Nitrate Modulates Fruit Lignification by Regulating CgLAC3 Expression in Pomelo
Abstract
1. Introduction
2. Results
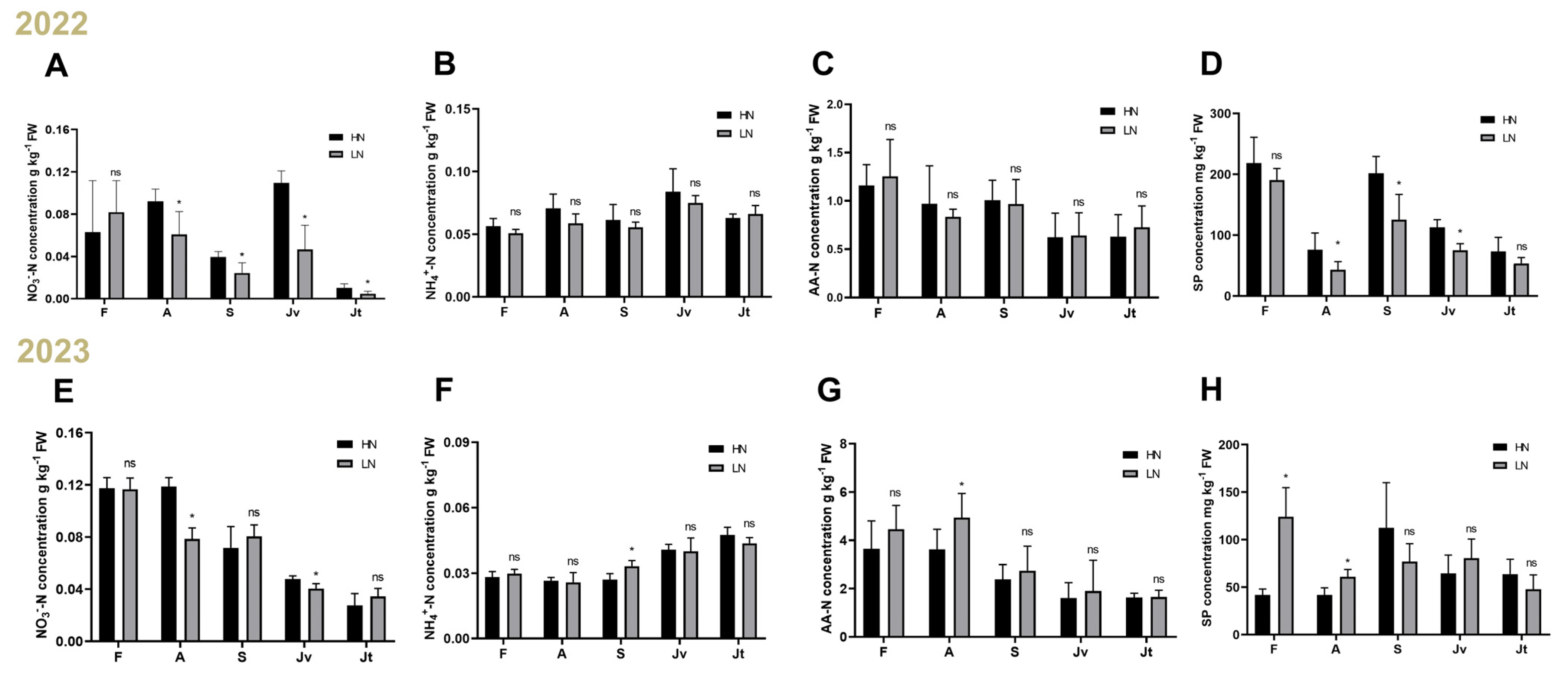
2.1. Nitrate Is an Important Determinant of Fruit Lignification
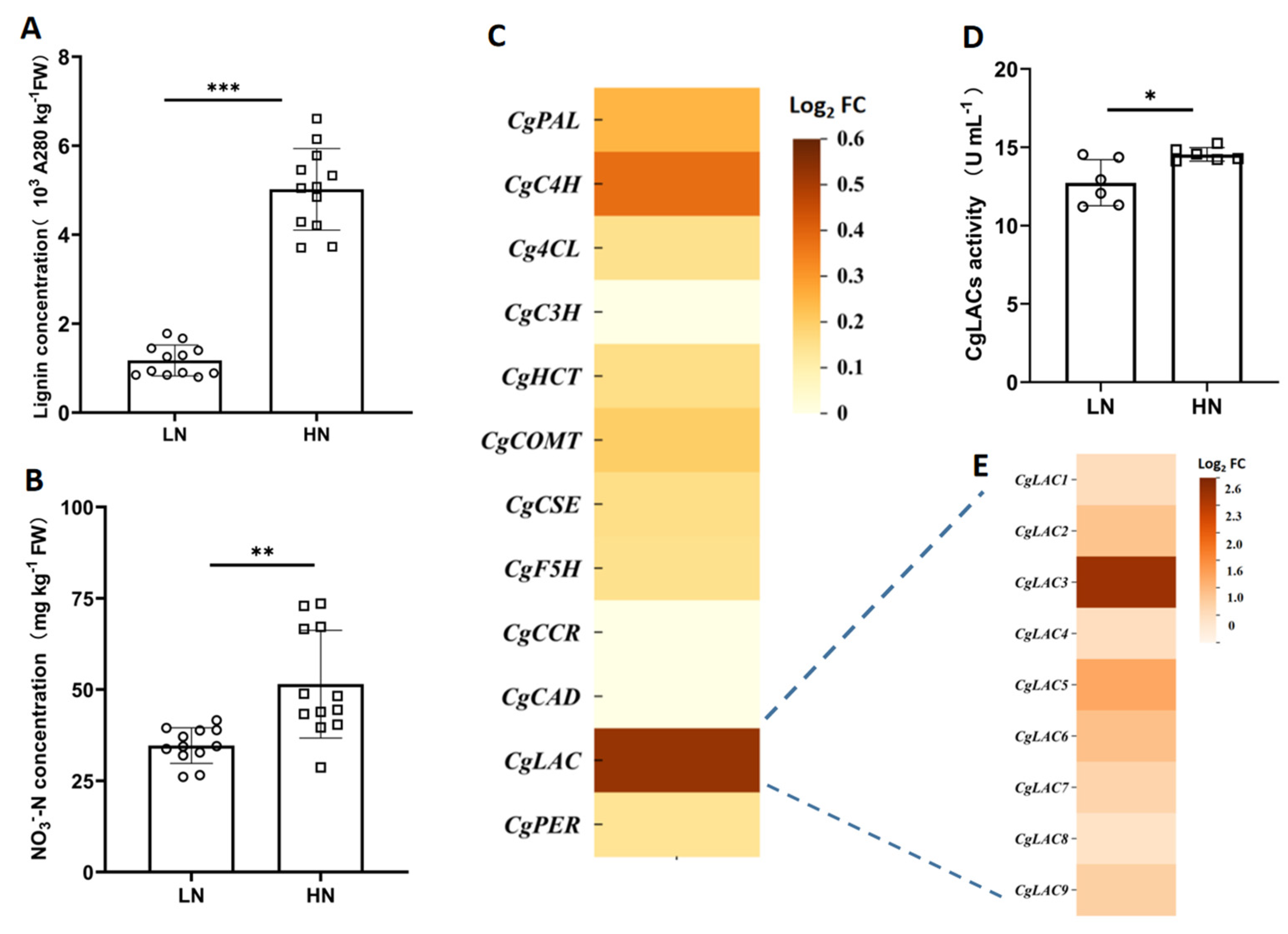
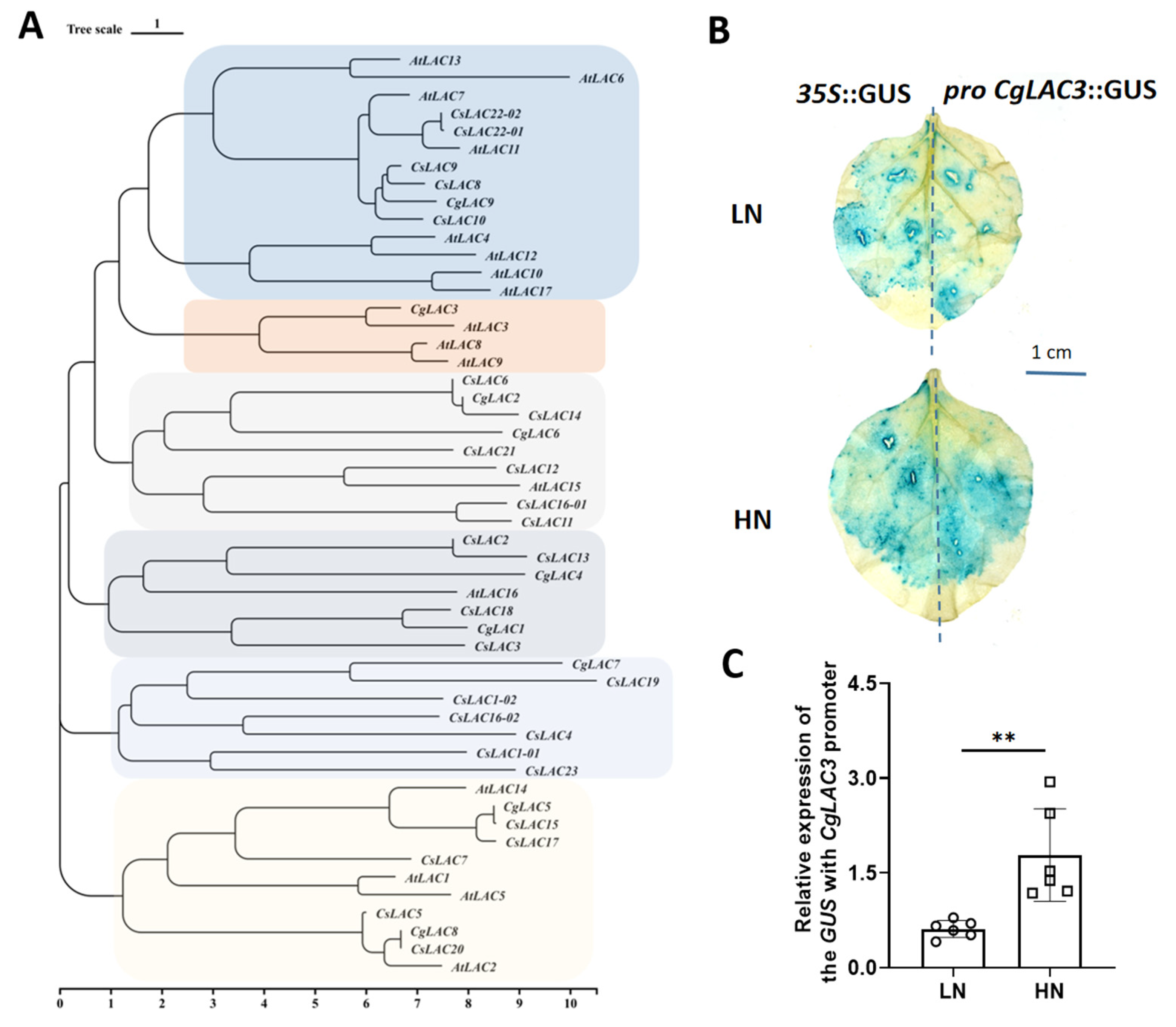
2.2. Candidate Genes Involved in Regulating Nitrate Effects on Lignification
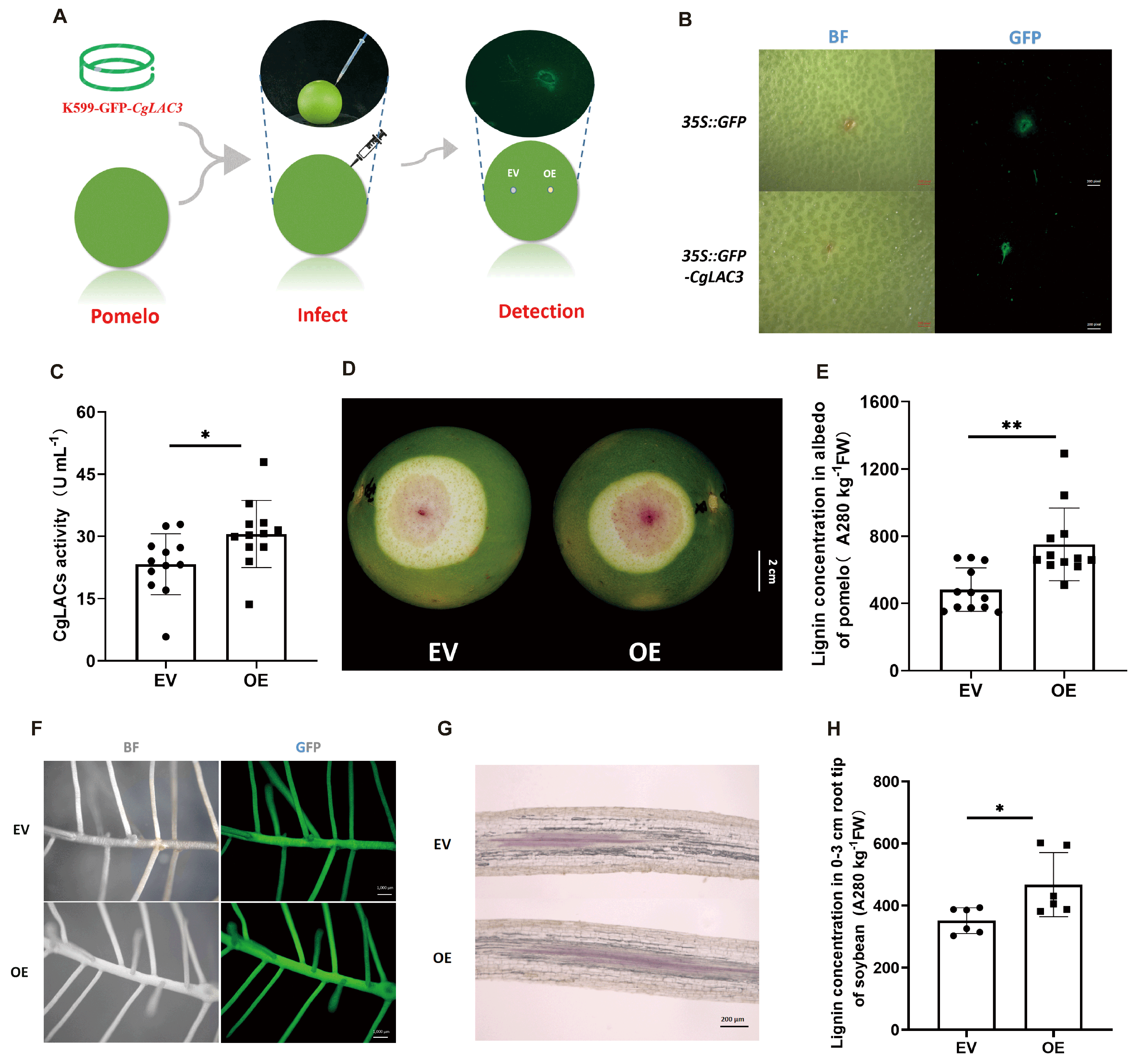
2.3. Over-Expression of CgLAC3 Promotes Lignification in Both Pomelo Albedo and Soybean Hairy Roots
3. Discussion
3.1. Nitrate Plays an Important Role in Regulation of Lignin Biosynthesis in Plants
3.2. CgLAC3 Is the Key Nitrate-Responsive Lignification Gene in Pomelo Fruits
4. Materials and Methods
4.1. Plant Materials
4.2. Determination of Fruit Indicators
4.2.1. Nutrient Determination
4.2.2. Lignin Staining and Lignin Determination
4.2.3. LAC Enzyme Activity Determination
4.3. RNA Isolation and qRT-PCR Analysis
4.4. Data Analysiss
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Food and Agriculture Organization. Food and Agriculture Data. 2022. Available online: https://www.fao.org/statistics/data-collection/general/en (accessed on 22 April 2025).
- China Statistics Press. China Statistical Yearbook; China Statistics Press: Beijing, China, 2022; pp. 372–402. Available online: https://www.stats.gov.cn/sj/ndsj/ (accessed on 22 April 2025). (In Chinese)
- Lai, C.H.; Hu, Y.C.; Li, P.J.; Xu, Y.J.; Wang, H.M.; Wang, H.X.; Liao, H. Nutrient allocation in pomelo fruits and its relationship with the quality of juice sac. South China Fruits 2024, 53, 66–72. (In Chinese) [Google Scholar]
- Lai, C.H.; Wang, H.X.; Hu, Y.C.; Xu, Y.J.; Liao, H. Analysis of structure feature of granulated juice sacs in pomelo fruits. South China Fruits 2024, 53, 44–49. (In Chinese) [Google Scholar]
- Zhang, X.; Zhuang, X.; Chen, M.; Wang, J.; Qiu, D.; Liu, Z.; Huang, Y.; Zhang, L.; Liu, Z. An environmentally friendly production method: The pectin and essential oil from the waste peel of juvenile pomelo (Citrus maxima ‘Shatian Yu’) were extracted simultaneously in one step with an acid-based deep eutectic solvent. LWT-Food Sci. Technol. 2024, 206, 116622. [Google Scholar] [CrossRef]
- Li, Q.; Yao, S.; Deng, L.; Zeng, K. Changes in biochemical properties and pectin nanostructures of juice sacs during the granulation process of pomelo fruit (Citrus grandis). Food Chem. 2022, 376, 131876. [Google Scholar] [CrossRef]
- Wu, J.; Pan, T.; Guo, Z.; Pan, M. Specific lignin accumulation in granulated juice sacs of Citrus maxima. J. Agric. Food Chem. 2014, 62, 12082–12089. [Google Scholar] [CrossRef]
- Shi, M.; Liu, X.; Zhang, H.; He, Z.; Yang, H.; Chen, J.; Feng, J.; Yang, W.; Jiang, Y.; Yao, J.L.; et al. The IAA- and ABA-responsive transcription factor CgMYB58 upregulates lignin biosynthesis and triggers juice sac granulation in pummelo. Hortic. Res. 2020, 7, 139. [Google Scholar] [CrossRef]
- Cai, Y.; Li, G.; Nie, J.; Lin, Y.; Nie, F.; Zhang, J. Study of the structure and biosynthetic pathway of lignin in stone cells of pear. Sci. Hortic. 2010, 125, 374–379. [Google Scholar] [CrossRef]
- Xue, C.; Yao, J.L.; Qin, M.F.; Zhang, M.Y.; Allan, A.C.; Wang, D.F.; Wu, J. PbrmiR397a regulates lignification during stone cell development in pear fruit. Plant Biotechnol. J. 2019, 17, 103–117. [Google Scholar] [CrossRef]
- Xu, S.; Sun, M.; Yao, J.L.; Liu, X.; Xue, Y.; Yang, G.; Zhu, R.; Jiang, W.; Wang, R.; Xue, C.; et al. Auxin inhibits lignin and cellulose biosynthesis in stone cells of pear fruit via the PbrARF13-PbrNSC-PbrMYB132 transcriptional regulatory cascade. Plant Biotechnol. J. 2023, 21, 1408–1425. [Google Scholar] [CrossRef]
- Xue, Y.; Shan, Y.; Yao, J.L.; Wang, R.; Xu, S.; Liu, D.; Ye, Z.; Lin, J.; Li, X.; Xue, C.; et al. The transcription factor PbrMYB24 regulates lignin and cellulose biosynthesis in stone cells of pear fruits. Plant Physiol. 2023, 192, 1997–2014. [Google Scholar] [CrossRef]
- Xu, Q.; Yin, X.R.; Zeng, J.K.; Ge, H.; Song, M.; Xu, C.J.; Li, X.; Ferguson, I.B.; Chen, K.S. Activator- and repressor-type MYB transcription factors are involved in chilling injury induced flesh lignification in loquat via their interactions with the phenylpropanoid pathway. J. Exp. Bot. 2014, 65, 4349–4359. [Google Scholar] [CrossRef]
- Zeng, J.K.; Li, X.; Xu, Q.; Chen, J.Y.; Yin, X.R.; Ferguson, I.B.; Chen, K.S. EjAP2-1, an AP2/ERF gene, is a novel regulator of fruit lignification induced by chilling injury, via interaction with EjMYB transcription factors. Plant Biotechnol. J. 2015, 13, 1325–1334. [Google Scholar] [CrossRef] [PubMed]
- Zhu, N.; Zhao, C.; Wei, Y.; Sun, C.; Wu, D.; Chen, K. Biosynthetic labeling with 3-O-propargylcaffeyl alcohol reveals in vivo cell-specific patterned lignification in loquat fruits during development and postharvest storage. Hortic. Res. 2021, 8, 61. [Google Scholar] [CrossRef] [PubMed]
- Jia, N.; Liu, J.; Sun, Y.; Tan, P.; Cao, H.; Xie, Y.; Wen, B.; Gu, T.; Liu, J.; Li, M.; et al. Citrus sinensis MYB transcription factors CsMYB330 and CsMYB308 regulate fruit juice sac lignification through fine-tuning expression of the Cs4CL1 gene. Plant Sci. 2018, 277, 334–343. [Google Scholar] [CrossRef]
- Jia, N.; Liu, J.; Tan, P.; Sun, Y.; Lv, Y.; Liu, J.; Sun, J.; Huang, Y.; Lu, J.; Jin, N.; et al. Citrus sinensis MYB transcription factor CsMYB85 induce fruit juice sac lignification through interaction with other CsMYB transcription factors. Front. Plant Sci. 2019, 10, 213. [Google Scholar] [CrossRef] [PubMed]
- Yao, S.X.; Cao, Q.; Xie, J.; Deng, L.; Zeng, K. Alteration of sugar and organic acid metabolism in postharvest granulation of Ponkan fruit revealed by transcriptome profiling. Postharvest Biol. Technol. 2018, 139, 2–11. [Google Scholar] [CrossRef]
- Miao, C.; Hu, Z.; Liu, X.; Ye, H.; Jiang, H.; Tan, J.; Chen, J. Transcriptome analysis of nitrate enhanced tobacco resistance to aphid infestation. Plant Physiol. Biochem. 2025, 220, 109514. [Google Scholar] [CrossRef]
- Boudet, A.M.; Kajita, S.; Grima-Pettenati, J.; Goffner, D. Lignins and lignocellulosics: A better control of synthesis for new and improved uses. Trends Plant Sci. 2003, 8, 576–581. [Google Scholar] [CrossRef]
- Raes, J.; Rohde, A.; Christensen, J.H.; Van-de-Peer, Y.; Boerjan, W. Genome-wide characterization of the lignification toolbox in Arabidopsis. Plant Physiol. 2003, 133, 1051–1071. [Google Scholar] [CrossRef]
- Hoengenaert, L.; Wouters, M.; Kim, H.; De Meester, B.; Morreel, K.; Vandersyppe, S.; Pollier, J.; Desmet, S.; Goeminne, G.; Ralph, J.; et al. Overexpression of the scopoletin biosynthetic pathway enhances lignocellulosic biomass processing. Sci. Adv. 2022, 8, eabo5738. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, L.; Wang, Z.; Zhang, R.; Liu, P.; Liu, M.; Liu, Z.; Zhao, Z.; Wang, L.; Chen, X.; et al. The regulation of cell wall lignification and lignin biosynthesis during pigmentation of winter jujube. Hortic. Res. 2021, 8, 238. [Google Scholar] [CrossRef]
- Zhao, Q.; Nakashima, J.; Chen, F.; Yin, Y.; Fu, C.; Yun, J.; Shao, H.; Wang, X.; Wang, Z.Y.; Dixon, R.A. Laccase is necessary and nonredundant with peroxidase for lignin polymerization during vascular development in Arabidopsis. Plant Cell 2013, 25, 3976–3987. [Google Scholar] [CrossRef]
- Li, W.; Sun, H.; Wang, G.; Sui, W.; Dai, L.; Si, C. Lignin as a green and multifunctional alternative to phenol for resin synthesis. Green Chem. 2023, 25, 2241–2261. [Google Scholar] [CrossRef]
- Zhuang, Y.; Zuo, D.; Tao, Y.; Cai, H.; Li, L. Laccase3-based extracellular domain provides possible positional information for directing Casparian strip formation in Arabidopsis. Proc. Natl. Acad. Sci. USA 2020, 117, 15400–15402. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Xiao, S.; Guan, Q.; Tu, L.; Sheng, F.; Du, X.; Zhang, X. The laccase gene GhLac1 modulates fiber initiation and elongation by coordinating jasmonic acid and flavonoid metabolism. Crop J. 2020, 8, 522–533. [Google Scholar] [CrossRef]
- Gong, Y.; Song, J.; Du, L.; Vinqvist, M.; Palmer, L.; Fillmore, S.; Pang, X.; Zhang, Z. Characterization of laccase from apple fruit during postharvest storage and its response to diphenylamine and 1-methylcyclopropene treatments. Food Chem. 2018, 253, 314–321. [Google Scholar]
- Zhao, G.; Xiang, F.; Zhang, S.; Song, J.; Li, X.; Song, L.; Zhai, R.; Yang, C.; Wang, Z.; Ma, F.; et al. PbLAC4-like, activated by PbMYB26, related to the degradation of anthocyanin during color fading in pear. BMC Plant Biol. 2021, 21, 469. [Google Scholar] [CrossRef]
- Berthet, S.; Demont-Caulet, N.; Pollet, B.; Bidzinski, P.; Cézard, L.; Le Bris, P.; Borrega, N.; Hervé, J.; Blondet, E.; Balzergue, S.; et al. Disruption of laccase 4 and 17 results in tissue-specific alterations to lignification of Arabidopsis thaliana stems. Plant Cell 2011, 23, 1124–1137. [Google Scholar] [CrossRef]
- Cheng, X.; Li, G.; Ma, C.; Abdullah, M.; Zhang, J.; Zhao, H.; Jin, Q.; Cai, Y.; Lin, Y. Comprehensive genome-wide analysis of the pear (Pyrus bretschneideri) laccase gene (PbLAC) family and functional identification of PbLAC1 involved in lignin biosynthesis. PLoS ONE 2019, 14, e0210892. [Google Scholar] [CrossRef]
- Dorairaj, D.; Ismail, M.R.; Sinniah, U.R.; Ban, T.K. Influence of silicon on growth, yield, and lodging resistance of MR219, a lowland rice of Malaysia. J. Plant Nutr. 2017, 40, 1111–1124. [Google Scholar] [CrossRef]
- Kuai, J.; Sun, Y.; Zhou, M.; Zhang, P.; Zuo, Q.; Wu, J.; Zhou, G. The effect of nitrogen application and planting density on the radiation use efficiency and the stem lignin metabolism in rapeseed (Brassica napus L.). Field Crop Res. 2016, 199, 89–98. [Google Scholar] [CrossRef]
- Zhang, J.; Lv, J.; Xie, J.; Gan, Y.; Coulter, J.A.; Yu, J.; Li, J.; Wang, J.; Zhang, X. Nitrogen fertilizer application affects lodging resistance by altering secondary cell wall synthesis in japonica rice (Oryza sativa). J. Plant Res. 2017, 130, 859–871. [Google Scholar] [CrossRef]
- Wang, C.; Hu, D.; Liu, X.; She, H.; Ruan, R.; Yang, H.; Yi, Z.; Wu, D. Effects of uniconazole on the lignin metabolism and lodging resistance of culm in common buckwheat (Fagopyrum esculentum M.). Field Crops Res. 2015, 180, 46–53. [Google Scholar] [CrossRef]
- Kong, L.; Sun, M.; Wang, F.; Liu, J.; Feng, B.; Si, J.; Zhang, B.; Li, S.; Li, H. Effects of high NH4+ on K+ uptake, culm mechanical strength and grain filling in wheat. Front. Plant Sci. 2014, 5, 703. [Google Scholar] [CrossRef] [PubMed]
- Qin, L.; Walk, T.C.; Han, P.; Chen, L.; Zhang, S.; Li, Y.; Hu, X.; Xie, L.; Yang, Y.; Liu, J.; et al. Adaption of roots to nitrogen deficiency revealed by 3D quantification and proteomic analysis. Plant Physiol. 2019, 179, 329–347. [Google Scholar] [CrossRef]
- Wang, X.; Chai, X.; Gao, B.; Deng, C.; Günther, C.S.; Wu, T.; Zhang, X.; Xu, X.; Han, Z.; Wang, Y. Multi-omics analysis reveals the mechanism of bHLH130 responding to low-nitrogen stress of apple rootstock. Plant Physiol. 2023, 191, 1305–1323. [Google Scholar] [CrossRef]
- Zhang, J.; Lv, J.; Xie, J.; Gan, Y.; Coulter, J.A.; Yu, J.; Li, J.; Wang, J.; Zhang, X. Nitrogen source affects the composition of metabolites in pepper (Capsicum annuum L.) and regulates the synthesis of capsaicinoids through the GOGAT-GS pathway. Foods 2020, 9, 150. [Google Scholar] [CrossRef]
- Xiong, H.; Ma, H.; Hu, B.; Zhao, H.; Wang, J.; Rennenberg, H.; Shi, X.; Zhang, Y. Nitrogen fertilization stimulates nitrogen assimilation and modifies nitrogen partitioning in the spring shoot leaves of citrus (Citrus reticulata Blanco) trees. J. Plant Physiol. 2021, 267, 153556. [Google Scholar] [CrossRef]
- O’Brien, J.A.; Vega, A.; Bouguyon, E.; Krouk, G.; Gojon, A.; Coruzzi, G.; Gutiérrez, R.A. Nitrate Transport, Sensing, and Responses in Plants. Mol. Plant 2016, 9, 837–856. [Google Scholar] [CrossRef]
- Hernández, V.; Hellín, P.; Fenoll, J.; Flores, P. Impact of nitrogen supply limitation on tomato fruit composition. Sci. Hortic. 2020, 264, 109173. [Google Scholar] [CrossRef]
- Huang, C.; Hou, J.; Huang, M.; Hu, M.; Deng, L.; Zeng, K.; Yao, S. A comprehensive review of segment drying (vesicle granulation and collapse) in citrus fruit: Current state and future directions. Sci. Hortic. 2023, 309, 11683. [Google Scholar] [CrossRef]
- Bai, B.; Li, X.; Gu, C.; Wu, S.; Chen, X.; Yang, G.; Liu, Y.; Sun, M.; Fei, Z.; Zhang, S.; et al. Genome-wide association studies provide insights into the genetic determination of fruit traits of pear. Nat. Commun. 2021, 12, 1144. [Google Scholar]
- Bonawitz, N.D.; Chapple, C. The genetics of lignin biosynthesis: Connecting genotype to phenotype. Annu. Rev. Genet. 2010, 44, 337–363. [Google Scholar] [CrossRef] [PubMed]
- Hoegger, P.J.; Kilaru, S.; James, T.Y.; Thacker, J.R.; Kües, U. Phylogenetic comparison and classification of laccase and related multicopper oxidase protein sequences. FEBS J. 2006, 273, 2308–2326. [Google Scholar] [CrossRef]
- Hu, Q.; Luo, C.; Zhang, Q.; Luo, Z. Isolation and characterization of a Laccase gene potentially involved in proanthocyanidin polymerization in oriental persimmon (Diospyros kaki Thunb.) fruit. Mol. Biol. Rep. 2013, 40, 2809–2820. [Google Scholar] [CrossRef]
- Bai, Y.; Ali, S.; Liu, S.; Zhou, J.; Tang, Y. Characterization of plant laccase genes and their functions. Gene 2023, 852, 147060. [Google Scholar] [CrossRef]
- Abdel-Ghany, S.E.; Pilon, M. MicroRNA-mediated systemic down-regulation of copper protein expression in response to low copper availability in Arabidopsis. J. Biol. Chem. 2008, 283, 15932–15945. [Google Scholar] [CrossRef]
- Cuperus, J.T.; Fahlgren, N.; Carrington, J.C. Evolution and functional diversification of MIRNA genes. Plant Cell 2021, 23, 431–442. [Google Scholar] [CrossRef]
- Pan, J.; Huang, D.; Guo, Z.; Kuang, Z.; Zhang, H.; Xie, X.; Ma, Z.; Gao, S.; Lerdau, M.T.; Chu, C.; et al. Overexpression of microRNA408 enhances photosynthesis, growth, and seed yield in diverse plants. J. Integr. Plant Biol. 2018, 60, 323–340. [Google Scholar] [CrossRef]
- Yang, Y.; Xu, L.; Hao, C.; Wan, M.; Tao, Y.; Zhuang, Y.; Su, Y.; Li, L. The microRNA408-plantacyanin module balances plant growth and drought resistance by regulating reactive oxygen species homeostasis in guard cells. Plant Cell 2024, 36, 4338–4355. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, D.; Wang, X.; Bai, F.; Li, R.; Zhou, R.; Wu, S.; Fang, Z.; Liu, W.; Huang, L.; et al. LACCASE35 enhances lignification and resistance against Pseudomonas syringae pv. actinidiae infection in kiwifruit. Plant Physiol. 2025, 197, kiaf040. [Google Scholar] [CrossRef] [PubMed]
- Meng, D.; Liu, X.; Cao, Y.; Cai, Y.; Duan, J. PbMADS49 Regulates Lignification During Stone Cell Development in ‘Dangshansuli’ (Pyrus bretschneideri) Fruit. Plant Cell Environ. 2025; advance online publication. [Google Scholar] [CrossRef]
- James, A.; Paul, J.Y.; Souvan, J.; Cooper, T.; Dale, J.; Harding, R.; Deo, P. Assessment of root-specific promoters in banana and tobacco and identification of a banana TIP2 promoter with strong root activity. Front. Plant Sci. 2022, 13, 1009487. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhou, H.; Cheng, L.; Ma, N.; Cui, B.; Wang, F.; Zhong, Y.; Liao, H. Shoot-to-root translocated GmNN1/FT2a triggers nodulation and regulates soybean nitrogen nutrition. PLoS Biol. 2022, 20, e3001739. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lai, C.; Zhou, H.; Liao, H. Nitrate Modulates Fruit Lignification by Regulating CgLAC3 Expression in Pomelo. Int. J. Mol. Sci. 2025, 26, 4158. https://doi.org/10.3390/ijms26094158
Lai C, Zhou H, Liao H. Nitrate Modulates Fruit Lignification by Regulating CgLAC3 Expression in Pomelo. International Journal of Molecular Sciences. 2025; 26(9):4158. https://doi.org/10.3390/ijms26094158
Chicago/Turabian StyleLai, Changhong, Huiwen Zhou, and Hong Liao. 2025. "Nitrate Modulates Fruit Lignification by Regulating CgLAC3 Expression in Pomelo" International Journal of Molecular Sciences 26, no. 9: 4158. https://doi.org/10.3390/ijms26094158
APA StyleLai, C., Zhou, H., & Liao, H. (2025). Nitrate Modulates Fruit Lignification by Regulating CgLAC3 Expression in Pomelo. International Journal of Molecular Sciences, 26(9), 4158. https://doi.org/10.3390/ijms26094158






