Tryptophan-Derived Metabolites and Glutamate Dynamics in Fatal Insulin Poisoning: Mendelian Randomization of Human Cohorts and Experimental Validation in Rat Models
Abstract
1. Background
2. Results
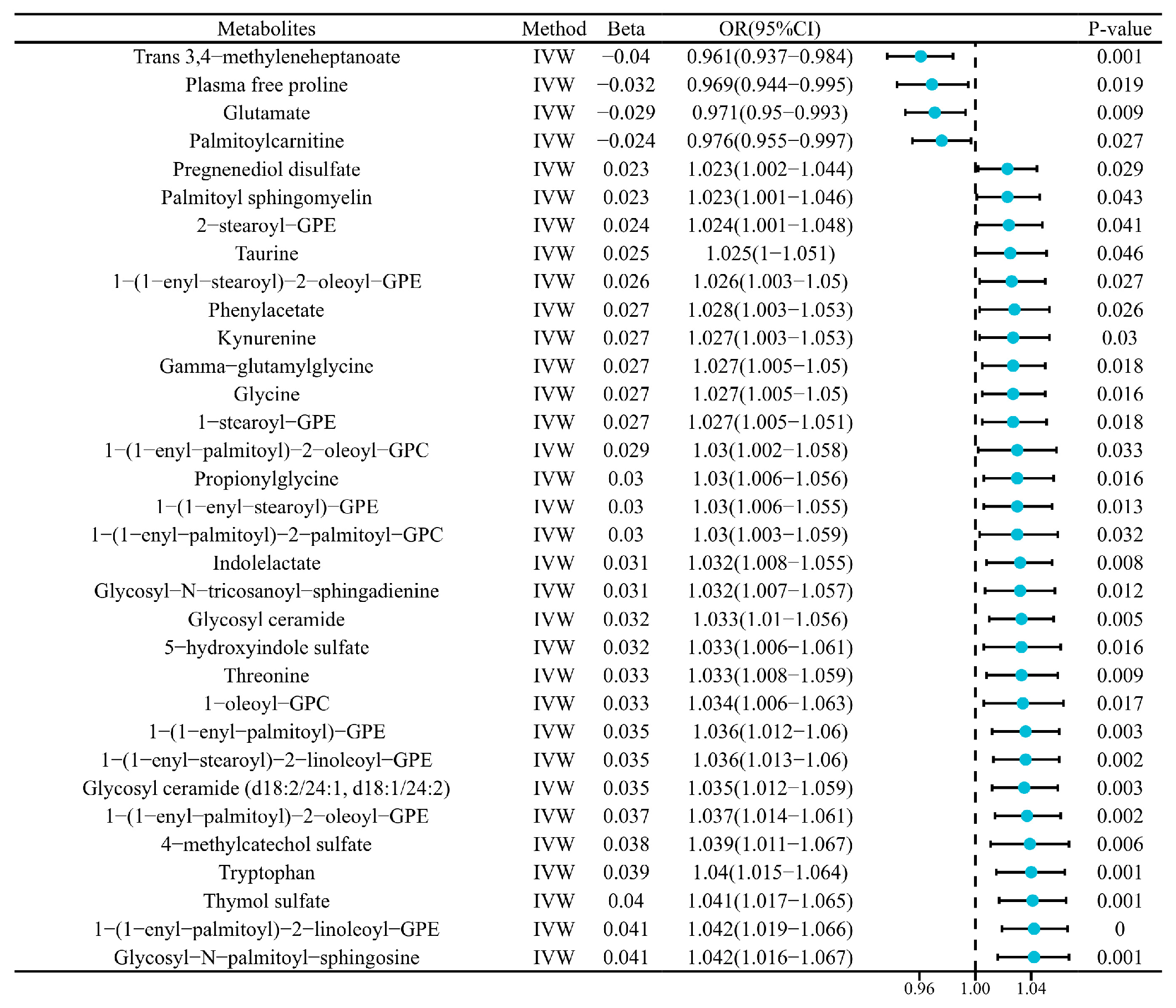
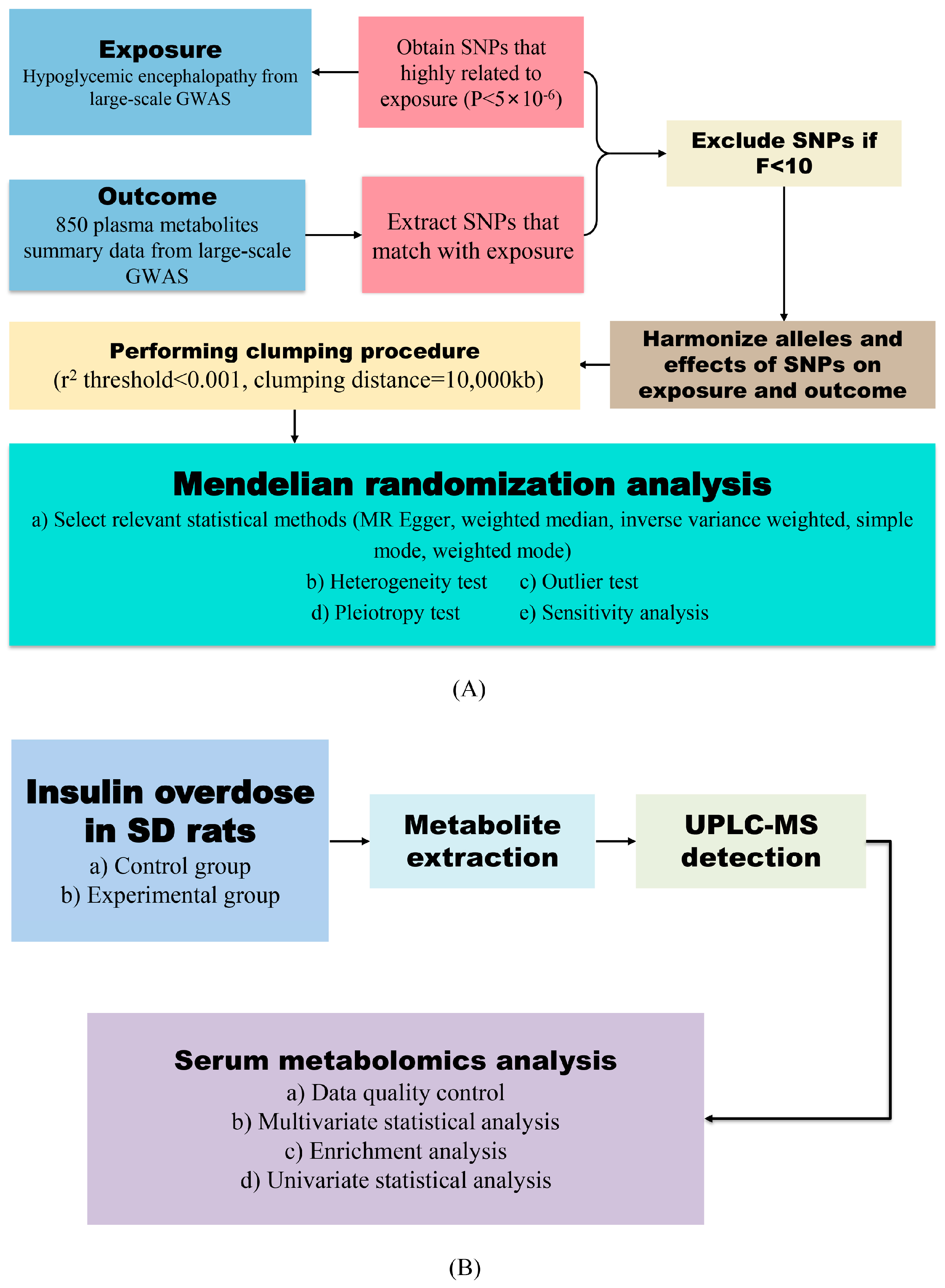
2.1. Causal Links Between Hypoglycemic Encephalopathy and Plasma Metabolites
2.2. Sensitivity Validation
2.3. General Condition of Hypoglycemic Encephalopathy Rats
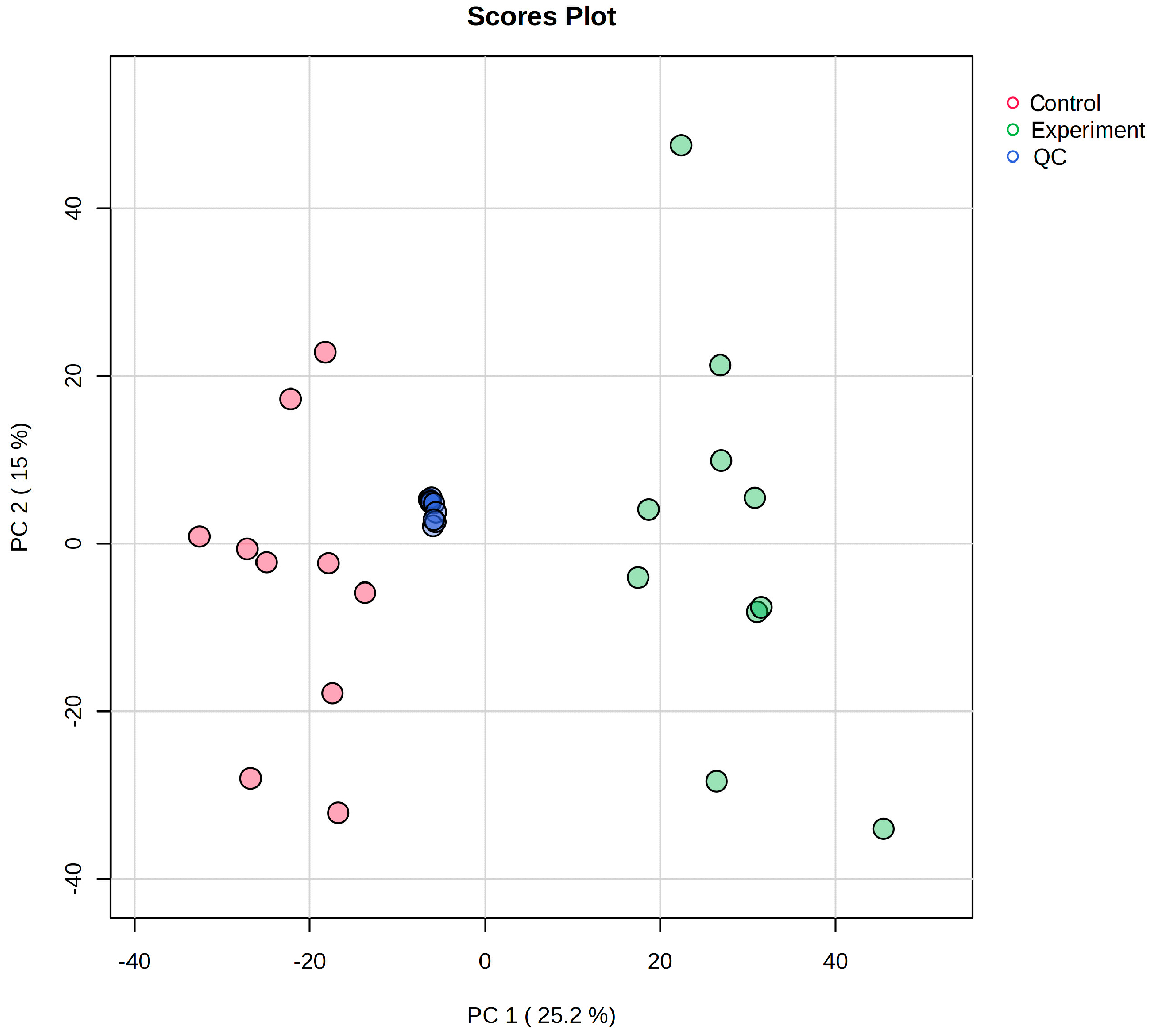
2.4. Data Quality Control
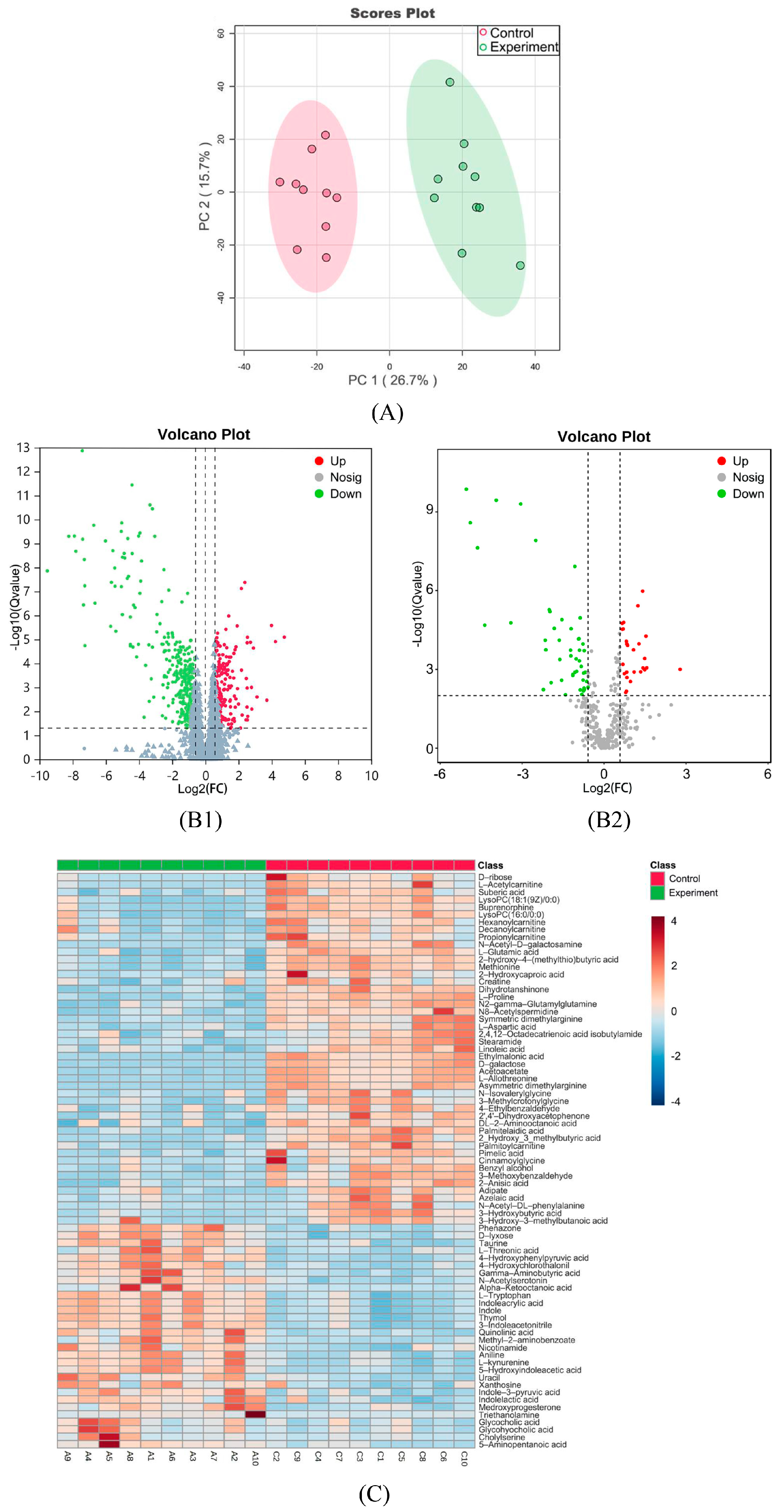
2.5. Multivariate Statistical Analysis
2.6. Univariate Statistical Analysis
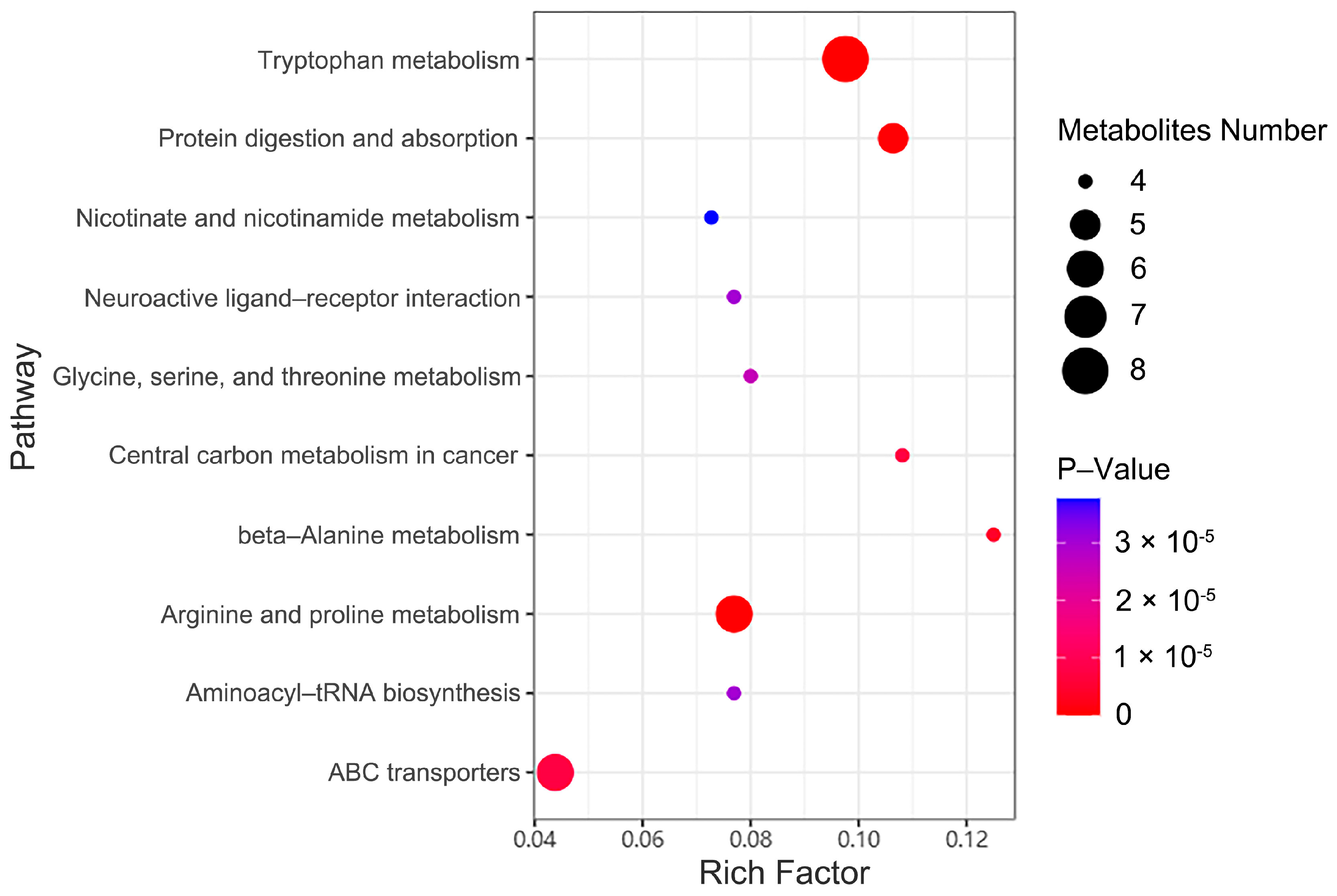
2.7. Metabolic Pathway Enrichment Analysis
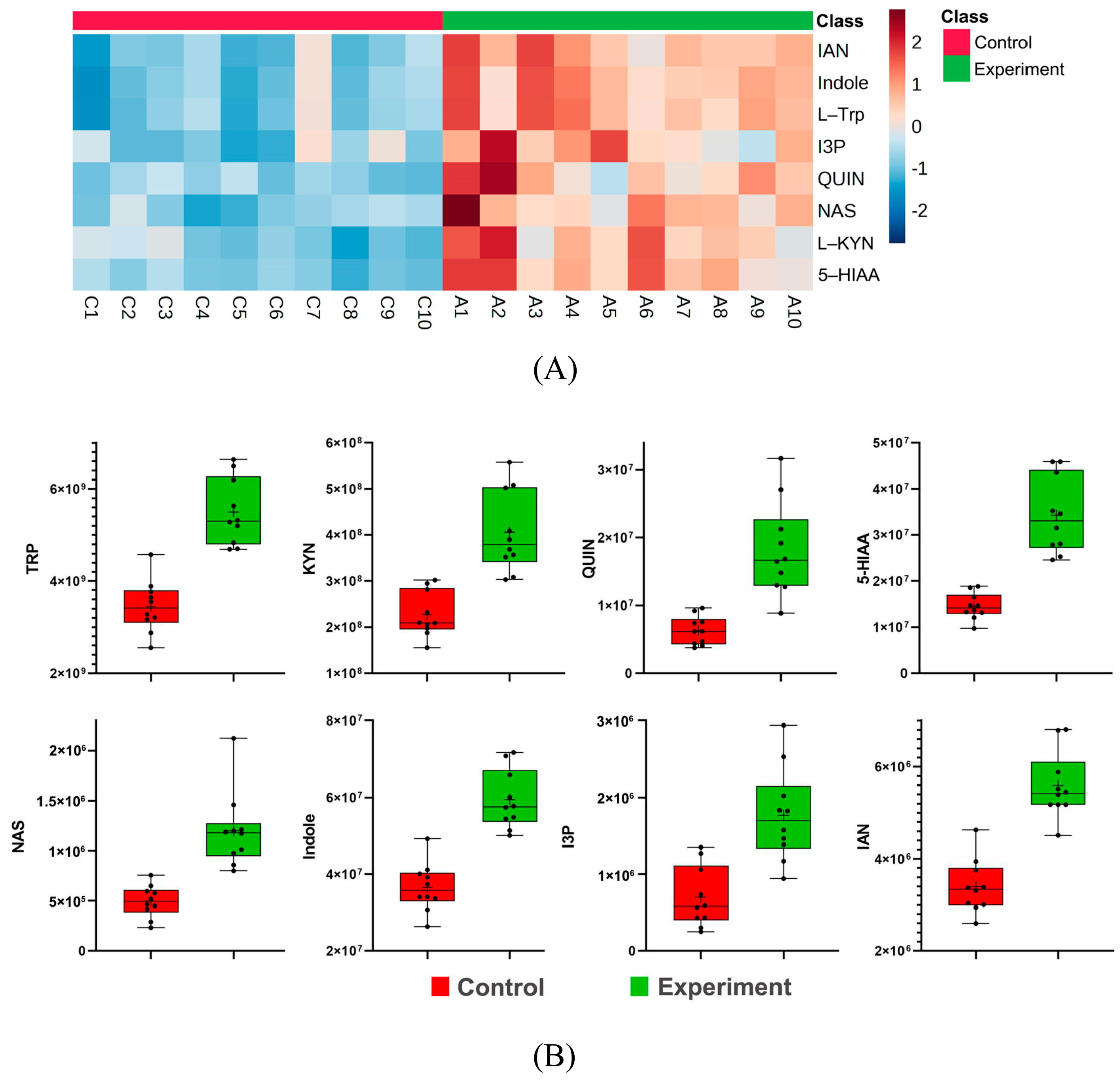
2.8. Tryptophan Metabolic Pathway Analysis
3. Discussion
4. Materials and Methods
4.1. GWAS Data from Human Plasma Metabolites
4.2. GWAS Data for Hypoglycemic Encephalopathy
4.3. Selection of Instrumental Variables
4.4. Statistical Methods
4.5. Experimental Animals and Grouping
4.6. Establishment of Insulin Overdose-Induced Hypoglycemic Encephalopathy Rat Model
4.7. Serum Sample Collection and Pretreatment
4.8. UPLC-MS/MS Analysis
4.9. Data Processing and Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Time (min) | Flow Velocity (mL/min) | A (%) | B (%) |
|---|---|---|---|
| 0 | 0.35 | 98.0 | 2.0 |
| 1 | 0.35 | 98.0 | 2.0 |
| 9 | 0.35 | 2.0 | 98.0 |
| 12 | 0.35 | 2.0 | 98.0 |
| 12.1 | 0.35 | 98.0 | 2.0 |
| 15 | 0.35 | 98.0 | 2.0 |
Appendix B
| Description | Parameter | |
|---|---|---|
| Scan range (m/z) | 70–1050 | |
| MS1 resolution | 120,000 | |
| AGC | 3 × 106 | |
| Maximum injection time (ms) | 100 | |
| MS2 resolution | 30,000 | |
| AGC | 1 × 105 | |
| Maximum injection time (ms) | 50 | |
| Collision energy (CE, eV) | 20, 40, 60 | |
| Sheath gas velocity (Arb) | 40 | |
| Auxiliary gas flow rate (Arb) | 10 | |
| Spray voltage (kV) | Positive ion mode | 3.80 |
| Spray voltage (kV) | Negative ion mode | 3.20 |
| Temperature of ion transport tube (°C) | 320 | |
| Auxiliary heating temperature (°C) | 350 | |
Appendix C
| Metabolite Classification | Metabolite Name | Trend |
|---|---|---|
| Amino acids and their derivatives | 2_Hydroxy_3_methylbutyric acid | Down |
| 3-Hydroxy-3-methylbutanoic acid | Down | |
| 3-Methylcrotonylglycine | Down | |
| Asymmetric dimethylarginine | Down | |
| Creatine | Down | |
| DL-2-Aminooctanoic acid | Down | |
| L-Allothreonine | Down | |
| L-Aspartic acid | Down | |
| L-Glutamic acid | Down | |
| L-Proline | Down | |
| Methionine | Down | |
| N2-gamma-Glutamylglutamine | Down | |
| N-Acetyl-DL-phenylalanine | Down | |
| N-Isovalerylglycine | Down | |
| Symmetric dimethylarginine | Down | |
| 3-Indoleacetonitrile | Up | |
| 4-Hydroxyphenylpyruvic acid | Up | |
| 5-Aminopentanoic acid | Up | |
| 5-Hydroxyindoleacetic acid | Up | |
| Alpha-Ketooctanoic acid | Up | |
| Gamma-Aminobutyric acid | Up | |
| Indole | Up | |
| Indole-3-pyruvic acid | Up | |
| Indoleacrylic acid | Up | |
| Indolelactic acid | Up | |
| L-kynurenine | Up | |
| L-Threonic acid | Up | |
| L-Tryptophan | Up | |
| N-Acetylserotonin | Up | |
| Quinolinic acid | Up | |
| Taurine | Up | |
| Fatty acids | 2,4,12-Octadecatrienoic acid isobutylamide | Down |
| 2-hydroxy-4-(methylthio)butyric acid | Down | |
| 2-Hydroxycaproic acid | Down | |
| Adipate | Down | |
| Decanoylcarnitine | Down | |
| Ethylmalonic acid | Down | |
| Hexanoylcarnitine | Down | |
| L-Acetylcarnitine | Down | |
| Linoleic acid | Down | |
| Palmitelaidic acid | Down | |
| Palmitoylcarnitine | Down | |
| Pimelic acid | Down | |
| Propionylcarnitine | Down | |
| Benzene and its derivatives | 3-Methoxybenzaldehyde | Down |
| 4-Ethylbenzaldehyde | Down | |
| Benzyl alcohol | Down | |
| Carbohydrate | D-galactose | Down |
| D-ribose | Down | |
| N-Acetyl-D-galactosamine | Down | |
| D-lyxose | Up | |
| Amine | N8-Acetylspermidine | Down |
| Nicotinamide | Up | |
| Stearamide | Down | |
| Triethanolamine | Up | |
| Bile acids and their derivatives | Cholylserine | Up |
| Glycocholic acid | Up | |
| Glycohyocholic acid | Up | |
| Ketones | 3-Hydroxybutyric acid | Down |
| Acetoacetate | Down | |
| Glycerophospholipids | LysoPC (16:0/0:0) | Down |
| LysoPC (18:1(9Z)/0:0) | Down | |
| Other metabolites | Uracil | Up |
| Xanthosine | Up |
References
- Norton, L.; Shannon, C.; Gastaldelli, A.; DeFronzo, R.A. Insulin: The master regulator of glucose metabolism. Metabolism 2022, 129, 155142. [Google Scholar] [CrossRef] [PubMed]
- Sims, E.K.; Carr, A.; Oram, R.A.; DiMeglio, L.A.; Evans-Molina, C. 100 years of insulin: Celebrating the past, present and future of diabetes therapy. Nat. Med. 2021, 27, 1154–1164. [Google Scholar] [CrossRef] [PubMed]
- Klein-Schwartz, W.; Stassinos, G.L.; Isbister, G.K. Treatment of sulfonylurea and insulin overdose. Br. J. Clin. Pharmacol. 2016, 81, 496–504. [Google Scholar] [CrossRef] [PubMed]
- Geller, A.I.; Shehab, N.; Lovegrove, M.C.; Kegler, S.R.; Weidenbach, K.N.; Ryan, G.J.; Budnitz, D.S. National estimates of insulin-related hypoglycemia and errors leading to emergency department visits and hospitalizations. JAMA Intern. Med. 2014, 174, 678–686. [Google Scholar] [CrossRef]
- Skrivarhaug, T.; Bangstad, H.J.; Stene, L.C.; Sandvik, L.; Hanssen, K.F.; Joner, G. Long-term mortality in a nationwide cohort of childhood-onset type 1 diabetic patients in Norway. Diabetologia 2006, 49, 298–305. [Google Scholar] [CrossRef]
- Yu, Z.; Yuan, Y.; Zhang, J.; Li, Y.; Wang, Z.; Wang, Y.; Duan, Y.; Zhou, Y. Review of the lethal mechanism of insulin poisoning and the characteristic of forensic identification. Leg. Med. 2024, 70, 102478. [Google Scholar] [CrossRef]
- Yuan, Y.; Yu, Z.; Tong, F.; Zhao, S.; Li, Y.; Shi, Q.; Zhou, Y. A retrospective study of 29 fatal cases of insulin overdose. Forensic Sci. Int. 2024, 361, 112126. [Google Scholar] [CrossRef]
- Li, Y.; Yu, Z.; Yuan, Y.; Zhang, J.; Zhao, S.; Duan, Y.; Zhou, Y. Two cases of fatal insulin homicide resolved using combined immunohistochemistry and immunofluorescence techniques. Forensic Sci. Med. Pathol. 2024. [Google Scholar] [CrossRef]
- Bottinelli, C.; Cartiser, N.; Bevalot, F.; Fanton, L.; Guitton, J. Is insulin intoxication still the perfect crime? Analysis and interpretation of postmortem insulin: Review and perspectives in forensic toxicology. Crit. Rev. Toxicol. 2020, 50, 324–347. [Google Scholar] [CrossRef]
- Rzepczyk, S.; Dolinska-Kaczmarek, K.; Uruska, A.; Zaba, C. The Other Face of Insulin-Overdose and Its Effects. Toxics 2022, 10, 123. [Google Scholar] [CrossRef]
- Tong, F.; Wu, R.; Huang, W.; Yang, Y.; Zhang, L.; Zhang, B.; Chen, X.; Tang, X.; Zhou, Y. Forensic aspects of homicides by insulin overdose. Forensic Sci. Int. 2017, 278, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Liu, Z.; Ma, L.; Yin, M.; Zhou, Y. Potential biomarkers in hypoglycemic brain injury. Forensic Sci. Med. Pathol. 2023, 20, 810–822. [Google Scholar] [CrossRef] [PubMed]
- Languren, G.; Montiel, T.; Julio-Amilpas, A.; Massieu, L. Neuronal damage and cognitive impairment associated with hypoglycemia: An integrated view. Neurochem. Int. 2013, 63, 331–343. [Google Scholar] [CrossRef]
- Auer, R.N. Hypoglycemic brain damage. Forensic Sci. Int. 2004, 146, 105–110. [Google Scholar] [CrossRef]
- Rehni, A.K.; Dave, K.R. Impact of Hypoglycemia on Brain Metabolism During Diabetes. Mol. Neurobiol. 2018, 55, 9075–9088. [Google Scholar] [CrossRef]
- Modoux, M.; Rolhion, N.; Mani, S.; Sokol, H. Tryptophan Metabolism as a Pharmacological Target. Trends Pharmacol. Sci. 2021, 42, 60–73. [Google Scholar] [CrossRef]
- Correia, A.S.; Vale, N. Tryptophan Metabolism in Depression: A Narrative Review with a Focus on Serotonin and Kynurenine Pathways. Int. J. Mol. Sci. 2022, 23, 8493. [Google Scholar] [CrossRef]
- Davidson, M.; Rashidi, N.; Nurgali, K.; Apostolopoulos, V. The Role of Tryptophan Metabolites in Neuropsychiatric Disorders. Int. J. Mol. Sci. 2022, 23, 9986. [Google Scholar] [CrossRef]
- Obara-Michlewska, M. The tryptophan metabolism, kynurenine pathway and oxidative stress—implications for glioma pathobiology. Neurochem. Int. 2022, 158, 105363. [Google Scholar] [CrossRef]
- Wang, S.; van Schooten, F.J.; Jin, H.; Jonkers, D.; Godschalk, R. The Involvement of Intestinal Tryptophan Metabolism in Inflammatory Bowel Disease Identified by a Meta-Analysis of the Transcriptome and a Systematic Review of the Metabolome. Nutrients 2023, 15, 2886. [Google Scholar] [CrossRef]
- Melhem, N.J.; Taleb, S. Tryptophan: From Diet to Cardiovascular Diseases. Int. J. Mol. Sci. 2021, 22, 9904. [Google Scholar] [CrossRef] [PubMed]
- Agus, A.; Planchais, J.; Sokol, H. Gut Microbiota Regulation of Tryptophan Metabolism in Health and Disease. Cell Host Microbe 2018, 23, 716–724. [Google Scholar] [CrossRef] [PubMed]
- Badawy, A.A. Kynurenine Pathway of Tryptophan Metabolism: Regulatory and Functional Aspects. Int. J. Tryptophan Res. 2017, 10, 1178646917691938. [Google Scholar] [CrossRef] [PubMed]
- Teunis, C.; Nieuwdorp, M.; Hanssen, N. Interactions between Tryptophan Metabolism, the Gut Microbiome and the Immune System as Potential Drivers of Non-Alcoholic Fatty Liver Disease (NAFLD) and Metabolic Diseases. Metabolites 2022, 12, 514. [Google Scholar] [CrossRef]
- Schwarcz, R.; Bruno, J.P.; Muchowski, P.J.; Wu, H.Q. Kynurenines in the mammalian brain: When physiology meets pathology. Nat. Rev. Neurosci. 2012, 13, 465–477. [Google Scholar] [CrossRef]
- Lapin, I.P. Stimulant and convulsive effects of kynurenines injected into brain ventricles in mice. J. Neural Transm. 1978, 42, 37–43. [Google Scholar] [CrossRef]
- Heyes, M.P.; Papagapiou, M.; Leonard, C.; Markey, S.P.; Auer, R.N. Brain and plasma quinolinic acid in profound insulin-induced hypoglycemia. J. Neurochem. 1990, 54, 1027–1033. [Google Scholar] [CrossRef]
- Chini, C.; Zeidler, J.D.; Kashyap, S.; Warner, G.; Chini, E.N. Evolving concepts in NAD+ metabolism. Cell Metab. 2021, 33, 1076–1087. [Google Scholar] [CrossRef]
- Wang, X.; Hu, X.; Zhang, L.; Xu, X.; Sakurai, T. Nicotinamide mononucleotide administration after sever hypoglycemia improves neuronal survival and cognitive function in rats. Brain Res. Bull. 2020, 160, 98–106. [Google Scholar] [CrossRef]
- Lenchner, J.R.; Santos, C. Biochemistry, 5 Hydroxyindoleacetic Acid; StatPearls: Tampa, FL, USA, 2025. [Google Scholar]
- Kang, J.H.; Guo, X.D.; Wang, Y.D.; Kang, X.W. Neuroprotective Effects of N-acetylserotonin and Its Derivative. Neuroscience 2023, 517, 18–25. [Google Scholar] [CrossRef]
- Lamas, B.; Richard, M.L.; Leducq, V.; Pham, H.P.; Michel, M.L.; Da, C.G.; Bridonneau, C.; Jegou, S.; Hoffmann, T.W.; Natividad, J.M.; et al. CARD9 impacts colitis by altering gut microbiota metabolism of tryptophan into aryl hydrocarbon receptor ligands. Nat. Med. 2016, 22, 598–605. [Google Scholar] [CrossRef] [PubMed]
- Wlodarska, M.; Luo, C.; Kolde, R.; D’Hennezel, E.; Annand, J.W.; Heim, C.E.; Krastel, P.; Schmitt, E.K.; Omar, A.S.; Creasey, E.A.; et al. Indoleacrylic Acid Produced by Commensal Peptostreptococcus Species Suppresses Inflammation. Cell Host Microbe 2017, 22, 25–37.e6. [Google Scholar] [CrossRef] [PubMed]
- Jennis, M.; Cavanaugh, C.R.; Leo, G.C.; Mabus, J.R.; Lenhard, J.; Hornby, P.J. Microbiota-derived tryptophan indoles increase after gastric bypass surgery and reduce intestinal permeability in vitro and in vivo. Neurogastroenterol. Motil. 2018, 30, e13178. [Google Scholar] [CrossRef] [PubMed]
- Suh, S.W.; Hamby, A.M.; Swanson, R.A. Hypoglycemia, brain energetics, and hypoglycemic neuronal death. Glia 2007, 55, 1280–1286. [Google Scholar] [CrossRef]
- Nellgard, B.; Wieloch, T. Cerebral protection by AMPA- and NMDA-receptor antagonists administered after severe insulin-induced hypoglycemia. Exp. Brain Res. 1992, 92, 259–266. [Google Scholar] [CrossRef]
- Vergun, O.; Han, Y.Y.; Reynolds, I.J. Glucose deprivation produces a prolonged increase in sensitivity to glutamate in cultured rat cortical neurons. Exp. Neurol. 2003, 183, 682–694. [Google Scholar] [CrossRef]
- Cardoso, S.; Carvalho, C.; Santos, R.; Correia, S.; Santos, M.S.; Seica, R.; Oliveira, C.R.; Moreira, P.I. Impact of STZ-induced hyperglycemia and insulin-induced hypoglycemia in plasma amino acids and cortical synaptosomal neurotransmitters. Synapse 2011, 65, 457–466. [Google Scholar] [CrossRef]
- Gundersen, V.; Fonnum, F.; Ottersen, O.P.; Storm-Mathisen, J. Redistribution of neuroactive amino acids in hippocampus and striatum during hypoglycemia: A quantitative immunogold study. J. Cereb. Blood Flow Metab. 2001, 21, 41–51. [Google Scholar] [CrossRef]
- Chen, Y.; Lu, T.; Pettersson-Kymmer, U.; Stewart, I.D.; Butler-Laporte, G.; Nakanishi, T.; Cerani, A.; Liang, K.; Yoshiji, S.; Willett, J.; et al. Genomic atlas of the plasma metabolome prioritizes metabolites implicated in human diseases. Nat. Genet. 2023, 55, 44–53. [Google Scholar] [CrossRef]
- Jiang, L.; Zheng, Z.; Fang, H.; Yang, J. A generalized linear mixed model association tool for biobank-scale data. Nat. Genet. 2021, 53, 1616–1621. [Google Scholar] [CrossRef]
- Slob, E.; Burgess, S. A comparison of robust Mendelian randomization methods using summary data. Genet. Epidemiol. 2020, 44, 313–329. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Zhu, Q.; Zhang, L.; Pei, Y.; Xu, X.; Liu, X.; Lu, G.; Pan, J.; Wang, Y. Causal relationship between gut microbiota and serum vitamin D: Evidence from genetic correlation and Mendelian randomization study. Eur. J. Clin. Nutr. 2022, 76, 1017–1023. [Google Scholar] [CrossRef] [PubMed]
- Song, K.; Ma, J.; Wang, B. The Causal Relationship Between Genetically Determined Plasma Metabolites and Rheumatoid Arthritis. Int. J. Rheum. Dis. 2024, 27, e15447. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhu, H.; Pan, L.; Zhang, M.; Wan, X.; Xu, H.; Hua, R.; Zhu, M.; Gao, P. Systemic inflammatory regulators and risk of acute-on-chronic liver failure: A bidirectional mendelian-randomization study. Front. Cell Dev. Biol. 2023, 11, 1125233. [Google Scholar] [CrossRef]
- Sankar, A.; Khodai, T.; McNeilly, A.D.; McCrimmon, R.J.; Luckman, S.M. Experimental Models of Impaired Hypoglycaemia-Associated Counter-Regulation. Trends Endocrinol. Metab. 2020, 31, 691–703. [Google Scholar] [CrossRef]
- Schymanski, E.L.; Jeon, J.; Gulde, R.; Fenner, K.; Ruff, M.; Singer, H.P.; Hollender, J. Identifying small molecules via high resolution mass spectrometry: Communicating confidence. Environ. Sci. Technol. 2014, 48, 2097–2098. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, Y.; Liu, Y.; Wang, S.; Zhang, J.; Gao, X.; Li, Y.; Yu, Z.; Zhou, Y. Tryptophan-Derived Metabolites and Glutamate Dynamics in Fatal Insulin Poisoning: Mendelian Randomization of Human Cohorts and Experimental Validation in Rat Models. Int. J. Mol. Sci. 2025, 26, 4152. https://doi.org/10.3390/ijms26094152
Yuan Y, Liu Y, Wang S, Zhang J, Gao X, Li Y, Yu Z, Zhou Y. Tryptophan-Derived Metabolites and Glutamate Dynamics in Fatal Insulin Poisoning: Mendelian Randomization of Human Cohorts and Experimental Validation in Rat Models. International Journal of Molecular Sciences. 2025; 26(9):4152. https://doi.org/10.3390/ijms26094152
Chicago/Turabian StyleYuan, Yuhao, Yu Liu, Shengnan Wang, Jiaxin Zhang, Xiangting Gao, Yiling Li, Zhonghao Yu, and Yiwu Zhou. 2025. "Tryptophan-Derived Metabolites and Glutamate Dynamics in Fatal Insulin Poisoning: Mendelian Randomization of Human Cohorts and Experimental Validation in Rat Models" International Journal of Molecular Sciences 26, no. 9: 4152. https://doi.org/10.3390/ijms26094152
APA StyleYuan, Y., Liu, Y., Wang, S., Zhang, J., Gao, X., Li, Y., Yu, Z., & Zhou, Y. (2025). Tryptophan-Derived Metabolites and Glutamate Dynamics in Fatal Insulin Poisoning: Mendelian Randomization of Human Cohorts and Experimental Validation in Rat Models. International Journal of Molecular Sciences, 26(9), 4152. https://doi.org/10.3390/ijms26094152





