Hypercapnia Increases Influenza A Virus Infection of Bronchial Epithelial Cells by Augmenting Cellular Cholesterol via mTOR and Akt
Abstract
1. Introduction
2. Results
2.1. Hypercapnia Increases Viral Protein Expression in Mouse Bronchial Epithelium Following IAV Infection In Vivo and in Human Bronchial Epithelial Cells Infected with IAV In Vitro
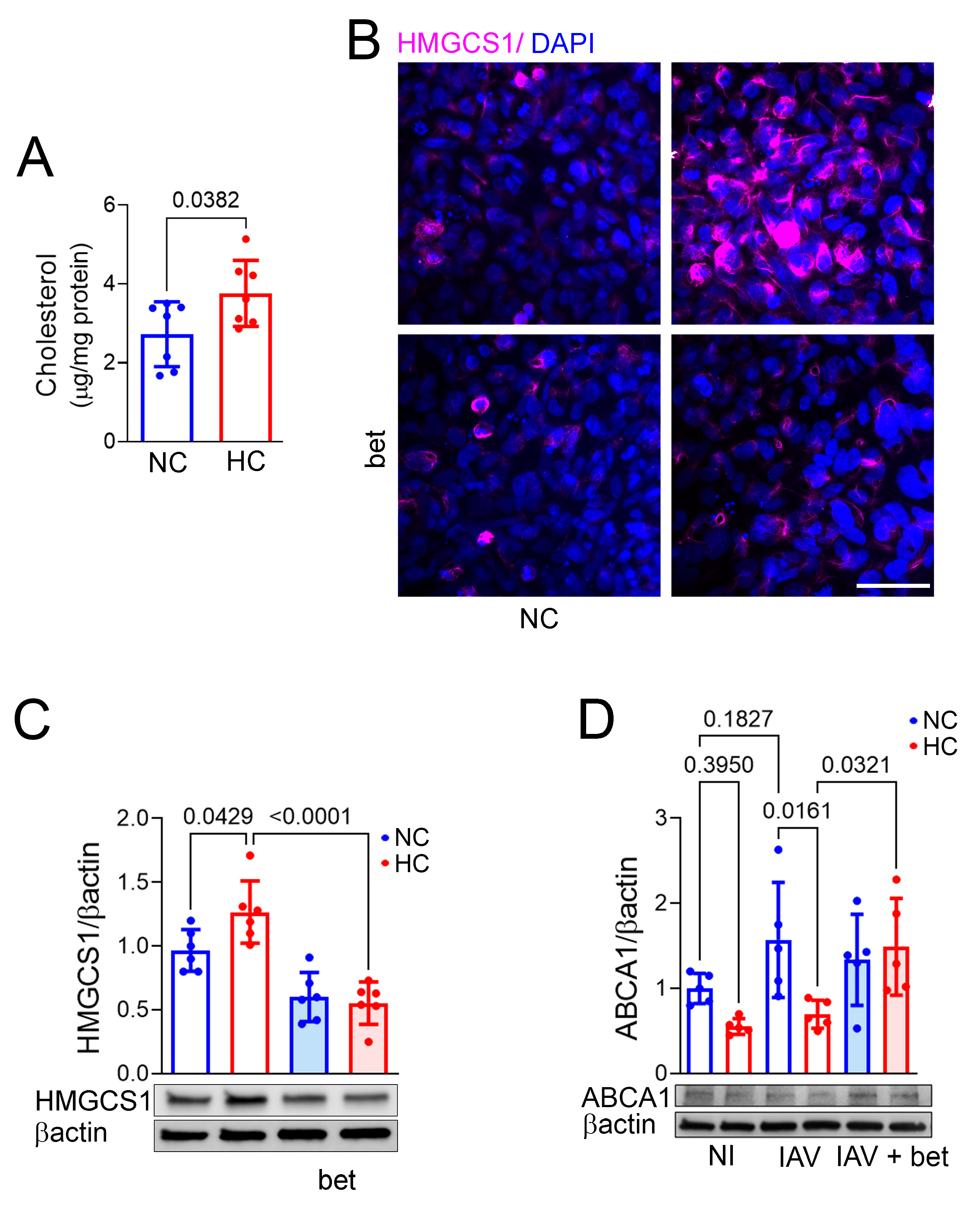
2.2. Hypercapnia Increases Cellular Cholesterol, and Inhibition of the Transcription Factor SREBP2 Blocks Hypercapnia-Induced Changes in Expression of the Cholesterol Synthesis Enzyme HMGCS1 and Efflux Transporter ABCA1
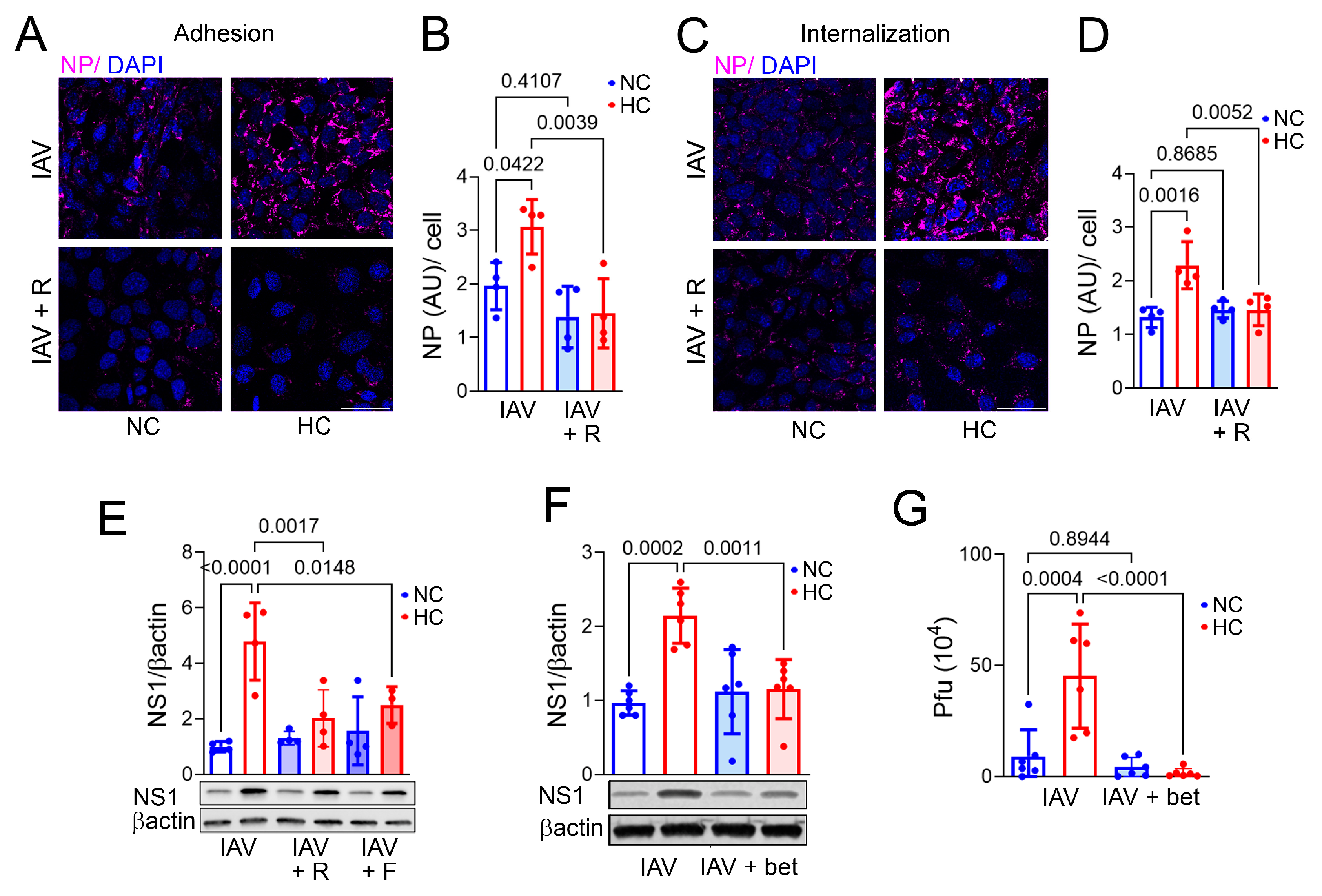
2.3. Inhibiting Cellular Cholesterol Accumulation Blocks Hypercapnia-Induced Increases in IAV Adhesion and Internalization, NS1 Expression, and Viral Replication in Bronchial Epithelial Cells
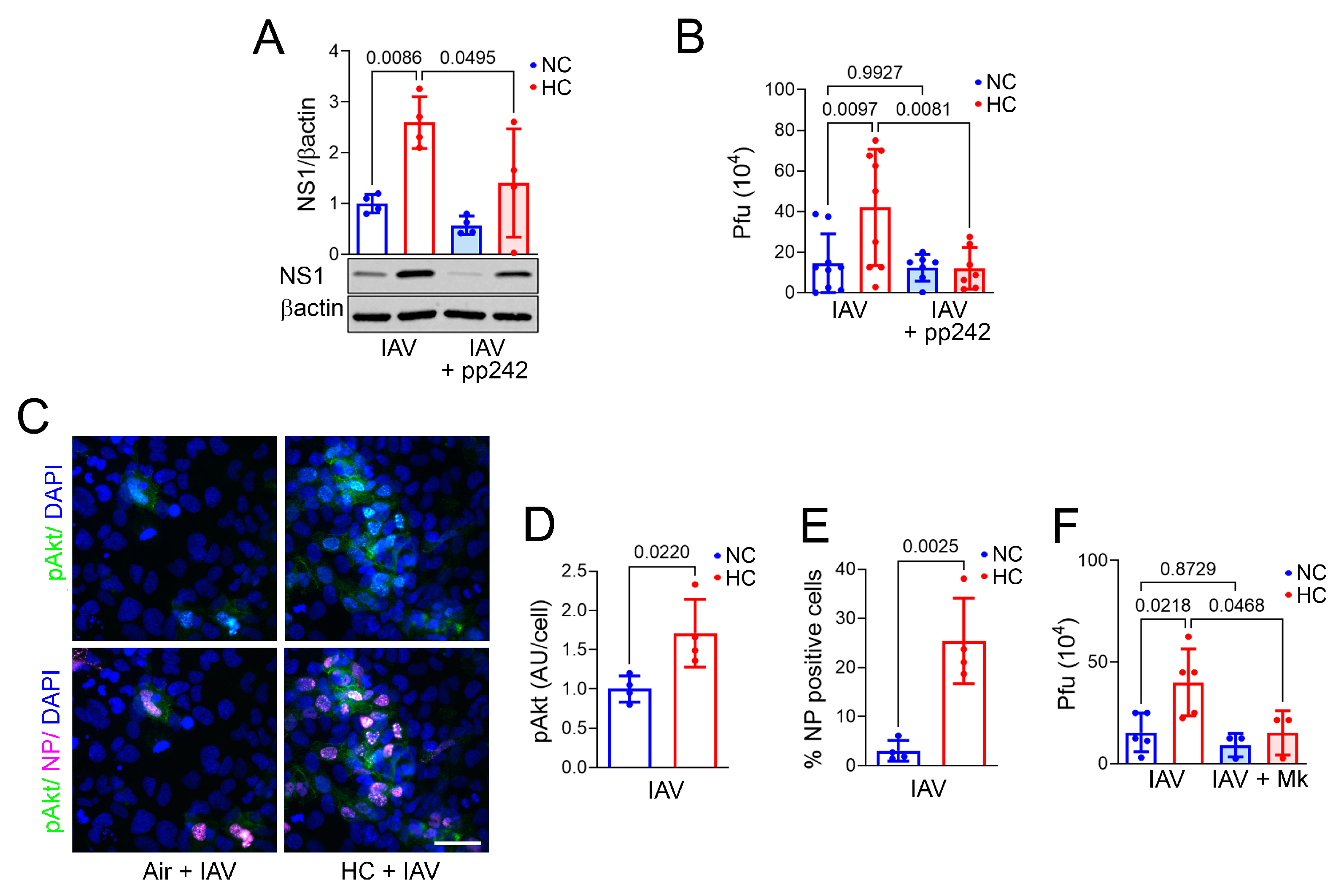
2.4. Inhibition of mTOR Signaling Blocks the Hypercapnia-Induced Increase in Activation of SREBP2, Viral Protein Expression, and IAV Replication
2.5. Hypercapnia Triggers mTOR Signaling, Which Activates S6K1 and Akt in IAV-Infected Bronchial Epithelial Cells
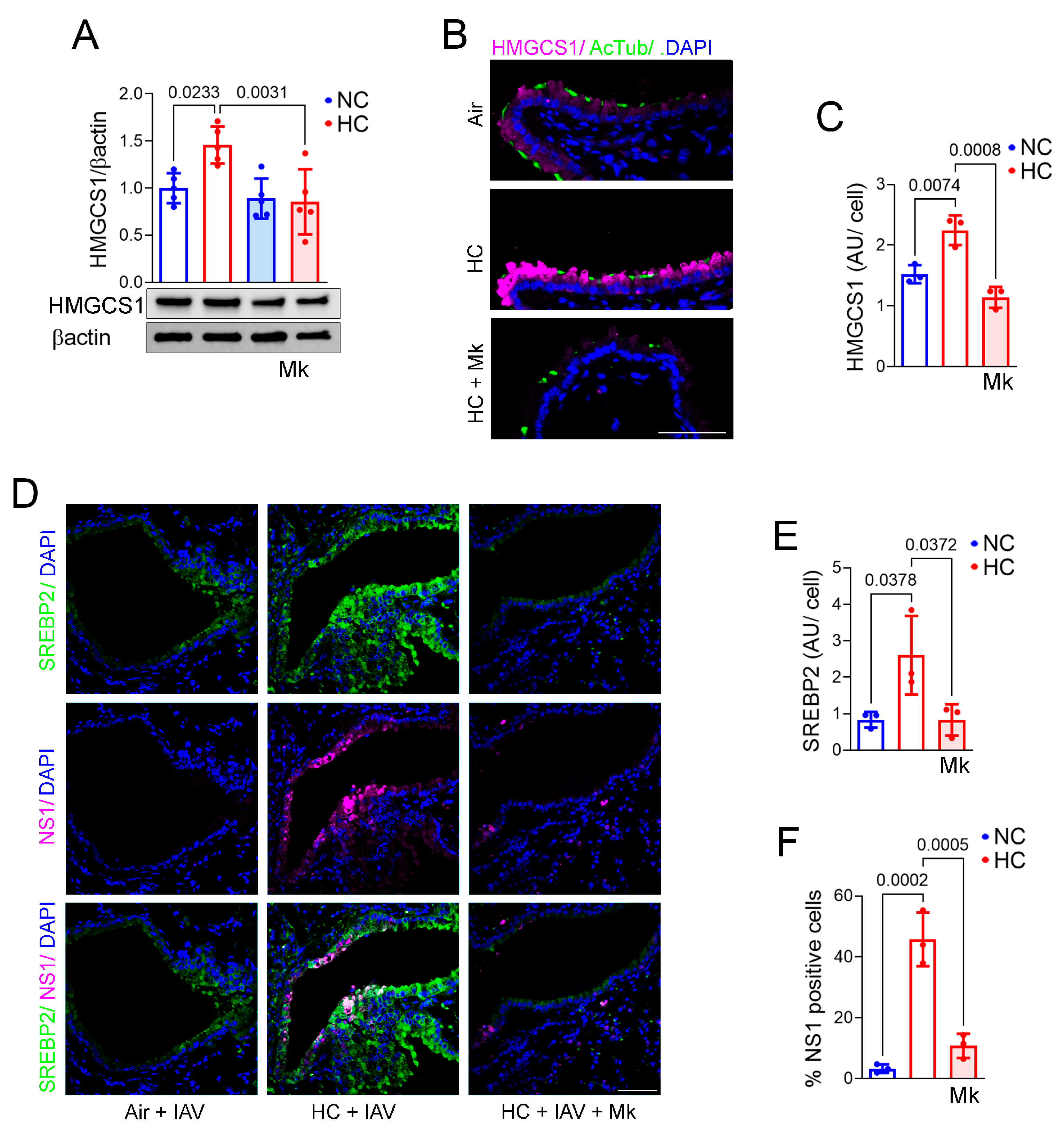
2.6. Inhibition of Akt Blocks Hypercapnia-Induced Increases in IAV Replication and HMGCS1 Expression in BEAS-2B Cells and Blocks HMGCS1 and SREBP2 Expression in Mouse Bronchial Epithelium
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Mice
Exposure of Mice to Hypercapnia and Influenza A Virus Infection
4.3. Cells
Exposure of Cells to Normocapnia and Hypercapnia and Infection with IAV
4.4. Immunofluorescence Microscopy in Tissue Sections and Cell Cultures
4.5. Immunoblotting
4.6. Viral Adhesion and Internalization
4.7. Cell Cholesterol
4.8. Viral Plaque Assay
4.9. Statistics
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Goel, A.; Pinckney, R.G.; Littenberg, B. APACHE II predicts long-term survival in COPD patients admitted to a general medical ward. J. Gen. Intern. Med. 2003, 18, 824–830. [Google Scholar] [CrossRef]
- Groenewegen, K.H.; Schols, A.M.; Wouters, E.F. Mortality and mortality-related factors after hospitalization for acute exacerbation of COPD. Chest 2003, 124, 459–467. [Google Scholar] [CrossRef] [PubMed]
- Martin, T.R.; Lewis, S.W.; Albert, R.K. The prognosis of patients with chronic obstructive pulmonary disease after hospitalization for acute respiratory failure. Chest 1982, 82, 310–314. [Google Scholar] [CrossRef]
- Mohan, A.; Premanand, R.; Reddy, L.N.; Rao, M.H.; Sharma, S.K.; Kamity, R.; Bollineni, S. Clinical presentation and predictors of outcome in patients with severe acute exacerbation of chronic obstructive pulmonary disease requiring admission to intensive care unit. BMC Pulm. Med. 2006, 6, 27. [Google Scholar] [CrossRef]
- Moser, K.M.; Shibel, E.M.; Beamon, A.J. Acute respiratory failure in obstructive lung disease. Long-term survival after treatment in an intensive care unit. JAMA 1973, 225, 705–707. [Google Scholar] [CrossRef] [PubMed]
- Sin, D.D.; Man, S.F.; Marrie, T.J. Arterial carbon dioxide tension on admission as a marker of in-hospital mortality in community-acquired pneumonia. Am. J. Med. 2005, 118, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Mallia, P.; Johnston, S.L. Influenza infection and COPD. Int. J. Chronic Obstr. Pulm. Dis. 2007, 2, 55–64. [Google Scholar] [CrossRef]
- De Serres, G.; Lampron, N.; La Forge, J.; Rouleau, I.; Bourbeau, J.; Weiss, K.; Barret, B.; Boivin, G. Importance of viral and bacterial infections in chronic obstructive pulmonary disease exacerbations. J. Clin. Virol. 2009, 46, 129–133. [Google Scholar] [CrossRef]
- Gerke, A.K.; Tang, F.; Yang, M.; Foster, E.D.; Cavanaugh, J.E.; Polgreen, P.M. Predicting chronic obstructive pulmonary disease hospitalizations based on concurrent influenza activity. COPD J. Chronic Obstr. Pulm. Dis. 2013, 10, 573–580. [Google Scholar] [CrossRef]
- Sethi, S.; Murphy, T.F. Infection in the pathogenesis and course of chronic obstructive pulmonary disease. N. Engl. J. Med. 2008, 359, 2355–2365. [Google Scholar] [CrossRef]
- Global-Initiative-for-Chronic-Obstructive-Lung-Disease. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease, Updated 2025. 2025. Available online: https://goldcopd.org/ (accessed on 22 April 2025).
- Laserna, E.; Sibila, O.; Aguilar, P.R.; Mortensen, E.M.; Anzueto, A.; Blanquer, J.M.; Sanz, F.; Rello, J.; Marcos, P.J.; Velez, M.I.; et al. Hypocapnia and hypercapnia are predictors for ICU admission and mortality in hospitalized patients with community-acquired pneumonia. Chest 2012, 142, 1193–1199. [Google Scholar] [CrossRef] [PubMed]
- Murtagh, P.; Giubergia, V.; Viale, D.; Bauer, G.; Pena, H.G. Lower respiratory infections by adenovirus in children. Clinical features and risk factors for bronchiolitis obliterans and mortality. Pediatr. Pulmonol. 2009, 44, 450–456. [Google Scholar] [CrossRef] [PubMed]
- Belkin, R.A.; Henig, N.R.; Singer, L.G.; Chaparro, C.; Rubenstein, R.C.; Xie, S.X.; Yee, J.Y.; Kotloff, R.M.; Lipson, D.A.; Bunin, G.R. Risk factors for death of patients with cystic fibrosis awaiting lung transplantation. Am. J. Respir. Crit. Care Med. 2006, 173, 659–666. [Google Scholar] [CrossRef]
- Tsonas, A.M.; Botta, M.; Horn, J.; Morales-Quinteros, L.; Artigas, A.; Schultz, M.J.; Paulus, F.; Neto, A.S.; PRoVENT-COVID Collaborative Group. Clinical characteristics, physiological features, and outcomes associated with hypercapnia in patients with acute hypoxemic respiratory failure due to COVID-19—insights from the PRoVENT-COVID study. J. Crit. Care 2022, 69, 154022. [Google Scholar] [CrossRef]
- Casalino-Matsuda, S.M.; Chen, F.; Gonzalez-Gonzalez, F.J.; Nair, A.; Dib, S.; Yemelyanov, A.; Gates, K.L.; Budinger, G.R.S.; Beitel, G.J.; Sporn, P.H.S. Hypercapnia Suppresses Macrophage Antiviral Activity and Increases Mortality of Influenza A Infection via Akt1. J. Immunol. 2020, 205, 489–501. [Google Scholar] [CrossRef]
- Casalino-Matsuda, S.M.; Chen, F.; Gonzalez-Gonzalez, F.J.; Matsuda, H.; Nair, A.; Abdala-Valencia, H.; Budinger, G.R.S.; Dong, J.T.; Beitel, G.J.; Sporn, P.H. Myeloid Zfhx3 deficiency protects against hypercapnia-induced suppression of host defense against influenza A virus. JCI Insight 2024, 9, e170316. [Google Scholar] [CrossRef]
- Casalino-Matsuda, S.M.; Wang, N.; Ruhoff, P.T.; Matsuda, H.; Nlend, M.C.; Nair, A.; Szleifer, I.; Beitel, G.J.; Sznajder, J.I.; Sporn, P.H.S. Hypercapnia Alters Expression of Immune Response, Nucleosome Assembly and Lipid Metabolism Genes in Differentiated Human Bronchial Epithelial Cells. Sci. Rep. 2018, 8, 13508. [Google Scholar] [CrossRef]
- Horton, J.D.; Goldstein, J.L.; Brown, M.S. SREBPs: Activators of the complete program of cholesterol and fatty acid synthesis in the liver. J. Clin. Investig 2002, 109, 1125–1131. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Matsuda, A.; Budinger, G.R.S.; Sporn, P.H.S.; Casalino-Matsuda, S.M. Hypercapnia increases ACE2 expression and pseudo-SARS-CoV-2 entry in bronchial epithelial cells by augmenting cellular cholesterol. Front. Immunol. 2023, 14, 1251120. [Google Scholar] [CrossRef]
- Goronzy, I.N.; Rawle, R.J.; Boxer, S.G.; Kasson, P.M. Cholesterol enhances influenza binding avidity by controlling nanoscale receptor clustering. Chem. Sci. 2018, 9, 2340–2347. [Google Scholar] [CrossRef]
- Scheiffele, P.; Roth, M.G.; Simons, K. Interaction of influenza virus haemagglutinin with sphingolipid-cholesterol membrane domains via its transmembrane domain. EMBO J. 1997, 16, 5501–5508. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.J.; Chen, C.Y.; Yang, J.H.; Chiu, Y.F. Modulating cholesterol-rich lipid rafts to disrupt influenza A virus infection. Front. Immunol. 2022, 13, 982264. [Google Scholar] [CrossRef]
- Bajimaya, S.; Frankl, T.; Hayashi, T.; Takimoto, T. Cholesterol is required for stability and infectivity of influenza A and respiratory syncytial viruses. Virology 2017, 510, 234–241. [Google Scholar] [CrossRef]
- Wang, B.T.; Ducker, G.S.; Barczak, A.J.; Barbeau, R.; Erle, D.J.; Shokat, K.M. The mammalian target of rapamycin regulates cholesterol biosynthetic gene expression and exhibits a rapamycin-resistant transcriptional profile. Proc. Natl. Acad. Sci. USA 2011, 108, 15201–15206. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Manning, B.D. A complex interplay between Akt, TSC2 and the two mTOR complexes. Biochem. Soc. Trans. 2009, 3 Pt 1, 217–222. [Google Scholar] [CrossRef]
- Gates, K.L.; Howell, H.A.; Nair, A.; Vohwinkel, C.U.; Welch, L.C.; Beitel, G.J.; Hauser, A.R.; Sznajder, J.I.; Sporn, P.H. Hypercapnia impairs lung neutrophil function and increases mortality in murine pseudomonas pneumonia. Am. J. Respir. Cell Mol. Biol. 2013, 49, 821–828. [Google Scholar] [CrossRef]
- Hale, B.G.; Randall, R.E.; Ortin, J.; Jackson, D. The multifunctional NS1 protein of influenza A viruses. J. Gen. Virol. 2008, 89 Pt 10, 2359–2376. [Google Scholar] [CrossRef] [PubMed]
- Schnell, J.R.; Chou, J.J. Structure and mechanism of the M2 proton channel of influenza A virus. Nature 2008, 451, 591–595. [Google Scholar] [CrossRef]
- Liu, K.N.; Boxer, S.G. Target Membrane Cholesterol Modulates Single Influenza Virus Membrane Fusion Efficiency but Not Rate. Biophys. J. 2020, 118, 2426–2433. [Google Scholar] [CrossRef]
- Eisfeld, A.J.; Neumann, G.; Kawaoka, Y. At the centre: Influenza A virus ribonucleoproteins. Nat. Rev. Microbiol. 2015, 13, 28–41. [Google Scholar] [CrossRef]
- Endo, A.; Kuroda, M.; Tsujita, Y. ML-236A, ML-236B, and ML-236C, new inhibitors of cholesterogenesis produced by Penicillium citrinium. J. Antibiot. 1976, 29, 1346–1348. [Google Scholar] [CrossRef] [PubMed]
- Istvan, E.S.; Deisenhofer, J. Structural mechanism for statin inhibition of HMG-CoA reductase. Science 2001, 292, 1160–1164. [Google Scholar] [CrossRef] [PubMed]
- Panwar, V.; Singh, A.; Bhatt, M.; Tonk, R.K.; Azizov, S.; Raza, A.S.; Sengupta, S.; Kumar, D.; Garg, M. Multifaceted role of mTOR (mammalian target of rapamycin) signaling pathway in human health and disease. Signal Transduct. Target. Ther. 2023, 8, 375. [Google Scholar] [CrossRef] [PubMed]
- Düvel, K.; Yecies, J.L.; Menon, S.; Raman, P.; Lipovsky, A.I.; Souza, A.L.; Triantafellow, E.; Ma, Q.; Gorski, R.; Cleaver, S.; et al. Activation of a metabolic gene regulatory network downstream of mTOR complex 1. Mol. Cell 2010, 39, 171–183. [Google Scholar] [CrossRef]
- Luu, W.; Sharpe, L.J.; Stevenson, J.; Brown, A.J. Akt acutely activates the cholesterogenic transcription factor SREBP-2. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2012, 1823, 458–464. [Google Scholar] [CrossRef]
- Eid, W.; Dauner, K.; Courtney, K.C.; Gagnon, A.; Parks, R.J.; Sorisky, A.; Zha, X. mTORC1 activates SREBP-2 by suppressing cholesterol trafficking to lysosomes in mammalian cells. Proc. Natl. Acad. Sci. USA 2017, 114, 7999–8004. [Google Scholar] [CrossRef]
- Apsel, B.; Blair, J.A.; Gonzalez, B.; Nazif, T.M.; Feldman, M.E.; Aizenstein, B.; Hoffman, R.; Williams, R.L.; Shokat, K.M.; Knight, Z.A. Targeted polypharmacology: Discovery of dual inhibitors of tyrosine and phosphoinositide kinases. Nat. Chem. Biol. 2008, 4, 691–699. [Google Scholar] [CrossRef]
- Vadlakonda, L.; Dash, A.; Pasupuleti, M.; Anil Kumar, K.; Reddanna, P. The Paradox of Akt-mTOR Interactions. Front. Oncol. 2013, 3, 165. [Google Scholar] [CrossRef]
- Denisova, O.V.; Söderholm, S.; Virtanen, S.; Von Schantz, C.; Bychkov, D.; Vashchinkina, E.; Desloovere, J.; Tynell, J.; Ikonen, N.; Theisen, L.L.; et al. Akt inhibitor MK2206 prevents influenza pH1N1 virus infection in vitro. Antimicrob. Agents Chemother. 2014, 58, 3689–3696. [Google Scholar] [CrossRef]
- Denney, L.; Ho, L.P. The role of respiratory epithelium in host defence against influenza virus infection. Biomed. J. 2018, 41, 218–233. [Google Scholar] [CrossRef]
- Fernández-Hernando, C.; Moore, K.J. MicroRNA modulation of cholesterol homeostasis. Arter. Thromb. Vasc. Biol. 2011, 31, 2378–2382. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.; Chu, H.; Chan, J.F.; Ye, Z.W.; Wen, L.; Yan, B.; Lai, P.M.; Tee, K.M.; Huang, J.; Chen, D.; et al. SREBP-dependent lipidomic reprogramming as a broad-spectrum antiviral target. Nat. Commun. 2019, 10, 120. [Google Scholar] [CrossRef] [PubMed]
- Branche, E.; Wang, Y.T.; Viramontes, K.M.; Valls Cuevas, J.M.; Xie, J.; Ana-Sosa-Batiz, F.; Shafee, N.; Duttke, S.H.; McMillan, R.E.; Clark, A.E.; et al. SREBP2-dependent lipid gene transcription enhances the infection of human dendritic cells by Zika virus. Nat. Commun. 2022, 13, 5341. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wu, Y.; Wang, Y.; Liu, P.; Liu, K.; Sun, J.; Zhang, P.; Wang, X.; Liu, X.; Xu, X. Influenza A virus infection activates STAT3 to enhance SREBP2 expression, cholesterol biosynthesis, and virus replication. iScience 2024, 27, 110424. [Google Scholar] [CrossRef]
- Zhang, J.; Pekosz, A.; Lamb, R.A. Influenza virus assembly and lipid raft microdomains: A role for the cytoplasmic tails of the spike glycoproteins. J. Virol. 2000, 74, 4634–4644. [Google Scholar] [CrossRef]
- Biswas, S.; Yin, S.R.; Blank, P.S.; Zimmerberg, J. Cholesterol promotes hemifusion and pore widening in membrane fusion induced by influenza hemagglutinin. J. Gen. Physiol. 2008, 131, 503–513. [Google Scholar] [CrossRef]
- Verma, D.K.; Gupta, D.; Lal, S.K. Host Lipid Rafts Play a Major Role in Binding and Endocytosis of Influenza A Virus. Viruses 2018, 10, 650. [Google Scholar] [CrossRef]
- Yang, Q.; Elz, A.E.; Panis, M.; Liu, T.; Nilsson-Payant, B.E.; Blanco-Melo, D. Modulation of Influenza A virus NS1 expression reveals prioritization of host response antagonism at single-cell resolution. Front. Microbiol. 2023, 14, 1267078. [Google Scholar] [CrossRef]
- Pavlova, N.I.; Savinova, O.V.; Nikolaeva, S.N.; Boreko, E.I.; Flekhter, O.B. Antiviral activity of betulin, betulinic and betulonic acids against some enveloped and non-enveloped viruses. Fitoterapia 2003, 74, 489–492. [Google Scholar] [CrossRef]
- Hong, E.H.; Song, J.H.; Kang, K.B.; Sung, S.H.; Ko, H.J.; Yang, H. Anti-Influenza Activity of Betulinic Acid from Zizyphus jujuba on Influenza A/PR/8 Virus. Biomol. Ther. 2015, 23, 345–349. [Google Scholar] [CrossRef]
- Mehrbod, P.; Hair-Bejo, M.; Tengku Ibrahim, T.A.; Omar, A.R.; El Zowalaty, M.; Ajdari, Z.; Ideris, A. Simvastatin modulates cellular components in influenza A virus-infected cells. Int. J. Mol. Med. 2014, 34, 61–73. [Google Scholar] [CrossRef] [PubMed]
- Radigan, K.A.; Urich, D.; Misharin, A.V.; Chiarella, S.E.; Soberanes, S.; Gonzalez, A.; Perlman, H.; Wunderink, R.G.; Budinger, G.R.; Mutlu, G.M. The effect of rosuvastatin in a murine model of influenza A infection. PLoS ONE 2012, 7, e35788. [Google Scholar] [CrossRef]
- Mehrbod, P.; Omar, A.R.; Hair-Bejo, M.; Haghani, A.; Ideris, A. Mechanisms of action and efficacy of statins against influenza. Biomed Res. Int. 2014, 2014, 872370. [Google Scholar] [CrossRef] [PubMed]
- Vahedian-Azimi, A.; Mannarino, M.R.; Shojaie, S.; Rahimibashar, F.; Galeh, H.E.G.; Banach, M.; Bianconi, V.; Pirro, M.; Sahebkar, A. The effect of statins on the prevalence and mortality of influenza virus infection: A systematic review and meta-analysis. Arch. Med. Sci. 2022, 18, 1513–1524. [Google Scholar] [CrossRef]
- Ma, K.L.; Liu, J.; Wang, C.X.; Ni, J.; Zhang, Y.; Wu, Y.; Lv, L.L.; Ruan, X.Z.; Liu, B.C. Activation of mTOR modulates SREBP-2 to induce foam cell formation through increased retinoblastoma protein phosphorylation. Cardiovasc. Res. 2013, 100, 450–460. [Google Scholar] [CrossRef]
- Fan, M.; Chen, Z.; Shao, W.; Chen, Y.; Lin, Z.; Yi, C.; Li, Y.; Lu, L.; Zhou, Y.; Lin, J. SREBP2 inhibitor betulin sensitizes hepatocellular carcinoma to lenvatinib by inhibiting the mTOR/IL-1β pathway. Acta Biochim. Biophys. Sin. 2023, 55, 1479–1486. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Kristiana, I.; Wong, J.; Brown, A.J. Involvement of Akt in ER-to-Golgi transport of SCAP/SREBP: A link between a key cell proliferative pathway and membrane synthesis. Mol. Biol. Cell 2006, 17, 2735–2745. [Google Scholar] [CrossRef]
- Kuss-Duerkop, S.K.; Wang, J.; Mena, I.; White, K.; Metreveli, G.; Sakthivel, R.; Mata, M.A.; Muñoz-Moreno, R.; Chen, X.; Krammer, F.; et al. Influenza virus differentially activates mTORC1 and mTORC2 signaling to maximize late stage replication. PLoS Pathog. 2017, 13, e1006635. [Google Scholar] [CrossRef]
- Baffi, T.R.; Lordén, G.; Wozniak, J.M.; Feichtner, A.; Yeung, W.; Kornev, A.P.; King, C.C.; Del Rio, J.C.; Limaye, A.J.; Bogomolovas, J.; et al. mTORC2 controls the activity of PKC and Akt by phosphorylating a conserved TOR interaction motif. Sci. Signal. 2021, 14, eabe4509. [Google Scholar] [CrossRef]
- Woodard, J.; Sassano, A.; Hay, N.; Platanias, L.C. Statin-dependent suppression of the Akt/mammalian target of rapamycin signaling cascade and programmed cell death 4 up-regulation in renal cell carcinoma. Clin. Cancer Res. 2008, 14, 4640–4649. [Google Scholar] [CrossRef]
- Casalino-Matsuda, S.M.; Monzón, M.E.; Forteza, R.M. Epidermal growth factor receptor activation by epidermal growth factor mediates oxidant-induced goblet cell metaplasia in human airway epithelium. Am. J. Respir. Cell Mol. Biol. 2006, 34, 581–591. [Google Scholar] [CrossRef] [PubMed]
- Stirling, D.R.; Swain-Bowden, M.J.; Lucas, A.M.; Carpenter, A.E.; Cimini, B.A.; Goodman, A. CellProfiler 4: Improvements in speed, utility and usability. BMC Bioinform. 2021, 22, 433. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Whittaker, G.R. Role for influenza virus envelope cholesterol in virus entry and infection. J. Virol. 2003, 77, 12543–12551. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, F.; Matsuda, A.; Sporn, P.H.S.; Casalino-Matsuda, S.M. Hypercapnia Increases Influenza A Virus Infection of Bronchial Epithelial Cells by Augmenting Cellular Cholesterol via mTOR and Akt. Int. J. Mol. Sci. 2025, 26, 4133. https://doi.org/10.3390/ijms26094133
Chen F, Matsuda A, Sporn PHS, Casalino-Matsuda SM. Hypercapnia Increases Influenza A Virus Infection of Bronchial Epithelial Cells by Augmenting Cellular Cholesterol via mTOR and Akt. International Journal of Molecular Sciences. 2025; 26(9):4133. https://doi.org/10.3390/ijms26094133
Chicago/Turabian StyleChen, Fei, Aiko Matsuda, Peter H. S. Sporn, and S. Marina Casalino-Matsuda. 2025. "Hypercapnia Increases Influenza A Virus Infection of Bronchial Epithelial Cells by Augmenting Cellular Cholesterol via mTOR and Akt" International Journal of Molecular Sciences 26, no. 9: 4133. https://doi.org/10.3390/ijms26094133
APA StyleChen, F., Matsuda, A., Sporn, P. H. S., & Casalino-Matsuda, S. M. (2025). Hypercapnia Increases Influenza A Virus Infection of Bronchial Epithelial Cells by Augmenting Cellular Cholesterol via mTOR and Akt. International Journal of Molecular Sciences, 26(9), 4133. https://doi.org/10.3390/ijms26094133






