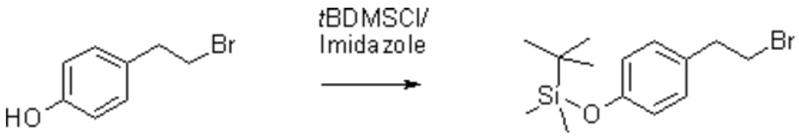
To 4-(2-bromoethyl)phenol [1] (28.75 g, 143 mmol) and tert-butyldimethylchlorosilane (23.64 g, 159 mmol) in dry THF (600 mL), imidazole (24.27 g, 357 mmol) was added at 15 °C over 10 min and stirred at ambient temperature. The mixture was filtered, the filtrate was concentrated in vacuo, and the residue was dissolved in Et2O (300 mL), washed with dil. AcOH (pH 5.5, 2 x 300 mL), water (2 x 300 mL), satd. NaHCO3 (1 x 250 mL), dried over Na2SO4, filtered and concentrated in vacuo. The residue was purified by column chromatography (250 g SiO2, petroleum ether : EtOAc = 95 : 5). Yield: colorless oil (32.92 g, 73%).
TLC: petroleum ether : EtOAc = 2 : 1, Rf = 0.95.
Anal. Calcd for C14H23BrOSi: C, 53.33; H, 7.35. Found: C, 53.56; H, 7.35.
1H NMR (CDCl3): d 7.10 (d, J = 8.0 Hz, 2H), 6.81 (d, J = 8.0 Hz, 2H), 3.53 (t, J = 6.7 Hz, 2H), 3.11 (t, J = 6.7 Hz, 2H), 1.04 (s, 9H), 0.22 (s, 6H).
13C NMR (CDCl3): d 154.5 (s), 131.6 (s), 129.5 (d), 120.1 (d), 38.7 (t), 33.1 (t), 25.6 (q), 18.1 (s), - 4.5 (q).
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3References and Notes
- Torssell, K.; Wahlberg, K. Isolation, structure, and synthesis of alkaloids from Valeriana officinalis. Acta Chem. Scand. 1967, 21, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Available from the auhtors.
© 2003 MDPI. All rights reserved.
