Abstract
We report the synthesis of N-{[(4-nitrophenyl)amino]methyl}benzamide from (benzamidomethyl)triethylammonium chloride and 4-nitroaniline in aqueous media. The structure of the newly synthesized compound was characterized on the basis of 1H-NMR, 13C-NMR and FTIR spectroscopy.
Introduction
In this study we report the synthesis of N-{[(4-nitrophenyl)amino]methyl}benzamide, that we have obtained in the course of our continuing study involving benzamidomethylation of N-containing compounds [1,2,3]. The incorporation of the benzamidomethyl moiety in various compounds has previously been reported due to its application in pro-drug design [4,5,6]. From the many methods for synthesis of a N-benzamidomethylated compound the most noted are the reaction of N-(chloromethyl)benzamide [7,8,9] or N-(hydroxylmethyl)benzamide [10,11] with a primary or secondary amine or the Mannich-Einhorn reactions [12,13,14] that utilize benzamide, an aldehyde and a primary or secondary amine. A possible outcome when using a more reactive benzamidomethylating reagent, such as N-(chloromethyl)benzamide, is the formation of dibenzamidomethylated products [7,9]. The title compound we synthesized using (benzamidomethyl)triethylammonium chloride as the benzamidomethylating reagent.
Results and Discussion
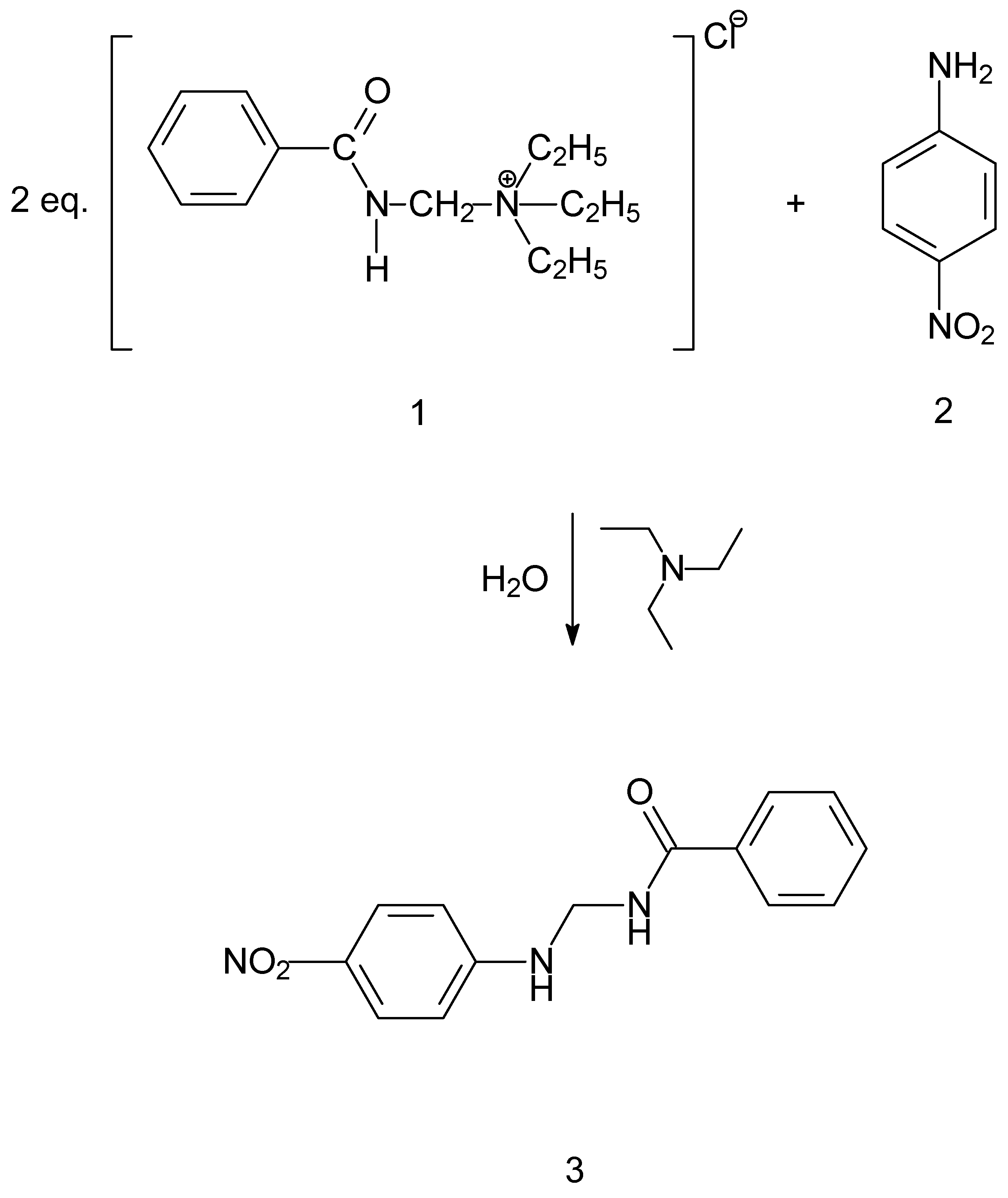
The title compound was prepared by reacting 4-nitroaniline (Figure 1-2) and (benzamidomethyl)triethylammonium chloride (Figure 1-1) in an aqueous phase. The main obstacle that was needed to be overcome was the low reactivity of the amine, that is 4-nitroaniline (Figure 1-2), due to the presence of the nitro group in the para position. This problem was resolved with applying an excess of the (benzamidomethyl)triethylammonium chloride salt (Figure 1-1) in the aqueous phase. The low solubility of the amine in water resulted witha slow release in the reaction medium that further assured a monobenzamidometylated product (Figure 1-3). The formation of a dibenzamidomethylated product was not observed, regardless of the quantity of (benzamidomethyl)triethylammonium chloride that was used. In the experimental protocols that were applied with ratios of up to 1:5, with favorance of the (benzamidomethyl)triethylammonium chloride salt, a dibenzamidomethylated product was not obtained. This can be explained with the very low reactivity of the title compound and its low solubility in water. After the reaction was completed (24 h), the product (Figure 1-3), was removed with vacuum filtration.
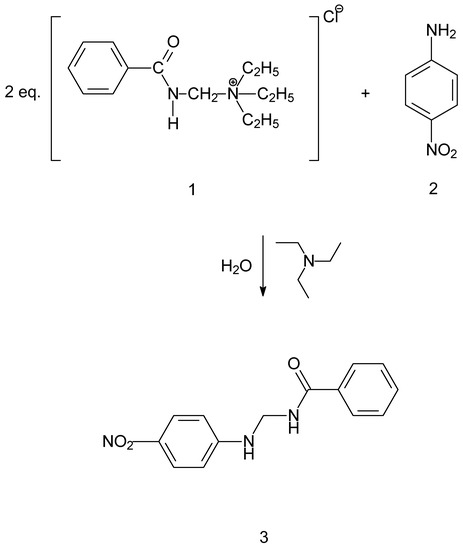
Figure 1.
Reaction scheme for the tittle compound.
The structure of the newly synthesized compound was confirmed by 1H-NMR, 13C-NMR, FTIR spectroscopy and HR-MS.
Experimental
Materials
All the reagents and solvents were obtained from commercial sources and used without further purification. The benzamidomethylation reagent, (benzamidomethyl)triethylammonium chloride, was prepared based on an already known method [2].
Instrumentation
Melting points were determined on a Mel-Temp II® and are uncorrected. The 1D (1H, 13C{1H}, DEPT) and 2D (COSY, HMQC, HMBC) NMR spectra were recorded on a Bruker 600 MHz instrument in DMSO-d6 as solvent and TMS as internal standard using standard sets of parameters.Infrared spectra (KBr pellets) were measured on a Perkin-Elmer System 2000 FT-IR. The HR mass spectra were made on FT-ICR Bruker Daltonics Apex II on ESI-MS High resolution mode.
Synthesis of N-{[(4-nitrophenyl)amino]methyl}benzamide
To an aqueous solution (50 mL) of 3.91 g (0.014 mol) of (benzamidomethyl)triethylammonium chloride and 0.2 mL of triethylamine, 1 g (0.0072 mol) of finely ground powder of 4-nitroaniline was added. The reaction mixture was left to stir at room temperature for 24 h. After the completion of the recation timea thick suspension of a yellow precipitate was observed. The precipitate (1.85 g, 94%) was removed from the solution via vacuum filtration. The product was purified with precipitation from ethanol with the addition of water.
The melting point of the purified product was 194–195 °C.
FTIR(KBr, cm−1): ν(N-H)amine 3412; ν(N-H)amide 3327; Amide I 1664; Amide II 1530; ν(N=O)asymmetric 1603; ν(N=O)symmetric 1326.
1H-NMR (600.13 MHz, DMSO-d6) δ (ppm): 4.75 (dd, J = 6.0 Hz, 2H, CH2 ), 6.87 (dd, J = 9.0 Hz, 2H, 4-NO2-ArH-2), 7.45 (dd, J = 8.0 Hz, 2H, ArH-3), 7.52 (dd, J = 8.0 Hz, 1H, ArH-4), 7.85 (d, J = 8.0 Hz, 2H, ArH-2), 7.92 (t, J = 8.0 Hz, 1H, Ar-NH), 8.01 (d, J = 9.0 Hz, 2H, 4-NO2-ArH-3), 9.22 (t, J = 6.0 Hz, 1H, CO-NH).
13C-NMR (150.91 MHz, DMSO-d6) δ (ppm): 47.8 CH2, 112.0 (4-NO2-ArC-2), 126.1 (4-NO2-ArC-3), 127.4 (ArC-2), 128.5 (ArC-3), 131.7 (ArC-4), 133.9 (ArC-1), 136.7 (4-NO2-ArC-4), 153.5 (4-NO2-ArC-1), 166.8 (C=O).
HRMS (ESI, pos) m/z calcd for C14H13N3NaO3 ([M+Na]+): 294.0849. Found: 294.0851.
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3Acknowledgments
This work was supported by the National Science Fund of Bulgaria (grant DRNF02/13).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Popovski, E. Synthesis of N-(N'-benzoylhydrazinomethyl)benzamide. Molbank 2007, M525. [Google Scholar] [CrossRef]
- Popovski, E. Synthesis of N-[N'-(2-hydroxy-2,2-diphenylacethyl)hydrazinomethyl]benzamide. Molbank 2007, M526. [Google Scholar] [CrossRef]
- Popovski, E.; Klisarova, L.; Vikic-Topic, D. Benzamidomethylation with (benzamidomethyl)triethylammonium chloride. 2. A simple method for benzamidomethylation of thiols, amines and carboxylic acids. Molecules 2000, 5, 927–936. [Google Scholar] [CrossRef]
- Schioppacassi, G.; Morvillo, E.; Della Bruna, C.; Franceschi, G.; Foglio, M. In vitro and in vivo evaluation of benzamidomethyl-benzylpenicillinate (fi7303). A new 'repository' form. Chemotherapy 1978, 24, 338–342. [Google Scholar] [CrossRef] [PubMed]
- Bundgaard, H.; Nielsen, N.M.; Buur, A. Aspirin prodrugs: Synthesis and hydrolysis of 2-acetoxybenzoate esters of various N-(hydroxyalkyl) amides. Int. J. Pharm. 1988, 44, 151–158. [Google Scholar] [CrossRef]
- Moreira, R.; Calheiros, T.; Cabrita, J.; Mendes, E.; Pimentel, M.; Iley, J. Acyloxymethyl as a drug protecting group. Part 3. Tertiary O-amidomethyl esters of penicillin g: Chemical hydrolysis and anti-bacterial activity. Pharm. Res. 1996, 13, 70–75. [Google Scholar] [CrossRef] [PubMed]
- Böhme, H.; Raude, E. Zur acylspaltung N-acylierter aminale. Chem. Ber. 1981, 114, 3421–3429. [Google Scholar] [CrossRef]
- Unterhalt, B.; Mohr, R.; Thamer, D. Nitramine, 17. Mitt. Alkyl-arylmethyl-nitramine. Arch. Pharm. 1985, 318, 878–882. [Google Scholar] [CrossRef]
- Schönenberger, H.; Petter, A.; Kuehling, V.; Bindl, L. Synthesis and testing the cardiocirculatory effects of N-(3'-methoxybenzamidomethyl)-D-norephedrine and analogous compounds. Arch. Pharm. 1976, 309, 289–301. [Google Scholar] [CrossRef]
- Haworth, R.D.; Peacock, D.H.; Smith, W.R.; MacGillivray, R. 569. The action of formaldehyde on proteins. Part II. Some reactions of N-hydroxymethylamides. J. Chem. Soc. 1952, 2972–2980. [Google Scholar] [CrossRef]
- Lazarevic, M.D.; Csanadi, J.; Klisarova, L. Synthesis of new benzotriazole derivatives. Bull. Chem. Technol. Macedonia 1995, 14, 19–22. [Google Scholar]
- Watase, Y.; Terao, Y.; Sekiya, M. Synthesis of N-(alkylaminomethyl) amides. Chem. Pharm. Bull. 1973, 21, 2775–2778. [Google Scholar] [CrossRef]
- Zanatta, N.; Rittner, R. Synthesis of 4-substituted N-[(dimethylamino)methyl]benzamides: New compounds. J. Pharm. Sci. 1983, 72, 831–832. [Google Scholar] [CrossRef] [PubMed]
- Zanatta, N.; Rittner, R. 13C NMR of 4-substituted N-[(dimethylamino)methyl]benzamides. Spectrosc. Lett. 1987, 20, 577–582. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).