Abstract
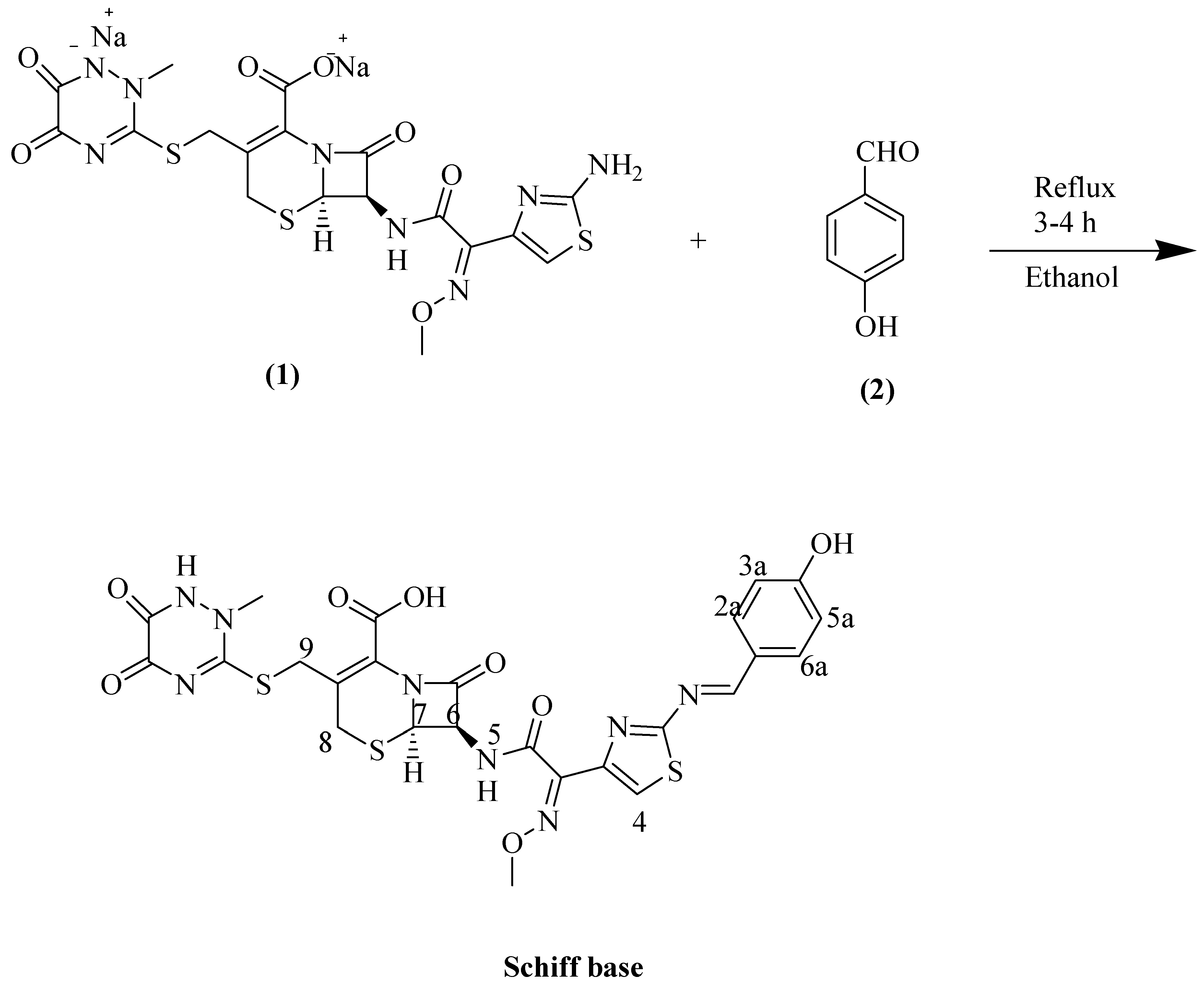
Novel 7-{[2-(4-hydroxyphenyl)methylidene]amino}-1,3-thiazol-4-yl)-2-(methoxyimino)acetyl]amino}-3-{[(2-methyl-5,6-dioxo-1,2,5,6-tetrahydro-1,2,4-triazin-3-yl)sulfanyl]methyl}-8-oxo-5-thia-1azabicyclo [4.2.0]oct-2-ene-2-carboxylic acid was prepared by condensation of ceftriaxone disodium (1) with 4-hydroxybenzaldehyde (2) in ethanol under reflux conditions for 3–4 h. The structure of synthesized compound was elucidated using LCMS, 1H-NMR, and CHN techniques.
Introduction
Since many years, ever-increasing resistance against human pathogens that cause serious infections is one of the main topics of attention for the medicinal chemist. Third generation cephalosporin antibiotic ceftriaxone has broad spectrum against human pathogens. Ceftriaxone has got high status among the class of cephalosporin but is no more effective than first and second generation cephalosporin. It has been used for the treatment of Lyme disease, typhoid fever, and gonorrhea. Ceftriaxone is effective in the treatment of various infections caused by pathogens including lower respiratory tract, abdomen, meninges, band and joint, pelvic area, urinary tract, skin and soft tissues. It is also commonly used in febrile infants of 4–8 weeks [1,2,3,4,5].
Result and Discussion
Ceftriaxone is susceptible for the synthesis of Schiff bases. Off-white colored Schiff base was obtained in ethanol under the reflux conditions for 3–4 h (Scheme 1).
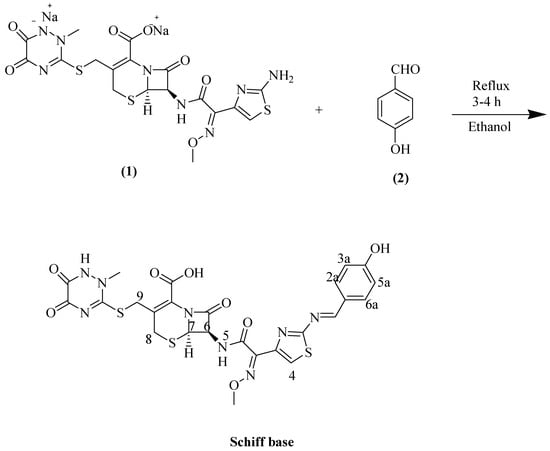
Scheme 1.
Reaction of ceftriaxone and 4-hydroxybenzaldehyde.
Experimental
The melting point was taken in capillary using Gallenkamp MF-370. The purity of compounds was determined by using TLC. 1H-NMR was recorded on Bruker AV-300 operating at 300 MHz. Mass was recorded on LCMS 2020 Shimadzu. CHN analysis was performed on a Carlo Erba Strumentazione-Mod-1106, Italy. Thin layer chromatography (TLC) was performed on pre-coated silica gel glass plates (Kieselgel 60, 254, E. Merck, Germany). Chromatograms were visualized by UV at 254 and 365 nm or by iodine vapors. All chemicals were of AR grade.
A mixture of 1 mmol (660 mg) ceftriaxone and 1 mmol (110 mg) 4-hydroxybenzaldehyde in 15 mL ethanol was taken in 50 mL conical flask and refluxed with stirring for 3–4 h. Reaction was monitored by thin layer chromatography. After completion of reaction, crushed ice was added. The precipitate was collected by filtration and washed several times with water. The product was dried and re-crystallized from cooled methanol to afford pure off-white Schiff base.
Yield = 85% (561 mg).
Melting Point = 235–237 °C.
LC-MS m/z (Rel intensity %) = 658.56 (M+, 100).
1H-NMR (DMSO-d6) δppm: 11.46 (s, 1H, N-H), 10.12 (br.s, 1H, COOH), 9.72 (s, 1H, OH) 8.71 (s, 1H, N=C-Ar), 7.91 (s, 1H, H-4), 7.58 (d, J = 7.3 Hz, 2H, H-2a / H-6a), 6.81(d, J = 7.3 Hz, 2H, H-5a / H-3a), 6.41 (s, 1H, H-6), 4.86 (s, 1H, H-7), 3.96 (s, 3H, -OCH3), 3.85 (s, 2H, H-9), 3.58 (s, 3H, N-CH3), 3.26 (s, 2H, H-8).
Elemental analysis Calculated for C25H22N8O8S3 = C (45.59%), H (3.37%), N (17.01%); Observed C (45.89%), H (3.29%), N (16.39%).
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3Acknowledgements
This work was supported by PCSIR Labs Complex, Karachi, Pakistan.
References
- Mandell, G.L.; Sande, M.A. Penicillin, Cephalosporins and other β-lactam antibiotics. In Goodman and Gilman’s the Pharmacological Basis of Therapeutics, 8th ed.; Gilman, A.G., Rale, T.W., Nies, A.S., Taylor, P., Eds.; Pergamon Press: New York, NY, USA, 1990; pp. 1065–1097. [Google Scholar]
- New, H.C. The New Beta-Lactamase-Stable Cephalosporins. Ann. Intern. Med. 1982, 97, 408–419. [Google Scholar]
- Garzone, P.; Lyon, J.; Yu, V.L. Third-generation and investigational cephalosporins: I. Structure-activity relationships and pharmacokinetic review. Drug Int. Clin. Pharm. 1983, 17, 507–515. [Google Scholar] [CrossRef]
- Balant, L.; Dayer, P.; Auckethaler, R. Clinical pharmacokinetics of the third generation cephalosporins. Clin. Pharmacokinet. 1985, 10, 101–143. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, J.E.; Parfitt, K.; Parsons, A.V.; Sweetman, S.C. Martindale the Extra Pharmacopoeia; The Pharmaceutical Press: London, UK, 1989; p. 167. [Google Scholar]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).