Supplementation of Forskolin and Linoleic Acid During IVC Improved the Developmental and Vitrification Efficiency of Bovine Embryos
Abstract
:1. Introduction
2. Results
2.1. Effect of Forskolin on the Developmental Ability and Survival Rate of Bovine Embryo
2.2. Effect of Linoleic Acid on the Developmental Ability and Survival Rate of Bovine Embryo
2.3. Effect of Forskolin and Linoleic Acid on the Developmental Ability and Survival Rate of Bovine Embryo
2.4. Effect of Forskolin and Linoleic Acid on Lipid Droplet Content in the Bovine Embryo
2.5. Metabolomic Analysis of Lipid Metabolites in Bovine Embryos Combined Treatment with Forskolin and Linoleic Acid
2.6. Differential Lipids Between F-L and Control Groups
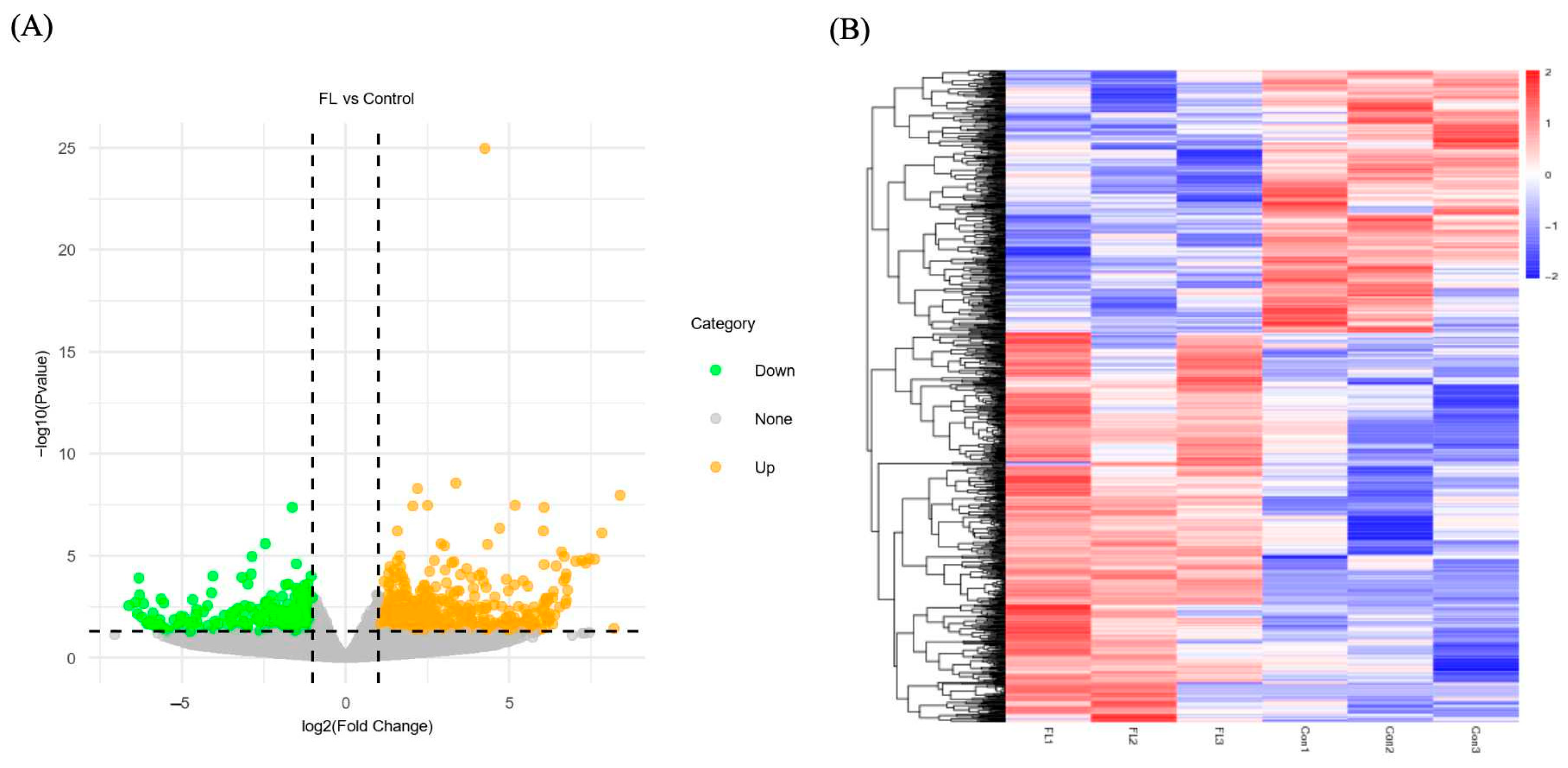
2.7. Differentially Expressed Genes (DEGs) in Forskolin and Linoleic Acid Treated Bovine Embryos
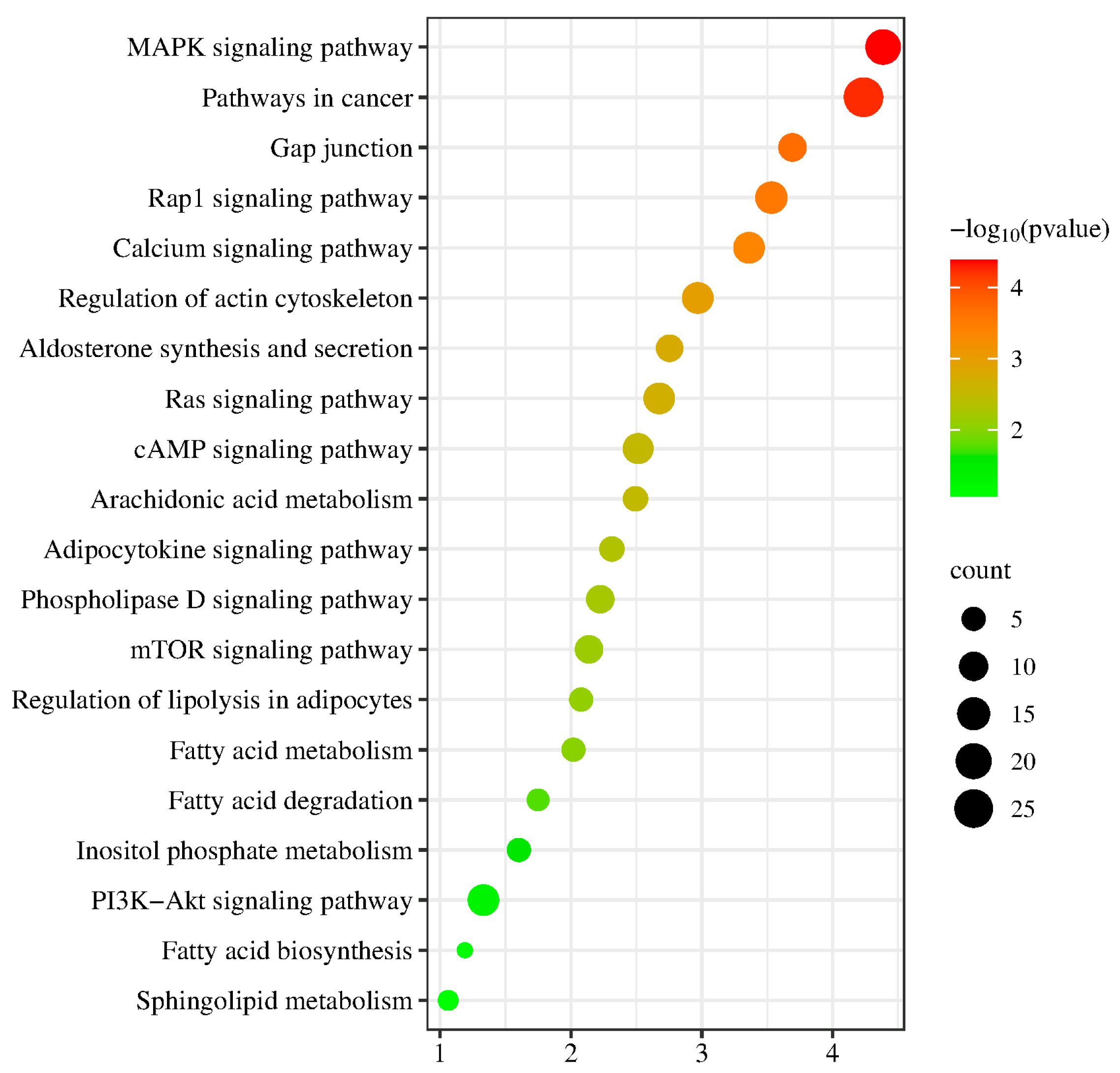
2.8. GO Enrichment Analysis of the DEGs
2.9. Pathway Enrichment Analysis of DEGs
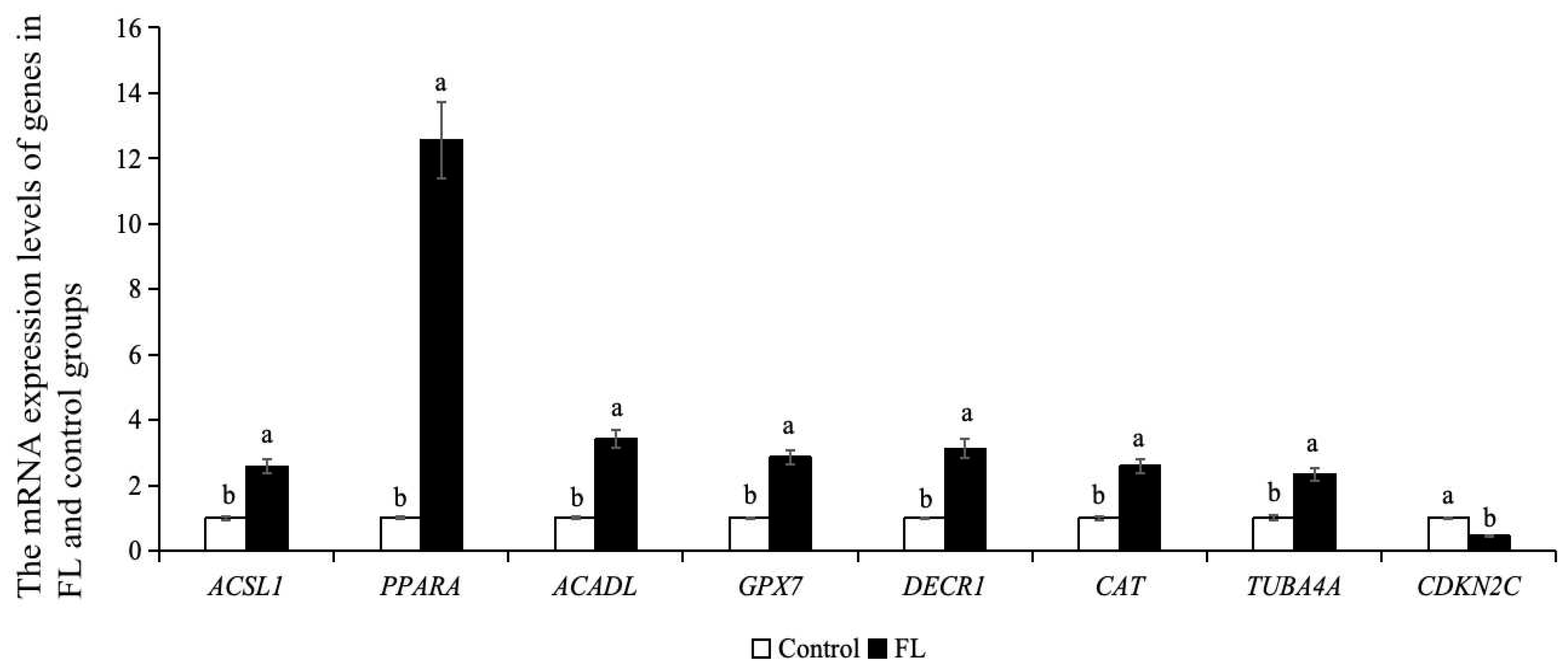
2.10. Effect of Forskolin and Linoleic Acid on Gene Expression in Bovine Blastocysts
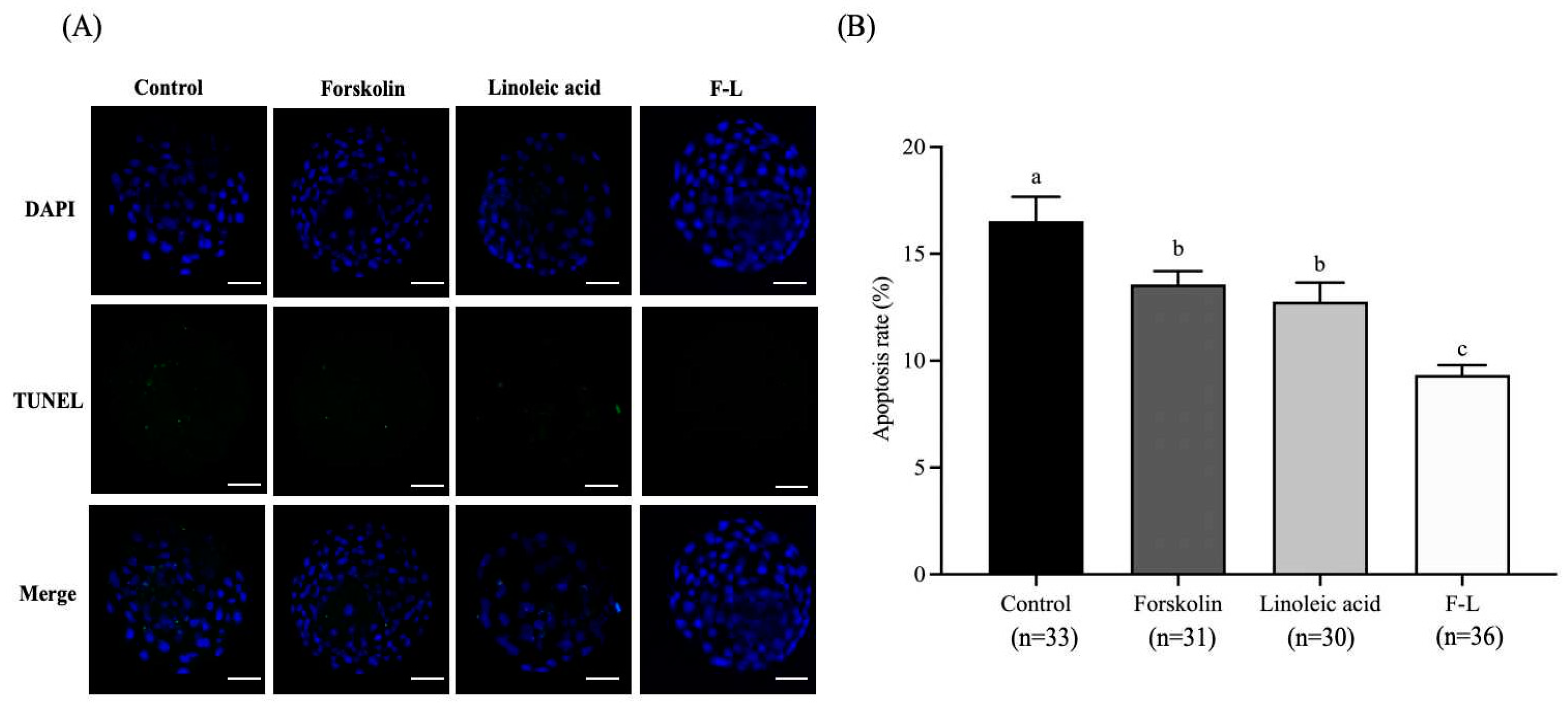
2.11. Effect of Forskolin and Linoleic Acid on the Apoptosis of Bovine Blastocysts
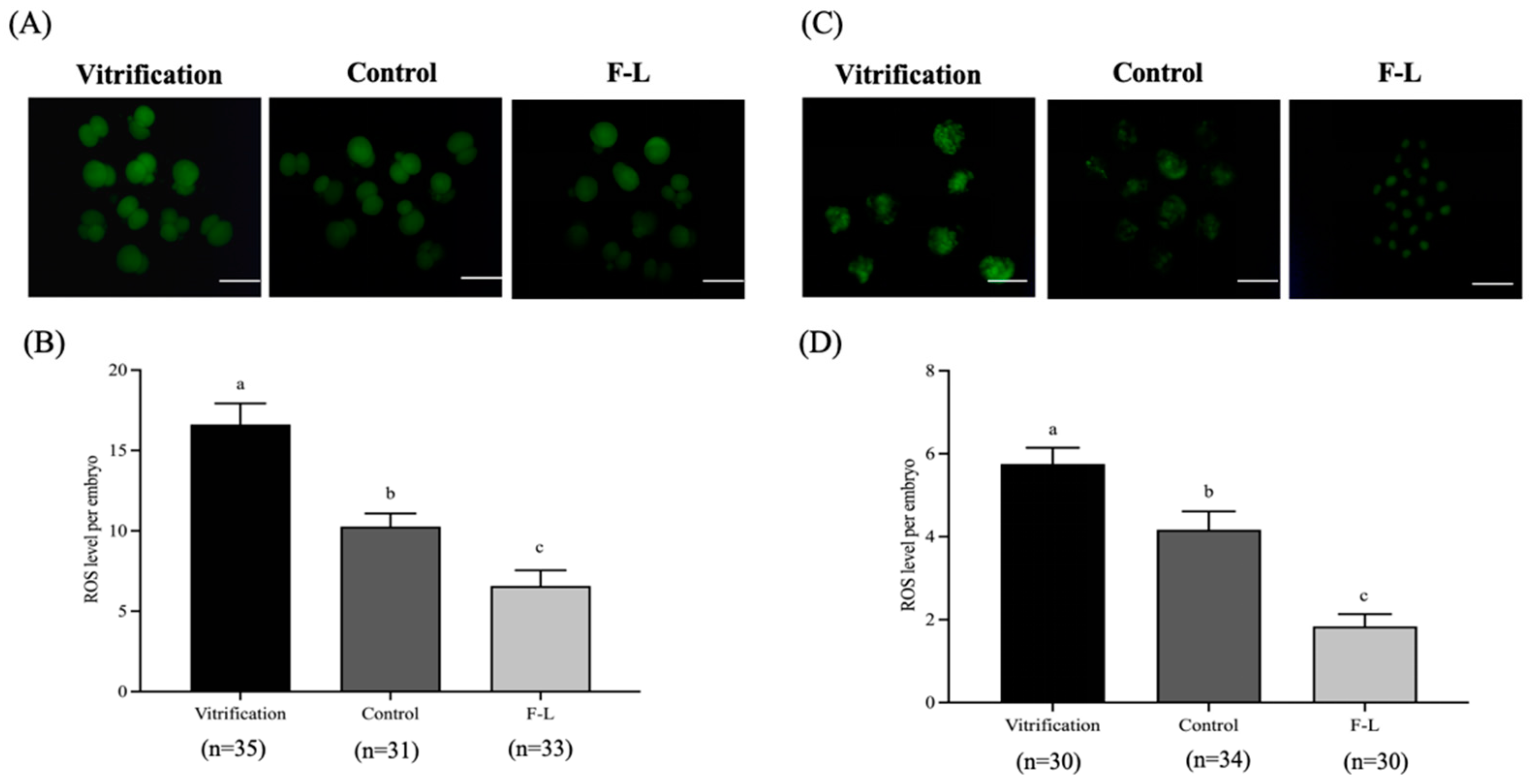
2.12. Linoleic Acid and Forskolin’s Effects on the ROS Levels of Bovine Blastocysts
2.13. Effect of Forskolin and Linoleic Acid on the Cytoskeleton of Bovine Blastocyst
3. Discussion
4. Materials and Methods
4.1. Oocytes In Vitro Maturation (IVM)
4.2. In Vitro Fertilization (IVF)
4.3. IVC
4.4. Vitrification and Warming
4.5. Embryo Lipid Droplet Staining
4.6. Assessment of the Apoptotic Index
4.7. Embryo ROS Staining
4.8. Embryo Cytoskeleton Staining
4.9. Untargeted Lipidomics Analysis
4.10. Sample Preparation, Library Construction, and RNA Sequencing
4.11. Functional Enrichment Analysis
4.12. Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR) of Genes
4.13. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kamoshita, M.; Sugita, H.; Kageyama, A.; Kawata, Y.; Ito, J.; Kashiwazaki, N. Recent advances of oocyte/embryo vitrification in mammals from rodents and large animals. Anim. Sci. J. 2024, 95, e13931. [Google Scholar] [CrossRef] [PubMed]
- Vining, L.M.; Zak, L.J.; Harvey, S.C.; Harvey, K.E. The role of apoptosis in cryopreserved animal oocytes and embryos. Theriogenology 2021, 173, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Practice Committees of the American Society for Reproductive Medicine and Society of Reproductive Biologists and Technologists. A review of best practices of rapid-cooling vitrification for oocytes and embryos: A committee opinion. Fertil. Steril. 2021, 115, 305–310. [CrossRef]
- Kurzella, J.; Miskel, D.; Rings, F.; Tholen, E.; Tesfaye, D.; Schellander, K.; Salilew-Wondim, D.; Held-Hoelker, E.; Groβe-Brinkhaus, C.; Hoelker, M. Mitochondrial bioenergetic profiles of warmed bovine blastocysts are typically altered after cryopreservation by slow freezing and vitrification. Theriogenology 2024, 214, 21–32. [Google Scholar] [CrossRef]
- Mathew, E.; Mathew, L. Conservation of Landraces and Indigenous Breeds: An Investment for the Future; Springer: Singapore, 2023. [Google Scholar]
- Zullo, G.; Albero, G.; Neglia, C.; De Canditiis, C.; Bifulco, G.; Campanile, G.; Gasparrini, B. L-ergothioneine supplementation during culture improves quality of bovine in vitro-produced embryos. Theriogenology 2016, 85, 688–697. [Google Scholar] [CrossRef]
- Abe, H.; Yamashita, S.; Satoh, T.; Hoshi, H. Accumulation of cytoplasmic lipid droplets in bovine embryos and cryotolerance of embryos developed in different culture systems using serum-free or serum-containing media. Mol. Reprod. Dev. 2002, 61, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Janati Idrissi, S.; Le Bourhis, D.; Lefevre, A.; Emond, P.; Le Berre, L.; Desnoës, O.; Joly, T.; Buff, S.; Maillard, V.; Schibler, L.; et al. Lipid profile of bovine grade-1 blastocysts produced either in vivo or in vitro before and after slow freezing process. Sci. Rep. 2021, 11, 11618. [Google Scholar] [CrossRef]
- Raza, S.H.A.; Abd El-Aziz, A.H.; Abdelnour, S.A.; Easa, A.A.; Alagawany, M.; Farag, M.R.; Al-Mutary, M.G.; Elfadadny, A.; Khan, R.; Quan, G.; et al. The role of forskolin as a lipolytic stimulator during in vitro oocyte maturation and the in vitro embryo production of livestock. Reprod. Domes. Anim. 2021, 56, 1486–1496. [Google Scholar] [CrossRef]
- Amstislavsky, S.; Mokrousova, V.; Brusentsev, E.; Okotrub, K.; Comizzoli, P. Influence of Cellular Lipids on Cryopreservation of Mammalian Oocytes and Preimplantation Embryos: A Review. Biopreserv. Biobank. 2019, 17, 76–83. [Google Scholar] [CrossRef]
- Bahmanpour, S.; Keshavarz, A.; Zarei Fard, N. Effect of Different Concentrations of Forskolin Along with Mature Granulosa Cell Co-Culturing on Mouse Embryonic Stem Cell Differentiation into Germ-Like Cells. Iran. Biomed. J. 2020, 24, 30–38. [Google Scholar] [CrossRef]
- Oliveira, C.S.; Feuchard, V.L.D.S.; Marques, S.C.S.; Saraiva, N.Z. Modulation of lipid metabolism through multiple pathways during oocyte maturation and embryo culture in bovine. Zygote 2022, 30, 258–266. [Google Scholar] [CrossRef]
- Panyaboriban, S.; Tharasanit, T.; Chankitisakul, V.; Swangchan-Uthai, T. Treatment with chemical delipidation forskolin prior to cryopreservation improves the survival rates of swamp buffalo (Bubalus bubalis) and bovine (Bos indicus) in vitro produced embryos. Cryobiology 2018, 84, 46–51. [Google Scholar] [CrossRef] [PubMed]
- Men, H.; Agca, Y.; Riley, L.K.; Critser, J.K. Improved survival of vitrified porcine embryos after partial delipation through chemically stimulated lipolysis and inhibition of apoptosis. Theriogenology 2006, 66, 2008–2016. [Google Scholar] [CrossRef] [PubMed]
- Park, B.; Lee, H.; Lee, Y.; Elahi, F.; Lee, J.; Lee, S.T.; Park, C.K.; Hyun, S.H.; Lee, E. Cilostamide and forskolin treatment during pre-IVM improves preimplantation development of cloned embryos by influencing meiotic progression and gap junction communication in pigs. Theriogenology 2016, 86, 757–765. [Google Scholar] [CrossRef]
- Razza, E.M.; Sudano, M.J.; Fontes, P.K.; Franchi, F.F.; Belaz, K.R.A.; Santos, P.H.; Castilho, A.C.S.; Rocha, D.F.O.; Eberlin, M.N.; Machado, M.F.; et al. Treatment with cyclic adenosine monophosphate modulators prior to in vitro maturation alters the lipid composition and transcript profile of bovine cumulus-oocyte complexes and blastocysts. Reprod. Fertil. Dev. 2018, 30, 1314–1328. [Google Scholar] [CrossRef]
- Metcalf, E.S.; Masterson, K.R.; Battaglia, D.; Thompson, J.G.; Foss, R.; Bech, R.; Cook, N.L.; O’ Leary, T. Conditions to optimise the developmental competence of immature equine oocytes. Reprod. Fertil. Dev. 2020, 32, 1012–1021. [Google Scholar] [CrossRef]
- Medina-Chávez, D.A.; Sánchez-Ajofrín, I.; Peris-Frau, P.; Maside, C.; Montoro, V.; Fernández-Santos, R.; Garde, J.J.; Soler, A.J. cAMP Modulators before In Vitro Maturation Decrease DNA Damage and Boost Developmental Potential of Sheep Oocytes. Animals 2021, 11, 2512. [Google Scholar] [CrossRef]
- Paschoal, D.M.; Sudano, M.J.; Guastali, M.D.; Dias Maziero, R.R.; Crocomo, L.F.; Oña Magalhães, L.C.; da Silva Rascado, T.; Martins, A.; da Cruz Landim-Alvarenga, F. Forskolin effect on the cryosurvival of in vitro-produced bovine embryos in the presence or absence of fetal calf serum. Zygote 2014, 22, 146–157. [Google Scholar] [CrossRef]
- Paschoal, D.M.; Sudano, M.J.; Schwarz, K.R.L.; Maziero, R.R.D.; Guastali, M.D.; Crocomo, L.F.; Magalhaes, L.C.O.; Martins, A., Jr.; Leal, C.L.V.; Landim-Alvarenga, F.D.C. Cell apoptosis and lipid content of in vitro-produced, vitrified bovine embryos treated with forskolin. Theriogenology 2017, 87, 108–114. [Google Scholar] [CrossRef]
- Pereira, R.M.; Baptista, M.C.; Vasques, M.I.; Horta, A.E.; Portugal, P.V.; Bessa, R.J.; Silva, J.C.; Pereira, M.S.; Marques, C.C. Cryosurvival of bovine blastocysts is enhanced by culture with trans-10 cis-12 conjugated linoleic acid (10t,12c CLA). Anim. Reprod. Sci. 2007, 98, 293–301. [Google Scholar] [CrossRef]
- Pereira, R.M.; Marques, C.C. Animal oocyte and embryo cryopreservation. Cell Tissue Bank. 2008, 9, 267–277. [Google Scholar] [CrossRef]
- Accorsi, M.F.; Leão, B.C.; Rocha-Frigoni, N.A.; Perri, S.H.; Mingoti, G.Z. Reduction in cytoplasmic lipid content in bovine embryos cultured in vitro with linoleic acid in semi-defined medium is correlated with increases in cryotolerance. Zygote 2016, 24, 485–494. [Google Scholar] [CrossRef]
- Karaşahin, T. The effect of oleic and linoleic acid addition to the culture media on bovine embryonic development following vitrification. Pol. J. Vet. Sci. 2019, 22, 661–666. [Google Scholar] [CrossRef] [PubMed]
- Lapa, M.; Marques, C.C.; Alves, S.P.; Vasques, M.I.; Baptista, M.C.; Carvalhais, I.; Silva Pereira, M.; Horta, A.E.; Bessa, R.J.; Pereira, R.M. Effect of trans-10 cis-12 conjugated linoleic acid on bovine oocyte competence and fatty acid composition. Reprod. Domest. Anim. 2011, 46, 904–910. [Google Scholar] [CrossRef]
- Matos, J.E.; Marques, C.C.; Moura, T.F.; Baptista, M.C.; Horta, A.E.; Soveral, G.; Pereira, R.M. Conjugated linoleic acid improves oocyte cryosurvival through modulation of the cryoprotectants influx rate. Reprod. Biol. Endocrinol. 2015, 13, 60. [Google Scholar] [CrossRef] [PubMed]
- Rakhmanova, T.; Mokrousova, V.; Okotrub, S.; Kizilova, E.; Brusentsev, E.; Amstislavsky, S. Effects of forskolin on cryopreservation and embryo development in the domestic cat. Theriogenology 2023, 210, 192–198. [Google Scholar] [CrossRef]
- Prates, E.G.; Marques, C.C.; Baptista, M.C.; Vasques, M.I.; Carolino, N.; Horta, A.E.; Charneca, R.; Nunes, J.T.; Pereira, R.M. Fat area and lipid droplet morphology of porcine oocytes during in vitro maturation with trans-10, cis-12 conjugated linoleic acid and forskolin. Animal 2013, 7, 602–609. [Google Scholar] [CrossRef] [PubMed]
- Leal, G.R.; Oliveira, T.A.; de Paula Guimarães, M.P.; Correia, L.F.L.; Vasconcelos, E.M.; Souza-Fabjan, J.M.G. Lipid modulation during IVM increases the metabolism and improves the cryosurvival of cat oocytes. Theriogenology 2024, 214, 33–42. [Google Scholar] [CrossRef]
- Fu, X.W.; Wu, G.Q.; Li, J.J.; Hou, Y.P.; Zhou, G.B.; Suo, L.; Wang, Y.P.; Zhu, S.E. Positive effects of Forskolin (stimulator of lipolysis) treatment on cryosurvival of in vitro matured porcine oocytes. Theriogenology 2011, 75, 268–275. [Google Scholar] [CrossRef]
- Sanches, B.V.; Marinho, L.S.; Filho, B.D.; Pontes, J.H.; Basso, A.C.; Meirinhos, M.L.; Silva-Santos, K.C.; Ferreira, C.R.; Seneda, M.M. Cryosurvival and pregnancy rates after exposure of IVF-derived Bos indicus embryos to forskolin before vitrification. Theriogenology 2013, 80, 372–377. [Google Scholar] [CrossRef]
- Roura, M.; Catalá, M.G.; Soto-Heras, S.; Hammami, S.; Izquierdo, D.; Fouladi-Nashta, A.; Paramio, M.T. Linoleic (LA) and linolenic (ALA) acid concentrations in follicular fluid of prepubertal goats and their effect on oocyte in vitro maturation and embryo development. Reprod. Fertil. Dev. 2018, 30, 286–296. [Google Scholar] [CrossRef] [PubMed]
- Ramos Leal, G.; Santos Monteiro, C.A.; Souza-Fabjan, J.M.G.; de Paula Vasconcelos, C.O.; Garcia Nogueira, L.A.; Reis Ferreira, A.M.; Varella Serapiao, R. Role of cAMP modulator supplementations during oocyte in vitro maturation in domestic animals. Anim. Reprod. Sci. 2018, 199, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.K.; Choi, K.H.; Oh, J.N.; Kim, S.H.; Lee, M.; Jeong, J.; Choe, G.C.; Lee, C.K. Linoleic acid reduces apoptosis via NF-κB during the in vitro development of induced parthenogenic porcine embryos. Theriogenology 2022, 187, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Marei, W.F.; Wathes, D.C.; Fouladi-Nashta, A.A. Impact of linoleic acid on bovine oocyte maturation and embryo development. Reproduction 2010, 139, 979–988. [Google Scholar] [CrossRef]
- Kim, J.Y.; Kinoshita, M.; Ohnishi, M.; Fukui, Y. Lipid and fatty acid analysis of fresh and frozen-thawed immature and in vitro matured bovine oocytes. Reproduction 2001, 122, 131–138. [Google Scholar] [CrossRef]
- Sturmey, R.G.; Reis, A.; Leese, H.J.; McEvoy, T.G. Role of fatty acids in energy provision during oocyte maturation and early embryo development. Reprod. Domest. Anim. 2009, 44, 50–58. [Google Scholar] [CrossRef]
- Rieger, D.; Luciano, A.M.; Modina, S.; Pocar, P.; Lauria, A.; Gandolfi, F. The effects of epidermal growth factor and insulin-like growth factor I on the metabolic activity, nuclear maturation and subsequent development of cattle oocytes in vitro. J. Reprod. Fertil. 1998, 112, 123–130. [Google Scholar] [CrossRef]
- Ferguson, E.M.; Leese, H.J. Triglyceride content of bovine oocytes and early embryos. J. Reprod. Fertil. 1999, 116, 373–378. [Google Scholar] [CrossRef]
- Li, M.; Li, W.; Wu, J.; Zheng, Y.; Shao, J.; Li, Q.; Kang, S.; Zhang, Z.; Yue, X.; Yang, M. Quantitative lipidomics reveals alterations in donkey milk lipids according to lactation. Food. Chem. 2020, 310, 125866. [Google Scholar] [CrossRef]
- Van Der Veen, J.N.; Kennelly, J.P.; Wan, S.; Vance, J.E.; Vance, D.E.; Jacobs, R.L. The critical role of phosphatidylcholine and phosphatidylethanolamine metabolism in health and disease. Biochim. Biophys. Acta Biomembr. 2017, 1859, 1558–1572. [Google Scholar] [CrossRef]
- Hamilton, J.A. New insights into the roles of proteins and lipids in membrane transport of fatty acids. Prostaglandins Leukot. Essent. Fatty Acids 2007, 77, 355–361. [Google Scholar] [CrossRef] [PubMed]
- Ellis, J.M.; Li, L.O.; Wu, P.C.; Koves, T.R.; Ilkayeva, O.; Stevens, R.D.; Watkins, S.M.; Muoio, D.M.; Coleman, R.A. Adipose acyl-CoA synthetase-1 directs fatty acids toward beta-oxidation and is required for cold thermogenesis. Cell Metab. 2010, 12, 53–64. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.S.; Tsai, C.T.; Huangfu, C.A.; Huang, W.Y.; Lei, H.Y.; Lin, C.F.; Su, I.J.; Chang, W.T.; Wu, P.H.; Chen, Y.T.; et al. ACSL3 and GSK-3β are essential for lipid upregulation induced by endoplasmic reticulum stress in liver cells. J. Cell Biochem. 2011, 112, 881–893. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Li, N.; Deng, L.; Jiang, X.; Zhang, Y.; Lee, L.T.O.; Zhang, H. ACSL1-induced ferroptosis and platinum resistance in ovarian cancer by increasing FSP1 N-myristylation and stability. Cell Death Discov. 2023, 9, 83. [Google Scholar] [CrossRef]
- Blomme, A.; Ford, C.A.; Mui, E.; Patel, R.; Ntala, C.; Jamieson, L.E.; Planque, M.; McGregor, G.H.; Peixoto, P.; Hervouet, E.; et al. 2,4-dienoyl-CoA reductase regulates lipid homeostasis in treatment-resistant prostate cancer. Nat. Commun. 2020, 11, 2508. [Google Scholar] [CrossRef]
- Cai, J.; Chen, T.; Jiang, Z.; Yan, J.; Ye, Z.; Ruan, Y.; Tao, L.; Shen, Z.; Liang, X.; Wang, Y.; et al. Bulk and single-cell transcriptome profiling reveal extracellular matrix mechanical regulation of lipid metabolism reprograming through YAP/TEAD4/ACADL axis in hepatocellular carcinoma. Int. J. Biol. Sci. 2023, 19, 2114–2131. [Google Scholar] [CrossRef]
- Pei, J.; Pan, X.; Wei, G.; Hua, Y. Research progress of glutathione peroxidase family (GPX) in redoxidation. Front. Pharmacol. 2023, 14, 1147414. [Google Scholar] [CrossRef]
- Chen, Y.I.; Wei, P.C.; Hsu, J.L.; Su, F.Y.; Lee, W.H. NPGPx (GPx7): A novel oxidative stress sensor/transmitter with multiple roles in redox homeostasis. Am. J. Transl. Res. 2016, 8, 1626–1640. [Google Scholar]
- Wei, P.C.; Hsieh, Y.H.; Su, M.I.; Jiang, X.; Hsu, P.H.; Lo, W.T.; Weng, J.Y.; Jeng, Y.M.; Wang, J.M.; Chen, P.L.; et al. Loss of the oxidative stress sensor NPGPx compromises GRP78 chaperone activity and induces systemic disease. Mol. Cell 2012, 48, 747–759. [Google Scholar] [CrossRef]
- Nihad, M.; Sen, U.; Chaudhury, D.; Das, U.N.; Shenoy, P.S.; Bose, B. Arachidonic acid modulates the cellular energetics of human pluripotent stem cells and protects the embryoid bodies from embryotoxicity effects in vitro. Reprod. Toxicol. 2023, 120, 108438. [Google Scholar] [CrossRef]
- Huang, N.; Liu, X.; Pei, X.; Peng, J.; Wei, H. The Quantitative Profiling of Oxylipins from Arachidonic Acid by LC-MS/MS in Feces at Birth 3 Days and 21 Days of Piglets. Metabolites 2022, 12, 702. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Suh, Y.; Choi, Y.M.; Chen, P.R.; Davis, M.E.; Lee, K. Differential Expression of Cell Cycle Regulators During Hyperplastic and Hypertrophic Growth of Broiler Subcutaneous Adipose Tissue. Lipids 2015, 50, 965–976. [Google Scholar] [CrossRef] [PubMed]
- Pereira, M.J.; Vranic, M.; Kamble, P.G.; Jernow, H.; Kristófi, R.; Holbikova, E.; Skrtic, S.; Kullberg, J.; Svensson, M.K.; Hetty, S.; et al. CDKN2C expression in adipose tissue is reduced in type II diabetes and central obesity: Impact on adipocyte differentiation and lipid storage? Transl. Res. 2022, 242, 105–121. [Google Scholar] [CrossRef]
- Gilchrist, R.B.; Luciano, A.M.; Richani, D.; Zeng, H.T.; Wang, X.; Vos, M.D.; Sugimura, S.; Smitz, J.; Richard, F.J.; Thompson, J.G. Oocyte maturation and quality: Role of cyclic nucleotides. Reproduction 2016, 152, R143–R157. [Google Scholar] [CrossRef]
- Appeltant, R.; Somfai, T.; Maes, D.; VAN Soom, A.; Kikuchi, K. Porcine oocyte maturation in vitro: Role of cAMP and oocyte-secreted factors—A practical approach. J. Reprod. Dev. 2016, 62, 439–449. [Google Scholar] [CrossRef]
- Richani, D.; Wang, X.; Zeng, H.T.; Smitz, J.; Thompson, J.G.; Gilchrist, R.B. Pre-maturation with cAMP modulators in conjunction with EGF-like peptides during in vitro maturation enhances mouse oocyte developmental competence. Mol. Reprod. Dev. 2014, 81, 422–435. [Google Scholar] [CrossRef]
- Buell, M.; Chitwood, J.L.; Ross, P.J. cAMP modulation during sheep in vitro oocyte maturation delays progression of meiosis without affecting oocyte parthenogenetic developmental competence. Anim. Reprod. Sci. 2015, 154, 16–24. [Google Scholar] [CrossRef]
- Ezoe, K.; Yabuuchi, A.; Tani, T.; Mori, C.; Miki, T.; Takayama, Y.; Beyhan, Z.; Kato, Y.; Okuno, T.; Kobayashi, T.; et al. Developmental Competence of Vitrified-Warmed Bovine Oocytes at the Germinal-Vesicle Stage is Improved by Cyclic Adenosine Monophosphate Modulators during In Vitro Maturation. PLoS ONE 2015, 10, e0126801. [Google Scholar] [CrossRef]
- Albuz, F.K.; Sasseville, M.; Lane, M.; Armstrong, D.T.; Thompson, J.G.; Gilchrist, R.B. Simulated physiological oocyte maturation (SPOM): A novel in vitro maturation system that substantially improves embryo yield and pregnancy outcomes. Hum. Reprod. 2010, 25, 2999–3011. [Google Scholar] [CrossRef]
- Huang, J.C.; Wun, W.S.; Goldsby, J.S.; Wun, I.C.; Noorhasan, D.; Wu, K.K. Stimulation of embryo hatching and implantation by prostacyclin and peroxisome proliferator-activated receptor delta activation: Implication in IVF. Hum. Reprod. 2007, 22, 807–814. [Google Scholar] [CrossRef]
- Yang, B.; An, Y.; Yang, Y.; Zhao, Y.; Yu, K.; Weng, Y.; Du, C.; Li, H.; Yu, B. The ERβ-cAMP signaling pathway regulates estradiol-induced ovine oocyte meiotic arrest. Theriogenology 2024, 214, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Márquez-Alvarado, Y.C.; Galina, C.S.; Castilla, B.; Leon, H.; Moreno-Mendoza, N. Evidence of damage in cryopreserved and fresh bovine embryos using the Tunel technique. Reprod. Domest. Anim. 2004, 39, 141–145. [Google Scholar] [CrossRef] [PubMed]
- Park, S.Y.; Kim, E.Y.; Cui, X.S.; Tae, J.C.; Lee, W.D.; Kim, N.H.; Park, S.P.; Lim, J.H. Increase in DNA fragmentation and apoptosis-related gene expression in frozen-thawed bovine blastocysts. Zygote 2006, 14, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Hayden, M.S.; Ghosh, S. Shared principles in NF-kappaB signaling. Cell 2008, 132, 344–362. [Google Scholar] [CrossRef]
- Su, L.J.; Zhang, J.H.; Gomez, H.; Murugan, R.; Hong, X.; Xu, D.; Jiang, F.; Peng, Z.Y. Reactive Oxygen Species-Induced Lipid Peroxidation in Apoptosis, Autophagy, and Ferroptosis. Oxidative Med. Cell. Longev. 2019, 2019, 5080843. [Google Scholar] [CrossRef]
- Castillo-Martín, M.; Bonet, S.; Morató, R.; Yeste, M. Supplementing culture and vitrification-warming media with l-ascorbic acid enhances survival rates and redox status of IVP porcine blastocysts via induction of GPX1 and SOD1 expression. Cryobiology 2014, 68, 451–458. [Google Scholar] [CrossRef]
- Guo, X.; Bai, X.; Li, L.; Li, J.; Wang, H. Forskolin protects against cisplatin-induced ototoxicity by inhibiting apoptosis and ROS production. Biomed. Pharmacother. 2018, 99, 530–536. [Google Scholar] [CrossRef]
- López, A.; Ducolomb, Y.; Casas, E.; Retana-Marquez, S.; Betancourt, M.; Casillas, F. Effects of Porcine Immature Oocyte Vitrification on Actin Microfilament Distribution and Chromatin Integrity During Early Embryo Development in vitro. Front. Cell Dev. Biol. 2021, 9, 636765. [Google Scholar] [CrossRef]
- Morató, R.; Izquierdo, D.; Albarracín, J.L.; Anguita, B.; Palomo, M.J.; Jimenez-Macedo, A.R.; Paramio, M.T.; Mogas, T. Effects of pre-treating in vitro-matured bovine oocytes with the cytoskeleton stabilizing agent taxol prior to vitrification. Mol. Reprod. Dev. 2008, 75, 191–201. [Google Scholar] [CrossRef]
- Perez, V.; Bouschet, T.; Fernandez, C.; Bockaert, J.; Journot, L. Dynamic reorganization of the astrocyte actin cytoskeleton elicited by cAMP and PACAP: A role for phosphatidylInositol 3-kinase inhibition. Eur. J. Neurosci. 2005, 21, 26–32. [Google Scholar] [CrossRef]
- Brackett, B.G.; Oliphant, G. Capacitation of rabbit spermatozoa in vitro. Biol. Reprod. 1975, 12, 260–274. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.M.; Hao, H.S.; Du, W.H.; Zhao, S.J.; Wang, H.Y.; Wang, N.; Wang, D.; Liu, Y.; Qin, T.; Zhu, H.B. Melatonin inhibits apoptosis and improves the developmental potential of vitrified bovine oocytes. J. Pineal Res. 2016, 60, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Buschiazzo, J.; Ríos, G.L.; Canizo, J.R.; Antollini, S.S.; Alberio, R.H. Free cholesterol and cholesterol esters in bovine oocytes: Implications in survival and membrane raft organization after cryopreservation. PLoS ONE 2017, 12, e0180451. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.M.; Idrees, M.; Perera, C.D.; Haider, Z.; Joo, M.D.; Kang, J.S.; Lee, S.H.; Kong, I.K. The effects of cycloastragenol on bovine embryo development, implantation potential and telomerase activity. Reprod. Fertil. Dev. 2023, 35, 527–538. [Google Scholar] [CrossRef]
- García-Martínez, T.; Vendrell-Flotats, M.; Martínez-Rodero, I.; Ordóñez-León, E.A.; Álvarez-Rodríguez, M.; López-Béjar, M.; Yeste, M.; Mogas, T. Glutathione ethyl Ester protects in vitro-maturing bovine oocytes against oxidative stress induced by subsequent vitrification/warming. Int. J. Mol. Sci. 2020, 21, 7547. [Google Scholar] [CrossRef]
- Cai, H.; Xiang, Y.; Zeng, Y.; Li, Z.; Zheng, X.; Luo, Q.; Zhu, H.; Gong, Q.; Gu, Z.; Liu, Y.; et al. Cathepsin B-responsive and gadolinium-labeled branched glycopolymer-PTX conjugate-derived nanotheranostics for cancer treatment. Acta Pharm. Sin. B 2021, 11, 544–559. [Google Scholar] [CrossRef]
- Rabotnick, M.H.; Haidari, A.; Dolinoy, D.C.; Meijer, J.L.; Harris, S.M.; Burant, C.F.; Padmanabhan, V.; Goodrich, J.M. Early pregnancy serum PFAS are associated with alterations in the maternal lipidome. Environ. Res. 2024, 263, 120183. [Google Scholar] [CrossRef]
- Gao, S.; Yan, L.; Wang, R.; Li, J.; Yong, J.; Zhou, X.; Wei, Y.; Wu, X.; Wang, X.; Fan, X.; et al. Tracing the temporal-spatial transcriptome landscapes of the human fetal digestive tract using single-cell RNA-sequencing. Nat. Cell Biol. 2018, 20, 721–734. [Google Scholar] [CrossRef]
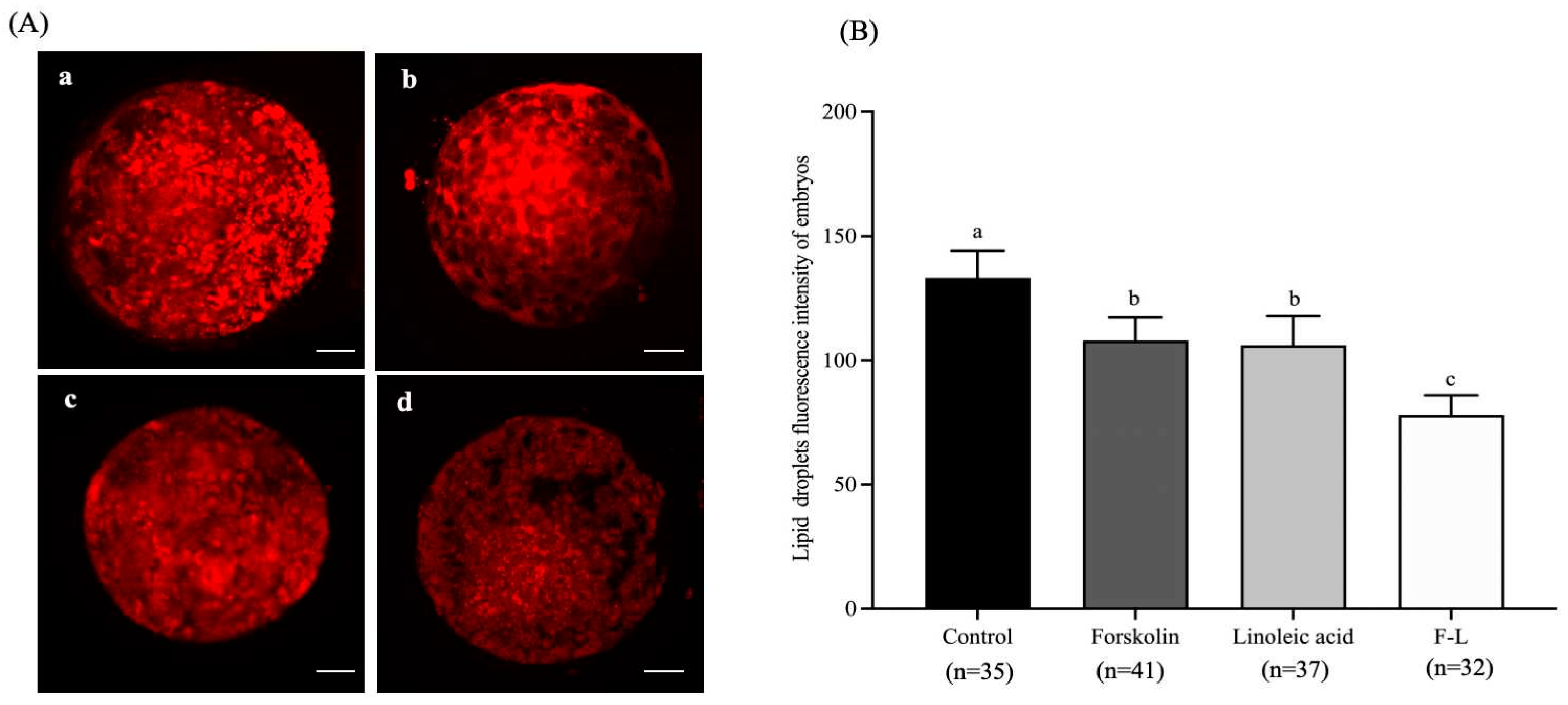
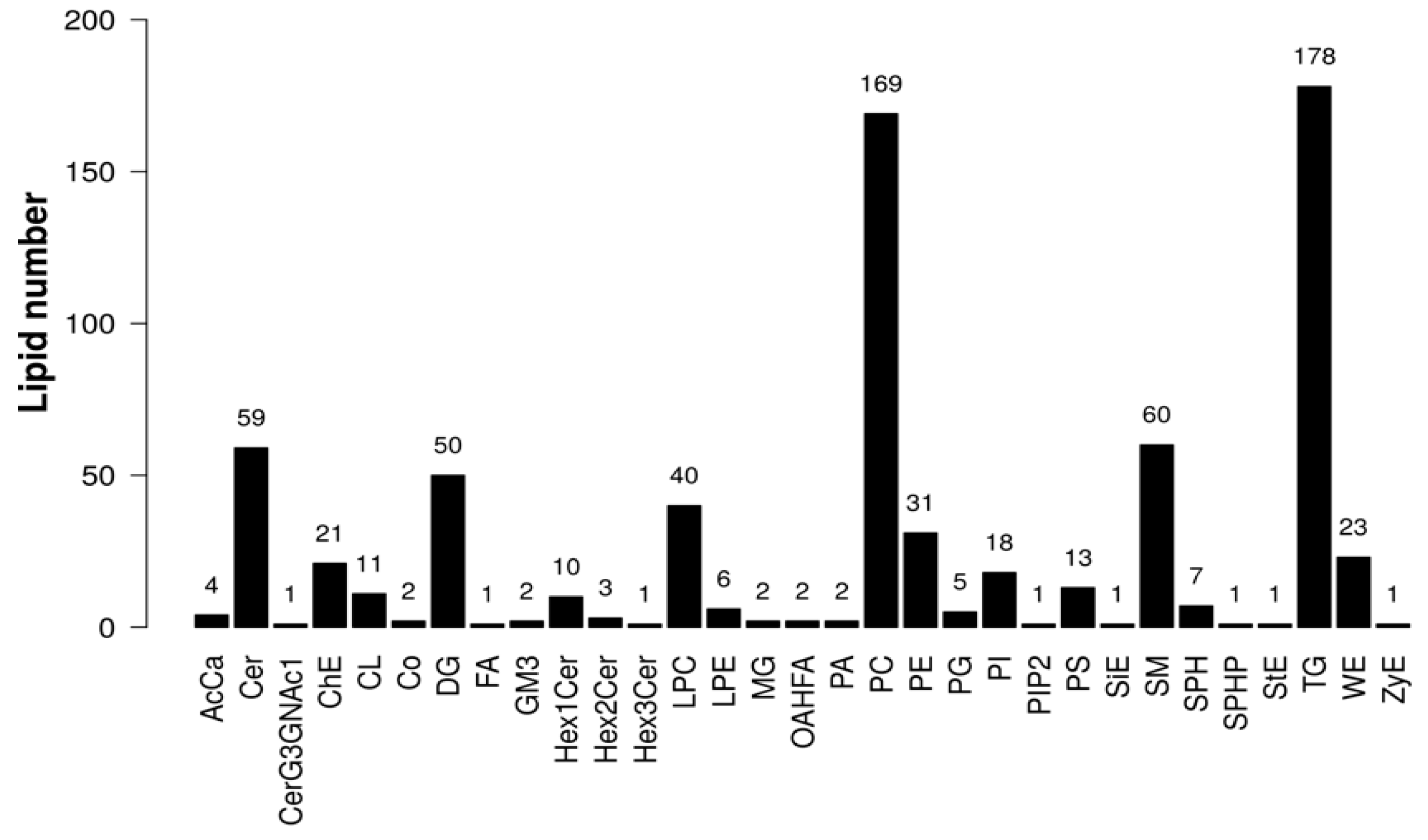
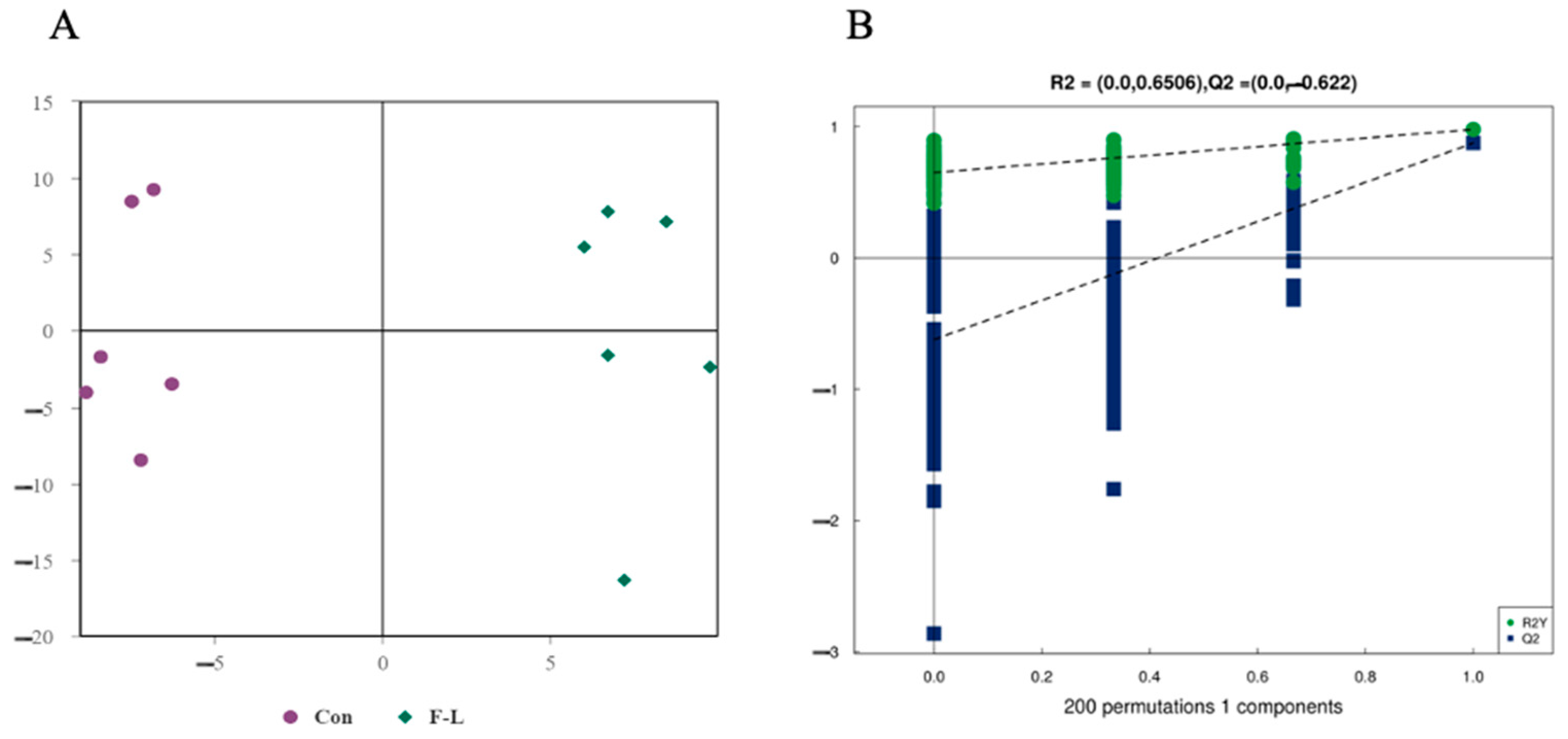
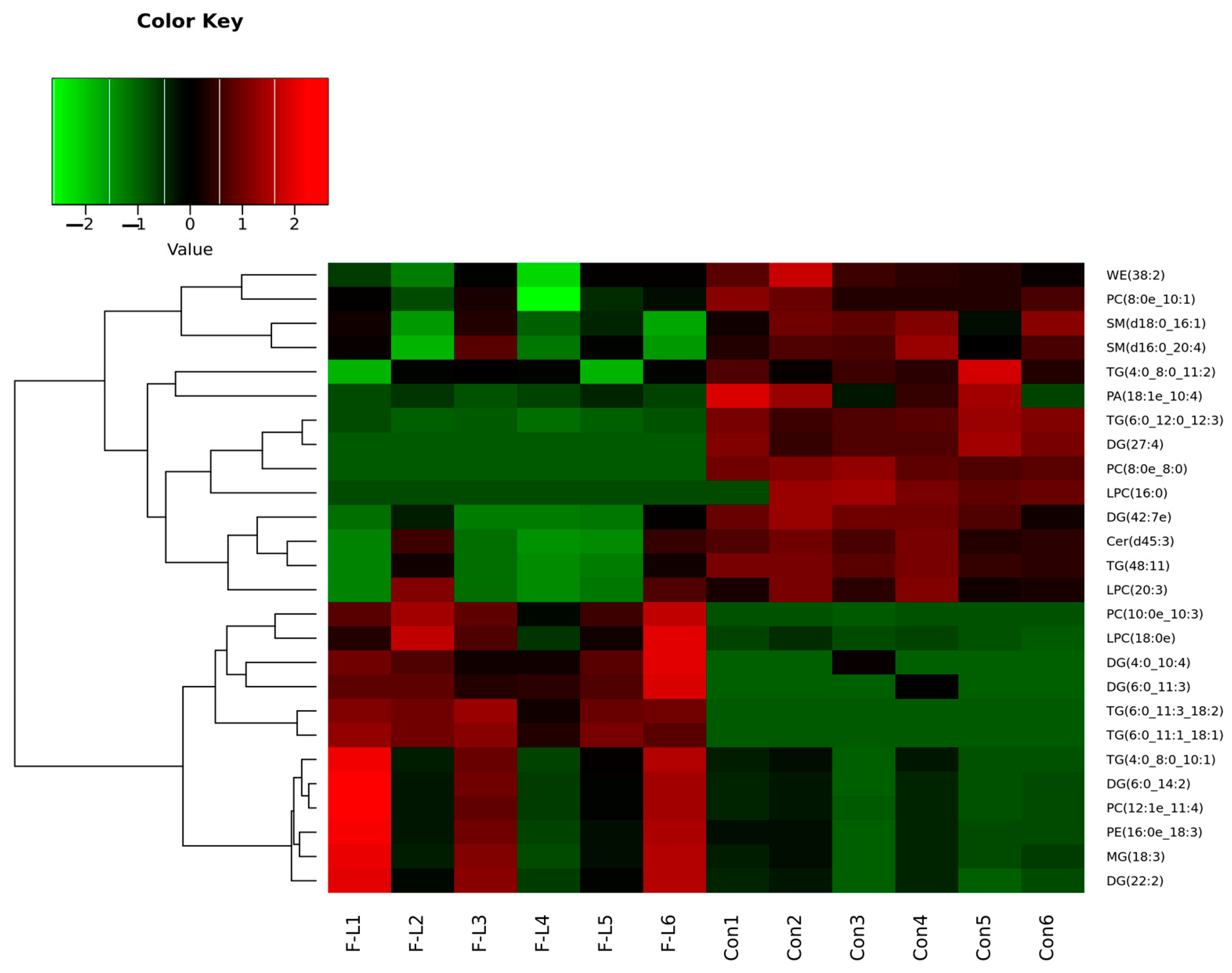


| Groups | No. COCs | Cleavage Rate | Blastocyst Rate | Survival Rate |
|---|---|---|---|---|
| 0 μM | 105 | 81.90 ± 2.57% (86/105) b | 34.89 ± 4.47% (30/86) b | 86.67 ± 6.59% (26/30) b |
| 5 μM | 119 | 84.87 ± 4.65% (101/119) b | 35.64 ± 4.70% (36/101) a | 88.89 ± 7.35% (32/36) b |
| 10 μM | 108 | 91.67 ± 3.71% (99/108) a | 42.42 ± 4.85% (42/99) a | 95.24 ± 7.23% (40/42) a |
| 15 μM | 134 | 64.18 ± 4.32% (86/134) c | 27.91 ± 3.81% (24/86) c | 83.33 ± 7.06% (20/24) c |
| Groups | No. COCs | Cleavage Rate | Blastocyst Rate | Survival Rate |
|---|---|---|---|---|
| 0 μM | 165 | 80.00 ± 6.59% (132/165) b | 33.33 ± 2.41% (44/132) b | 86.36 ± 7.15% (38/44) b |
| 50 μM | 188 | 61.17 ± 5.85% (115/188) c | 27.83 ± 1.68% (32/115) c | 81.25 ± 7.46% (26/32) c |
| 100 μM | 151 | 88.74 ± 6.73% (134/151) a | 38.81 ± 2.55% (52/134) a | 96.15 ± 8.53% (50/52) a |
| 500 μM | 147 | 60.54 ± 5.68% (89/147) c | 29.21 ± 1.84% (26/89) c | 80.77 ± 7.21% (21/26) c |
| Groups | No. COCs | Cleavage Rate | Blastocyst Rate | Survival Rate |
|---|---|---|---|---|
| Control | 262 | 75.57 ± 7.46% (198/262) c | 30.30 ± 2.65% (60/198) c | 80.65 ± 7.68% (25/31) b |
| Forskolin (10 μM) | 284 | 82.39 ± 8.52% (234/284) b | 39.74 ± 4.12% (93/234) b | 90.24 ± 7.31% (37/41) b |
| Linoleic acid (100 μM) | 260 | 83.08 ± 8.46% (216/260) b | 37.97 ± 4.03% (82/216) b | 89.47 ± 8.15% (34/38) b |
| F-L | 306 | 89.54 ± 7.62% (274/306) a | 45.26 ± 5.24% (124/274) a | 98.18 ± 8.64% (54/55) a |
| Number | the Adduct Ion Form of Lipid | Cal m/z | (t/min) | VIP | FC | p-Value |
|---|---|---|---|---|---|---|
| 1 | PA (18:1e_10:4) − 2H | 283.169 | 4.75 | 1.64 | 0.47 | <0.01 |
| 2 | DG (4:0_10:4) + H | 309.170 | 1.18 | 1.70 | 9.25 | <0.01 |
| 3 | DG (6:0_11:3) + Na | 375.214 | 1.17 | 6.92 | 11.21 | <0.01 |
| 4 | DG (6:0_14:2) + NH4 | 414.321 | 1.88 | 2.82 | 2.42 | 0.03 |
| 5 | DG (22:2) + NH4 | 442.353 | 1.88 | 1.80 | 2.47 | 0.02 |
| 6 | DG (27:4) + H | 491.373 | 1.46 | 3.21 | 0.01 | <0.01 |
| 7 | DG (42:7e) + NH4 | 698.608 | 13.38 | 1.73 | 0.36 | <0.01 |
| 8 | LPC (16:0) + H | 496.340 | 3.56 | 9.43 | 0.01 | <0.01 |
| 9 | LPC (18:0e) + H | 510.392 | 3.64 | 1.02 | 2.15 | <0.01 |
| 10 | LPC (20:3) + H | 546.355 | 3.03 | 1.08 | 0.77 | 0.04 |
| 11 | Car (d45:3) + Na | 710.642 | 14.89 | 2.50 | 0.48 | <0.01 |
| 12 | MG (18:3) + NH4 | 370.295 | 1.93 | 1.18 | 2.10 | 0.04 |
| 13 | PC (8:0e_8:0) + H | 496.340 | 3.59 | 11.20 | 0.01 | <0.01 |
| 14 | PC (8:0e_10:1) + H | 522.355 | 2.20 | 2.50 | 0.83 | 0.02 |
| 15 | PC (10:0e_10:3) + H | 546.355 | 3.62 | 1.35 | 12.56 | <0.01 |
| 16 | PC (12:1e_11:4) + Na | 606.353 | 1.89 | 1.10 | 2.74 | 0.03 |
| 17 | PE (16:0e_18:3) + Na | 722.510 | 1.61 | 1.87 | 2.31 | 0.04 |
| 18 | SM (d18:0_16:1) + H | 703.575 | 9.68 | 6.67 | 0.72 | <0.01 |
| 19 | SM (d16:0_20:4) + H | 725.559 | 9.12 | 1.51 | 0.80 | 0.02 |
| 20 | TG (4:0_8:0_10:1) + NH4 | 458.348 | 1.84 | 3.90 | 2.44 | 0.03 |
| 21 | TG (4:0_8:0_11:2) + NH4 | 470.348 | 1.23 | 1.41 | 0.47 | 0.01 |
| 22 | TG (6:0_12:0_12:3) + H | 549.415 | 1.61 | 2.34 | 0.08 | <0.01 |
| 23 | TG (6:0_11:1_18:1) + NH4 | 638.535 | 6.47 | 1.72 | 188.65 | <0.01 |
| 24 | TG (6:0_11:3_18:2) + H | 615.462 | 6.44 | 2.83 | 237.56 | <0.01 |
| 25 | TG (48:11) + NH4 | 802.598 | 7.37 | 1.03 | 0.38 | <0.01 |
| 26 | WE (38:2) + H | 561.561 | 16.33 | 2.24 | 0.86 | 0.01 |
| Gene | Sample | Up/Down | |||||
|---|---|---|---|---|---|---|---|
| FL 1 | FL 2 | FL 3 | Con 1 | Con 2 | Con 3 | ||
| LRPAP1 | 2467 | 1571 | 1888 | 1006 | 996 | 1040 | up |
| APRT | 580 | 462 | 498 | 117 | 420 | 127 | up |
| NFKBIA | 603 | 988 | 835 | 315 | 420 | 453 | up |
| CRISP2 | 250 | 281 | 328 | 127 | 59 | 127 | up |
| NDRG4 | 187 | 74 | 256 | 67 | 62 | 29 | up |
| TGM1 | 227 | 1319 | 456 | 250 | 342 | 189 | up |
| ACSL1 | 1688 | 945 | 913 | 664 | 308 | 540 | up |
| CASP7 | 479 | 285 | 257 | 171 | 132 | 146 | up |
| SEC22B | 1494 | 1150 | 984 | 558 | 716 | 771 | up |
| CDC25B | 560 | 535 | 234 | 190 | 95 | 81 | up |
| MX2 | 54 | 46 | 33 | 6 | 25 | 5 | up |
| EPHA4 | 288 | 131 | 180 | 105 | 86 | 45 | up |
| ACADL | 289 | 135 | 140 | 66 | 99 | 44 | up |
| SLC38A5 | 26 | 15 | 98 | 10 | 1 | 13 | up |
| CLDN1 | 1868 | 1435 | 647 | 302 | 920 | 602 | up |
| TBC1D1 | 691 | 581 | 682 | 381 | 253 | 285 | up |
| NSUN3 | 560 | 363 | 339 | 229 | 241 | 193 | up |
| CREM | 42 | 9 | 89 | 7 | 13 | 6 | up |
| BMP7 | 107 | 35 | 82 | 41 | 6 | 10 | up |
| IFIT5 | 452 | 170 | 291 | 124 | 85 | 91 | up |
| PPARA | 37 | 35 | 31 | 2 | 0 | 7 | up |
| IRF8 | 1238 | 1299 | 420 | 653 | 388 | 189 | up |
| NR2F2 | 79 | 7 | 110 | 8 | 9 | 4 | up |
| SLC2A4 | 141 | 100 | 29 | 38 | 37 | 9 | up |
| GPX7 | 92 | 94 | 99 | 31 | 20 | 39 | up |
| ADCY8 | 28 | 6 | 0 | 0 | 1 | 0 | up |
| DECR1 | 256 | 389 | 215 | 171 | 142 | 111 | up |
| TGFBR2 | 379 | 142 | 281 | 70 | 173 | 71 | up |
| IDH1 | 9515 | 7924 | 6175 | 5525 | 3145 | 4063 | up |
| CAT | 7923 | 8367 | 5654 | 3618 | 3614 | 4907 | up |
| DCAF11 | 866 | 680 | 665 | 394 | 480 | 354 | up |
| TUBA4A | 855 | 597 | 787 | 377 | 247 | 500 | up |
| ARID1A | 391 | 324 | 367 | 516 | 516 | 483 | down |
| KAT6B | 711 | 641 | 639 | 892 | 653 | 1606 | down |
| ACSF3 | 56 | 91 | 22 | 93 | 133 | 143 | down |
| GLS2 | 11 | 25 | 11 | 78 | 58 | 35 | down |
| CDKN2C | 2 | 7 | 9 | 17 | 25 | 24 | down |
| PLPP3 | 183 | 139 | 74 | 280 | 308 | 363 | down |
| DDX54 | 1533 | 538 | 850 | 1523 | 1532 | 1475 | down |
| PLN | 27 | 6 | 7 | 81 | 25 | 49 | down |
| TIAM1 | 458 | 531 | 619 | 783 | 874 | 783 | down |
| Gene | NCBI Accession | Primer (3′–5′) | Size (bp) |
|---|---|---|---|
| ACSL1 | NM_001076085 | TTTGTCCACGGAGAGAGCTT | 108 |
| TTCAAAGGAGCCCACAATGC | |||
| DECR1 | NM_001075423 | ACAACTTGTCTGTCCAGCCT | 136 |
| GGTCCCTCACATCACACTGA | |||
| ACADL | XM_006935526 | TGATTCCTCACCACGCAGAA | 85 |
| CCGAGAAGTCCTTGTTTGCC | |||
| GPX7 | NM_001101113 | CGACAGCAACAAGGAGATCG TGATTTCCTCCACCGACACA | 224 |
| PPARA | NM_001034036 | TTCCCTCTTTGTGGCTGCTA | 112 |
| TAGGTGGAGTTTGAGCACGT | |||
| TUBA4A | XM_045034511 | TGGAACATGGGATTCAGCCT | 152 |
| ATCAATCACAGTGGGCTCCA | |||
| CAT | NM_001035386 | CTGTGAACTGTCCCTACCGT | 159 |
| CAGAGAAGTGGGTCCTGTGT | |||
| CDKN2C | NM_001101054 | CTGCAATGAATGTGGGGAGG | 229 |
| TGAGACTGGCAAAGGGAGAG | |||
| β-ACTIN | NM_173979 | CAAGTACCCCATTGAGCACG | 159 |
| GTCATCTTCTCACGGTTGGC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, P.; Zhang, H.; Shahzad, M.; Kolachi, H.A.; Li, Y.; Sheng, H.; Zhang, X.; Wan, P.; Zhao, X. Supplementation of Forskolin and Linoleic Acid During IVC Improved the Developmental and Vitrification Efficiency of Bovine Embryos. Int. J. Mol. Sci. 2025, 26, 4151. https://doi.org/10.3390/ijms26094151
Zhang P, Zhang H, Shahzad M, Kolachi HA, Li Y, Sheng H, Zhang X, Wan P, Zhao X. Supplementation of Forskolin and Linoleic Acid During IVC Improved the Developmental and Vitrification Efficiency of Bovine Embryos. International Journal of Molecular Sciences. 2025; 26(9):4151. https://doi.org/10.3390/ijms26094151
Chicago/Turabian StyleZhang, Peipei, Hang Zhang, Muhammad Shahzad, Hubdar Ali Kolachi, Yupeng Li, Hui Sheng, Xiaosheng Zhang, Pengcheng Wan, and Xueming Zhao. 2025. "Supplementation of Forskolin and Linoleic Acid During IVC Improved the Developmental and Vitrification Efficiency of Bovine Embryos" International Journal of Molecular Sciences 26, no. 9: 4151. https://doi.org/10.3390/ijms26094151
APA StyleZhang, P., Zhang, H., Shahzad, M., Kolachi, H. A., Li, Y., Sheng, H., Zhang, X., Wan, P., & Zhao, X. (2025). Supplementation of Forskolin and Linoleic Acid During IVC Improved the Developmental and Vitrification Efficiency of Bovine Embryos. International Journal of Molecular Sciences, 26(9), 4151. https://doi.org/10.3390/ijms26094151






