Abstract
A novel compound, 1-[3-(2-methyl-4-phenylquinolin-3-yl)-5-phenyl-4,5-dihydro-1H-pyrazol-1-yl]-propane-1-one (3) has been synthesized by cyclocondensation of (E)-1-(2-methyl-4-phenylquinolin-3-yl)-3-phenylprop-2-en-1-one (2) and hydrazine hydrate in propionic acid. The structure of this compound was established by elemental analysis, IR, 1H-NMR, 13C-NMR and MS data.
Quinoline and its derivatives have been widely found in natural products and synthetic pharmaceuticals, which are associated with a broad spectrum of biological activities [1,2,3,4,5]. On the other hand, pyrazolines are important nitrogen-containing five-membered heterocyclic compounds, and their derivatives have been found to possess a broad spectrum of biological activities, such as antimalarial, antitrypanosomal, antitumor, and antidepressant activities [6,7,8,9,10,11]. The coupling of this chemical entity with a quinoline unit might be considered to have some biological activities. In this context, we report in this paper the synthesis of a novel 3-(quinolin-3-yl)-2-pyrazoline by cyclocondensation of the known (E)-1-(2-methyl-4-phenylquinolin-3-yl)-3-phenylprop-2-en-1-one (2) with hydrazine hydrate.
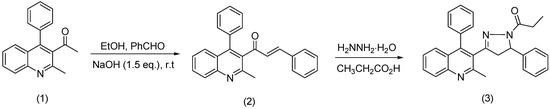
Scheme 1.
The synthesis of 1-[3-(2-methyl-4-phenylquinolin-3-yl)-5-phenyl-4,5-dihydro-1H-pyrazol-1-yl]-propane-1-one.
Scheme 1.
The synthesis of 1-[3-(2-methyl-4-phenylquinolin-3-yl)-5-phenyl-4,5-dihydro-1H-pyrazol-1-yl]-propane-1-one.
Experimental Section
Synthesis of 1-[3-(2-Methyl-4-phenylquinolin-3-yl)-5-phenyl-4,5-dihydro-1H-pyrazol-1-yl]-propane-1-one (3)
A mixture of 3-acetyl-2-methyl-4-phenylquinoline (2.6 g, 0.01 mol) (1), benzaldehyde (1.06 g, 0.01 mol) and 0.6 g of NaOH (0.015 mol) in 40 mL of ethanol was stirred at room temperature for 12 h. The resulting mixture was concentrated, then poured onto ice and neutralized with acetic acid. The resultant solid was filtered, dried and purified by column chromatography on silica gel using a mixture of ethyl acetate and petroleum ether (1:1). Recrystallization from the mixture of petroleum ether/acetone (8:4) gives the intermediate compound (2), m.p.: 149–150 °C, yield: 82%. Compound 2 is known in literature [12,13] and its structure has been confirmed by spectroscopic methods. Next, the title compound (3) was prepared by heating (E)-1-(2-methyl-4-phenylquinolin-3-yl)-3-phenylprop-2-en-1-one 2(0.5 g, 0.0014 mol) and hydrazine hydrate (0.070 g, 0.0014 mol) in propionic acid (20 mL) at 115–120 °C for 4 h. After completion, the solution was concentrated, cooled, and then poured onto ice. The resulting solid was filtered, washed with water, dried, and then recrystallized from the ethanol/petroleum ether mixture (2:1). The starting materials were generally used as received (Acros, Aldrich) without any further purification. Melting points were determined on an Electrothermal Digital Melting Point Apparatus (IA 9200) and are uncorrected. 1H-NMR and 13C-NMR spectra were recorded on VARIAN Mercury 300 spectrometers, CSIC, Spain.
White crystals; Yield: 92%; m.p. = 210 °C.
HRMS (ESI): [M + H]+ calculated for C28H26N3O = 420.2076; found 420.2092.
IR (KBr) vmax cm−1: 1666.8 (C=O ketone), 1618.0 (C=N pyrazoline)
1H-NMR (300 MHz, CDCl3) δ: 8.13 (d, J = 8.3 Hz, 1H, H-8), 7.75 (td, J = 8.3 Hz, J = 1.8 Hz, 1H, H-7), 7.53–7.25 (m, 10H, H-Ar), 6.90-6.84 (m, 2H, H-Ar), 5.35 (dd, J = 11.9 Hz, J = 5.1 Hz, 1H, H-5 pyrazoline), 3.44 (dd, J = 18.6 Hz, J = 12.0 Hz, 1H, H-4 pyrazoline), 2.82 (s, 3H, CH3), 2.73 (q, J = 7.6 Hz, 2H, CH2), 2.53 (dd, J = 18.5 Hz, J = 5.1 Hz, 1H, H-4ʹ pyrazoline), 1.18 (t, J = 7.5 Hz, 3H, CH3).
13C-NMR (75.4 MHz, CDCl3) δ: 172.50 (CO), 156.57 (C, C3 pyrazoline), 153.82, 148.28, 147.55, 141.62, 135.77, 130.40, 130.09, 123.84, 128.87, 128.70, 128.57, 127.54, 126.58, 126.42, 125.80, 125.62, 124.74 (C, CH phenyl and quinoline, 21C), 59.69 (CH, C5 pyrazoline), 46.51 (CH2, C4 pyrazoline), 27.83 (CH2), 24.81 (CH3), 9.2 (CH3).
Anal. calcd. For C28H25N3O: C, 80.16; H, 6.01; N, 10.02; Found C, 79.92; H, 6.19; N, 9.96.
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3Author Contributions
Allaoua Kedjadja and Rachid Merdes have contributed to the experimental part of this work. Experimental characterization was made by Elhadj Kolli. Abdelmalek Bouraiou contributed to the preparation of the manuscript. All authors read and approve the final manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Morimoto, Y.; Matsuda, F.; Shirahama, H. Total Synthesis of (±)-Virantmycin and Determination of Its Stereochemistry. Synlett 1991, 1991, 202–203. [Google Scholar] [CrossRef]
- Markees, D.G.; Dewey, V.C.; Kidder, G.W. Antiprotozoal 4-aryloxy-2-aminoquinolines and related compounds. J. Med. Chem. 1970, 13, 324–326. [Google Scholar] [CrossRef] [PubMed]
- Campbell, F.S.; Hardstone, J.D.; Palmer, J.M. 2,4-Diamino-6,7-dimethoxyquinoline derivatives as .alpha.1-adrenoceptor antagonists and antihypertensive agents. J. Med. Chem. 1988, 31, 1031–1035. [Google Scholar] [CrossRef] [PubMed]
- Maguire, M.P.; Sheets, K.R.; Zilberstein, A.A. New Series of PDGF Receptor Tyrosine Kinase Inhibitors: 3-Substituted Quinoline Derivatives. J. Med. Chem. 1994, 37, 2129–2137. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.L.; Fang, K.C.; Sheu, J.Y.; Hsu, S.L.; Tzeng, C.C. Synthesis and Antibacterial Evaluation of Certain Quinolone Derivatives. J. Med. Chem. 2001, 44, 2374–2377. [Google Scholar] [CrossRef] [PubMed]
- Insuasty, B.; Ramírez, J.; Becerra, D.; Echeverry, C.; Quiroga, J.; Abonia, R.; Robledo, S.M.; Darío Vélez, I.; Upegui, Y.; Muňoz, J.A.; et al. An efficient synthesis of new caffeine-based chalcones, pyrazolines and pyrazolo[3,4-b][1,4]diazepines as potential antimalarial, antitrypanosomal and antileishmanial agents. Eur. J. Med. Chem. 2015, 93, 401–413. [Google Scholar] [CrossRef] [PubMed]
- Insuasty, B.; Montoya, A.; Becerra, D.; Quiroga, J.; Abonia, R.; Robledo, S.M.; Darío Vélez, I.; Upegui, Y.; Muňoz, J.A.; Nogueras, M.; et al. Synthesis of novel analogs of 2-pyrazoline obtained from [(7-chloroquinolin-4-yl)amino]chalcones and hydrazine as potential antitumor and antimalarial agents. Eur. J. Med. Chem. 2013, 67, 252–262. [Google Scholar] [CrossRef] [PubMed]
- Insuasty, B.; Tigreros, A.; Orozco, F.; Quiroga, J.; Abonia, R.; Nogueras, M.; Sanchez, A.; Cobo, J. Synthesis of novel pyrazolic analogues of chalcones and their 3-aryl-4-(3-aryl-4,5-dihydro-1H-pyrazol-5-yl)-1-phenyl-1H-pyrazole derivatives as potential antitumor agents. Bioorg. Med. Chem. 2010, 18, 4965–4974. [Google Scholar] [CrossRef] [PubMed]
- Sarveswari, S.; Vijayakumar, V. An Efficient Microwave Assisted Eco-friendly Synthesis of 6-Chloro-3-(3-arylacryloyl)-2-methyl-4-phenylquinolines and their Conversion to 6-Chloro-3-(1-phenyl-5-aryl-4,5-dihydro-1H-pyrazol-3-yl)-2-methyl-4-phenylquinolines. J. Chin. Chem. Soc. 2012, 59, 66–71. [Google Scholar] [CrossRef]
- Prasad, Y.R.; Rao, A.L.; Prasoona, L.; Murali, K.; Kumar, P.R. Synthesis and antidepressant activity of some 1,3,5-triphenyl-2-pyrazolines and 3-(2″-hydroxynaphthalen-1″-yl)-1,5-diphenyl-2-pyrazolines. Bioorg. Med. Chem. Lett. 2005, 15, 5030–5034. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Negi, J.S.; Pant, G.J.N.; Rawat, M.S.M.; Budakoti, A. Synthesis and Characterization of a Novel 2-Pyrazoline. Molbank 2009, 2009, M614. [Google Scholar] [CrossRef]
- Loh, W.-S.; Fun, H.-K.; Prasath, R.; Sarveswari, S.; Vijayakumar, V. (E)-1-(2-Methyl-4-phenylquinolin-3-yl)-3-phenylprop-2-en-1-one. Acta Crystallogr. 2011, E67, o764–o765. [Google Scholar] [CrossRef] [PubMed]
- Kotra, V.; Ganapaty, S.; Adapa, S.R. Synthesis of new series of quinolinyl chalcones as anticancer and anti-inflammatory agents. Indian J. Chem. Sec. B 2010, 49, 1109–1116. [Google Scholar]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
