The Virulence of Metarhizium rileyi to Locusta migratoria Is Determined by the Ability of the Fungus to Respond to Carbon and Nitrogen Sources
Abstract
:1. Introduction
2. Results
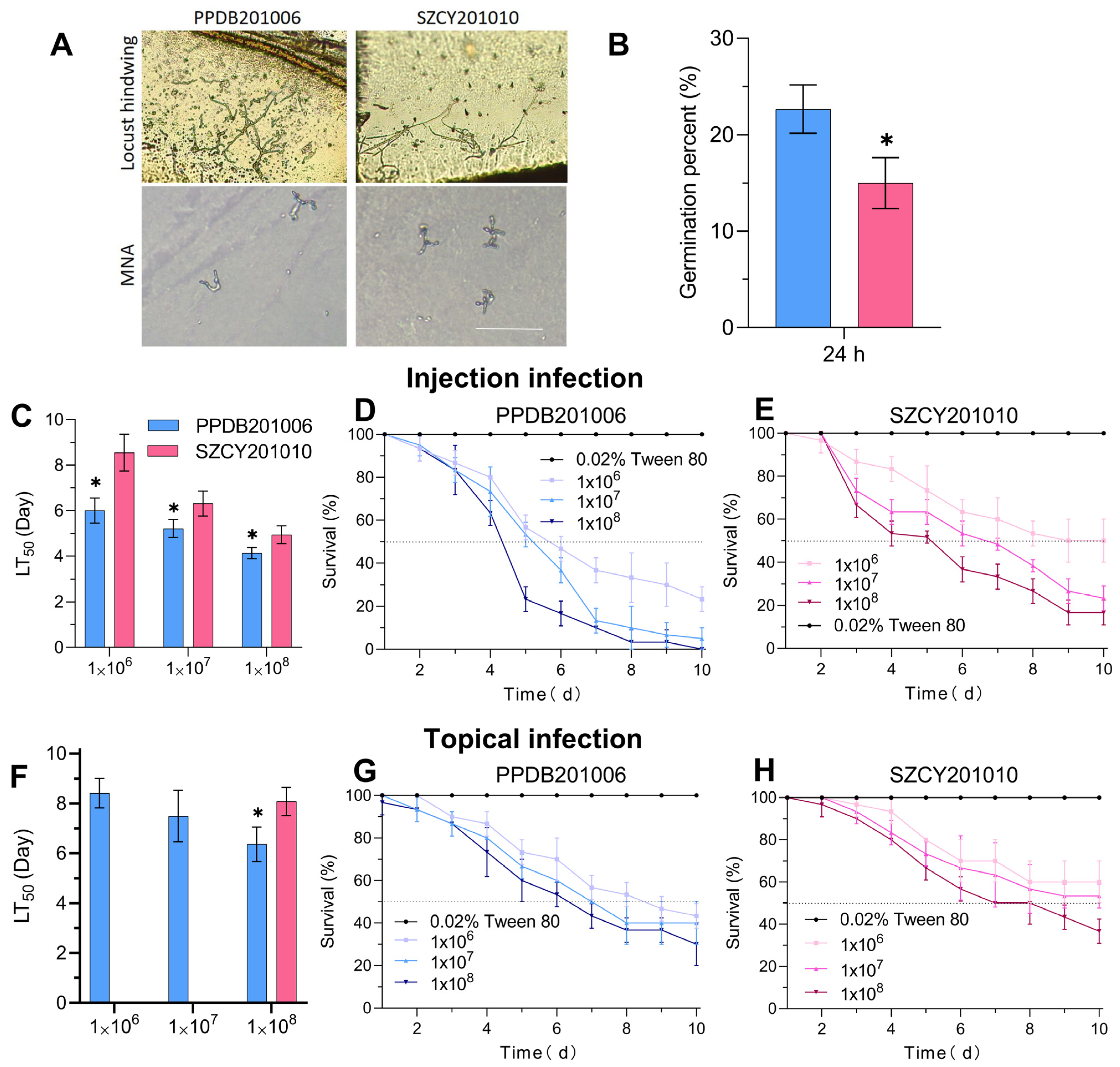
2.1. The Pathogenic Fungi PPDB201006 and SZCY201010 Were Identified as M. rileyi
2.2. Virulence of L. migratoria Infected by M. rileyi PPDB201006 and SZCY201010 by Different Methods
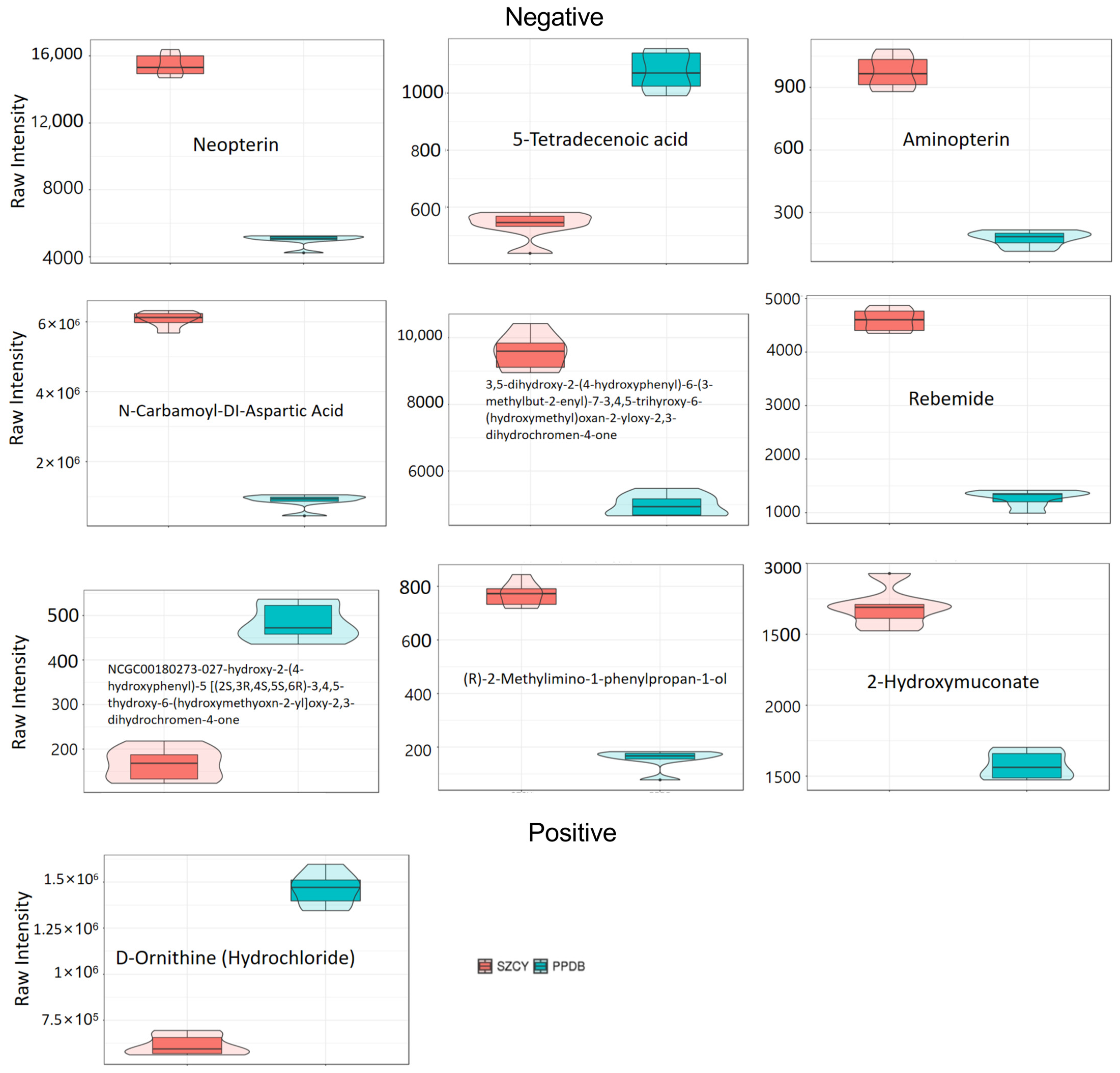
2.3. Effects of M. rileyi PPDB201006 and SZCY201010 on Metabolism of L. migratoria
2.4. Analysis of Differentially Expressed Metabolite Pathways and Enrichment Analysis
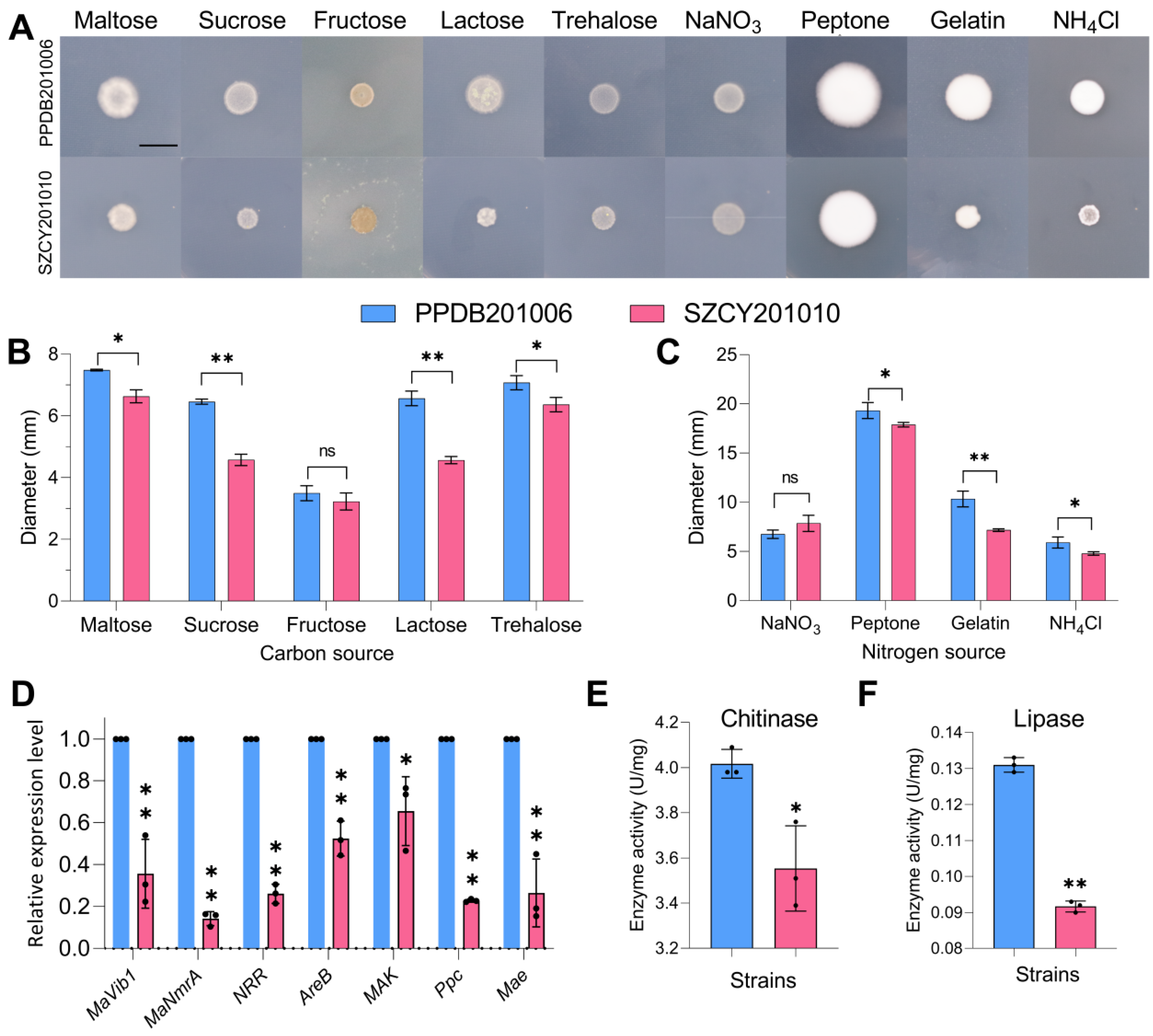
2.5. PPDB201006 and SZCY201010 Showed Significant Differences in Response to Carbon Sources and Nitrogen Sources
3. Discussion
4. Materials and Methods
4.1. Strains, Medium and Insect
4.2. Determination of Fungal Growth Phenotype
4.3. Phenotypic Determination of Conidial Germination
4.4. Virulence Test
4.5. Determining Chitinase and Lipase Activity in Fungi
4.6. Nontargeted Metabolomics Analysis of Hemolymph of L. migratoria
4.7. Fungal RNA Extraction and RT-qPCR
4.8. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xie, J.; Li, S.; Zhang, W.; Xia, Y. RNAi-knockdown of the Locusta migratoria nuclear export factor protein results in insect mortality and alterations in gut microbiome. Pest. Manag. Sci. 2019, 75, 1383–1390. [Google Scholar] [CrossRef]
- Zhang, L.; Lecoq, M.; Latchininsky, A.; Hunter, D. Locust and Grasshopper Management. Annu. Rev. Entomol. 2019, 64, 15–34. [Google Scholar] [CrossRef]
- Chapuis, M.P.; Loiseau, A.; Michalakis, Y.; Lecoq, M.; Franc, A.; Estoup, A. Outbreaks, gene flow and effective population size in the migratory locust, Locusta migratoria: A regional-scale comparative survey. Mol. Ecol. 2009, 18, 792–800. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Zhang, J.; Zhu, K.Y.; Xuan, T.; Liu, X.; Guo, Y.; Ma, E. Increased activity and reduced sensitivity of acetylcholinesterase associated with malathion resistance in a field population of the oriental migratory locust, Locusta migratoria manilensis (Meyen). Pestic. Biochem. Phys. 2008, 91, 32–38. [Google Scholar] [CrossRef]
- Abdelatti, Z.A.S.; Hartbauer, M. Plant oil mixtures as a novel botanical pesticide to control gregarious locusts. J. Pest. Sci. 2020, 93, 341–353. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, J.; Liu, J.; Li, Y.; Liu, X.; Wu, H.; Ma, E.; Zhang, J. Knockdown of NADPH-cytochrome P450 reductase increases the susceptibility to carbaryl in the migratory locust, Locusta migratoria. Chemosphere 2017, 188, 517–524. [Google Scholar] [CrossRef]
- Quesada-Moraga, E.; González-Mas, N.; Yousef-Yousef, M.; Garrido-Jurado, I.; Fernández-Bravo, M. Key role of environmental competence in successful use of entomopathogenic fungi in microbial pest control. J. Pest. Sci. 2024, 97, 1–15. [Google Scholar] [CrossRef]
- Furst, M.A.; McMahon, D.P.; Osborne, J.L.; Paxton, R.J.; Brown, M.J. Disease associations between honeybees and bumblebees as a threat to wild pollinators. Nature 2014, 506, 364–366. [Google Scholar] [CrossRef]
- Jiang, W.; Peng, Y.; Ye, J.; Wen, Y.; Liu, G.; Xie, J. Effects of the Entomopathogenic Fungus Metarhizium anisopliae on the Mortality and Immune Response of Locusta migratoria. Insects 2019, 11, 36. [Google Scholar] [CrossRef]
- Shah, P.A.; Pell, J.K. Entomopathogenic fungi as biological control agents. Appl. Microbiol. Biotechnol. 2003, 61, 413–423. [Google Scholar] [CrossRef]
- Zhu, S.; Feng, X.; Keyhani, N.O.; Liu, Y.; Jin, D.; Tong, S.; Pei, Y.; Fan, Y. Manipulation of host ecdysteroid hormone levels facilitates infection by the fungal insect pathogen, Metarhizium rileyi. Environ. Microbiol. 2021, 23, 5087–5101. [Google Scholar] [CrossRef] [PubMed]
- Holder, D.J.; Keyhani, N.O. Adhesion of the entomopathogenic fungus Beauveria (Cordyceps) bassiana to substrata. Appl. Environ. Microbiol. 2005, 71, 5260–5266. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.L.; Yang, K.H.; Wang, S.S.; Li, X.L.; Liu, J.; Yu, Y.X.; Liu, X.S. Infection of the entomopathogenic fungus Metarhizium rileyi suppresses cellular immunity and activates humoral antibacterial immunity of the host Spodoptera frugiperda. Pest. Manag. Sci. 2022, 78, 2828–2837. [Google Scholar] [CrossRef]
- Tokarev, Y.S.; Levchenko, M.V.; Naumov, A.M.; Senderskiy, I.V.; Lednev, G.R. Interactions of two insect pathogens, Paranosema locustae (Protista: Microsporidia) and Metarhizium acridum (Fungi: Hypocreales), during a mixed infection of Locusta migratoria (Insecta: Orthoptera) nymphs. J. Invertebr. Pathol. 2011, 106, 336–338. [Google Scholar] [CrossRef]
- Jia, H.; Camara, I.; Zhang, Z.; Gao, Y.; Yang, X.; Sangbaramou, R.; Zhen, C.; Shi, W.; Tan, S. Effect of ultraviolet radiation on Beauveria bassiana virulence and development of protective formulations. Arch. Microbiol. 2023, 205, 112. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.; Hu, X.; Tang, J.; Wang, L.; Liu, X.; Peng, Y.; Xia, Y.; Xie, J. The symbiont Acinetobacter baumannii enhances the insect host resistance to entomopathogenic fungus Metarhizium anisopliae. Commun. Biol. 2024, 7, 1184. [Google Scholar] [CrossRef]
- Huang, Z.; Wang, Q.; Li, Y.; Huang, P.; Liao, J.; Wang, J.; Li, H.; Cai, Y.; Wang, J.; Liu, X.; et al. A multilayered regulatory network mediated by protein phosphatase 4 controls carbon catabolite repression and de-repression in Magnaporthe oryzae. Commun. Biol. 2025, 8, 130. [Google Scholar] [CrossRef]
- Keller, N.P. Fungal secondary metabolism: Regulation, function and drug discovery. Nat. Rev. Microbiol. 2019, 17, 167–180. [Google Scholar] [CrossRef]
- Su, X.; Liu, H.; Xia, Y.; Cao, Y. Transcription Factor Mavib-1 Negatively Regulates Conidiation by Affecting Utilization of Carbon and Nitrogen Source in Metarhizium acridum. J. Fungi 2022, 8, 594. [Google Scholar] [CrossRef]
- Luo, Z.; Chen, Q.; Su, Y.; Hu, S.; Keyhani, N.O.; Wang, J.; Zhu, C.; Zhou, T.; Pan, Y.; Bidochka, M.J.; et al. The AreA Nitrogen Catabolite Repression Activator Balances Fungal Nutrient Utilization and Virulence in the Insect Fungal Pathogen Beauveria bassiana. J. Agric. Food Chem. 2023, 71, 646–659. [Google Scholar] [CrossRef]
- Wang, G.; Chen, B.; Zhang, X.; Du, G.; Han, G.; Liu, J.; Peng, Y. The basic leucine zipper domain (bZIP) transcription factorBbYap1 promotes evasion of host humoral immunity and regulates lipid homeostasis contributing to fungal virulence in Beauveria bassiana. Msphere 2024, 9, e00351-24. [Google Scholar] [CrossRef]
- Peng, Y.; Wang, J.; Lin, H.; Ding, J.; Feng, M.; Ying, S. HapX, an Indispensable bZIP Transcription Factor for Iron Acquisition, Regulates Infection Initiation by Orchestrating Conidial Oleic Acid Homeostasis and Cytomembrane Functionality in Mycopathogen Beauveria bassiana. Msystems 2020, 5, 10–1128. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Zhang, H.; Wang, G.; Feng, M.; Ying, S. MARVEL family proteins contribute to vegetative growth, development, and virulence of the insect fungal pathogen Beauveria bassiana. J. Invertebr. Pathol. 2024, 203, 108076. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, G.; Chen, B.; Peng, Y. The virulence contribution of the CFEM family genes of Beauveria bassiana is closely influenced by the external iron environment. Microbiol. Spectrum 2025, e03096-24. [Google Scholar] [CrossRef]
- Peng, Y.J.; Hou, J.; Zhang, H.; Lei, J.H.; Lin, H.Y.; Ding, J.L.; Feng, M.G.; Ying, S.H. Systematic contributions ofCFEM domain-containing proteins to iron acquisition are essential for interspecies interaction of the filamentous pathogenic fungus Beauveria bassiana. Environ. Microbiol. 2022, 24, 3693–3704. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Chen, B. Role of cell membrane homeostasis in the pathogenicity of pathogenic filamentous fungi. Virulence 2024, 15, 2299183. [Google Scholar] [CrossRef]
- Camara, I.; Yin, Y.; Cao, K.; Sangbaramou, R.; Cao, C.; Tan, S.; Shi, W.; Shi, W. Effect of antipyretic phenidone on the nodulation response and mortality of locust infected with fungus. Biol. Control 2022, 170, 104933. [Google Scholar] [CrossRef]
- Tan, S.Q.; Yin, Y.; Cao, K.L.; Zhao, X.X.; Wang, X.Y.; Zhang, Y.X.; Shi, W.P. Effects of a combined infection with Paranosema locustae and Beauveria bassiana on Locusta migratoria and its gut microflora. Insect Sci. 2021, 28, 347–354. [Google Scholar] [CrossRef]
- Bitsadze, N.; Jaronski, S.; Khasdan, V.; Abashidze, E.; Abashidze, M.; Latchininsky, A.; Samadashvili, D.; Sokhadze, I.; Rippa, M.; Ishaaya, I.; et al. Joint action of Beauveria bassiana and the insect growth regulators diflubenzuron and novaluron, on the migratory locust, Locusta migratoria. J. Pest. Sci. 2013, 86, 293–300. [Google Scholar] [CrossRef]
- Fernandez, J.; Wright, J.D.; Hartline, D.; Quispe, C.F.; Madayiputhiya, N.; Wilson, R.A. Principles of carbon catabolite repression in the rice blast fungus: Tps1, Nmr1-3, and a MATE-family pump regulate glucose metabolism during infection. Plos Genet. 2012, 8, e1002673. [Google Scholar] [CrossRef]
- Divon, H.H.; Ziv, C.; Davydov, O.; Yarden, O.; Fluhr, R. The global nitrogen regulator, FNR1, regulates fungal nutrition-genes and fitness during Fusarium oxysporum pathogenesis. Mol. Plant Pathol. 2006, 7, 485–497. [Google Scholar] [CrossRef] [PubMed]
- Jia, M.; Cao, G.; Li, Y.; Tu, X.; Wang, G.; Nong, X.; Whitman, D.W.; Zhang, Z. Biochemical basis of synergism between pathogenic fungus Metarhizium anisopliae and insecticide chlorantraniliprole in Locusta migratoria (Meyen). Sci. Rep. 2016, 6, 28424. [Google Scholar] [CrossRef]
- Yi, H.Y.; Chowdhury, M.; Huang, Y.D.; Yu, X.Q. Insect antimicrobial peptides and their applications. Appl. Microbiol. Biotechnol. 2014, 98, 5807–5822. [Google Scholar] [CrossRef]
- Nagata, S. Feeding modulation in insects through factors in the hemolymph. Biosci. Biotechnol. Biochem. 2019, 83, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Pedrini, N. Molecular interactions between entomopathogenic fungi (Hypocreales) and their insect host: Perspectives from stressful cuticle and hemolymph battlefields and the potential of dual RNA sequencing for future studies. Fungal Biol. 2018, 122, 538–545. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Yao, Y.; Pang, J.; Di, T.; Du, G.; Chen, B. Genetic Diversity and Virulence Variation of Metarhizium rileyi from Infected Spodoptera frugiperda in Corn Fields. Microorganisms 2024, 12, 264. [Google Scholar] [CrossRef]
- Pang, J.; Peng, Y.; Di, T.; Du, G.; Chen, B. Virulence of Metarhizium rileyi Is Determined by Its Growth and Antioxidant Stress and the Protective and Detoxifying Enzymes of Spodoptera frugiperda. Insects 2023, 14, 260. [Google Scholar] [CrossRef]
- Wang, G.; Xu, S.; Chen, L.; Zhan, T.; Zhang, X.; Liang, H.; Chen, B.; Peng, Y. Gut Microbial Diversity Reveals Differences in Pathogenicity between Metarhizium rileyi and Beauveria bassiana during the Early Stage of Infection in Spodoptera litura Larvae. Microorganisms 2024, 12, 1129. [Google Scholar] [CrossRef]



| Primer Name | Primer Sequence (5′-3′) | Accession Number | Purpose |
|---|---|---|---|
| MrVib1 | F: TTCTTATCGGAGATGCCCGC R: TCGTTGGCCTCAGTAACCAC | OAA45487.1 | RT-qPCR |
| MrNmrA | F: GAAGTTCCAGCACCTGGCTA R: CCGTTGGACATGCACTTGGA | TWU77448.1 | RT-qPCR |
| MrNRR | F: CAGCAACTGCCAAACCAACA R: TCGGGCGGTTATGTCTGATG | OAA42774.1 | RT-qPCR |
| MrAreB | F: AGAGTCGCAACCGGGTAAAG R: TCGACACCAGTTACGTCAGC | OAA47622.1 | RT-qPCR |
| MrMAK | F: GCTTCGGCAGTGTTGTTCTG R: TGATGGCCACAACTGAACCG | OAA44248.1 | RT-qPCR |
| MrPpc | F: AGAACGTTGTCTTCGACCCC R: CATCGGGTGTTCTCGGTCAA | OAA50610.1 | RT-qPCR |
| MrMae | F: ATGGTCGAGGTCCATCCTCA R: ACGTTGTCTGAAGTCGTCCC | OAA47740.1 | RT-qPCR |
| ACTIN | F: CTTTTAATCGGCGCACGGAG R: CGAAGCTTGGCGCTATTGTC | OAA40333.1 | RT-qPCR |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yao, Y.; Li, M.; Liu, Q.; Huang, Q.; Yang, S.; Chen, B.; Peng, Y. The Virulence of Metarhizium rileyi to Locusta migratoria Is Determined by the Ability of the Fungus to Respond to Carbon and Nitrogen Sources. Int. J. Mol. Sci. 2025, 26, 4156. https://doi.org/10.3390/ijms26094156
Yao Y, Li M, Liu Q, Huang Q, Yang S, Chen B, Peng Y. The Virulence of Metarhizium rileyi to Locusta migratoria Is Determined by the Ability of the Fungus to Respond to Carbon and Nitrogen Sources. International Journal of Molecular Sciences. 2025; 26(9):4156. https://doi.org/10.3390/ijms26094156
Chicago/Turabian StyleYao, Yunhao, Mei Li, Qingqing Liu, Qiuyue Huang, Shuo Yang, Bin Chen, and Yuejin Peng. 2025. "The Virulence of Metarhizium rileyi to Locusta migratoria Is Determined by the Ability of the Fungus to Respond to Carbon and Nitrogen Sources" International Journal of Molecular Sciences 26, no. 9: 4156. https://doi.org/10.3390/ijms26094156
APA StyleYao, Y., Li, M., Liu, Q., Huang, Q., Yang, S., Chen, B., & Peng, Y. (2025). The Virulence of Metarhizium rileyi to Locusta migratoria Is Determined by the Ability of the Fungus to Respond to Carbon and Nitrogen Sources. International Journal of Molecular Sciences, 26(9), 4156. https://doi.org/10.3390/ijms26094156







