Abstract
Prolactin-releasing peptide (PrRP) has a regulatory role in both acute and chronic stress, suggesting its potential contribution to stress-related disorders such as depression. However, not all individuals with depression respond equally to stressors. We aimed to determine whether the PrRP system could underlie stress coping, an important aspect of depression. The forced swim test was used both as a stressor and as a method to assess coping strategy. Based on immobility time, active coping and passive coping subgroups were identified, and 10 brain regions were studied using qPCR to measure the mRNA expression levels of PrRP and its receptors (specific: GPR10; non-specific: NPFFR2). Passive coping animals spent more time in an immobile posture and exhibited altered mRNA expression levels in the medullary A1 region, the habenula, and the arcuate nucleus than control or active coping rats. Additionally, we identified corticotropin-releasing hormone and vesicular glutamate transporter 2 positive neurons in the A1 medullary region that contained Prrp, suggesting a modulatory role of PrRP in these excitatory neurons involved in stress regulation. Our findings reinforce the hypothesis that PrRP plays a role in stress coping, a process closely linked to depression. However its effect is brain region-specific.
1. Introduction
With increasing life expectancy, mental health and quality of life are becoming increasingly important. Stress-related disorders, like anxiety and depression, have a devastating impact on individuals, families, and society [1]. The COVID-19 pandemic has further worsened mental health outcomes [2,3,4,5]. By 2030, the World Health Organization predicts that depression, which very often involves a passive coping strategy, like avoidance or rumination [6,7,8], will be the leading cause of disability-adjusted life years lost [9]. Understanding its molecular mechanism is crucial for developing effective, personalized treatments, as current therapies, including selective serotonin reuptake inhibitors and serotonin and norepinephrine reuptake inhibitors, fail in about 30% of patients. Even augmentation (e.g., lithium, T3, antipsychotics) or drug repurposing (e.g., ketamine, cyclooxygenase 2 inhibitors, psilocybin, infliximab) is still ineffective in one-third of the treatment-resistant patients [10], highlighting the need for new therapeutic targets.
Prolactin-releasing peptide (PrRP), a member of the RFamide neuropeptide family, has recently gained attention for its role in stress and related pathologies [11]. Its involvement in depression is particularly relevant, as altered PrRP mRNA expression has been observed in the brains of suicidal individuals [12]. Indeed, PrRP plays a key role in regulating acute and chronic stress [13,14], making it significant in stress-related psychopathologies. Primarily expressed in the noradrenergic A1 and A2 cell groups of the medulla oblongata (in co-expression with noradrenaline) [15,16], PrRP transmits stress-related information from the brainstem to the hypothalamus, including the paraventricular nucleus (PVN), the center of the hypothalamic–pituitary–adrenocortical (HPA) axis [17]. Lower PrRP expression is also found in the dorsomedial nucleus of the hypothalamus (DMN) in non-noradrenergic neurons [12]. PrRP fibers primarily target the hypothalamus, particularly the PVN, lateral hypothalamus, and DMN [12,18]. PrRP binds to the GPR10 receptor [19,20] but also acts as an agonist for the neuropeptide FF receptor 2 (NPFFR2) [21], both of which are widely distributed in brain regions associated with depression, such as the PVN [22] and amygdala [23]. Intracerebroventricular PrRP administration activated the HPA axis, leading to increased plasma adrenocorticotropin (ACTH) and corticosterone levels via the PVN [24,25,26,27]. It also raised arterial blood pressure, reflecting sympathetic activation [24,25,26,27]. Chronic stress, such as repeated restraint, increased Prrp mRNA expression in the brainstem, shifting the PrRP/tyrosine hydroxylase (TH) ratio in favor of PrRP [13]. This suggests a crucial role for PrRP in adapting to chronic stress, potentially protecting against the mental health consequences of prolonged stress [28].
Here, we investigated changes in PrRP and its receptors at the mRNA level in a preclinical model widely used to assess passive coping strategies closely associated with depression. Given individual differences in stress susceptibility, which may impact treatment strategies, we compared active coping (A) and passive coping (P) animals. Although depressive-like symptoms can be studied in animals using many methods (see Supplementary Table S1), we chose the forced swim test (FST, Porsolt test), validated for both rats and mice [29,30]. Despite intensive debate [31,32,33,34], FST has some validity in predicting a compound’s antidepressant potential. The major criticism is that it measures coping strategy rather than a depressive-like state. However, the passive coping observed in FST was linked to depression in many studies [6,7,8]. Moreover, FST is a short, not severe stress [35], it is easy to conduct, and active and passive coping animals can be easily separated [36]. To minimize variability from female cyclic changes [37] and sex-dependent stress-induced PrRP alterations [13], only male rats were included. Medullary PrRP-positive cells in the A1 region were analyzed using immunohistochemistry (protein detection) combined with RNAscope in situ hybridization (mRNA detection).
2. Results
2.1. FST Increased Passive Coping in Vulnerable Animals
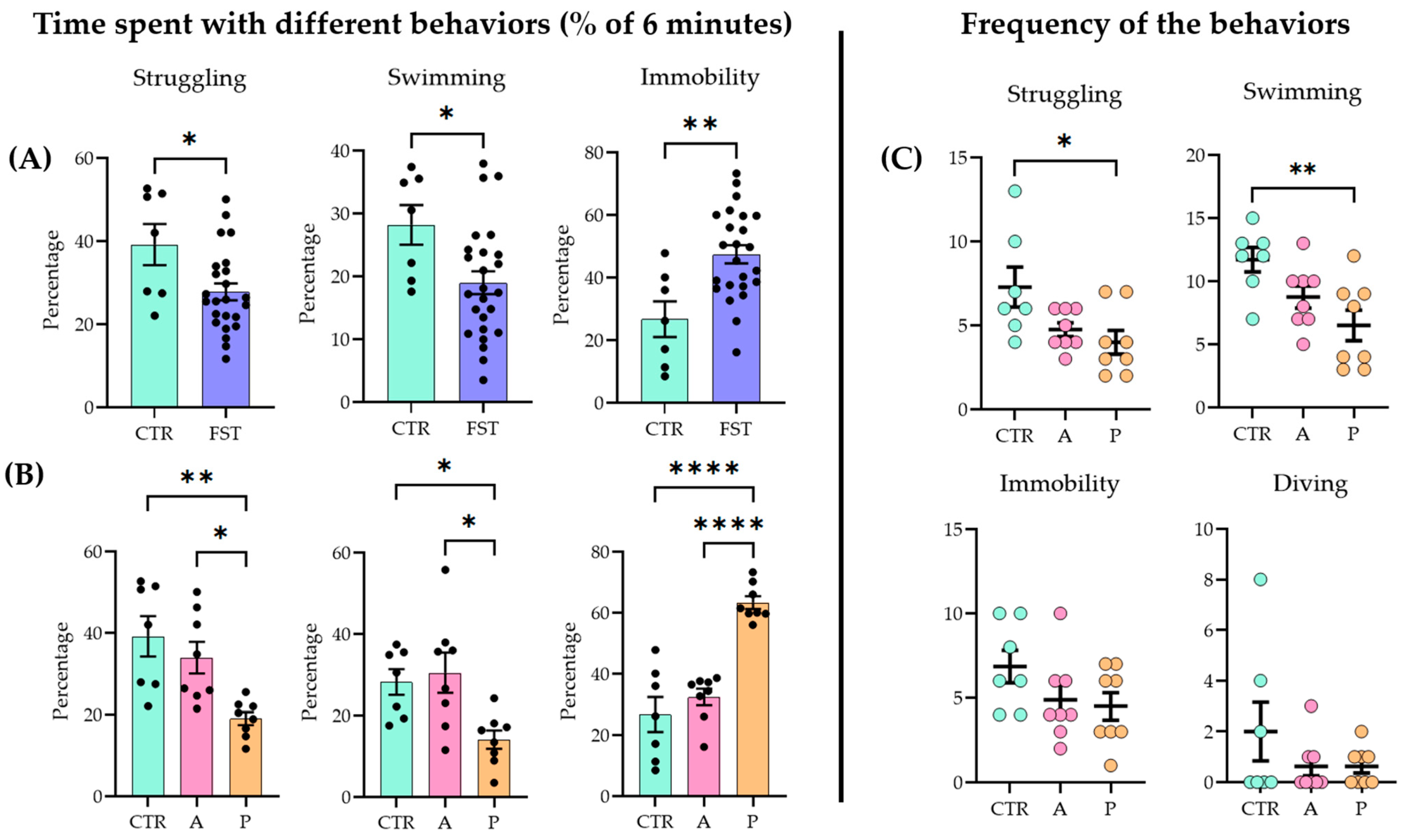
When naïve and previously stressed (FST 24 h earlier) animals were subjected to a 6 min FST, all the main parameters (struggling, swimming, and immobilization) showed significant differences between the two groups (unpaired two-tailed t-test: struggling p = 0.019, t = 4.488, df = 29; swimming p = 0.0222, t = 2.411, df = 30; immobility p = 0.002, t = 3.327, df = 29; control (CTR) n = 7; FST n = 24) (Figure 1A; for raw data, see Supplementary Table S2; for detailed statistics, see Supplementary Table S3).
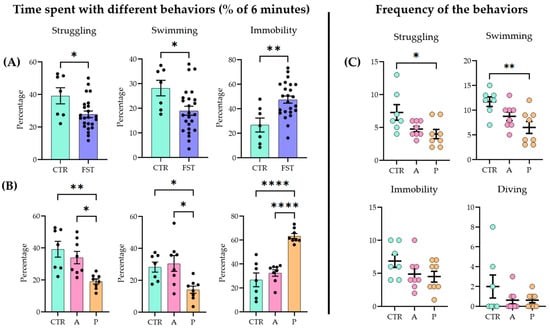
Figure 1.
Behavioral outcomes in the forced swim test (FST) (A) One day after a 15 min forced swim session, the animals exhibited an increased passive coping strategy compared to naïve controls (CTRs) during a 6 min test. (B) When the FST group was divided further into active coping (A) and passive coping (P) subgroups based on immobility time, the A group showed no significant difference from the controls (CTRs), while the P group differed from both the CTR and A groups. (C) Active coping animals displayed active behaviors (struggling and swimming) more frequently than the other groups. Data are expressed as mean ± SEM. Dots represent individual data points. * p < 0.05, ** p < 0.01, and **** p < 0.0001 vs. the labeled group.
Active (A) and passive (P) coping subgroups were separated based on the behavioral data (see Section 4.2). Upon analysis (ANOVA and Tukey’s post hoc test), the A group did not differ significantly from the controls (CTRs), while both the CTR and the A groups showed significant differences from the P group (struggling F(2, 20) = 8.354, p = 0.002; swimming F(2, 20) = 6.069, p = 0.0087; immobility F(2, 20) = 29.51, p < 0.0001) (Figure 1B). In terms of frequency (how many times the animal started the behavior), P animals began the two active behaviors fewer times than the other two groups (struggling F(2, 20) = 4.488, p = 0.0245; swimming F(2, 20) = 6.165, p = 0.0082). There were no differences among groups in immobility (F(2, 20) = 1.195, p = 0.1622) and diving frequencies (F(2, 20) = 1.359, p = 0.2797) (Figure 1C).
2.2. A1, Arcuate Nucleus, and Habenula Showed mRNA Expression Differences in the PrRP System Parallel with FST Sensitivity
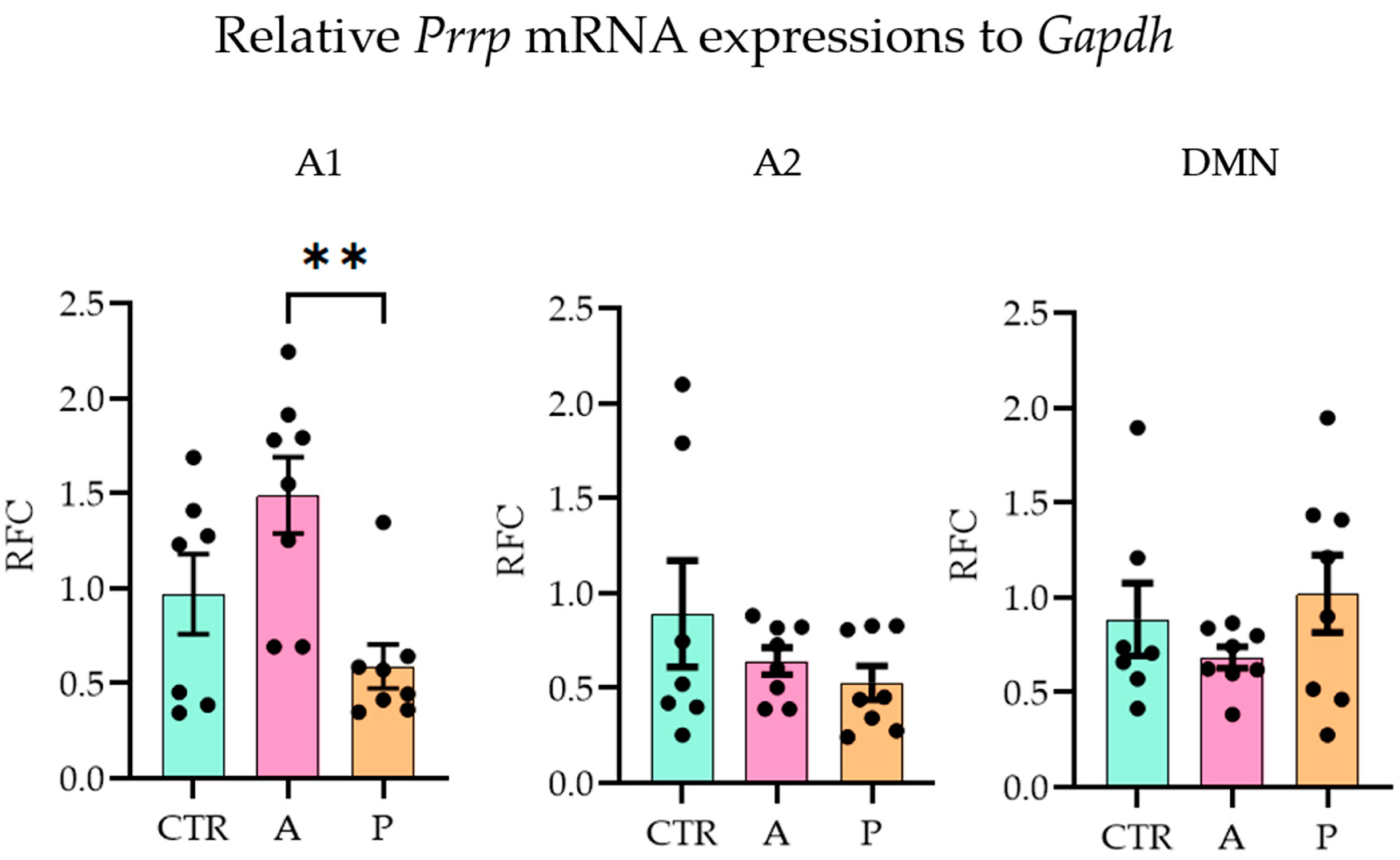
With the quantitative polymerase chain reaction (qPCR), we examined the three major sites of PrRP expression (A1, A2 (part of the nucleus tractus solitarius, NTS [38]), DMN [39]) and ten possible target brain areas (receptor expressions, see Section 4.3) on the mRNA level (one-way ANOVA with Tukey’s multiple comparison) (threshold cycle (CT) values are available in Supplementary Table S4). In A animals, Prrp expression in the A1 region was significantly higher than in P group (ANOVA F(2,20) = 6.705, p = 0.006; Tukey’s multiple comparison p = 0.004 between A and P), while the CTR and P groups did not differ from each other, indicating an inadequate response of the P animals to the repeated stress. The other two brain areas, A2 and DMN, did not show group differences (Figure 2).
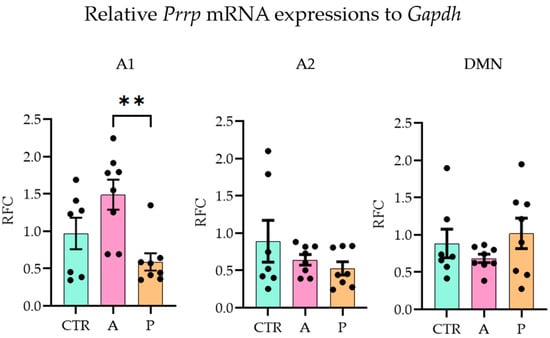
Figure 2.
Relative Prrp mRNA expression level changes in the three Prrp-producing brain regions. Active coping animals displayed a higher expression of Prrp than passive coping animals in the medullary A1 region. Abbreviations: A1 and A2—medullary noradrenergic nuclei, A—active coping group, CTR—control group, DMN—dorsomedial nucleus of the hypothalamus, Gapdh—glyceraldehyde 3-phosphate dehydrogenase, P—passive coping group, RFC—relative fold change. Data are expressed as mean ± SEM. Dots represent individual data points. ** p < 0.01, vs. the labeled group.
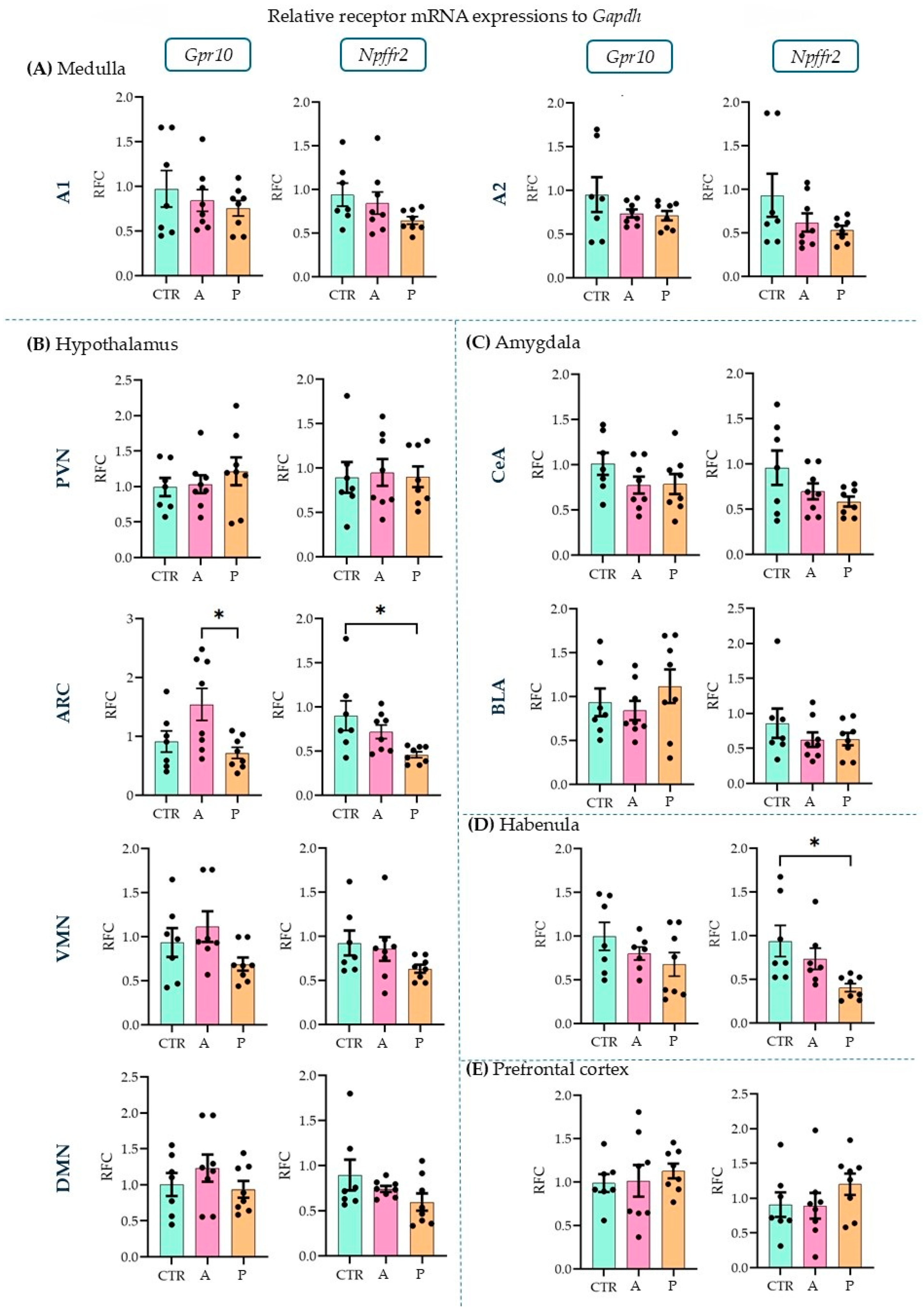
Upon investigating the receptors, in the ARC (Figure 3B), we detected a higher expression level of Gpr10 in P than in A animals (ANOVA F(2, 20) = 4.856, p = 0.019; Tukey’s multiple comparison p = 0.019 between A and P). P animals, however, had lower Npffr2 expression levels here than CTRs (ANOVA F(2, 20) = 4.736, p = 0.021; Tukey’s multiple comparison p = 0.017 between CTR and P) and the same difference was found in the habenula (HAB, Figure 3D) (ANOVA F(2, 19) = 5.001, p = 0.018; Tukey’s multiple comparison p = 0.015 between CTR and P). This suggests that PrRP signaling may be insufficient in these areas in response to repeated stressors. However, the other sevenbrain areas did not show alterations between the groups (Figure 3A–E). The relative fold changes and ANOVA results for all the regions are summarized in Table 1. The expression of both receptors has been previously described in the medulla, hypothalamus, and amygdala [11] but not in the HAB or prefrontal cortex (PFC). Here, we report the detectable mRNA expression levels of the receptors in both areas (Table 2).
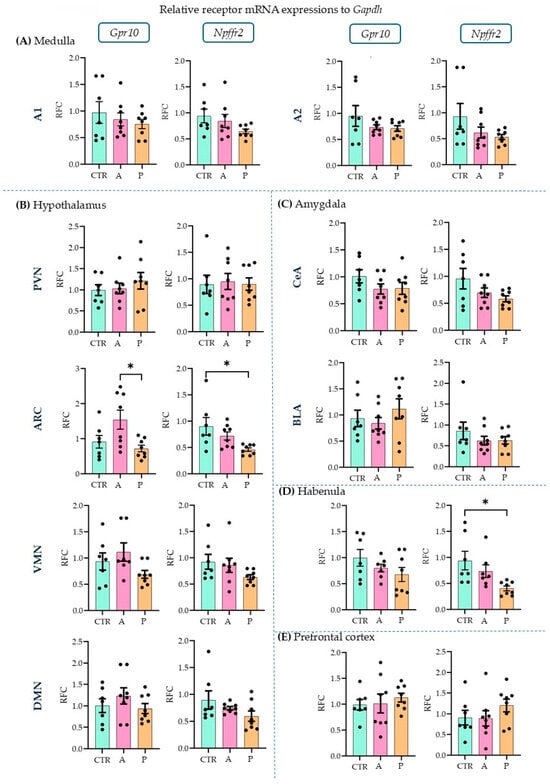
Figure 3.
Relative mRNA expression changes in the Gpr10 and Npffr2 levels. A higher Gpr10 expression was found in the arcuate nucleus in active compared to control and passive coping animals. Passive coping animals displayed a lower expression of Npffr2 than controls in both the arcuate nucleus and habenula. mRNA abbreviations: Gpr10—specific receptor to PrRP and Npff2—neuropeptide FF receptor 2; groups: CTR—control, A—active coping, and P—passive coping; brain regions: A1 and A2—noradrenergic nuclei of the medulla, VMN—ventromedial nucleus of the hypothalamus, DMN—dorsomedial nucleus of the hypothalamus, PVN—paraventricular nucleus of the hypothalamus, ARC—arcuate nucleus, CEA—central amygdala, BLA—basolateral amygdala, HAB—habenula, and PFC—prefrontal cortex. Data are expressed as mean ± SEM. Dots represent individual data points. * p < 0.05, vs. the labeled group.

Table 1.
Summary of qPCR data.

Table 2.
Excerpt from Supplementary Table S4: threshold cycle values in habenula (HAB) and the prefrontal cortex (PFC).
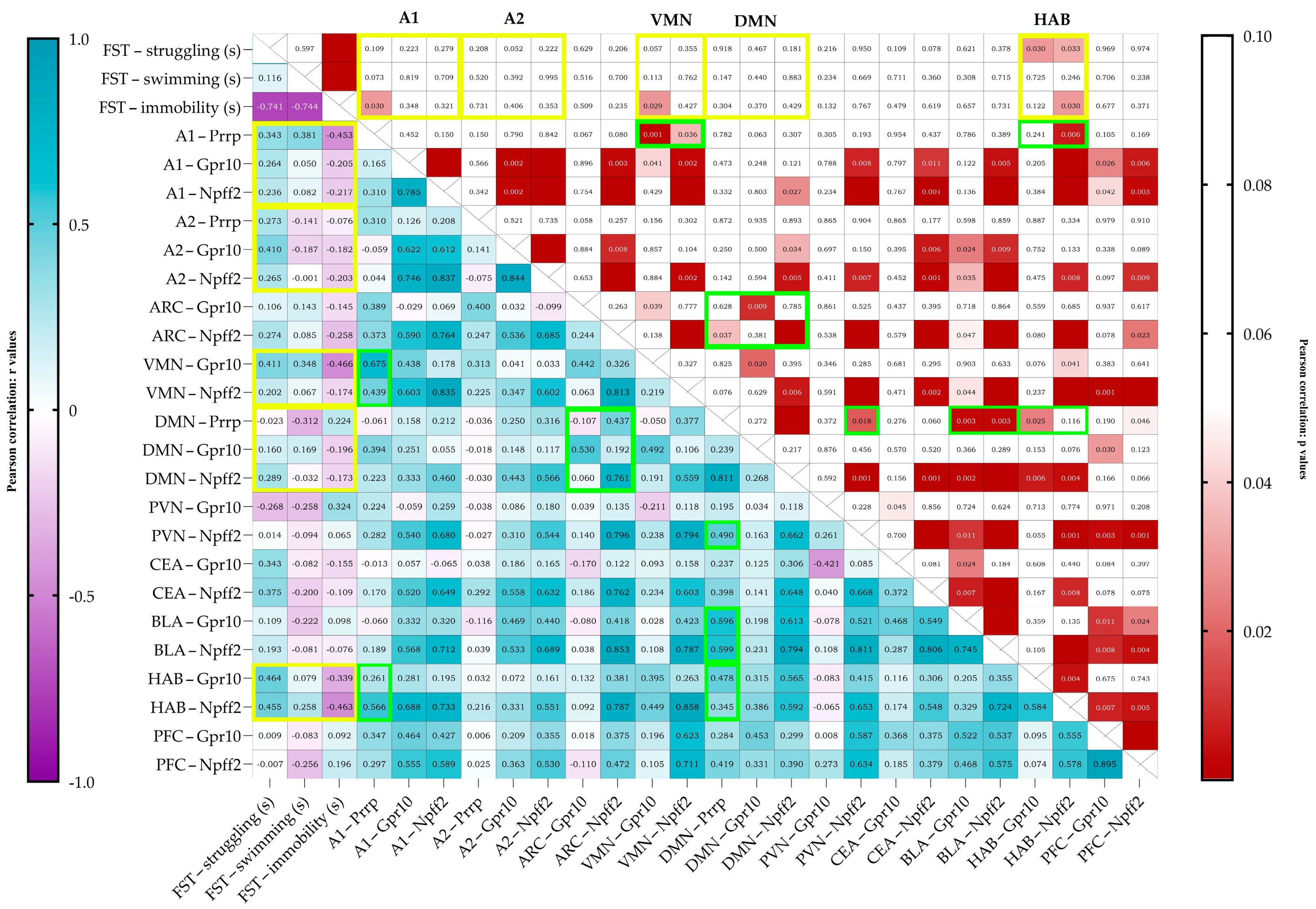
Upon correlating the results of the behavioral test and the mRNA expressions (Pearson correlation), immobility negatively correlated with Prrp production in the A1 region (r = −0.453, p = 0.030) (Figure 4). The struggling behavior of the animals positively correlated with the relative expression of both receptors’ mRNA in the HAB (Gpr10 r = 0.464, p = 0.030, Npff2r r = 0.455, p = 0.033). Immobility correlated negatively with the receptor expressions in the HAB; however, only with Npff2r did it reach significance (Gpr10 r = −0.339, p = 0.122, Npff2r r = −0.463, p = 0.030). Immobility also correlated negatively with the Gpr10 expression in the VMN (r = −0.466, p = 0.029).
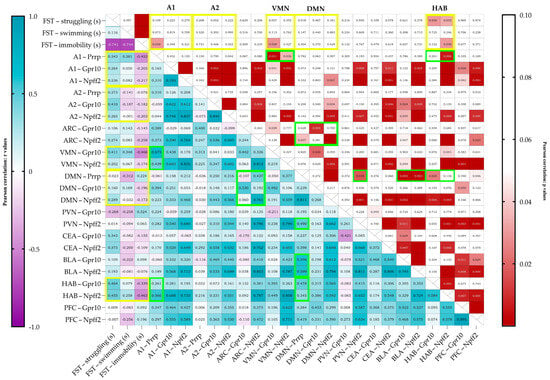
Figure 4.
Pearson’s correlation data visualized on a heatmap with r values on the left and p values on the right. On the left, a strong positive correlation is shown in darker shades of blue, and a strong negative correlation is shown in purple. Boxes are labeled with an r value of up to 3 decimals. On the right, cells with values over 0.05 are left blank (white), and under 0.05, the lower the p value, the darker the shade of red shown. The cells are labeled with the exact p values, and if the cell is empty, the p value is below 0.001. FST data are given in seconds (FST—forced swim test). Yellow frames represent the important correlations between behavior and mRNA expression, and green frames represent the noteworthy correlations between Prrp production and receptor expression. mRNA abbreviations: Prrp—prolactin-releasing peptide, Gpr10—specific receptor to PrRP, and Npff2—neuropeptide FF receptor 2; brain regions: A1 and A2—noradrenergic nuclei of the medulla, VMN—ventromedial nucleus of the hypothalamus, DMN—dorsomedial nucleus of the hypothalamus, PVN—paraventricular nucleus of the hypothalamus, ARC—arcuate nucleus, CEA—central amygdala, BLA—basolateral amygdala, HAB—habenula, and PFC—prefrontal cortex.
There were also several positive correlations between PrRP production and receptor expressions. Prrp mRNA levels in the medullary A1 region correlated with the receptor levels in the VMN, showing a moderate correlation with Gpr10 (r = 0.675, p = 0.001) and a low correlation with Npff2r (r = 0.439, p = 0.036). A1 Prrp levels showed a moderate correlation with Npff2 (r = 0.566, p = 0.006) levels in the HAB. Prrp mRNA expression in the medullary A2 region did not correlate with any other site’s Prrp nor with any receptor mRNA expressions. Prrp mRNA expression levels and Npff2r expression in the DMN showed a high correlation (r = 0.811, p < 0.0001). DMN’s Prrp expression showed low-to-moderate correlation to Npff2r levels both in the ARC and the PVN (ARC Npffr2 r = 0.437, p = 0.037; PVN Npff2r r = 0.490, r = 0.018). Interestingly, Prrp expression in the DMN showed moderate positive correlation to all receptor expression levels in the BLA (Gpr10 r = 0.596, p = 0.003; Npff2 r = 0.599, p = 0.003) but not in the CEA (Gpr10 r = 0.237, p = 0.276; Npff2 r = 0.389, p = 0.059). Positive correlation with Prrp production in DMN was also found in the HAB’s and PFC’s receptor expressions; specifically, in the HAB, the Gpr10, while in the PFC, the Npff2r expression was significant (Gpr10 in HAB r = 0.478, p = 0.025; Npff2r in PFC r = 0.419, p = 0.046).
2.3. In Situ Hybridization Reveals New Characteristics of Medullary A1 Cells
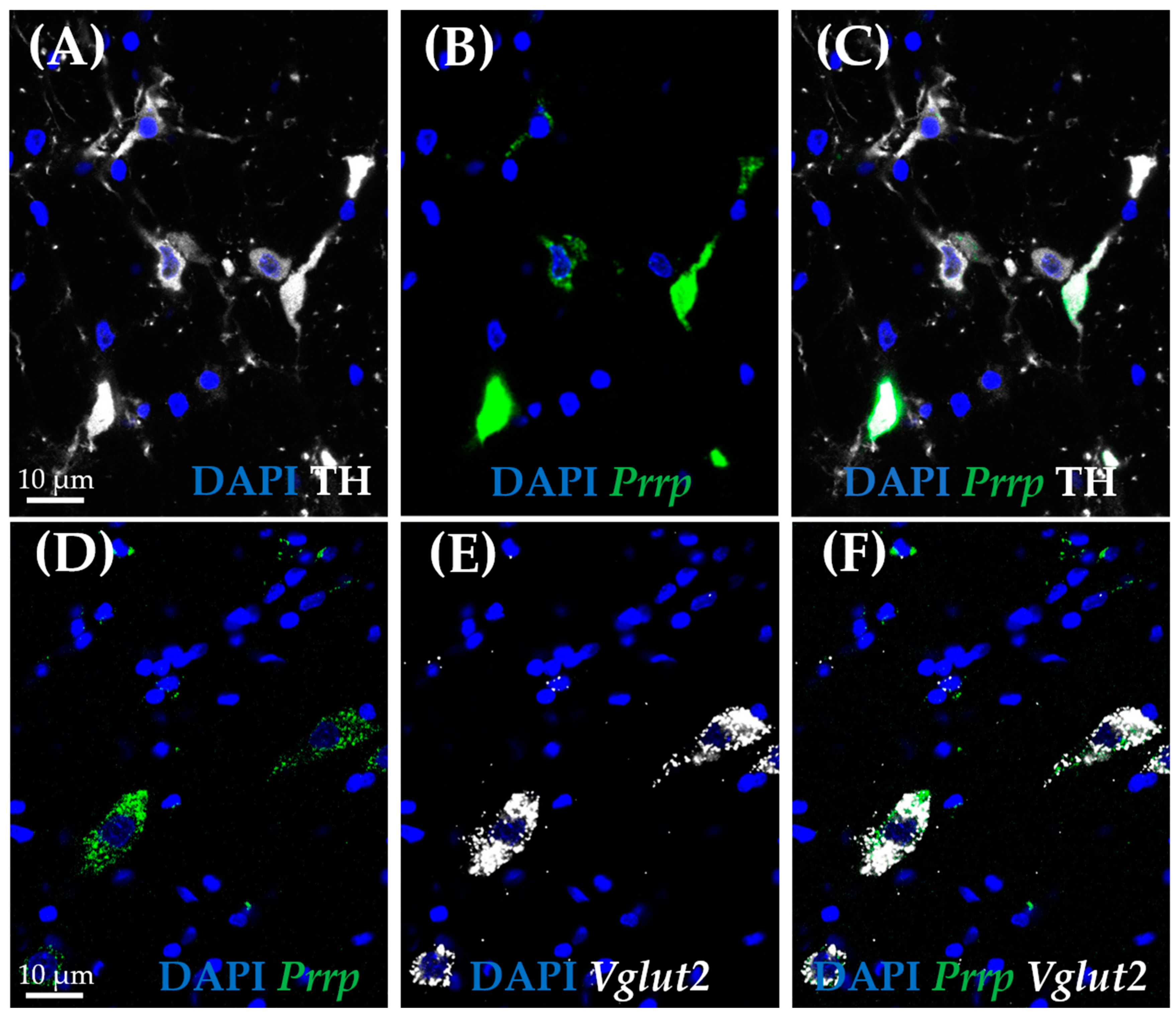
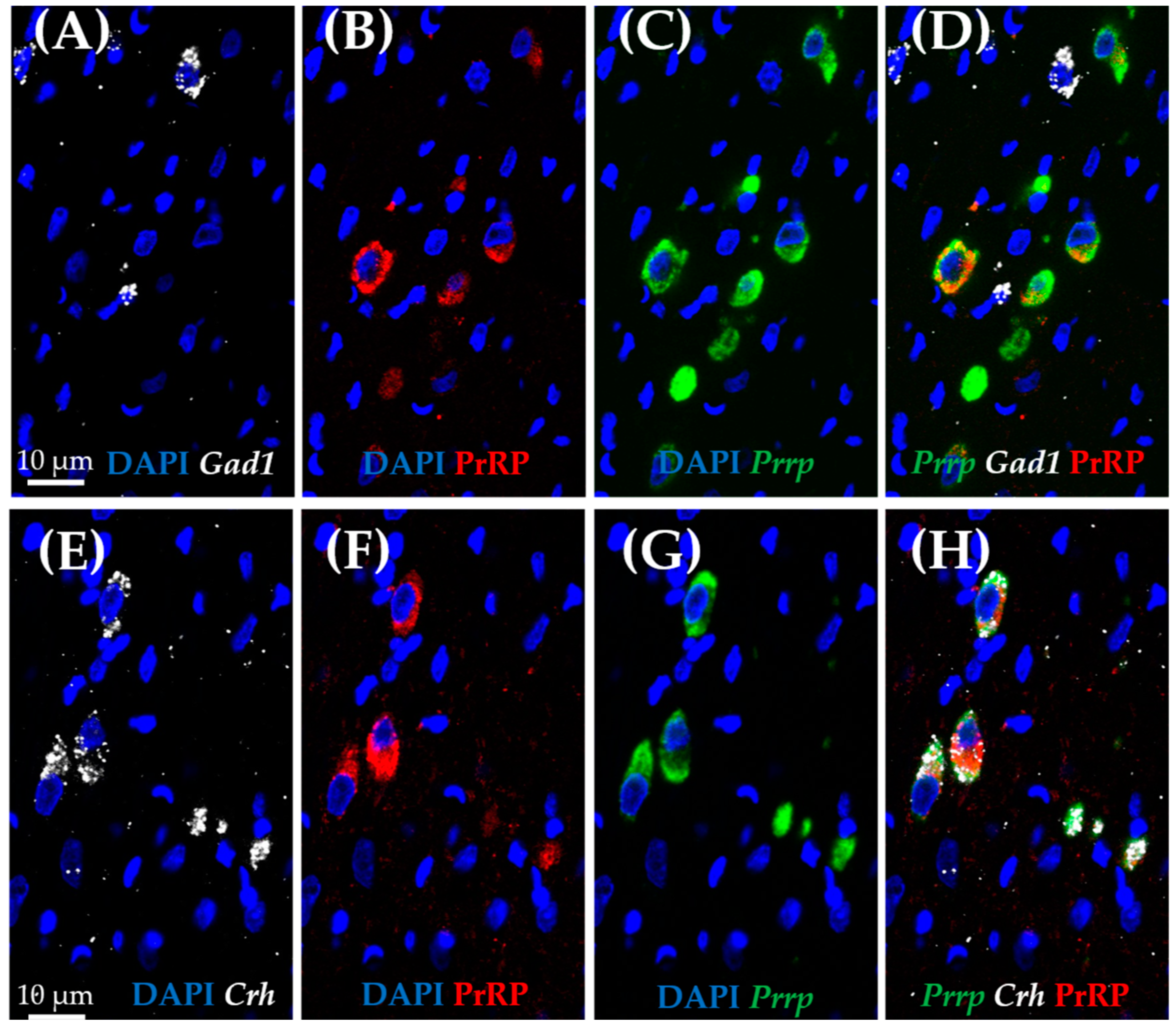
As medullary A1 neurons were deeply implicated in active coping, resembling resiliency to depression-like behavior, we used the RNAscope in situ hybridization (ISH) technique to characterize these neurons. In concordance with the literature, Prrp mRNA-containing neurons can be found in this region, and they proved to be noradrenergic, staining positive with tyrosine–hydroxylase antibody (TH) (Figure 5A–C). More precisely, 85.71% of TH-positive neurones were also positive for Prrp (Supplementary Table S6). To further characterize these cells, we hybridized vesicular glutamate transporter 2 (Vglut2) (Figure 5D–F) or glutamate decarboxylase (Gad1) mRNA probes alongside the Prrp probe and PrRP antibody (Figure 6A–D). As expected, the PrRP proteins were found in 100% of Prrp mRNA-positive cells. The mRNA copy number was very high, forming a confluent mRNA signal in many cases (e.g., Figure 5B, green). The positivity for Vglut2 labels glutamatergic, excitatory neurons, while Gad1 positivity labels GABA-ergic (γ-aminobutyric acid), inhibitory neurons. We found Prrp mRNA co-expression in Vglut2-positive (72% of Vglut2-positive cells) but not Gad1-positive (0%,) cells in the A1 area (Supplementary Table S6). Additionally, we examined the well-known stress hormone, corticotropin-releasing hormone (Crh) mRNA, which also showed co-localization with the Prrp probe (100%, Supplementary Table S6) as well as with the PrRP antibody (Figure 6E–H).
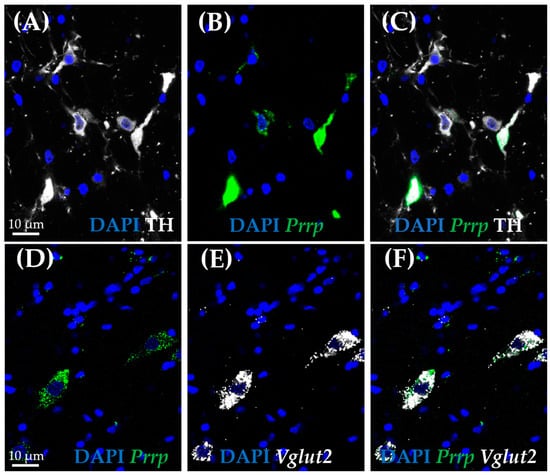
Figure 5.
Co-expression analysis of the prolactin-releasing peptide-positive neurons in the A1 cell group by RNAscope in situ hybridization combined with immunostaining. Representative fluorescence images showing the co-localization of prolactin-releasing peptide mRNA (B,D; Prrp, green) with vesicular glutamate transporter 2 mRNA (E,F; Vglut2, white), as well as tyrosine hydroxylase (A,C; TH, white). Nuclei were stained with 4′,6-diamidino-2-phenylindole (DAPI, blue).
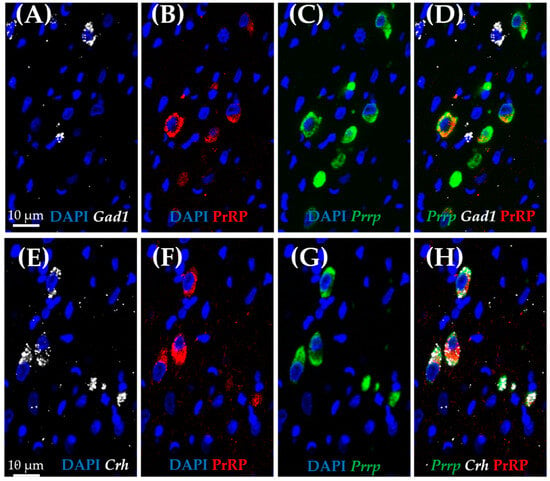
Figure 6.
Characterization of prolactin-releasing peptide-positive neurons in the medullary A1 region by RNAscope in situ hybridization combined with immunostaining. Representative fluorescence images showing the co-localization of prolactin-releasing peptide (B,F; PrRP, red) and mRNA (C,G; Prrp, green) with corticotropin-releasing hormone mRNA (E,H; Crh, white). Note that there is no co-localization with glutamate decarboxylase 1 mRNA (A,D; Gad1, white). Nuclei were stained with 4′,6-diamidino-2-phenylindole (DAPI, blue).
3. Discussion
We confirmed that behavioral passive coping in the FST model was accompanied by lower Prrp mRNA levels in the medullary A1 region, as well as lower specific Gpr10 receptor levels in the ARC and lower Npffr2 mRNA (non-specific receptor) concentration both in the HAB and the ARC. In accordance with the literature, the RNAscope ISH in naïve animals confirmed Prrp expression in the noradrenergic neurons of the medullary A1 region. Moreover, the Prrp mRNA as well as protein signal was present in Vglut2- and Crh-positive A1 medullary neurons, highlighting PrRP’s role in neuronal excitation, which might be especially important during stress.
PrRP has a role in regulating the HPA axis during both acute and chronic stress [13,40,41], and our previous results also suggested its role in depression using human samples [12]. In our FST model, out of the three main PrRP-producing brain regions (A1, A2/NTS, DMN), only the medullary A1 region showed different Prrp mRNA expression in A than P individuals. Moreover, the immobility time negatively correlated with the A1 Prrp mRNA levels, suggesting that an increased passive coping strategy is accompanied by lower Prrp. As all animals underwent the 6 min FST section, we can exclude swimming-induced changes. Thus, the observed Prrp mRNA difference might be a possible determinant of the stress vulnerability of the rats. This is in line with our previous findings on the importance of the A1 region in stress adaptation [13,14]. Moreover, high Prrp mRNA content was also detected in the A1 region of P rats using another depression model, the learned helplessness [12]. However, in that case, the Prrp difference in the DMN also underlined the vulnerability. The present FST is a short intervention, while the learned helplessness procedure is more severe (electric foot shock for 40 min) and prolonged (tested 7 days after training). We might assume that regulating the A1, as one of the centers of the sympathetic system [42], is more important during short interventions, while the role of DMN might come into focus during chronic situations [43]. However, it is clear that DMN has an important regulatory role during an acute stress situation as well, e.g., by increasing cardiovascular sympathetic activity [44] or regulating respiration [45]. Previously, the DMN PrRP neurons were implicated in feeding [46]. We might assume that learned helplessness-induced anhedonia [47] influenced food intake, thereby having a stronger impact on DMN than on A1. Although there was no significant difference in the Prrp mRNA level between P and A individuals, the DMN-PrRP levels positively correlated with the receptor levels on many stress-related areas like PVN and BLA as well as HAB and PFC. These correlations support that DMN-PrRP might form stress adaptation, as we have seen in the learned helplessness model [12]. However, it was also suggested that Prrp expression correlates with stress rather than with the individual’s vulnerability to develop depression [11].
We further characterized the medullary A1 neurons as a major PrRP-dependent determinant of stress vulnerability in FST. We found that these TH-positive noradrenergic neurons, besides containing Prrp mRNA and PrRP peptide [14], were also positive for Vglut2 and Crh mRNA. Interestingly, PrRP receptors share similarity to AMPA (α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid, a glutamate receptor) suggesting their interaction in signalization [48]. It is well known that the noradrenergic A1 cells [49,50] project to the PVN, the center of the HPA axis, where they might regulate CRH synthesis and release. Interestingly, CRH production was found in catecholaminergic brainstem nuclei most probably transmitting systemic stressor information (e.g., blood loss, pain, hypoglycemia) to the PVN [51]. Although, in the PVN, CRH can be found in excitatory VGluT2-positive cells, but in other brain areas, e.g., in the central amygdala (CeA) and bed nucleus of the stria terminalis (BNST), it is produced in GABAergic neurons [52]. Here, we confirmed its presence in excitatory Vglut2-positive A1 neurons, which makes these brainstem CRH cells more similar to the PVN-CRH neurons. We might assume that together with noradrenaline and PrRP, glutamate and CRH will also be transported to the PVN and participate in the fine-tuning of the stress response. In line with this assumption, the DMN Vglut2=positive neurons (which might contain PrRP as well) were previously confirmed to regulate CRH release [53]. Thus, we hypothesize that the noradrenergic PrRP-expressing neurons in the medullary A1 region through PVN innervation will influence glucocorticoid production, thereby coping with the stressors. Indeed, glucocorticoid elevation has been described to have a positive correlation with the vulnerable phenotype [54,55]. However, we cannot dismiss the possibility that the noradrenergic innervation of the CRHergic BNST is responsible for the different stress coping, as this brain area might be responsible for behavioral alterations as well as for transmitting information to the PVN [56]. In supporting studies, it has been previously shown that GPR10 is co-expressed extensively with CRH in the BNST [57].
In addition to these HPA axis effects, the cortico-habenular circuit has also been suggested as an important modifier of the stress response [58,59,60]. HAB dysfunction has been implicated in the pathomechanism of depression [61] and in vulnerability to stress [62]. Our passive coping rats showed reduced PrRP receptor signaling in this brain area, highlighting this circuit’s role in stress regulation. As additional support, Npff2r signaling in the HAB negatively correlated with immobility and positively with struggling time, and there was a positive correlation between A1 Prrp and HAB Npffr2 expression as well. In mice, a GABA-ergic (inhibitory) signal from the lateral septum increased immobility in the tail suspension test through a parallel influence on HAB and DMN [63]. The positive correlation between HAB Gpr10 receptor mRNA and DMN Prrp mRNA expression in our hands suggests that PrRP might be an important coordinator of stress coping.
In the ARC, both the specific and non-specific Prrp receptor mRNA levels were lower in passive coping individuals than in controls, suggesting lower PrRP signaling in this brain area. Silencing or overproducing interleukin 11 in the proopiomelanocortin (POMC)-positive cells of the ARC influenced depressive-like behavior in a mouse chronic unpredictable stress model [64]. The chronic chemogenetic stimulation of these POMC-ARC cells led to anhedonia and behavioral despair (i.e., increased immobility in FST), however, only in male but not female mice [65]. This suggests that POMC-ARC is a key hub regulating depression, including hypophagia and anhedonia [66]. PrRP might regulate the gonadotropin-releasing hormone secretion [67], which might contribute to sexual problems in depression, although this was linked more to the preoptic hypothalamic area than to ARC. However, the PrRP innervation of dopaminergic ARC neurons might regulate prolactin release and, in this way, influence sexual functions [68].
Interestingly, the specific PrRP receptor Gpr10 expression in the VMN negatively correlated with immobility time and showed a positive correlation with A1 PrRP expression, similarly to HAB, however, without significant direct differences between the R and V groups. VMN is also a known satiety center [69]; thus, its role in stress adaptation might be related to energy homeostasis.
Our results strengthen the theory about PrRP’s role in stress adaptation, emphasizing its brain region specificity, with the most important role of medullary A1 cells. We confirmed the possible utility of the PrRP system as a therapeutic target in depression.
4. Materials and Methods
4.1. Animals
Male Wistar rats from the local colony were used (University of Pécs, Institute of Physiology, facility license number: BAHU0140L 17). All animals were housed individually, and tap water and standard chow were available ad libitum. The facility maintained a 12/12 dark–light cycle, experiments were conducted between 10 am and 3 pm during the animals’ dark/active period. For FST and quantitative PCR, 8-week-old adolescent rats (250–350 g) were used, while for the RNAscope, we used 6-month-old adult rats (450–500 g, n = 2). For qPCR, the brains were harvested by decapitation 30 min after the 6 min swimming session. The brains were snap-frozen on dry ice and stored at −80 °C. Animals for RNAscope were deeply anesthetized with 26% urethane (0.5 mL/100 g in body weight). The depth of anesthesia was determined by the diminished corneal reflex. These animals were perfused transcardially (with 0.1 M, pH = 7.4 PBS and 4% paraformaldehyde solution in the same PBS).
4.2. Forced Swim Test (FST)
First day (training): From the 31 animals, 7 were randomly selected and omitted this day (control group). The other 24 animals completed a 15 min forced swim session. We examined 3 animals at the same time in separate glass cylinders (high: 61 cm, diameter: 18 cm, filled up to 30 cm with tap water 24 ± 1 °C). The sessions were recorded with a digital camera.
Second day: All 31 animals completed a 6 min forced swimming session 24 h ± 20 min after their first swimming (protocol has been adjusted to include the controls). The session was recorded by a digital camera and later analyzed manually with the open access SolomonCoder behavioral coding program (https://solomon.andraspeter.com/, downloaded on 30 September 2022). Four behaviors were coded and the time (expressed as % of 6 min) and frequency of each behavior was used for further analysis.
We defined the four distinct behaviors as follows:
- Struggling (also called climbing), when the animal is almost in a vertical position, uses hard strokes and the front paws reach above the water line;
- Swimming, when the animal is in a more horizontal position and does not reach above the water with front paws as it swims around;
- Immobility (or floating), when the animal is almost still and only uses little movements to keep its head above the water;
- Diving, when the animal is fully immersed, and usually it dives to the bottom of the cylinder.
To clearly distinguish between stress-sensitive and resistant animals, we divided the trained group into three parts and considered the 1/3 group that floated the most as passive coping (P), while the group that floated the least was considered active coping (A) [33]. The middle 1/3 gray zone was not considered. The significant difference between the A and P groups supported our choice (Figure 1).
4.3. Quantitative PCR
To collect the samples from 10 brain areas, the brain was manually cut into 1 mm slices, and a tissue cylinder with a diameter of 1 mm was cut out with a tissue sampler over dry ice according to the Paxinos & Watson brain atlas [70] (Figure 7). All equipment was cleaned with 70% alcohol between animals. Samples were placed directly into the first buffer solution of the RNA isolation kit (RNeasy Mini Kit, Quiagen, Venlo, The Netherlands cat.no.: 74104). Total RNA isolation was carried out following the manufacturer’s protocol. RNA samples were stored at −80 °C. A High-Capacity RNA-to-DNA kit (by Thermo Fischer Scientific, Braunschweig, Germany, cat. no.: 4387406) was used for RT-PCR with 1 µg of isolated RNA, 1 µL of enzyme mix and 10 µL of buffer solution for each sample. The cDNA solution was diluted 2.5×. Primers designed with BLAST (https://www.ncbi.nlm.nih.gov/tools/primer-blast/ accessed on 17 November 2022) and manufactured by Integrated DNA Technologies (see sequences below) were validated in silico (with BLAST) and used in a 10× dilution. Gapdh (glyceraldehyde 3-phosphate dehydrogenase) was used as reference gene (Table 3). The qPCR master mix was prepared with 10 µL of SYBR GREEN (Meridian, cat.no.: BIO-94020), 1-1 µL of diluted primers, 3 µL of RNase-free water, and 3 µL of cDNA sample. Every new batch of master mix was tested as no-template controls, and fluorescence did not reach the detection threshold. Samples were run in duplicates on 96-well plates with the Applied Biosystems QuantStudio 3 Real-Time PCR System. The qPCR setup is disclosed in Supplementary Table S5. For data analysis, the ΔΔCT method was used [71].
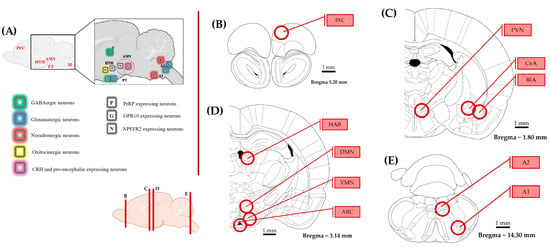
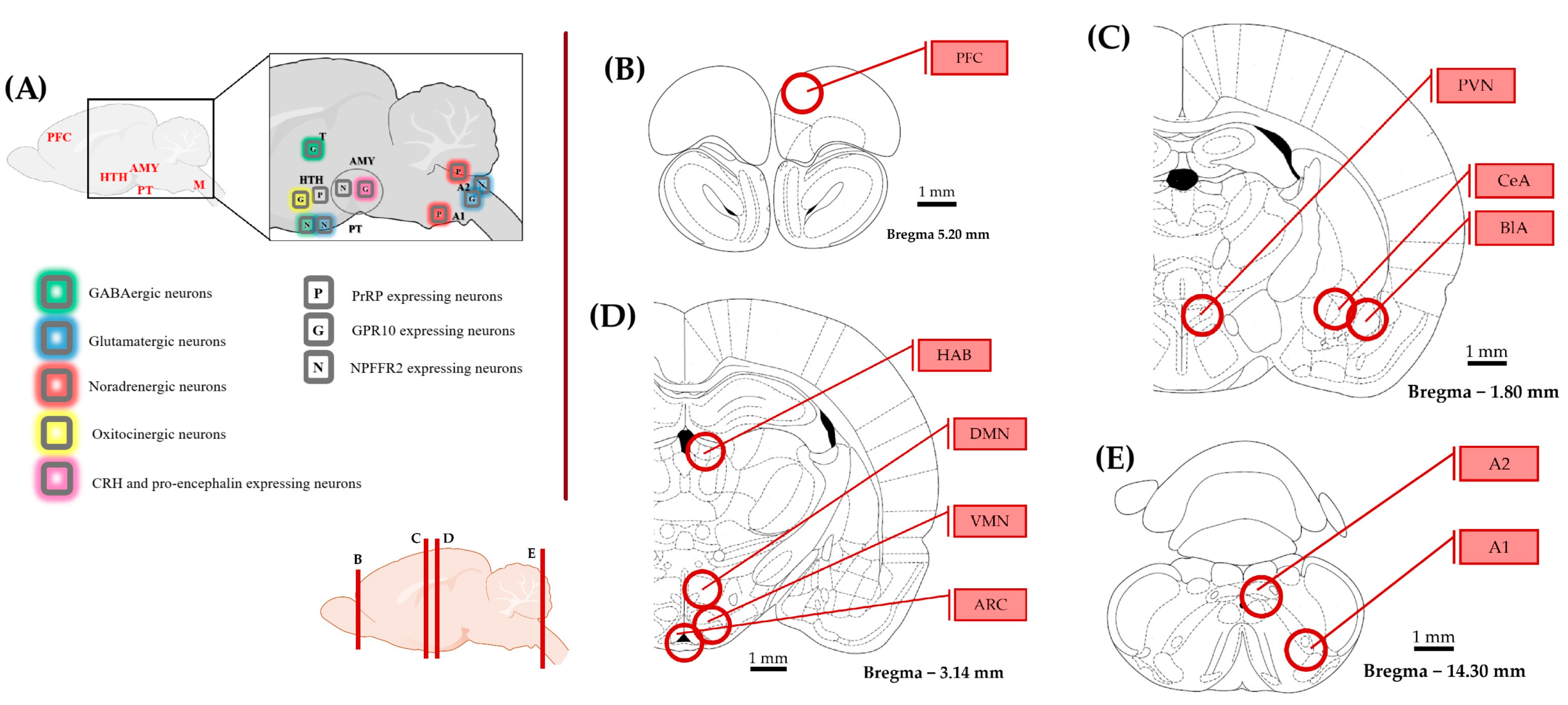
Figure 7.
Representation of the brain areas relevant to this study. The schematics were made according to the rat brain atlas [70]. (A) Distribution of prolactin-releasing peptide (PrRP) and its receptors with a focus on brain areas implicated in stress coping. (B–E) The examined brain regions (circles) are illustrated on coronal sections from rostral to caudal. The circles represent the location and size of the punched tissue samples. The rostro-caudal position of the coronal sections is shown at the bottom. Abbreviations: A1, A2—noradrenergic cell groups in the medulla oblongata, AMY—amygdala, ARC—arcuate nucleus, BLA—basolateral amygdala, CeA—central amygdala, CRH—corticotropin releasing hormone, DMN—dorsomedial nucleus of the hypothalamus, G—GPR10 expressing neuron, GABA—gamma-amino-butyric acid, HAB—habenula, HTH—hypothalamus, M—medulla oblongata, N—Neuropeptide FF receptor 2 expression, P—PrRP, PT—pituitary, PFC—prefrontal cortex, PVN—paraventricular nucleus of the hypothalamus, T—thalamus, and VMN—ventromedial nucleus of the hypothalamus.

Table 3.
Primer sequences.
4.4. RNAscope In Situ Hybridization Combined with Immunostaining
4.4.1. Sample Collection and Preparation
Brains from the transcardially perfused animals were collected and subjected to 2 × 24 h post fixation in 4% PFA solution. For cryoprotection, they were immersed in 30% sucrose solution for another 2 × 24 h at 4 °C. While frozen on dry ice, 30 µm thick coronal slices were cut with a manually operated sliding microtome (Leica SM2010R, Leica Biosystems, Deer Park, TX, USA). Sections were stored in antifreeze solution at −20 °C.
4.4.2. RNAscope and IHC
The RNAscope was carried out as described previously [72,73]. Briefly, an endogenous peroxidase-blocking pretreatment was applied with 1% hydrogen–peroxide for 30 min in free-floating, then the slices were mounted on SuperFrost Ultra Plus adhesion slides (Thermo Fischer Scientific, Braunschweig, Germany, cat.no.: 10417002). The slides were baked at 60 °C for 1 h, then were postfixed with 4% PFA both before and after a proteinase-K treatment. Rat-specific probes Prrp (Bio-Techne, Minneapolis, MN, USA, cat. no.: 518741), Crh (cat. no.: 318931-C3), Vglut2 (cat. no.: 317011-C2), and Gad1 (cat. no.: 316401-C2) were hybridized with the samples. The assay (signal amplification and sequential channel development) was completed with RNAscope Multiplex Fluorescent Reagent Kit v.2 (Advanced Cell Diagnostics, Newark, CA, USA) according to the manufacturer’s protocol. 4′,6-diamidino-2-phenylindole (DAPI, cat. no.: 323108, Advanced Cell Diagnostics, Newark, CA, USA) was used to detect cell nuclei. Sections were cover-slipped with ProLong Antifade Mountant (Thermo Fischer Scientific) for confocal microscopy.
The sections with combined immunostaining were washed in PBS for 2 × 15 min after the channel development was complete and incubated overnight with polyclonal rabbit anti-PrRP31 (Phoenix Pharmaceuticals, Bulingame, CA, USA, cat. no.: H-008-52; diluted 1:2000 with 2% normal goat serum) or anti-TH (Abcam, Cambridge, UK, cat. no.: ab112; diluted 1:2000 with 2% normal goat serum). Then, sections were washed for 2 × 15 min in PBS and treated in Cyanine3-conjugated donkey anti-rabbit (Jackson Immunoresearch, St Thomas’ Place, UK, cat. no.: 711–165-152) or Alexa Fluor 647-conjugated donkey anti-rabbit (Jackson Immunoresearch, cat. no.: 711–605-152) secondary antibody, respectively, diluted 1:500 in PBS with 2% normal goat serum for 3 h at RT. After rinses, cell nuclei were stained with DAPI, and sections were covered with the mentioned mountant.
To obtain fluorescent images, an Olympus Fluoview FV-1000 laser scanning confocal microscope and FluoView FV-1000S-IX81 image acquisition software system (Olympus, Tokyo, Japan) were used. The confocal aperture was set to 80 µm. We conducted the analog sequential scanning with a 60 × objective lens (NA: 1.49 oil). We applied an optical thickness of 1 µm, and the resolution was set to 1024 × 1024 pixels. The excitation time was set to 4 µs per pixel. The following virtual colors were used for the fluorescent signals: blue (DAPI), red (Cy3), green (Fluorescein), and white (Cy5 and Alexa 647).
4.5. Statistics and Software
Raw data were organized in Microsoft Excel and outliers (outside 3 standard deviations) were winsorized. Preparing graphs as well as one-way ANOVA, followed by Tukey’s multiple comparisons test, were performed using GraphPad Prism version 10.4.1 for Windows, GraphPad Software, Boston, MA, USA. Figures were prepared in Microsoft Power Point with the help of BioRender online application and flaticon.com (both accessed on 25 April 2025). Data are represented as mean ± SEM.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms26094155/s1.
Author Contributions
Conceptualization D.Z., Z.E.T., V.K., and A.K.; investigation E.S., V.K., A.K., and D.Z.; data analysis, E.S.; writing—original draft preparation E.S.; writing—review and editing V.K., D.Z., Z.E.T., and A.K.; visualization E.S. and V.K.; supervision D.Z. and A.K.; funding acquisition D.Z. and A.K. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the PTE-AOK-KA-2022 project of the University of Pécs, the National Research Development and Innovation Office of Hungary (grant numbers K141934, K138763, and K146086); the ÚNKP-23-2-III-PTE-2068 National Excellence Program of the Ministry for Innovation and Technology; the National Brain Research Program (NAP 3.0) of the Hungarian Academy of Sciences; and the Thematic Excellence Program 2021 Health Subprogram of the Ministry for Innovation and Technology in Hungary within the framework of the TKP2021-EGA-16 project of Pécs University and TKP2021-EGA-25 project of Semmelweis University. V.K. was supported by the János Bolyai Research Scholarship of the Hungarian Academy of Sciences (BO/00750/22/5).
Institutional Review Board Statement
All experiments were approved by the Workplace Animal Welfare Committee of the University of Pécs and the National Scientific Ethical Committee on Animal Experimentation of Hungary (BA/73/00244-7/2023, Approval date: 30.03.2023) and performed according to the European Communities Council Directive recommendations for the care and use of laboratory animals (2010/63/EU). The authors complied with the ARRIVE guidelines.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data are available in the Supplementary Material.
Acknowledgments
The authors would like to thank the István Ábrahám Nano-Bioimaging Core Facility for their support in microscopy.
Conflicts of Interest
The authors declare that they have no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Liu, Q.; He, H.; Yang, J.; Feng, X.; Zhao, F.; Lyu, J. Changes in the Global Burden of Depression from 1990 to 2017: Findings from the Global Burden of Disease Study. J. Psychiatr. Res. 2020, 126, 134–140. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.; Krishan, P.; Goodwin, L.; Iduye, D.; de Los Godos, E.F.; Fryer, J.; Gallagher, K.; Hair, K.; O’Connell, E.; Ogarrio, K.; et al. Impact of Covid-19 Mitigations on Anxiety and Depression Amongst University Students: A Systematic Review and Meta-Analysis. J. Glob. Health 2023, 13, 06035. [Google Scholar] [CrossRef] [PubMed]
- Radulovic, D.; Masic, S.; Stanisavljevic, D.; Bokonjic, D.; Radevic, S.; Rajovic, N.; Milic, N.V.; Vukomanovic, I.S.; Mijovic, B.; Vukovic, M.; et al. A Complex Relationship between Quality of Life, Anxiety, and Depression among General Population during Second Year of Covid-19 Pandemic: A Population-Based Study. J. Clin. Med. 2024, 13, 3874. [Google Scholar] [CrossRef] [PubMed]
- Lenzo, V.; Sardella, A.; Musetti, A.; Quattropani, M.C.; Franceschini, C. Longitudinal Associations between Resilience and Mental Health during the Covid-19 Pandemic. Clin. Neuropsychiatry 2024, 21, 189–194. [Google Scholar] [CrossRef]
- Chang, O.; Levitt, A.; Khalid, M.; Kodeeswaran, S.; Markoulakis, R. The Prevalence of Mental Health and Addiction Concerns and Factors Associated with Depression and Anxiety during the Covid-19 Pandemic in Ontario, Canada: A Cross-Sectional Study. PLoS ONE 2024, 19, e0305229. [Google Scholar] [CrossRef]
- Compas, B.E.; Jaser, S.S.; Bettis, A.H.; Watson, K.H.; Gruhn, M.A.; Dunbar, J.P.; Williams, E.; Thigpen, J.C. Coping, Emotion Regulation, and Psychopathology in Childhood and Adolescence: A Meta-Analysis and Narrative Review. Psychol. Bull. 2017, 143, 939–991. [Google Scholar] [CrossRef]
- Holahan, C.J.; Moos, R.H. Personal and Contextual Determinants of Coping Strategies. J. Pers. Soc. Psychol 1987, 52, 946–955. [Google Scholar] [CrossRef]
- Billings, A.G.; Moos, R.H. Coping, Stress, and Social Resources among Adults with Unipolar Depression. J. Pers. Soc. Psychol 1984, 46, 877–891. [Google Scholar] [CrossRef]
- World Health, Assembly. Global Burden of Mental Disorders and the Need for a Comprehensive, Coordinated Response from Health and Social Sectors at the Country Level: Report by the Secretariat; World Health Organization: Geneva, Switzerland, 2012. [Google Scholar]
- Voineskos, D.; Daskalakis, Z.J.; Blumberger, D.M. Management of Treatment-Resistant Depression: Challenges and Strategies. Neuropsychiatr. Dis. Treat. 2020, 16, 221–234. [Google Scholar] [CrossRef]
- Kovacs, A.; Szabo, E.; Laszlo, K.; Kertes, E.; Zagoracz, O.; Mintal, K.; Toth, A.; Galosi, R.; Berta, B.; Lenard, L.; et al. Brain Rfamide Neuropeptides in Stress-Related Psychopathologies. Cells 2024, 13, 1097. [Google Scholar] [CrossRef]
- Vas, S.; Papp, R.S.; Konczol, K.; Bogathy, E.; Papp, N.; Adori, C.; Durst, M.; Sipos, K.; Ocskay, K.; Farkas, I.; et al. Prolactin-Releasing Peptide Contributes to Stress-Related Mood Disorders and Inhibits Sleep/Mood Regulatory Melanin-Concentrating Hormone Neurons in Rats. J. Neurosci. 2023, 43, 846–862. [Google Scholar] [CrossRef] [PubMed]
- Toth, Z.E.; Zelena, D.; Mergl, Z.; Kirilly, E.; Varnai, P.; Mezey, E.; Makara, G.B.; Palkovits, M. Chronic Repeated Restraint Stress Increases Prolactin-Releasing Peptide/Tyrosine-Hydroxylase Ratio with Gender-Related Differences in the Rat Brain. J. Neurochem. 2008, 104, 653–666. [Google Scholar] [CrossRef]
- Matuska, R.; Zelena, D.; Konczol, K.; Papp, R.S.; Durst, M.; Guba, D.; Torok, B.; Varnai, P.; Toth, Z.E. Colocalized Neurotransmitters in the Hindbrain Cooperate in Adaptation to Chronic Hypernatremia. Brain Struct. Funct. 2020, 225, 969–984. [Google Scholar] [CrossRef] [PubMed]
- Fujii, R.; Fukusumi, S.; Hosoya, M.; Kawamata, Y.; Habata, Y.; Hinuma, S.; Sekiguchi, M.; Kitada, C.; Kurokawa, T.; Nishimura, O.; et al. Tissue Distribution of Prolactin-Releasing Peptide (Prrp) and Its Receptor. Regul. Pept. 1999, 83, 1–10. [Google Scholar] [CrossRef]
- Chen, C.; Dun, S.L.; Dun, N.J.; Chang, J.K. Prolactin-Releasing Peptide-Immunoreactivity in A1 and A2 Noradrenergic Neurons of the Rat Medulla. Brain Res. 1999, 822, 276–279. [Google Scholar] [CrossRef]
- Pacak, K.; Palkovits, M.; Kopin, I.J.; Goldstein, D.S. Stress-Induced Norepinephrine Release in the Hypothalamic Paraventricular Nucleus and Pituitary-Adrenocortical and Sympathoadrenal Activity: In Vivo Microdialysis Studies. Front. Neuroendocrinol. 1995, 16, 89–150. [Google Scholar] [CrossRef]
- Dodd, G.T.; Luckman, S.M. Physiological Roles of Gpr10 and Prrp Signaling. Front. Endocrinol. 2013, 4, 20. [Google Scholar] [CrossRef]
- Hinuma, S.; Habata, Y.; Fujii, R.; Kawamata, Y.; Hosoya, M.; Fukusumi, S.; Kitada, C.; Masuo, Y.; Asano, T.; Matsumoto, H.; et al. A Prolactin-Releasing Peptide in the Brain. Nature 1998, 393, 272–276. [Google Scholar] [CrossRef] [PubMed]
- Langmead, C.J.; Szekeres, P.G.; Chambers, J.K.; Ratcliffe, S.J.; Jones, D.N.; Hirst, W.D.; Price, G.W.; Herdon, H.J. Characterization of the Binding of [(125)I]-Human Prolactin Releasing Peptide (Prrp) to Gpr10, a Novel G Protein Coupled Receptor. Br. J. Pharmacol. 2000, 131, 683–688. [Google Scholar] [CrossRef]
- Ma, L.; MacTavish, D.; Simonin, F.; Bourguignon, J.J.; Watanabe, T.; Jhamandas, J.H. Prolactin-Releasing Peptide Effects in the Rat Brain Are Mediated through the Neuropeptide Ff Receptor. Eur. J. Neurosci. 2009, 30, 1585–1593. [Google Scholar] [CrossRef]
- Raadsheer, F.C.; van Heerikhuize, J.J.; Lucassen, P.J.; Hoogendijk, W.J.; Tilders, F.J.; Swaab, D.F. Corticotropin-Releasing Hormone Mrna Levels in the Paraventricular Nucleus of Patients with Alzheimer’s Disease and Depression. Am. J. Psychiatry 1995, 152, 1372–1376. [Google Scholar] [CrossRef]
- Yoshida, M.; Takayanagi, Y.; Onaka, T. The Medial Amygdala-Medullary Prrp-Synthesizing Neuron Pathway Mediates Neuroendocrine Responses to Contextual Conditioned Fear in Male Rodents. Endocrinology 2014, 155, 2996–3004. [Google Scholar] [CrossRef] [PubMed]
- Maruyama, M.; Matsumoto, H.; Fujiwara, K.; Noguchi, J.; Kitada, C.; Hinuma, S.; Onda, H.; Nishimura, O.; Fujino, M.; Higuchi, T.; et al. Central Administration of Prolactin-Releasing Peptide Stimulates Oxytocin Release in Rats. Neurosci. Lett. 1999, 276, 193–196. [Google Scholar] [CrossRef] [PubMed]
- Samson, W.K.; Resch, Z.T.; Murphy, T.C. A Novel Action of the Newly Described Prolactin-Releasing Peptides: Cardiovascular Regulation. Brain Res. 2000, 858, 19–25. [Google Scholar] [CrossRef]
- Matsumoto, H.; Maruyama, M.; Noguchi, J.; Horikoshi, Y.; Fujiwara, K.; Kitada, C.; Hinuma, S.; Onda, H.; Nishimura, O.; Inoue, K.; et al. Stimulation of Corticotropin-Releasing Hormone-Mediated Adrenocorticotropin Secretion by Central Administration of Prolactin-Releasing Peptide in Rats. Neurosci. Lett. 2000, 285, 234–238. [Google Scholar] [CrossRef] [PubMed]
- Mera, T.; Fujihara, H.; Kawasaki, M.; Hashimoto, H.; Saito, T.; Shibata, M.; Saito, J.; Oka, T.; Tsuji, S.; Onaka, T.; et al. Prolactin-Releasing Peptide Is a Potent Mediator of Stress Responses in the Brain through the Hypothalamic Paraventricular Nucleus. Neuroscience 2006, 141, 1069–1086. [Google Scholar] [CrossRef]
- Bao, A.M.; Swaab, D.F. The Human Hypothalamus in Mood Disorders: The Hpa Axis in the Center. IBRO Rep. 2019, 6, 45–53. [Google Scholar] [CrossRef]
- Porsolt, R.D.; Anton, G.; Blavet, N.; Jalfre, M. Behavioural Despair in Rats: A New Model Sensitive to Antidepressant Treatments. Eur. J. Pharmacol. 1978, 47, 379–391. [Google Scholar] [CrossRef]
- Porsolt, R.D.; Bertin, A.; Jalfre, M. Behavioral Despair in Mice: A Primary Screening Test for Antidepressants. Arch. Int. Pharmacodyn. Ther. 1977, 229, 327–336. [Google Scholar] [CrossRef]
- Molendijk, M.L.; de Kloet, E.R. Forced Swim Stressor: Trends in Usage and Mechanistic Consideration. Eur. J. Neurosci. 2022, 55, 2813–2831. [Google Scholar] [CrossRef]
- Nestler, E.J.; Hyman, S.E. Animal Models of Neuropsychiatric Disorders. Nat. Neurosci. 2010, 13, 1161–1169. [Google Scholar] [CrossRef] [PubMed]
- Molendijk, M.L.; de Kloet, E.R. Immobility in the Forced Swim Test Is Adaptive and Does Not Reflect Depression. Psychoneuroendocrinology 2015, 62, 389–391. [Google Scholar] [CrossRef] [PubMed]
- de Kloet, E.R.; Molendijk, M.L. Coping with the Forced Swim Stressor: Towards Understanding an Adaptive Mechanism. Neural Plast 2016, 2016, 6503162. [Google Scholar] [CrossRef] [PubMed]
- Becker, L.; Mallien, A.S.; Pfeiffer, N.; Brandwein, C.; Talbot, S.R.; Bleich, A.; Palme, R.; Potschka, H.; Gass, P. Evidence-Based Severity Assessment of the Forced Swim Test in the Rat. PLoS ONE 2023, 18, e0292816. [Google Scholar] [CrossRef]
- Ruiz-Sanchez, E.; Lopez-Ramirez, A.M.; Ruiz-Chow, A.; Calvillo, M.; Resendiz-Albor, A.A.; Anguiano, B.; Rojas, P. Variability in Behavioral Phenotypes after Forced Swimming-Induced Stress in Rats Is Associated with Expression of the Glucocorticoid Receptor, Nurr1, and Il-1beta in the Hippocampus. Int. J. Mol. Sci. 2021, 22, 12700. [Google Scholar] [CrossRef]
- Kokras, N.; Antoniou, K.; Mikail, H.G.; Kafetzopoulos, V.; Papadopoulou-Daifoti, Z.; Dalla, C. Forced Swim Test: What About Females? Neuropharmacology 2015, 99, 408–421. [Google Scholar] [CrossRef]
- Bundzikova-Osacka, J.; Ghosal, S.; Packard, B.A.; Ulrich-Lai, Y.M.; Herman, J.P. Role of Nucleus of the Solitary Tract Noradrenergic Neurons in Post-Stress Cardiovascular and Hormonal Control in Male Rats. Stress 2015, 18, 221–232. [Google Scholar] [CrossRef]
- Anderson, S.T.; Kokay, I.C.; Lang, T.; Grattan, D.R.; Curlewis, J.D. Quantification of Prolactin-Releasing Peptide (Prrp) Mrna Expression in Specific Brain Regions of the Rat during the Oestrous Cycle and in Lactation. Brain Res. 2003, 973, 64–73. [Google Scholar] [CrossRef]
- Maruyama, M.; Matsumoto, H.; Fujiwara, K.; Noguchi, J.; Kitada, C.; Fujino, M.; Inoue, K. Prolactin-Releasing Peptide as a Novel Stress Mediator in the Central Nervous System. Endocrinology 2001, 142, 2032–2038. [Google Scholar] [CrossRef]
- Morales, T.; Hinuma, S.; Sawchenko, P.E. Prolactin-Releasing Peptide Is Expressed in Afferents to the Endocrine Hypothalamus, but Not in Neurosecretory Neurones. J. Neuroendocrinol. 2000, 12, 131–140. [Google Scholar] [CrossRef]
- Granata, A.R.; Numao, Y.; Kumada, M.; Reis, D.J. A1 Noradrenergic Neurons Tonically Inhibit Sympathoexcitatory Neurons of C1 Area in Rat Brainstem. Brain Res. 1986, 377, 127–146. [Google Scholar] [CrossRef] [PubMed]
- Brouillard, C.; Carrive, P.; Camus, F.; Benoliel, J.J.; Sevoz-Couche, C. Vulnerability to Stress Consequences Induced by Repeated Social Defeat in Rats: Contribution of the Angiotensin Ii Type 1 Receptor in Cardiovascular Alterations Associated to Low Brain Derived Neurotrophic Factor. Eur. J. Pharmacol. 2019, 861, 172595. [Google Scholar] [CrossRef] [PubMed]
- Zahner, M.R.; Hillard, K.J.; Chandley, M.C. The Role of the Dorsomedial Hypothalamus in the Cardiogenic Sympathetic Reflex in the Sprague Dawley Rat. Front. Physiol. 2024, 15, 1479892. [Google Scholar] [CrossRef]
- Bondarenko, E.; Beig, M.I.; Hodgson, D.M.; Braga, V.A.; Nalivaiko, E. Blockade of the Dorsomedial Hypothalamus and the Perifornical Area Inhibits Respiratory Responses to Arousing and Stressful Stimuli. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2015, 308, R816–R822. [Google Scholar] [CrossRef]
- Renner, E.; Szabo-Meltzer, K.I.; Puskas, N.; Toth, Z.E.; Dobolyi, A.; Palkovits, M. Activation of Neurons in the Hypothalamic Dorsomedial Nucleus Via Hypothalamic Projections of the Nucleus of the Solitary Tract Following Refeeding of Fasted Rats. Eur. J. Neurosci. 2010, 31, 302–314. [Google Scholar] [CrossRef]
- Teng, T.; Fan, L.; Yan, W.; Li, X.; Zhang, Y.; Xiang, Y.; Jiang, Y.; Yuan, K.; Yin, B.; Shi, L.; et al. A Diathesis-Stress Rat Model Induced Suicide-Implicated Endophenotypes and Prefrontal Cortex Abnormalities in the Pka and Gaba Receptor Signaling Pathways. Prog. Neuropsychopharmacol. Biol. Psychiatry 2022, 116, 110538. [Google Scholar] [CrossRef]
- Lin, S.H.; Arai, A.C.; Wang, Z.; Nothacker, H.P.; Civelli, O. The Carboxyl Terminus of the Prolactin-Releasing Peptide Receptor Interacts with Pdz Domain Proteins Involved in Alpha-Amino-3-Hydroxy-5-Methylisoxazole-4-Propionic Acid Receptor Clustering. Mol. Pharmacol. 2001, 60, 916–923. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, E.T.; Sawchenko, P.E. Anatomical Specificity of Noradrenergic Inputs to the Paraventricular and Supraoptic Nuclei of the Rat Hypothalamus. J. Comp. Neurol. 1988, 274, 60–76. [Google Scholar] [CrossRef]
- Kannan, H.; Kasai, M.; Osaka, T.; Yamashita, H. Neurons in the Paraventricular Nucleus Projecting to the Median Eminence: A Study of Their Afferent Connections from Peripheral Baroreceptors, and from the A1-Catecholaminergic Area in the Ventrolateral Medulla. Brain Res. 1987, 409, 358–363. [Google Scholar] [CrossRef]
- Aguilera, G.; Liu, Y. The Molecular Physiology of Crh Neurons. Front. Neuroendocrinol. 2012, 33, 67–84. [Google Scholar] [CrossRef]
- Chaves, T.; Fazekas, C.L.; Horvath, K.; Correia, P.; Szabo, A.; Torok, B.; Banrevi, K.; Zelena, D. Stress Adaptation and the Brainstem with Focus on Corticotropin-Releasing Hormone. Int. J. Mol. Sci. 2021, 22, 9090. [Google Scholar] [CrossRef]
- Ramirez-Plascencia, O.D.; De Luca, R.; Machado, N.L.S.; Eghlidi, D.; Khanday, M.A.; Bandaru, S.S.; Raffin, F.; Vujovic, N.; Arrigoni, E.; Saper, C.B. A Hypothalamic Circuit for Circadian Regulation of Corticosterone Secretion. Res. Sq. 2024, 3, 4718850. [Google Scholar] [CrossRef]
- Levit-Binnun, N.; Golland, Y. Finding Behavioral and Network Indicators of Brain Vulnerability. Front. Hum. Neurosci. 2011, 6, 10. [Google Scholar] [CrossRef] [PubMed]
- Cattane, N.; Mazzelli, M.; Begni, V.; Mombelli, E.; Papp, M.; Maj, C.; Riva, M.A.; Cattaneo, A. Molecular Mechanisms Underlying Stress Vulnerability and Resilience in the Chronic Mild Stress Model: New Insights from Mrna and Mirnas Data Combining. Brain Behav. Immun. 2024, 121, 340–350. [Google Scholar] [CrossRef] [PubMed]
- Halladay, L.R.; Herron, S.M. Lasting Impact of Postnatal Maternal Separation on the Developing Bnst: Lifelong Socioemotional Consequences. Neuropharmacology 2023, 225, 109404. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.H.; Leslie, F.M.; Civelli, O. Neurochemical Properties of the Prolactin Releasing Peptide (Prrp) Receptor Expressing Neurons: Evidence for a Role of Prrp as a Regulator of Stress and Nociception. Brain Res. 2002, 952, 15–30. [Google Scholar] [CrossRef]
- Vadovicova, K. Affective and Cognitive Prefrontal Cortex Projections to the Lateral Habenula in Humans. Front. Hum. Neurosci. 2014, 8, 819. [Google Scholar] [CrossRef]
- Benekareddy, M.; Stachniak, T.J.; Bruns, A.; Knoflach, F.; von Kienlin, M.; Kunnecke, B.; Ghosh, A. Identification of a Corticohabenular Circuit Regulating Socially Directed Behavior. Biol. Psychiatry 2018, 83, 607–617. [Google Scholar] [CrossRef]
- Mathis, V.P.; Williams, M.; Fillinger, C.; Kenny, P.J. Networks of Habenula-Projecting Cortical Neurons Regulate Cocaine Seeking. Sci. Adv. 2021, 7, eabj2225. [Google Scholar] [CrossRef]
- Taraku, B.; Loureiro, J.R.; Sahib, A.K.; Zavaliangos-Petropulu, A.; Al-Sharif, N.; Leaver, A.M.; Wade, B.; Joshi, S.; Woods, R.P.; Espinoza, R.; et al. Modulation of Habenular and Nucleus Accumbens Functional Connectivity by Ketamine in Major Depression. Brain Behav. 2024, 14, e3511. [Google Scholar] [CrossRef]
- Zhukovskaya, A.; Zimmerman, C.A.; Willmore, L.; Vazquez, A.P.; Janarthanan, S.; Lynch, L.A.; Falkner, A.L.; Witten, I.B. Heightened Lateral Habenula Activity during Stress Produces Brainwide and Behavioral Substrates of Susceptibility. Neuron 2024, 112, 3940–3956.e10. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Li, P.; Li, Z.; da Silva, B.S.; Zheng, W.; Xiang, Z.; He, Y.; Xu, T.; Cordeiro, C.; Deng, L.; et al. Lateral Septum Adenosine a(2a) Receptors Control Stress-Induced Depressive-Like Behaviors Via Signaling to the Hypothalamus and Habenula. Nat. Commun. 2023, 14, 1880. [Google Scholar] [CrossRef]
- Yang, M.; Tian, S.; Han, X.; Xu, L.; You, J.; Wu, M.; Cao, Y.; Jiang, Y.; Zheng, Z.; Liu, J.; et al. Interleukin-11ralpha2 in the Hypothalamic Arcuate Nucleus Affects Depression-Related Behaviors and the Akt-Bdnf Pathway. Gene 2025, 933, 148966. [Google Scholar] [CrossRef]
- Fang, X.; Chen, Y.; Wang, J.; Zhang, Z.; Bai, Y.; Denney, K.; Gan, L.; Guo, M.; Weintraub, N.L.; Lei, Y.; et al. Increased Intrinsic and Synaptic Excitability of Hypothalamic Pomc Neurons Underlies Chronic Stress-Induced Behavioral Deficits. Mol. Psychiatry 2023, 28, 1365–1382. [Google Scholar] [CrossRef]
- Qu, N.; He, Y.; Wang, C.; Xu, P.; Yang, Y.; Cai, X.; Liu, H.; Yu, K.; Pei, Z.; Hyseni, I.; et al. A Pomc-Originated Circuit Regulates Stress-Induced Hypophagia, Depression, and Anhedonia. Mol. Psychiatry 2020, 25, 1006–1021. [Google Scholar] [CrossRef] [PubMed]
- Watanobe, H. In Vivo Release of Prolactin-Releasing Peptide in Rat Hypothalamus in Association with Luteinizing Hormone and Prolactin Surges. Neuroendocrinology 2001, 74, 359–366. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.F.; Yang, S.C.; Pan, J.T. Effects of Prolactin-Releasing Peptide on Tuberoinfundibular Dopaminergic Neuronal Activity and Prolactin Secretion in Estrogen-Treated Female Rats. J. Biomed. Sci. 2002, 9, 112–118. [Google Scholar] [CrossRef]
- Segi-Nishida, E.; Sukeno, M.; Imoto, Y.; Kira, T.; Sakaida, M.; Tsuchiya, S.; Sugimoto, Y.; Okuno, Y. Electroconvulsive Seizures Activate Anorexigenic Signals in the Ventromedial Nuclei of the Hypothalamus. Neuropharmacology 2013, 71, 164–173. [Google Scholar] [CrossRef]
- Paxinos, G.; Watson, C. The Rat Brain in Stereotaxic Coordinates; Academic Press: New York, NY, USA, 2014. [Google Scholar]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative Pcr and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Konkoly, J.; Kormos, V.; Gaszner, B.; Sandor, Z.; Kecskes, A.; Alomari, A.; Szilagyi, A.; Szilagyi, B.; Zelena, D.; Pinter, E. The Role of Trpa1 Channels in the Central Processing of Odours Contributing to the Behavioural Responses of Mice. Pharmaceuticals 2021, 14, 1336. [Google Scholar] [CrossRef]
- Nemes, B.; Bolcskei, K.; Kecskes, A.; Kormos, V.; Gaszner, B.; Aczel, T.; Hegedus, D.; Pinter, E.; Helyes, Z.; Sandor, Z. Human Somatostatin Sst(4) Receptor Transgenic Mice: Construction and Brain Expression Pattern Characterization. Int. J. Mol. Sci. 2021, 22, 3758. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).