Abstract
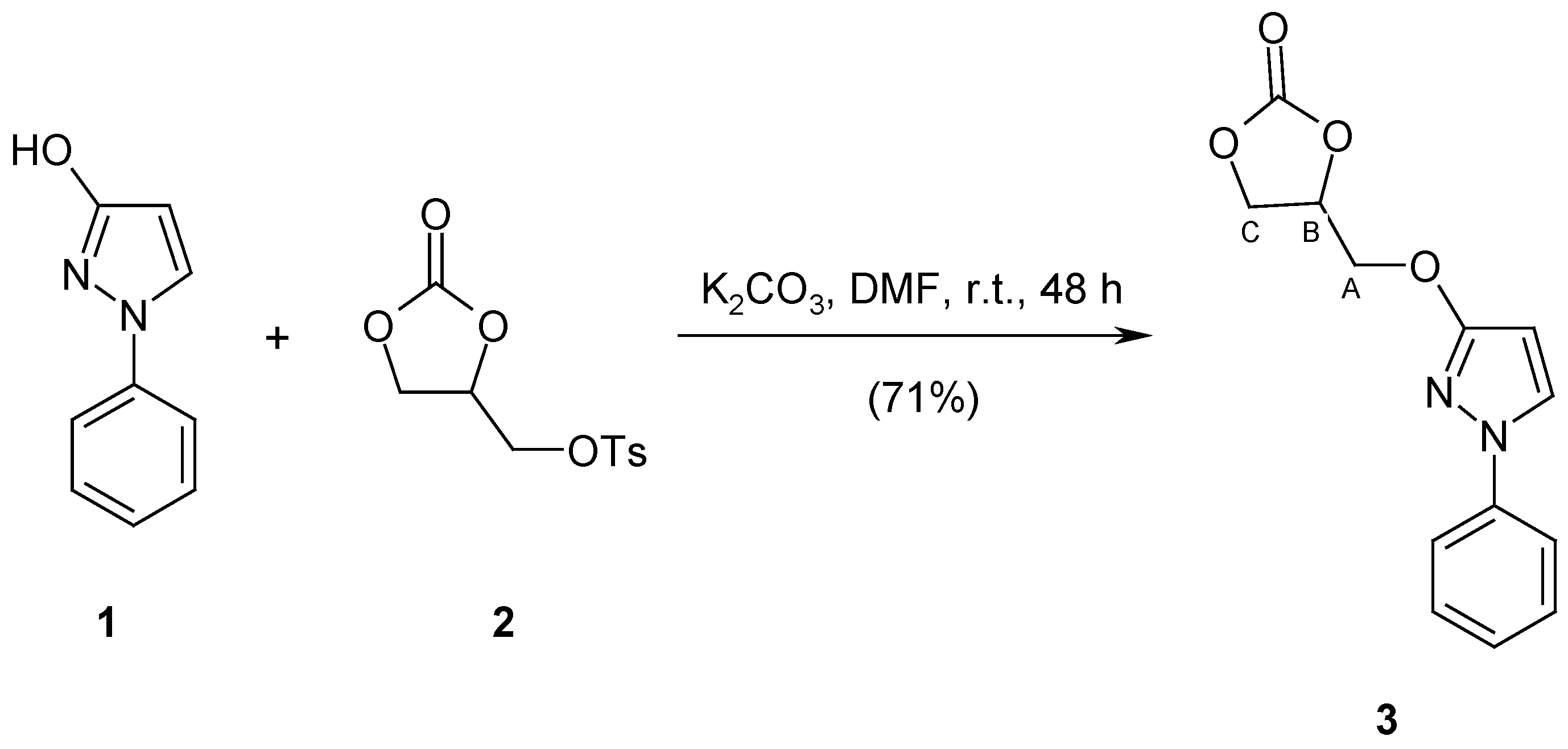
The title compound was obtained by the reaction of tosylated glycerol carbonate with 1-phenyl-1H-pyrazol-3-ol in a good 71% yield. Detailed spectroscopic data (1H-NMR, 13C-NMR, 15N-NMR, IR, MS) are presented.
1-Phenylpyrazole derivatives are known to have a broad spectrum of biological activities [1,2,3,4,5,6]. Recently, 1-phenyl-1H-pyrazol-3-ol was used as a versatile synthon for the preparation of various (het)aryl- and carbo-functionally substituted pyrazole derivatives employing Pd-catalyzed cross-coupling reactions [7,8]. In the present work, functionalization of 1-phenyl-1H-pyrazol-3-ol with tosylated glycerol 1,2-carbonate (TGC) was investigated. TGC is relatively new and efficient reagent, which have found application as an initiator of cationic ring-opening polymerization [9] and as a versatile bis-electrophile to access new functionalized glycidol derivatives [10,11]. TGC can be easily obtained by tosylation of glycerol carbonate (4-(hydroxymethyl)-1,3-dioxan-2-one) [10], the latter is an industrial product of glycerol valorization [12].
It is known that TGC reacts with 4-methoxyphenol in DMF in the presence of K2CO3 to afford O-alkylated product, 4-(3-methoxyphenoxy)methyl-1,3-dioxolan-2-one, in only 41% yield [11], while 55% of the arylsulfanyl analogue is obtained in analogous conditions from m-methoxythiophenol [10]. The reaction of 1-phenyl-1H-pyrazol-3-ol 1 with TGC 2 was carried out in DMF in the presence of K2CO3 and gave chemoselectively 4-{[(1-phenyl-1H-pyrazol-3-yl)oxy]methyl}-1,3-dioxolan-2-one 3 in 71% isolated yield. The structure of compound 3 was confirmed by its spectroscopic data (1H NMR, 13C and 15N NMR, IR, MS) as well as by elemental analysis.
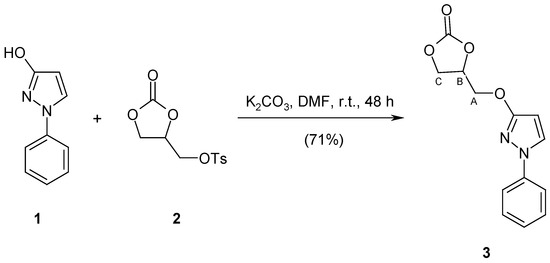
Scheme 1.
Synthesis of the title compound 3.
Scheme 1.
Synthesis of the title compound 3.
Experimental
The melting point was determined on a Reichert–Kofler hot-stage microscope and is uncorrected. Mass spectrum: Shimadzu QP 1000 instrument (EI, 70 eV). IR spectrum: Perkin-Elmer FTIR Spectrum 1605 spectrophotometer (KBr-disc). The elemental analysis was performed at the Microanalytical Laboratory, University of Vienna. NMR spectra were recorded from CDCl3 solutions on a Bruker Avance 500 instrument with a ‘directly’ detecting broadband observe probe (BBFO) at 298 K (500.13 MHz for 1H, 125.76 MHz for 13C, 50.68 MHz for 15N). The centre of the solvent signal was used as an internal standard which was related to TMS with δ = 7.26 ppm (1H in CDCl3) and δ = 77.0 ppm (13C in CDCl3). The digital resolutions were 0.2 Hz/data point in the 1H and 0.4 Hz/data point in the 1H-coupled 13C-NMR spectra (gated decoupling). The 15N-NMR spectrum (gradient-selected 15N, 1H-HMBC) was referenced against external nitromethane.
4-{[(1-Phenyl-1H-pyrazol-3-yl)oxy]methyl}-1,3-dioxolan-2-one (3)
To a solution of 1-phenyl-1H-pyrazol-3-ol (1) (1.6 g, 1.0 mmol) in DMF (15 mL) K2CO3 (2.76 g, 2.0 mmol) and tosylate (2) (2.72 g, 1.0 mmol) were added. The mixture was stirred at r.t. for 48 h (TLC control, eluent: ethyl acetate–n-hexane, 1:2; Rf 0.25). Then, 50 mL of water were added and the mixture was extracted with 3 × 60 mL of ethyl acetate. The organic layers were combined, washed with brine, dried over anhydrous Na2SO4 and filtered. The solvent was evaporated under reduced pressure and the residue was purified by column chromatography (silica gel, eluent: ethyl acetate–n-hexane, 1:2) to give pure 3 as yellowish crystals, m.p. 95–96 °C. Yield: 1.86 g (71%).
IR (KBr) ν (cm−1): 1786 (C=O), 1600, 1546, 1475, 1396, 1315, 1186, 1094, 989, 774, 751, 682.
MS (EI, 70 eV): (m/z, %) 260 (M+, 29), 160 (93), 77 (100), 51 (35), 43 (20).
1H-NMR (CDCl3): (ppm) 4.48 (dd, 1H, 2J(H1A,H2A) = 11.9 Hz, 3J(H1A,HB) = 4.0 Hz, H1A), 4.51 (dd, 1H, 2J(H1C,H2C) = 8.5 Hz, 3J(HB,H1C) = 6.1 Hz, H1C), 4.54 (dd, 1H, 2J(H1A,H2A) = 11.9 Hz, 3J(H2A,HB) = 3.9 Hz, H2A), 4.60 (t, 1H, 2J(H1C,H2C) = 8.5 Hz, 3J(HB,H2C) = 8.5 Hz, H2C), 5.07 (dddd, 1H, 3J(HB,H2C) = 8.5 Hz, 3J(HB,H1C) = 6.1 Hz, 3J(H1A,HB) = 4.0 Hz, 3J(H2A,HB) = 3.9 Hz, HB), 5.92 (d, 1H, 3J(4-H,5-H) = 2.6 Hz, 4-H), 7.22 (m, 1H, Ph 4-H), 7.41 (m, 2H, Ph 3,5-H), 7.57 (m, 2H, Ph 2,6-H), 7.74 (d, 1H, 3J(4-H,5-H) = 2.6 Hz, 5-H).
13C-NMR (CDCl3): δ (ppm) 66.1 (CC), 67.4 (CA), 74.2 (CB), 93.9 (C-4, 1J(C-4,4-H) = 180.6 Hz, 2J(C-4,5-H) = 8.1 Hz), 117.8 (Ph C-2,6), 125.6 (Ph C-4), 128.2 (C-5, 1J(C-5,5-H) = 187.0 Hz, 2J(C-5,4-H) = 8.3 Hz), 129.4 (Ph C-3,5), 139.8 (Ph C-1), 154.7 (C=O), 163.3 (C-3, 2J(C-3,4-H) = 2.2 Hz, 3J(C-3,5-H) = 10.5 Hz, 3J(C-3,OCH2) = 2.2 Hz).
15N-NMR (CDCl3): δ (ppm) −185.5 (N-1), N-2 was not found.
Anal. Calcd for C13H12N2O4: C, 60.00%; H, 4.65%; N, 10.76%. Found: C, 59.78%; H, 4.50%; N, 10.74%.
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3References and Notes
- Ha-Duong, N.-T.; Dijols, S.; Marques-Soares, C.; Minoletti, C.; Dansette, P.M.; Mansuy, D. Synthesis of Sulfaphenazole Derivatives and Their Use as Inhibitors and Tools for Comparing the Active Sites of Human Liver Cytochromes P450 of the 2C Subfamily. J. Med. Chem. 2001, 44, 3622–3631. [Google Scholar] [CrossRef] [PubMed]
- de Paulis, T.; Hemstapat, K.; Chen, Y.; Zhang, Y.; Saleh, S.; Alagille, D.; Baldwin, R.M.; Tamagnan, G.D.; Conn, P.J. Substituent Effects of N-(1,3-Diphenyl-1H-pyrazol-5-yl)benzamides on Positive Allosteric Modulation of the Metabotropic Glutamate-5 Receptor in Rat Cortical Astrocytes. J. Med. Chem. 2006, 49, 3332–3344. [Google Scholar] [CrossRef] [PubMed]
- Bebernitz, G.R.; Argentieri, G.; Battle, B.; Brennan, C.; Balkan, B.; Burkey, B.F.; Eckhardt, M.; Gao, J.; Kapa, P.; Strohschein, R.J.; et al. The Effect of 1,3-Diaryl-[1H]-pyrazole-4-acetamides on Glucose Utilization in ob/ob Mice. J. Med. Chem. 2001, 44, 2601–2611. [Google Scholar] [CrossRef] [PubMed]
- Raufl, M.; König, W. Effects of the nonsteroidal anti-inflammatory compounds Lonazolac Ca, indomethacin and NDGA on inflammatory mediator generation and release from various cells. Immunopharmacology 1990, 19, 103–111. [Google Scholar]
- Ahrens, H.; Koch, H.; Schröder, E.; Biere, H.; Kapp, J.-F. Novel Anti-Inflammatory Pyrazole Derivatives and Preparation Thereof. U.S. Patent 4,042,706, 16 August 1977. [Google Scholar]
- Oh, L.M. Synthesis of celecoxib via 1,3-dipolar cycloaddition. Tetrahedron Lett. 2006, 47, 7943–7946. [Google Scholar] [CrossRef]
- Arbačiauskienė, E.; Vilkauskaitė, G.; Eller, G.A.; Holzer, W.; Šačkus, A. Pd-catalyzed cross-coupling reactions of halogenated 1-phenylpyrazol-3-ols and related triflates. Tetrahedron 2009, 65, 7817–7824. [Google Scholar] [CrossRef]
- Arbačiauskienė, E.; Martynaitis, V.; Krikštolaitytė, S.; Holzer, W.; Šačkus, A. Synthesis of 3-substituted 1-phenyl-1H-pyrazole-4-carbaldehydes and the corresponding ethanones by Pd-catalysed cross-coupling reactions. ARKIVOC 2011, xi, 1–21. [Google Scholar]
- Giardi, C.; Lapinte, V.; Nielloud, F.; Devoisselle, J.-M.; Robin, J.-J. Synthesis of polyoxazolines using glycerol carbonate derivative and end chains functionalization via carbonate and isocyanate routes. J. Polym. Sci. Pol. Chem. 2010, 48, 4027–4035. [Google Scholar] [CrossRef]
- Simão, A.-C.; Lynikaitė-Puklevičienė, B.; Rousseau, C.; Tatibouët, A.; Cassel, S.; Šačkus, A.; Rauter, A.P.; Rollin, P. 1,2-Glycerol Carbonate: A Versatile Renewable Synthon. Lett. Org. Chem. 2006, 3, 744–748. [Google Scholar]
- Rousseau, J.; Rousseau, C.; Lynikaitė, B.; Šačkus, A.; de Leon, C.; Rollin, P.; Tatibouët, A. Tosylated glycerol carbonate, a versatile bis-electrophile to access new functionalized glycidol derivatives. Tetrahedron 2009, 65, 8571–8581. [Google Scholar] [CrossRef]
- Bai, R.; Wang, Y.; Wang, S.; Mei, F.; Li, T.; Li, G. Synthesis of glycerol carbonate from glycerol and dimethyl carbonate catalyzed by NaOH/γ-Al2O3. Fuel Process. Technol. 2012, in press. Available online: http://dx.doi.org/10.1016/j.fuproc.2012.07.027 (accessed on 7 November 2012). [Google Scholar] and references cited therein.
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
