. Dentinogenesis. Crit. Rev. Oral Biol. Med. Off. Publ. Am. Assoc. Oral Biol. 1993, 4, 679–728. [Google Scholar] [CrossRef]
- Wang, F.; Flanagan, J.; Su, N.; Wang, L.C.; Bui, S.; Nielson, A.; Wu, X.; Vo, H.T.; Ma, X.J.; Luo, Y. RNAscope: A novel in situ RNA analysis platform for formalin-fixed, paraffin-embedded tissues. J. Mol. Diagn. JMD 2012, 14, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Baker, M. Reproducibility crisis: Blame it on the antibodies. Nature 2015, 521, 274–276. [Google Scholar] [CrossRef] [PubMed]
- Bradbury, A.; Plückthun, A. Reproducibility: Standardize antibodies used in research. Nature 2015, 518, 27–29. [Google Scholar] [CrossRef] [PubMed]
- Liang, T.; Smith, C.E.; Hu, Y.; Zhang, H.; Zhang, C.; Xu, Q.; Lu, Y.; Qi, L.; Hu, J.C.; Simmer, J.P. Dentin defects caused by a Dspp(-1) frameshift mutation are associated with the activation of autophagy. Sci. Rep. 2023, 13, 6393. [Google Scholar] [CrossRef]
- Sherwood, D.R. Basement membrane remodeling guides cell migration and cell morphogenesis during development. Curr. Opin. Cell Biol. 2021, 72, 19–27. [Google Scholar] [CrossRef]
- Naylor, R.W.; Morais, M.; Lennon, R. Complexities of the glomerular basement membrane. Nat. Rev. Nephrol. 2021, 17, 112–127. [Google Scholar] [CrossRef]
- Sugawara, K.; Tsuruta, D.; Kobayashi, H.; Ikeda, K.; Hopkinson, S.B.; Jones, J.C.; Ishii, M. Spatial and temporal control of laminin-332 (5) and -511 (10) expression during induction of anagen hair growth. J. Histochem. Cytochem. Off. J. Histochem. Soc. 2007, 55, 43–55. [Google Scholar] [CrossRef]
- Wazen, R.M.; Viegas-Costa, L.C.; Fouillen, A.; Moffatt, P.; Adair-Kirk, T.L.; Senior, R.M.; Nanci, A. Laminin γ2 knockout mice rescued with the human protein exhibit enamel maturation defects. Matrix Biol. J. Int. Soc. Matrix Biol. 2016, 52–54, 207–218. [Google Scholar] [CrossRef]
- Ronnholm, E. The amelogenesis of human teeth as revealed by electron microscopy. II. The development of the enamel crystallites. J. Ultrastruct. Res. 1962, 6, 249–303. [Google Scholar] [CrossRef]
- Chavda, N.D.; Sari, B.; Asiri, F.M.; Hamill, K.J. Laminin N-terminus (LaNt) proteins, laminins and basement membrane regulation. Biochem. Soc. Trans. 2022, 50, 1541–1553. [Google Scholar] [CrossRef]
- Akter, L.; Flechsig, H.; Marchesi, A.; Franz, C.M. Observing Dynamic Conformational Changes within the Coiled-Coil Domain of Different Laminin Isoforms Using High-Speed Atomic Force Microscopy. Int. J. Mol. Sci. 2024, 25, 1951. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.S.; Champliaud, M.F.; Burgeson, R.E.; Marinkovich, M.P.; Yurchenco, P.D. Self-assembly of laminin isoforms. J. Biol. Chem. 1997, 272, 31525–31532. [Google Scholar] [CrossRef] [PubMed]
- Walko, G.; Castañón, M.J.; Wiche, G. Molecular architecture and function of the hemidesmosome. Cell Tissue Res. 2015, 360, 363–378. [Google Scholar] [CrossRef] [PubMed]
- Pulkkinen, L.; Rouan, F.; Bruckner-Tuderman, L.; Wallerstein, R.; Garzon, M.; Brown, T.; Smith, L.; Carter, W.; Uitto, J. Novel ITGB4 mutations in lethal and nonlethal variants of epidermolysis bullosa with pyloric atresia: Missense versus nonsense. Am. J. Hum. Genet. 1998, 63, 1376–1387. [Google Scholar] [CrossRef]
- Schumann, H.; Kiritsi, D.; Pigors, M.; Hausser, I.; Kohlhase, J.; Peters, J.; Ott, H.; Hyla-Klekot, L.; Gacka, E.; Sieron, A.L.; et al. Phenotypic spectrum of epidermolysis bullosa associated with α6β4 integrin mutations. Br. J. Dermatol. 2013, 169, 115–124. [Google Scholar] [CrossRef]
- Dos Santos Neves, J.; Wazen, R.M.; Kuroda, S.; Francis Zalzal, S.; Moffatt, P.; Nanci, A. Odontogenic ameloblast-associated and amelotin are novel basal lamina components. Histochem. Cell Biol. 2012, 137, 329–338. [Google Scholar] [CrossRef]
- Fouillen, A.; Dos Santos Neves, J.; Mary, C.; Castonguay, J.D.; Moffatt, P.; Baron, C.; Nanci, A. Interactions of AMTN, ODAM and SCPPPQ1 proteins of a specialized basal lamina that attaches epithelial cells to tooth mineral. Sci. Rep. 2017, 7, 46683. [Google Scholar] [CrossRef]
- Hormia, M.; Owaribe, K.; Virtanen, I. The dento-epithelial junction: Cell adhesion by type I hemidesmosomes in the absence of a true basal lamina. J. Periodontol. 2001, 72, 788–797. [Google Scholar] [CrossRef]
- Oksonen, J.; Sorokin, L.M.; Virtanen; Hormia, M. The junctional epithelium around murine teeth differs from gingival epithelium in its basement membrane composition. J. Dent. Res. 2001, 80, 2093–2097. [Google Scholar] [CrossRef]
- Nouri, S.; Holcroft, J.; Caruso, L.L.; Vuong, T.V.; Simmons, C.A.; Master, E.R.; Ganss, B. An SCPPPQ1/LAM332 protein complex enhances the adhesion and migration of oral epithelial cells: Implications for dentogingival regeneration. Acta Biomater. 2022, 147, 209–220. [Google Scholar] [CrossRef]
- Decker, J.D. A light and electron microscope study of the rat molar enamel organ. Arch. Oral Biol. 1963, 8, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Galea, I. The blood-brain barrier in systemic infection and inflammation. Cell Mol. Immunol. 2021, 18, 2489–2501. [Google Scholar] [CrossRef] [PubMed]
- Rayagiri, S.S.; Ranaldi, D.; Raven, A.; Mohamad Azhar, N.I.F.; Lefebvre, O.; Zammit, P.S.; Borycki, A.G. Basal lamina remodeling at the skeletal muscle stem cell niche mediates stem cell self-renewal. Nat. Commun. 2018, 9, 1075. [Google Scholar] [CrossRef] [PubMed]
- Kiyozumi, D.; Nakano, I.; Sato-Nishiuchi, R.; Tanaka, S.; Sekiguchi, K. Laminin is the ECM niche for trophoblast stem cells. Life Sci Alliance 2020, 3, e201900515. [Google Scholar] [CrossRef]
- Yap, L.; Tay, H.G.; Nguyen, M.T.X.; Tjin, M.S.; Tryggvason, K. Laminins in Cellular Differentiation. Trends Cell Biol. 2019, 29, 987–1000. [Google Scholar] [CrossRef]
- Kvist, A.J.; Nyström, A.; Hultenby, K.; Sasaki, T.; Talts, J.F.; Aspberg, A. The major basement membrane components localize to the chondrocyte pericellular matrix--a cartilage basement membrane equivalent? Matrix Biol. J. Int. Soc. Matrix Biol. 2008, 27, 22–33. [Google Scholar] [CrossRef]
- Yuasa, K.; Fukumoto, S.; Kamasaki, Y.; Yamada, A.; Fukumoto, E.; Kanaoka, K.; Saito, K.; Harada, H.; Arikawa-Hirasawa, E.; Miyagoe-Suzuki, Y.; et al. Laminin alpha2 is essential for odontoblast differentiation regulating dentin sialoprotein expression. J. Biol. Chem. 2004, 279, 10286–10292. [Google Scholar] [CrossRef]
- Behrens, D.T.; Villone, D.; Koch, M.; Brunner, G.; Sorokin, L.; Robenek, H.; Bruckner-Tuderman, L.; Bruckner, P.; Hansen, U. The epidermal basement membrane is a composite of separate laminin- or collagen IV-containing networks connected by aggregated perlecan, but not by nidogens. J. Biol. Chem. 2012, 287, 18700–18709. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, T.; Zhang, H.; Hu, Y.; Solanki, M.; Zhang, C.; Sasaki, T.; Smith, C.E.; Hu, J.C.-C.; Simmer, J.P. Localizations of Laminin Chains Suggest Their Multifaceted Functions in Mouse Tooth Development. Int. J. Mol. Sci. 2025, 26, 4134. https://doi.org/10.3390/ijms26094134
Liang T, Zhang H, Hu Y, Solanki M, Zhang C, Sasaki T, Smith CE, Hu JC-C, Simmer JP. Localizations of Laminin Chains Suggest Their Multifaceted Functions in Mouse Tooth Development. International Journal of Molecular Sciences. 2025; 26(9):4134. https://doi.org/10.3390/ijms26094134
Chicago/Turabian StyleLiang, Tian, Hong Zhang, Yuanyuan Hu, Mansi Solanki, Chuhua Zhang, Takako Sasaki, Charles E. Smith, Jan C.-C. Hu, and James P. Simmer. 2025. "Localizations of Laminin Chains Suggest Their Multifaceted Functions in Mouse Tooth Development" International Journal of Molecular Sciences 26, no. 9: 4134. https://doi.org/10.3390/ijms26094134
APA StyleLiang, T., Zhang, H., Hu, Y., Solanki, M., Zhang, C., Sasaki, T., Smith, C. E., Hu, J. C.-C., & Simmer, J. P. (2025). Localizations of Laminin Chains Suggest Their Multifaceted Functions in Mouse Tooth Development. International Journal of Molecular Sciences, 26(9), 4134. https://doi.org/10.3390/ijms26094134




