Egg Overactivation—An Overlooked Phenomenon of Gamete Physiology
Abstract
1. Introduction
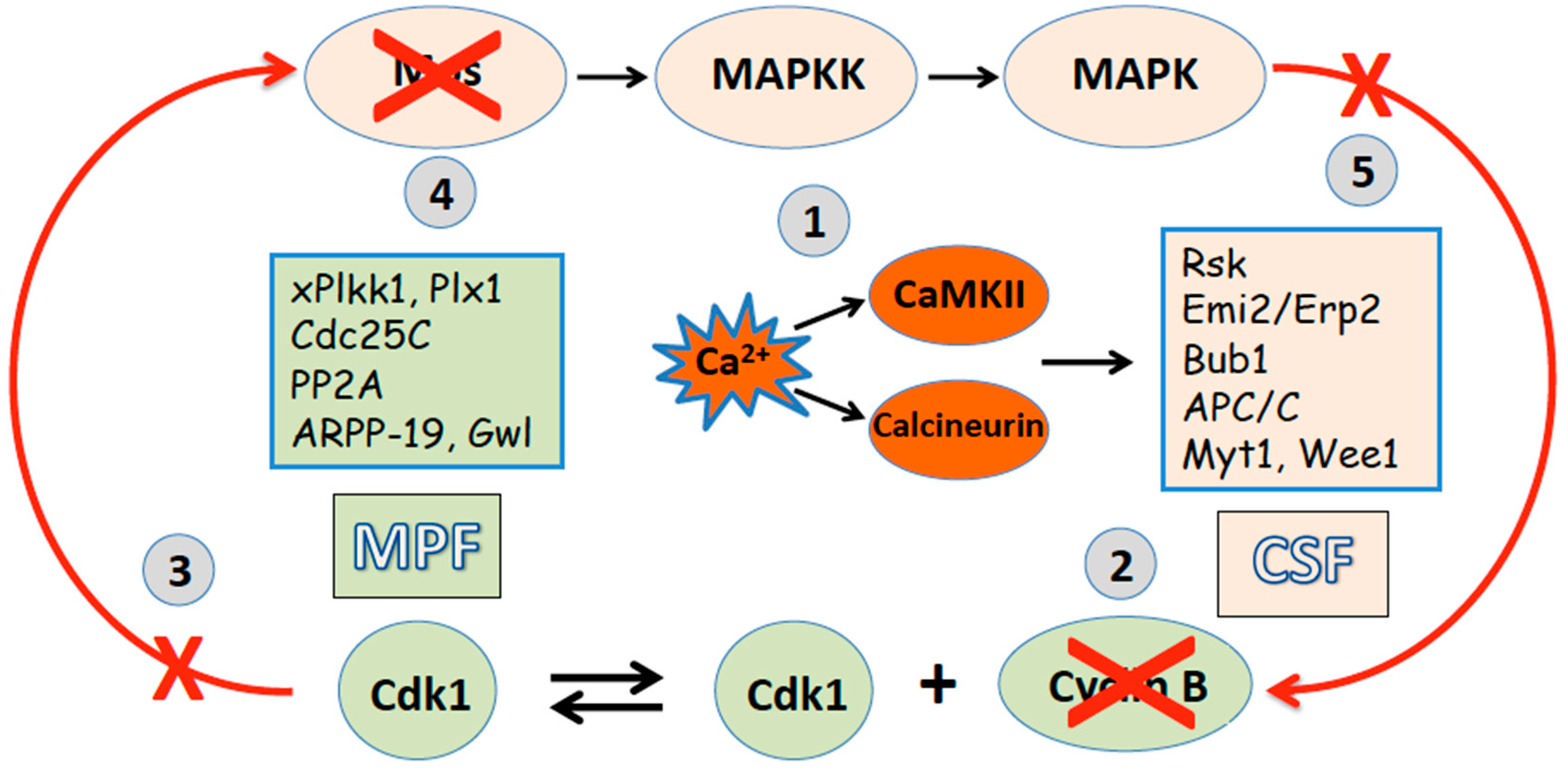
2. Egg Maturation and Metaphase II Arrest
3. Egg Activation and Exit from the Meiotic Metaphase Arrest
4. Egg Overactivation, Its Inducers and Hallmarks
5. Different Fates of Activated and Overactivated Eggs
6. Physiological Relevance of Egg Overactivation
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Miao, Y.-L.; Kikuchi, K.; Sun, Q.-Y.; Schatten, H. Oocyte aging: Cellular and molecular changes, developmental potential and reversal possibility. Hum. Reprod. Update 2009, 15, 573–585. [Google Scholar] [CrossRef]
- Prasad, S.; Tiwari, M.; Koch, B.; Chaube, S.K. Morphological, cellular and molecular changes during postovulatory egg aging in mammals. J. Biomed. Sci. 2015, 22, 36. [Google Scholar] [CrossRef] [PubMed]
- Tokmakov, A.A.; Sato, K.I.; Stefanov, V.E. Postovulatory cell death: Why eggs die via apoptosis in biological species with external fertilization. J. Reprod. Dev. 2018, 64, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Perez, G.I.; Tao, X.J.; Tilly, J.L. Fragmentation and death (a.k.a. apoptosis) of ovulated oocytes. Mol. Hum. Reprod. 1999, 5, 414–420. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, A.; Chaube, S.K. High cytosolic free calcium level signals apoptosis through mitochondria-caspase mediated pathway in rat eggs cultured in vitro. Apoptosis 2012, 17, 439–448. [Google Scholar] [CrossRef]
- Houel-Renault, L.; Philippe, L.; Piquemal, M.; Ciapa, B. Autophagy is used as a survival program in unfertilized sea urchin eggs that are destined to die by apoptosis after inactivation of MAPK1/3 (ERK2/1). Autophagy 2013, 9, 1527–1539. [Google Scholar] [CrossRef]
- Philippe, L.; Tosca, L.; Zhang, W.L.; Piquemal, M.; Ciapa, B. Different routes lead to apoptosis in unfertilized sea urchin eggs. Apoptosis 2014, 19, 436–450. [Google Scholar] [CrossRef]
- Tokmakov, A.A.; Iguchi, S.; Iwasaki, T.; Fukami, Y. Unfertilized frog eggs die by apoptosis following meiotic exit. BMC Cell Biol. 2011, 12, 56. [Google Scholar] [CrossRef]
- Iguchi, S.; Iwasaki, T.; Fukami, Y.; Tokmakov, A.A. Unlaid Xenopus eggs degrade by apoptosis in the genital tract. BMC Cell Biol. 2013, 14, 11. [Google Scholar] [CrossRef]
- Du Pasquier, D.; Dupré, A.; Jessus, C. Unfertilized Xenopus eggs die by Bad-dependent apoptosis under the control of Cdk1 and JNK. PLoS ONE 2011, 6, e23672. [Google Scholar] [CrossRef]
- Tokmakov, A.A.; Teranishi, R.; Sato, K.I. Spontaneous Overactivation of Xenopus Frog Eggs Triggers Necrotic Cell Death. Int. J. Mol. Sci. 2024, 25, 5321. [Google Scholar] [CrossRef] [PubMed]
- Masui, Y.; Markert, C.L. Cytoplasmic control of nuclear behavior during meiotic maturation of frog oocytes. J. Exp. Zool. 1971, 177, 129–145. [Google Scholar] [CrossRef] [PubMed]
- Masui, Y. A cytostatic factor in amphibian oocytes: Its extraction and partial characterization. J. Exp. Zool. 1974, 187, 141–147. [Google Scholar] [CrossRef]
- Sagata, N.; Watanabe, N.; vande Woude, G.F.; Ikawa, Y. The c-mos proto-oncogene product is a cytostatic factor responsible for meiotic arrest in vertebrate eggs. Nature 1989, 342, 512–518. [Google Scholar] [CrossRef] [PubMed]
- Mueller, P.R.; Coleman, T.R.; Kumagai, A.; Dunphy, W.G. Myt1: A membrane-associated inhibitory kinase that phosphorylates Cdc2 on both threonine-14 and tyrosine-15. Science 1995, 270, 86–90. [Google Scholar] [CrossRef]
- Murakami, M.S.; vande Woude, G.F. Analysis of the early embryonic cell cycles of Xenopus; regulation of cell cycle length by Xe-wee1 and Mos. Development 1998, 125, 237–248. [Google Scholar] [CrossRef]
- Nakajo, N.; Yoshitome, S.; Iwashita, J.; Iida, M.; Uto, K.; Ueno, S.; Okamoto, K.; Sagata, N. Absence of Wee1 ensures the meiotic cell cycle in Xenopus oocytes. Genes Dev. 2000, 14, 328–338. [Google Scholar] [CrossRef]
- Duckworth, B.C.; Weaver, J.S.; Ruderman, J.V. G2 arrest in Xenopus oocytes depends on phosphorylation of cdc25 by protein kinase A. Proc. Natl. Acad. Sci. USA 2002, 99, 16794–16799. [Google Scholar] [CrossRef]
- Castro, A.; Peter, M.; Magnaghi-Jaulin, L.; Vigneron, S.; Galas, S.; Lorca, T.; Labbé, J.C. Cyclin B/cdc2 induces c-Mos stability by direct phosphorylation in Xenopus oocytes. Mol. Biol. Cell. 2001, 12, 2660–2671. [Google Scholar] [CrossRef]
- Howard, E.L.; Charlesworth, A.; Welk, J.; MacNicol, A.M. The mitogen-activated protein kinase signaling pathway stimulates mos mRNA cytoplasmic polyadenylation during Xenopus oocyte maturation. Mol. Cell. Biol. 1999, 19, 1990–1999. [Google Scholar] [CrossRef]
- Palmer, A.; Gavin, A.C.; Nebreda, A.R. A link between MAP kinase and p34(cdc2)/cyclin B during oocyte maturation: p90(rsk) phosphorylates and inactivates the p34(cdc2) inhibitory kinase Myt1. EMBO J. 1998, 17, 5037–5047. [Google Scholar] [CrossRef] [PubMed]
- Mueller, P.R.; Coleman, T.R.; Dunphy, W.G. Cell cycle regulation of a Xenopus wee1-like kinase. Mol. Biol. Cell. 1995, 6, 119–134. [Google Scholar] [CrossRef] [PubMed]
- Schwab, M.S.; Roberts, B.T.; Gross, S.D.; Tunquist, B.J.; Taieb, F.E.; Lewellyn, A.L.; Maller, J.L. Bub1 is activated by the protein kinase p90 (Rsk) during Xenopus oocyte maturation. Curr. Biol. 2001, 11, 141–150. [Google Scholar] [CrossRef]
- Nishiyama, T.; Ohsumi, K.; Kishimoto, T. Phosphorylation of Erp1 by p90rsk is required forcytostatic factor arrest in Xenopus laevis eggs. Nature 2007, 446, 1096–1099. [Google Scholar] [CrossRef] [PubMed]
- Inoue, D.; Ohe, M.; Kanemori, Y.; Nobui, T.; Sagata, N. A direct link of the Mos–MAPK pathway to Erp1/Emi2 in meiotic arrest of Xenopus laevis eggs. Nature 2007, 446, 1100–1104. [Google Scholar] [CrossRef]
- Tung, J.J.; Padmanabhan, K.; Hansen, D.V.; Richter, J.D.; Jackson, P.K. Translational unmasking of Emi2 directs cytostatic factor arrest in meiosis II. Cell Cycle 2007, 6, 725–731. [Google Scholar] [CrossRef]
- Schmidt, A.; Duncan, P.I.; Rauh, N.R.; Sauer, G.; Fry, A.M.; Nigg, E.A.; Mayer, T.U. Xenopus polo-like kinase Plx1 regulates XErp1, a novel inhibitor of APC/C activity. Genes Dev. 2005, 19, 502–513. [Google Scholar] [CrossRef]
- Tung, J.J.; Hansen, D.V.; Ban, K.H.; Loktev, A.V.; Summers, M.K.; Adler, J.R., 3rd; Jackson, P.K. A role for the anaphase-promoting complex inhibitor Emi2/XErp1, a homolog of early mitotic inhibitor 1, in cytostatic factor arrest of Xenopus eggs. Proc. Natl. Acad. Sci. USA 2005, 102, 4318–4323. [Google Scholar] [CrossRef]
- Shoji, S.; Yoshida, N.; Amanai, M.; Ohgishi, M.; Fukui, T.; Fujimoto, S.; Nakano, Y.; Kajikawa, E.; Perry, A.C. Mammalian Emi2 mediates cytostatic arrest and transduces the signal for meiotic exit via Cdc20. EMBO J. 2006, 25, 834–845. [Google Scholar] [CrossRef]
- Musacchio, A. The molecular biology of spindle assembly checkpoint signaling dynamics. Curr. Biol. 2015, 25, R1002–R1018. [Google Scholar] [CrossRef]
- Santaguida, S.; Vernieri, C.; Villa, F.; Ciliberto, A.; Musacchio, A. Evidence that Aurora B is implicated in spindle checkpoint signalling independently of error correction. EMBO J. 2011, 30, 1508–1519. [Google Scholar] [CrossRef] [PubMed]
- Blengini, C.S.; Nguyen, A.L.; Aboelenain, M.; Schindler, K. Age-dependent integrity of the meiotic spindle assembly checkpoint in females requires Aurora kinase B. Aging Cell 2021, 20, e13489. [Google Scholar] [CrossRef]
- Abrieu, A.; Brassac, T.; Galas, S.; Fisher, D.; Labbé, J.C.; Dorée, M. The Polo-like kinase Plx1 is a component of the MPF amplification loop at the G2/M-phase transition of the cell cycle in Xenopus eggs. J. Cell Sci. 1998, 111, 1751–1757. [Google Scholar] [CrossRef] [PubMed]
- Gavin, A.C.; Ni Ainle, A.; Chierici, E.; Jones, M.; Nebreda, A.R. A p90(rsk) mutant constitutively interacting with MAP kinase uncouples MAP kinase from p34(cdc2)/cyclin B activation in Xenopus oocytes. Mol. Biol. Cell 1999, 10, 2971–2986. [Google Scholar] [CrossRef] [PubMed]
- Kumagai, A.; Dunphy, W.G. Purification and molecular cloning of Plx1, a Cdc25-regulatory kinase from Xenopus egg extracts. Science 1996, 273, 1377–1380. [Google Scholar] [CrossRef]
- Qian, Y.W.; Erikson, E.; Maller, J.L. Purification and cloning of a protein kinase that phosphorylates and activates the polo-like kinase Plx1. Science 1998, 282, 1701–1704. [Google Scholar] [CrossRef]
- Mochida, S.; Ikeo, S.; Gannon, J.; Hunt, T. Regulated activity of PP2A-B55 delta is crucial for controlling entry into and exit from mitosis in Xenopus egg extracts. EMBO J. 2009, 28, 2777–2785. [Google Scholar] [CrossRef]
- Yu, J.; Zhao, Y.; Li, Z.; Galas, S.; Goldberg, M.L. Greatwall kinase participates in the Cdc2 autoregulatory loop in Xenopus egg extracts. Mol. Cell. 2006, 22, 83–91. [Google Scholar] [CrossRef]
- Castilho, P.V.; Williams, B.C.; Mochida, S.; Zhao, Y.; Goldberg, M.L. The M phase kinase Greatwall (Gwl) promotes inactivation of PP2A/B55delta, a phosphatase directed against CDK phosphosites. Mol. Biol. Cell. 2009, 20, 4777–4789. [Google Scholar] [CrossRef]
- Vigneron, S.; Brioudes, E.; Burgess, A.; Labbé, J.C.; Lorca, T.; Castro, A. Greatwall maintains mitosis through regulation of PP2A. EMBO J. 2009, 28, 2786–2793. [Google Scholar] [CrossRef]
- Mochida, S.; Maslen, S.L.; Skehel, M.; Hunt, T. Greatwall phosphorylates an inhibitor of protein phosphatase 2A that is essential for mitosis. Science 2010, 330, 1670–1673. [Google Scholar] [CrossRef] [PubMed]
- Gharbi-Ayachi, A.; Labbé, J.C.; Burgess, A.; Vigneron, S.; Strub, J.M.; Brioudes, E.; Van-Dorsselaer, A.; Castro, A.; Lorca, T. The substrate of Greatwall kinase, Arpp19, controls mitosis by inhibiting protein phosphatase 2A. Science 2010, 330, 1673–1677. [Google Scholar] [CrossRef]
- Hara, M.; Abe, Y.; Tanaka, T.; Yamamoto, T.; Okumura, E.; Kishimoto, T. Greatwall kinase and cyclin B-Cdk1 are both critical constituents of M-phase-promoting factor. Nat. Commun. 2012, 3, 1059. [Google Scholar] [CrossRef]
- Dupré, A.; Buffin, E.; Roustan, C.; Nairn, A.C.; Jessus, C.; Haccard, O. The phosphorylation ofARPP19 by Greatwall renders the auto-amplification of MPF independently of PKA in Xenopus oocytes. J. Cell Sci. 2013, 126, 3916–3926. [Google Scholar]
- De Moor, C.H.; Richter, J.D. The Mos pathway regulates cytoplasmic polyadenylation in Xenopus oocytes. Mol. Cell. Biol. 1997, 17, 6419–6426. [Google Scholar] [CrossRef] [PubMed]
- Whitaker, M. Control of meiotic arrest. Rev. Reprod. 1996, 1, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Stricker, S.A. Comparative biology of calcium signaling during fertilization and egg activation in animals. Dev. Biol. 1999, 211, 157–176. [Google Scholar] [CrossRef]
- Ramos, I.; Wessel, G.M. Calcium pathway machinery at fertilization in echinoderms. Cell Calcium 2013, 53, 16–23. [Google Scholar] [CrossRef]
- Stein, P.; Savy, V.; Williams, A.M.; Williams, C.J. Modulators of calcium signalling at fertilization. Open Biol. 2020, 10, 200118. [Google Scholar] [CrossRef]
- Miao, Y.L.; Stein, P.; Jefferson, W.N.; Padilla-Banks, E.; Williams, C.J. Calcium influx-mediated signaling is required for complete mouse egg activation. Proc. Natl. Acad. Sci. USA 2012, 109, 4169–4174. [Google Scholar] [CrossRef]
- Xu, Y.R.; Yang, W.X. Calcium influx and sperm-evoked calcium responses during oocyte maturation and egg activation. Oncotarget 2017, 8, 89375–89390. [Google Scholar] [CrossRef] [PubMed]
- Runft, L.L.; Watras, J.; Jaffe, L.A. Calcium release at fertilization of Xenopus eggs requires type I IP(3) receptors, but not SH2 domain-mediated activation of PLCgamma or G(q)-mediated activation of PLCβ. Dev. Biol. 1999, 214, 399–411. [Google Scholar] [CrossRef] [PubMed]
- Dupont, G.; Goldbeter, A. Properties of intracellular Ca2+ waves generated by a model based on Ca2+-induced Ca2+ release. Biophys. J. 1994, 67, 2191–2204. [Google Scholar] [CrossRef] [PubMed]
- Wagner, J.; Li, Y.X.; Pearson, J.; Keizer, J. Simulation of the fertilization Ca2+ wave in Xenopus laevis eggs. Biophys. J. 1998, 75, 2088–2097. [Google Scholar] [CrossRef]
- Chebotareva, T.; Taylor, J.; Mullins, J.J.; Wilmut, I. Rat eggs cannot wait: Spontaneous exit from meiotic metaphase-II arrest. Mol. Reprod. Dev. 2011, 78, 795–807. [Google Scholar] [CrossRef]
- Premkumar, K.V.; Chaube, S.K. RyR channel-mediated increase of cytosolic free calcium level signals cyclin B1 degradation during abortive spontaneous egg activation in rat. Vitr. Cell. Dev. Biol. Anim. 2014, 50, 640–647. [Google Scholar] [CrossRef]
- Xu, Z.; Abbott, A.; Kopf, G.S.; Schultz, R.M.; Ducibella, T. Spontaneous activation of ovulated mouse eggs: Time-dependent effects on M-phase exit, cortical granule exocytosis, maternal messenger ribonucleic acid recruitment, and inositol 1,4,5-trisphosphate sensitivity. Biol. Reprod. 1997, 57, 743–750. [Google Scholar] [CrossRef]
- Ma, W.; Zhang, D.; Hou, Y.; Li, Y.H.; Sun, Q.Y.; Sun, X.F.; Wang, W.H. Reduced expression of MAD2, BCL2, and MAP kinase activity in pig oocytes after in vitro aging are associated with defects in sister chromatid segregation during meiosis II and embryo fragmentation after activation. Biol. Reprod. 2005, 72, 373–383. [Google Scholar] [CrossRef]
- Santos, H.B.; Sato, Y.; Moro, L.; Bazzoli, N.; Rizzo, E. Relationship among follicular apoptosis, integrin beta1 and collagen type IV during early ovarian regression in the teleost Prochilodus argenteus after induced spawning. Cell Tissue Res. 2008, 332, 159–170. [Google Scholar] [CrossRef]
- Rauh, N.R.; Schmidt, A.; Bormann, J.; Nigg, E.A.; Mayer, T.U. Calcium triggers exit from meiosis II by targeting the APC/C inhibitor XErp1 for degradation. Nature 2005, 437, 1048–1052. [Google Scholar] [CrossRef]
- Hansen, D.V.; Tung, J.J.; Jackson, P.K. CaMKII and polo-like kinase 1 sequentially phosphorylate the cytostatic factor Emi2/XErp1 to trigger its destruction and meiotic exit. Proc. Natl. Acad. Sci. USA 2006, 103, 608–613. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Maller, J.L. Calcium elevation at fertilization coordinates phosphorylation of XErp1/Emi2 by Plx1 and CaMK II to release metaphase arrest by cytostatic factor. Curr. Biol. 2005, 15, 1458–1468. [Google Scholar] [CrossRef]
- Wu, J.Q.; Kornbluth, S. Across the meiotic divide—CSF activity in the post-Emi2/XErp1 era. J. Cell Sci. 2008, 121, 3509–3514. [Google Scholar] [CrossRef] [PubMed]
- Chung, E.; Chen, R.H. Phosphorylation of Cdc20 is required for its inhibition by the spindle checkpoint. Nat. Cell Biol. 2003, 5, 748–753. [Google Scholar] [CrossRef] [PubMed]
- Ferrell, J.E., Jr. Self-perpetuating states in signal transduction: Positive feedback, double-negative feedback and bistability. Curr. Opin. Cell Biol. 2002, 14, 140–148. [Google Scholar] [CrossRef]
- Nishizawa, M.; Furuno, N.; Okazaki, K.; Tanaka, H.; Ogawa, Y.; Sagata, N. Degradation of Mos by the N-terminal proline (Pro2)-dependent ubiquitin pathway on fertilization of Xenopus eggs: Possible significance of natural selection for Pro2 in Mos. EMBO J. 1993, 12, 4021–4027. [Google Scholar] [CrossRef]
- Tokmakov, A.A.; Stefanov, V.E.; Iwasaki, T.; Sato, K.; Fukami, Y. Calcium signaling and meiotic exit at fertilization in Xenopus egg. Int. J. Mol. Sci. 2014, 15, 18659–18676. [Google Scholar] [CrossRef]
- Levasseur, M.; Dumollard, R.; Chambon, J.P.; Hebras, C.; Sinclair, M.; Whitaker, M.; McDougall, A. Release from meiotic arrest in ascidian eggs requires the activity of two phosphatases but not CaMKII. Development 2013, 140, 4583–4593. [Google Scholar] [CrossRef]
- Madgwick, S.; Levasseur, M.; Jones, K.T. Calmodulin-dependent protein kinase II, and not protein kinase C, is sufficient for triggering cell-cycle resumption in mammalian eggs. J. Cell Sci. 2005, 118, 3849–3859. [Google Scholar] [CrossRef]
- Suzuki, T.; Suzuki, E.; Yoshida, N.; Kubo, A.; Li, H.; Okuda, E.; Amanai, M.; Perry, A.C. Mouse Emi2 as a distinctive regulatory hub in second meiotic metaphase. Development 2010, 137, 3281–3291. [Google Scholar] [CrossRef]
- Tokmakov, A.A.; Awamura, M.; Sato, K.I. Biochemical Hallmarks of Oxidative Stress-Induced Overactivation of Xenopus Eggs. Biomed. Res. Int. 2019, 2019, 7180540. [Google Scholar] [CrossRef]
- Tokmakov, A.A.; Morichika, Y.; Teranishi, R.; Sato, K.I. Oxidative Stress-Induced Overactivation of Frog Eggs Triggers Calcium-Dependent Non-Apoptotic Cell Death. Antioxidants 2022, 11, 2433. [Google Scholar] [CrossRef]
- Sato, K.; Ogawa, K.; Tokmakov, A.A.; Iwasaki, T.; Fukami, Y. Hydrogen peroxide induces Src family tyrosine kinase-dependent activation of Xenopus eggs. Dev. Growth Differ. 2001, 43, 55–72. [Google Scholar] [CrossRef] [PubMed]
- Sato, K.; Tokmakov, A.A.; Iwasaki, T.; Fukami, Y. Tyrosine kinase-dependent activation of phospholipase Cgamma is required for calcium transient in Xenopus egg fertilization. Dev. Biol. 2000, 224, 453–469. [Google Scholar] [CrossRef] [PubMed]
- Nikoletopoulou, V.; Markaki, M.; Palikaras, K.; Tavernarakis, N. Crosstalk between apoptosis, necrosis and autophagy. Biochim. Biophys. Acta 2013, 1833, 3448–3459. [Google Scholar] [CrossRef] [PubMed]
- Kung, G.; Konstantinidis, K.; Kitsis, R.N. Programmed necrosis, not apoptosis, in the heart. Circ. Res. 2011, 108, 1017–1036. [Google Scholar] [CrossRef]
- Galluzzi, L.; Vitale, I.; Aaronson, S.A.; Abrams, J.M.; Adam, D.; Agostinis, P.; Alnemri, E.S.; Altucci, L.; Amelio, I.; Andrews, D.W.; et al. Molecular mechanisms of cell death: Recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ. 2018, 25, 486–541. [Google Scholar]
- Xiong, W.; Ferrell, J.E., Jr. A positive-feedback-based bistable memory module’ that governs a cell fate decision. Nature 2003, 426, 460–465. [Google Scholar] [CrossRef]
- Watanabe, N.; Hunt, T.; Ikawa, Y.; Sagata, N. Independent inactivation of MPF and cytostatic factor (Mos) upon fertilization of Xenopus eggs. Nature 1991, 352, 247–248. [Google Scholar] [CrossRef]
- Eguchi, Y.; Shimizu, S.; Tsujimoto, Y. Intracellular ATP levels determine cell death fate by apoptosis or necrosis. Cancer Res. 1997, 57, 1835–1840. [Google Scholar]
- Huang, F.; Vemuri, M.C.; Schneider, J.S. Modulation of ATP levels alters the mode of hydrogen peroxide-induced cell death in primary cortical cultures: Effects of putative neuroprotective agents. Brain Res. 2004, 997, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Miyoshi, N.; Watanabe, E.; Osawa, T.; Okuhira, M.; Murata, Y.; Ohshima, H.; Nakamura, Y. ATP depletion alters the mode of cell death induced by benzyl isothiocyanate. Biochim. Biophys. Acta 2008, 1782, 566–573. [Google Scholar] [CrossRef] [PubMed]
- Barros, L.F.; Hermosilla, T.; Castro, J. Necrotic volume increase and the early physiology of necrosis. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2001, 130, 401–409. [Google Scholar] [CrossRef]
- Karch, J.; Molkentin, J.D. Regulated necrotic cell death: The passive aggressive side of Bax and Bak. Circ. Res. 2015, 116, 1800–1809. [Google Scholar] [CrossRef] [PubMed]
- Witschi, E. Overripeness of the egg as a cause of twinning and teratogenesis: A review. Cancer Res. 1952, 12, 763–786. [Google Scholar]
- Méndez-Tepepa, M.; Morales-Cruz, C.; García-Nieto, E.; Anaya-Hernández, A. A review of the reproductive system in anuran amphibians. Zool. Lett. 2023, 9, 3. [Google Scholar] [CrossRef]


| Stimulus | Oxidative Stress | Mechanical Stress | Spontaneous Overactivation | |
|---|---|---|---|---|
| Features | ||||
| Irreversible cortical contraction | + | + | + | |
| Non-compensted rise of intracellular calcium | + | ? | ? | |
| Cyclin degradation | + | + | + | |
| MAPK dephosphorylation | ? | − | + | |
| ATP depletion | + | + | + | |
| Increase in ADP/ATP ratio | ? | + | + | |
| Leakage of ATP and ADP | ? | + | + | |
| Termination of protein synthesis | + | ? | ? | |
| Lipofuscin accumulation | + | ? | ? | |
| Decline of MMP | + | ? | ? | |
| Endosomal acidification | + | ? | ? | |
| Decrease in soluble protein content | + | ? | ? | |
| Increase in egg size | + | + | + | |
| Caspase activation | − | − | − | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tokmakov, A.A.; Sato, K.-I. Egg Overactivation—An Overlooked Phenomenon of Gamete Physiology. Int. J. Mol. Sci. 2025, 26, 4163. https://doi.org/10.3390/ijms26094163
Tokmakov AA, Sato K-I. Egg Overactivation—An Overlooked Phenomenon of Gamete Physiology. International Journal of Molecular Sciences. 2025; 26(9):4163. https://doi.org/10.3390/ijms26094163
Chicago/Turabian StyleTokmakov, Alexander A., and Ken-Ichi Sato. 2025. "Egg Overactivation—An Overlooked Phenomenon of Gamete Physiology" International Journal of Molecular Sciences 26, no. 9: 4163. https://doi.org/10.3390/ijms26094163
APA StyleTokmakov, A. A., & Sato, K.-I. (2025). Egg Overactivation—An Overlooked Phenomenon of Gamete Physiology. International Journal of Molecular Sciences, 26(9), 4163. https://doi.org/10.3390/ijms26094163






