StarD5 Plays a Critical Role in the Hepatocyte ER Stress Survival Response
Abstract
:1. Introduction
2. Results
2.1. Tunicamycin Injection Induces StarD5 Expression in Livers
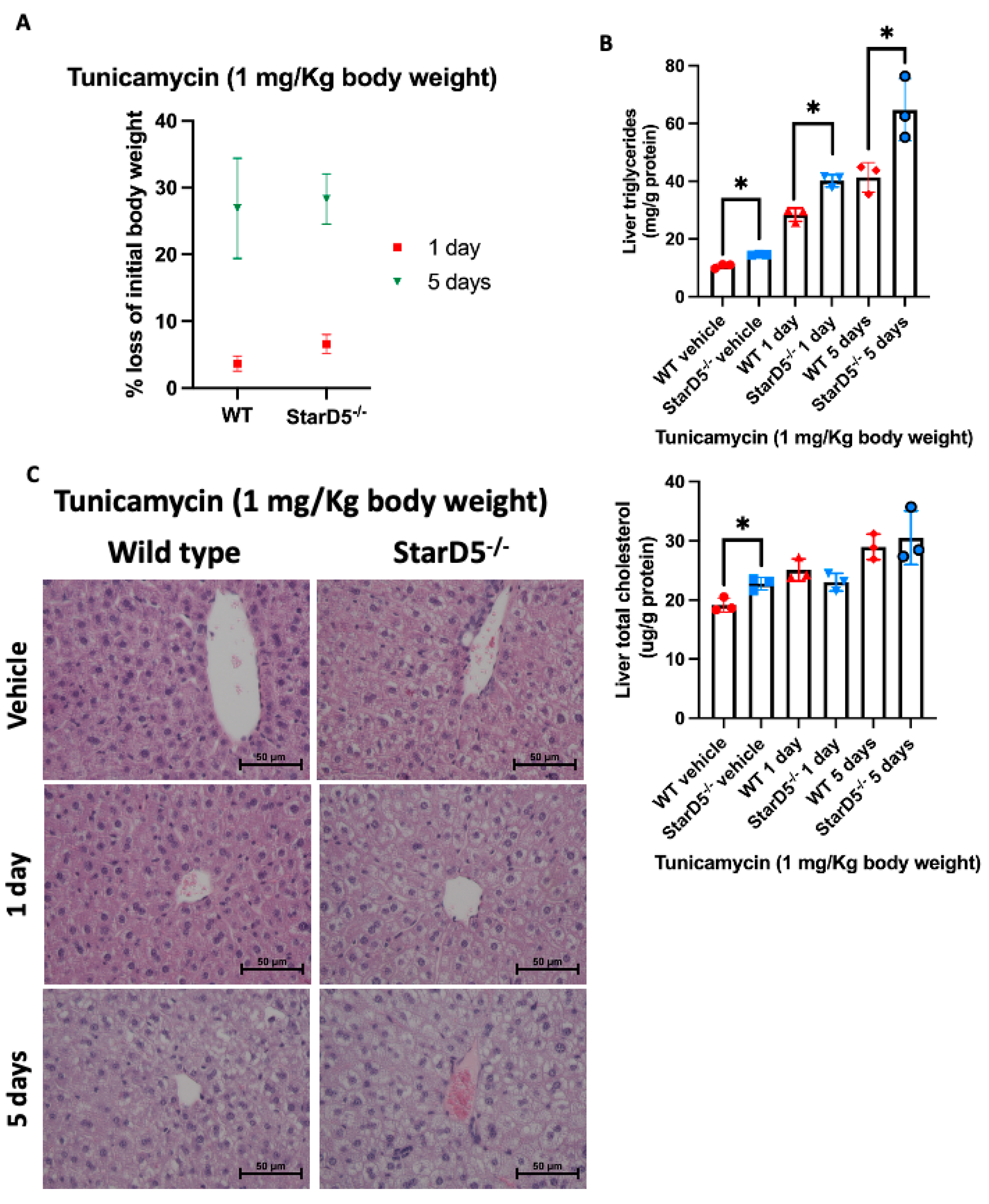
2.2. Tunicamycin-Induced ER Stress In Vivo Leads to Weight Loss While Increasing Lipid Accumulation in Livers
2.3. Tunicamycin-Induced ER Stress In Vivo Leads to an Increase in Apoptosis in Livers of StarD5−/− Mice Compared to Wild Type Livers
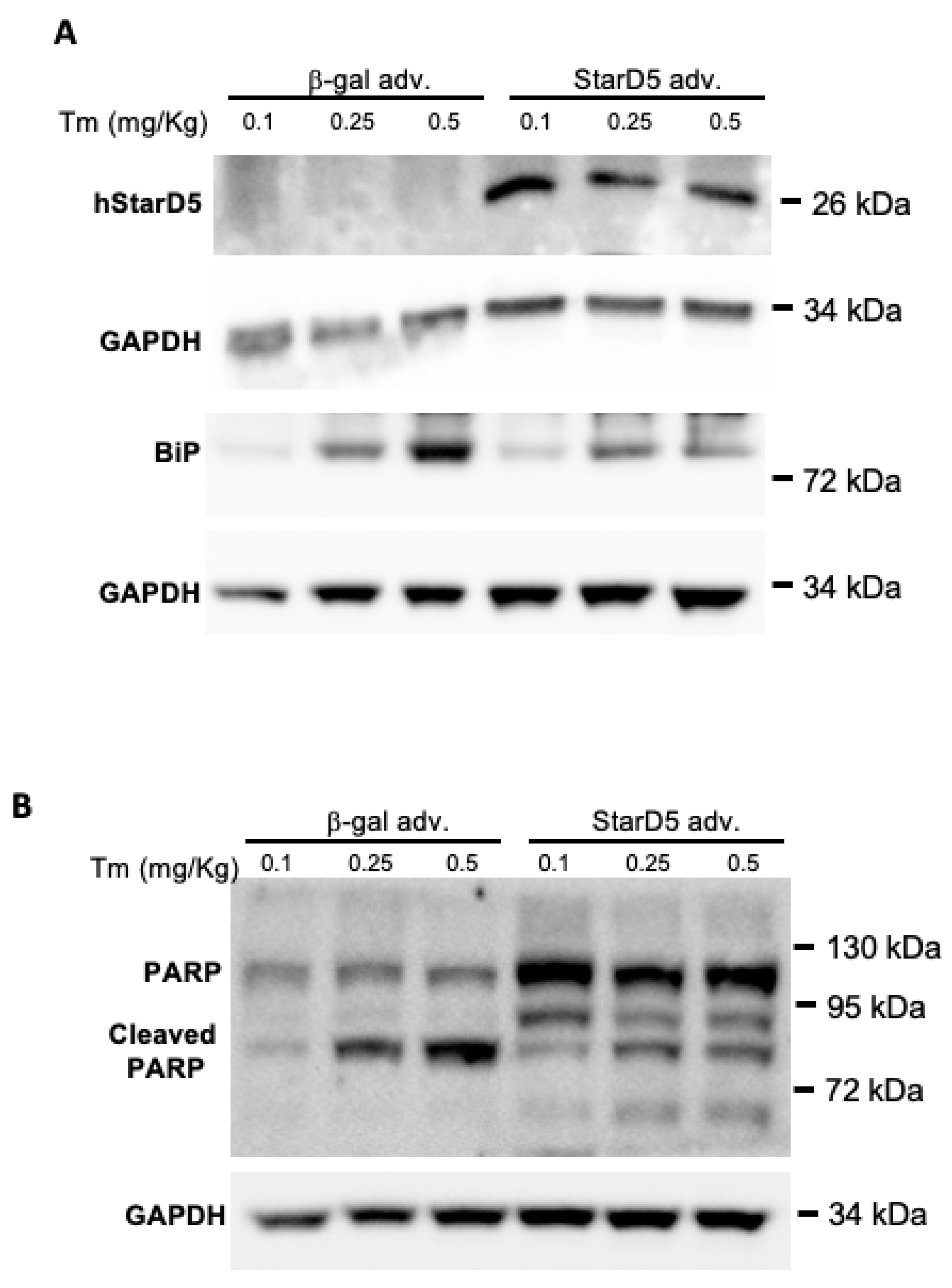
2.4. Restoring Expression of StarD5 Reduces ER Stress and Apoptosis in StarD5−/− Mice
3. Discussion
4. Materials and Methods
4.1. Animal Studies
4.2. Generation of StarD5 Knockout (StarD5−/−) Mice, Preparation and Injection of Tunicamycin Solution
4.3. Histological Analysis
4.4. Cholesterol and Triglyceride Quantification
4.5. Immunoblots
4.6. Data Reproducibility and Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ATF6 | activating transcription factor 6 |
| ER | endoplasmic reticulum |
| FAS | fatty acid synthase |
| GRP78/BiP | glucose-regulated protein 78 |
| HMG-CoA | 3-hydroxy-3-methylglutaryl coenzyme A |
| HRP | horseradish peroxidase |
| IRE | inositol-requiring enzyme I |
| PARP | poly (ADP-ribose) polymerase |
| PERK | protein kinase-like ER kinase |
| PM | plasma membrane |
| StarD | steroidogenic acute regulatory-related lipid transfer domain |
| TG | Triglyceride |
| Tm | Tunicamycin |
| UPR | unfolded protein response |
| WD | Western diet |
| WT | wild type |
References
- Cnop, M.; Foufelle, F.; Velloso, L.A. Endoplasmic reticulum stress, obesity and diabetes. Trends Mol. Med. 2012, 18, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Schroder, M.; Kaufman, R.J. ER stress and the unfolded protein response. Mutat. Res./Fundam. Mol. Mech. Mutagen. 2005, 569, 29–63. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, R.J. Orchestrating the unfolded protein response in health and disease. J. Clin. Investig. 2002, 110, 1389–1398. [Google Scholar] [CrossRef]
- Zhang, K.; Kaufman, R.J. Signaling the unfolded protein response from the endoplasmic reticulum. J. Biol. Chem. 2004, 279, 25935–25938. [Google Scholar] [CrossRef] [PubMed]
- Walter, P.; Ron, D. The unfolded protein response: From stress pathway to homeostatic regulation. Science 2011, 334, 1081–1086. [Google Scholar] [CrossRef]
- Colgan, S.M.; Hashimi, A.A.; Austin, R.C. Endoplasmic reticulum stress and lipid dysregulation. Expert. Rev. Mol. Med. 2011, 13, e4. [Google Scholar] [CrossRef]
- Rao, R.V.; Ellerby, H.M.; Bredesen, D.E. Coupling endoplasmic reticulum stress to the cell death program. Cell Death Differ. 2004, 11, 372–380. [Google Scholar] [CrossRef]
- van Meer, G.; Voelker, D.R.; Feigenson, G.W. Membrane lipids: Where they are and how they behave. Nat. Rev. Mol. Cell Biol. 2008, 9, 112–124. [Google Scholar] [CrossRef]
- Li, Y.; Ge, M.; Ciani, L.; Kuriakose, G.; Westover, E.J.; Dura, M.; Covey, D.F.; Freed, J.H.; Maxfield, F.R.; Lytton, J.; et al. Enrichment of endoplasmic reticulum with cholesterol inhibits sarcoplasmic-endoplasmic reticulum calcium ATPase-2b activity in parallel with increased order of membrane lipids: Implications for depletion of endoplasmic reticulum calcium stores and apoptosis in cholesterol-loaded macrophages. J. Biol. Chem. 2004, 279, 37030–37039. [Google Scholar] [CrossRef]
- Rodriguez-Agudo, D.; Calderon-Dominguez, M.; Medina, M.A.; Ren, S.; Gil, G.; Pandak, W.M. ER stress increases StarD5 expression by stabilizing its mRNA and leads to relocalization of its protein from the nucleus to the membranes. J. Lipid Res. 2012, 53, 2708–2715. [Google Scholar] [CrossRef]
- Rodriguez-Agudo, D.; Malacrida, L.; Kakiyama, G.; Sparrer, T.; Fortes, C.; Maceyka, M.; Subler, M.A.; Windle, J.J.; Gratton, E.; Pandak, W.M.; et al. StarD5: An ER stress protein regulates plasma membrane and intracellular cholesterol homeostasis. J. Lipid Res. 2019, 60, 1087–1098. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Mendez, R.; Heng, H.H.; Yang, Z.Q.; Zhang, K. Pharmacological ER stress promotes hepatic lipogenesis and lipid droplet formation. Am. J. Transl. Res. 2012, 4, 102–113. [Google Scholar] [PubMed]
- Gomez, J.A.; Rutkowski, D.T. Experimental reconstitution of chronic ER stress in the liver reveals feedback suppression of BiP mRNA expression. eLife 2016, 5, e20390. [Google Scholar] [CrossRef]
- Soccio, R.E.; Adams, R.M.; Maxwell, K.N.; Breslow, J.L. Differential gene regulation of StarD4 and StarD5 cholesterol transfer proteins. Activation of StarD4 by sterol regulatory element-binding protein-2 and StarD5 by endoplasmic reticulum stress. J. Biol. Chem. 2005, 280, 19410–19418. [Google Scholar] [CrossRef]
- Zhang, K.; Kaufman, R.J. Identification and characterization of endoplasmic reticulum stress-induced apoptosis in vivo. Methods Enzym. 2008, 442, 395–419. [Google Scholar] [CrossRef]
- Chikka, M.R.; McCabe, D.D.; Tyra, H.M.; Rutkowski, D.T. C/EBP homologous protein (CHOP) contributes to suppression of metabolic genes during endoplasmic reticulum stress in the liver. J. Biol. Chem. 2013, 288, 4405–4415. [Google Scholar] [CrossRef]
- DeZwaan-McCabe, D.; Sheldon, R.D.; Gorecki, M.C.; Guo, D.F.; Gansemer, E.R.; Kaufman, R.J.; Rahmouni, K.; Gillum, M.P.; Taylor, E.B.; Teesch, L.M.; et al. ER Stress Inhibits Liver Fatty Acid Oxidation while Unmitigated Stress Leads to Anorexia-Induced Lipolysis and Both Liver and Kidney Steatosis. Cell Rep. 2017, 19, 1794–1806. [Google Scholar] [CrossRef]
- Kakiyama, G.; Minoiwa, K.; Bai-Kamara, N.; Hashiguchi, T.; Pandak, W.M.; Rodriguez-Agudo, D. StarD5 levels of expression correlate with onset and progression of steatosis and liver fibrosis. Am. J. Physiol. 2024, 326, G747–G761. [Google Scholar] [CrossRef]
- Pincus, D.; Chevalier, M.W.; Aragon, T.; van Anken, E.; Vidal, S.E.; El-Samad, H.; Walter, P. BiP binding to the ER-stress sensor Ire1 tunes the homeostatic behavior of the unfolded protein response. PLoS Biol. 2010, 8, e1000415. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, R.; Ni, M.; Gill, P.; Lee, A.S. Cell surface relocalization of the endoplasmic reticulum chaperone and unfolded protein response regulator GRP78/BiP. J. Biol. Chem. 2010, 285, 15065–15075. [Google Scholar] [CrossRef]
- Han, J.; Back, S.H.; Hur, J.; Lin, Y.H.; Gildersleeve, R.; Shan, J.; Yuan, C.L.; Krokowski, D.; Wang, S.; Hatzoglou, M.; et al. ER-stress-induced transcriptional regulation increases protein synthesis leading to cell death. Nat. Cell Biol. 2013, 15, 481–490. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhyay, P.; Horvath, B.; Rajesh, M.; Varga, Z.V.; Gariani, K.; Ryu, D.; Cao, Z.; Holovac, E.; Park, O.; Zhou, Z.; et al. PARP inhibition protects against alcoholic and non-alcoholic steatohepatitis. J. Hepatol. 2017, 66, 589–600. [Google Scholar] [CrossRef] [PubMed]
- Gariani, K.; Ryu, D.; Menzies, K.J.; Yi, H.S.; Stein, S.; Zhang, H.; Perino, A.; Lemos, V.; Katsyuba, E.; Jha, P.; et al. Inhibiting poly ADP-ribosylation increases fatty acid oxidation and protects against fatty liver disease. J. Hepatol. 2017, 66, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Kakiyama, G.; Marques, D.; Martin, R.; Takei, H.; Rodriguez-Agudo, D.; LaSalle, S.A.; Hashiguchi, T.; Liu, X.; Green, R.M.; Erickson, S.K.; et al. Insulin resistance dysregulates CYP7B1 leading to oxysterol accumulation: A pathway for NAFL to NASH transition. J. Lipid Res. 2020, 61, 1629–1644. [Google Scholar] [CrossRef]
- Minowa, K.; Rodriguez-Agudo, D.; Suzuki, M.; Muto, Y.; Hirai, S.; Wang, Y.; Su, L.; Zhou, H.; Chen, Q.; Lesnefsky, E.J.; et al. Insulin dysregulation drives mitochondrial cholesterol metabolite accumulation: Initiating hepatic toxicity in nonalcoholic fatty liver disease. J. Lipid Res. 2023, 64, 100363. [Google Scholar] [CrossRef]


| Antibody | Company Name (Reference) | Dilution |
|---|---|---|
| Anti-HMGCR | Abcam (ab174830) | 1:1000 |
| Anti-FLAG | Sigma (F7425) | 1:4000 |
| Anti-PARP | Cell Signaling (9542) | 1:1000 |
| Anti-FAS | Abcam (ab22759) | 1:1000 |
| Anti-StarD5 | Santa Cruz (sc-514236) | 1:400 |
| Anti-GRP78/BiP | Abcam (ab21865) | 1:3000 |
| Anti-GAPDH | Cell Signaling (2118S) | 1:2000 |
| Anti-β-actin | Sigma (A5441) | 1:3000 |
| Goat anti-rabbit HRP-conjugated IgG | BioRad (170-6515) | 1:2000 |
| Goat anti-mouse HRP-conjugated IgG | BioRad (170-6516) | 1:2000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pandak, W.M.; Kakiyama, G.; Rodriguez-Agudo, D. StarD5 Plays a Critical Role in the Hepatocyte ER Stress Survival Response. Int. J. Mol. Sci. 2025, 26, 4157. https://doi.org/10.3390/ijms26094157
Pandak WM, Kakiyama G, Rodriguez-Agudo D. StarD5 Plays a Critical Role in the Hepatocyte ER Stress Survival Response. International Journal of Molecular Sciences. 2025; 26(9):4157. https://doi.org/10.3390/ijms26094157
Chicago/Turabian StylePandak, William M., Genta Kakiyama, and Daniel Rodriguez-Agudo. 2025. "StarD5 Plays a Critical Role in the Hepatocyte ER Stress Survival Response" International Journal of Molecular Sciences 26, no. 9: 4157. https://doi.org/10.3390/ijms26094157
APA StylePandak, W. M., Kakiyama, G., & Rodriguez-Agudo, D. (2025). StarD5 Plays a Critical Role in the Hepatocyte ER Stress Survival Response. International Journal of Molecular Sciences, 26(9), 4157. https://doi.org/10.3390/ijms26094157





