Abstract
4-Methoxy-3-(3-methylbut-2-en-1-yl)-7-[(3-methylbut-2-en-1-yl)oxy]quinolin-2(1H)-one (1) was isolated from the leaves of Melicope moluccana T.G. Hartley. The chemical structure of 1 was elucidated using mainly UV, IR, HRESIMS, 1D and 2D-NMR spectroscopy.
1. Introduction
Melicope is one of the genus of the family Rutaceae, consisting of about 320 species growing in the world [1]. Phytochemical studies have shown that the species produce a variety of alkaloids [2,3], flavonoids [4,5], coumarins [6], acetophenones [7], and lignans [8], which exhibit various biological activities, including antioxidant [8,9], anticancer [10,11], and antiinflammatory [12]. This study is part of our research on the chemical constituents of Melicope species found in Indonesia. In continuation of our research for alkaloid compounds in this medicinal plant, we report the isolation of 4-methoxy-3-(3-methylbut-2-en-1-yl)-7-[(3-methylbut-2-en-1-yl)oxy]quinolin-2(1H)-one (1) from the methanol extract of the leaves of Melicope moluccana T.G. Hartley. The chemical structure of compound 1 were established by UV, IR, HRESIMS, 1D and 2D-NMR, and by comparison with those related compounds previously reported. Cytotoxic and antiplasmodial activities of isolated compound from this species are also briefly described.
2. Result and Discussion
The dried and powdered leaves of M. moluccana (2.0 kg) were extracted with methanol. The residue was partitioned with n-hexane. The methanol extract was then adjusted to pH 3–4 with 3% sulfuric acid and partitioned with ethyl acetate to separate the non-alkaloid compound. Acid extracts were basified with ammonia solution (pH 8–9) and partitioned with ethyl acetate to give the crude alkaloid.
The crude alkaloid (5 g) was fractionated by column chromatography on silica gel eluted with mixtures of n-hexane-ethyl acetate (9:1 to 1:1) to give four major fractions A–D. Fraction A (325 mg) was further separated by radial chromatography to yield two sub-fractions (A1, A2). Sub-fraction A1 was purified using radial chromatography eluted with n hexane-chloroform (from 1:1 to 3:7) to give compound 1 (Figure 1).
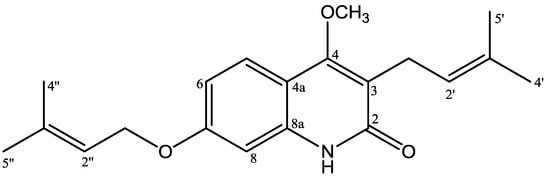
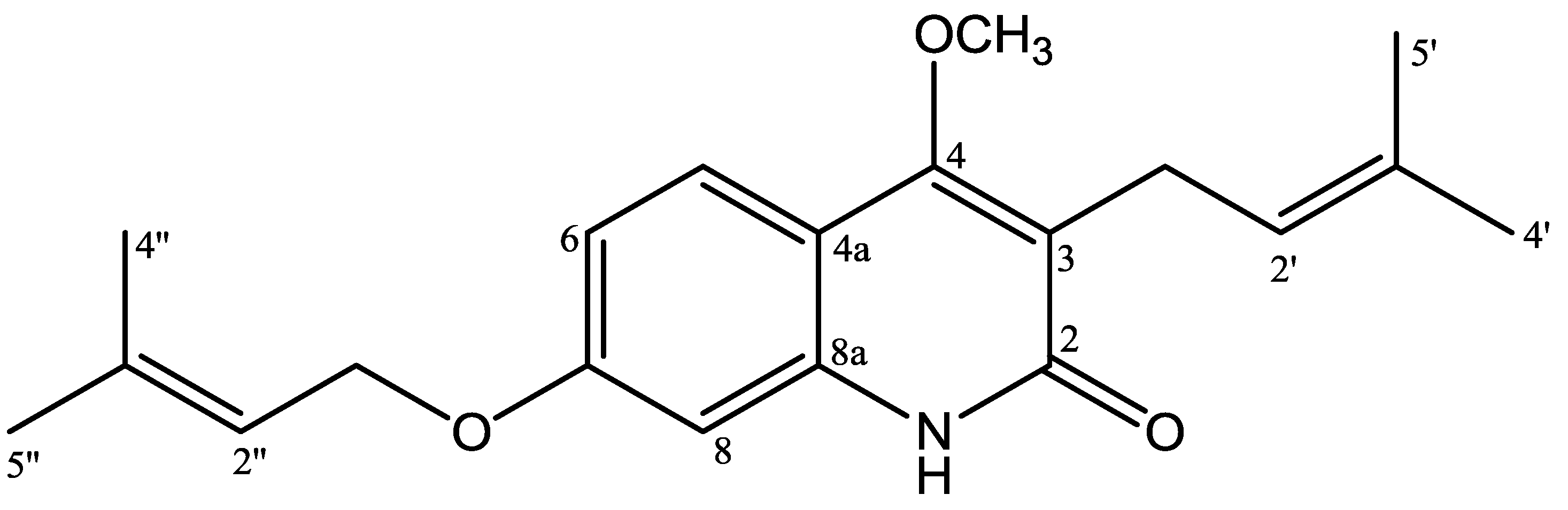
Figure 1.
Structures of 4-methoxy-3-(3-methylbut-2-en-1-yl)-7-[(3-methylbut-2-en-1-yl)oxy]quinolin-2(1H)-one.
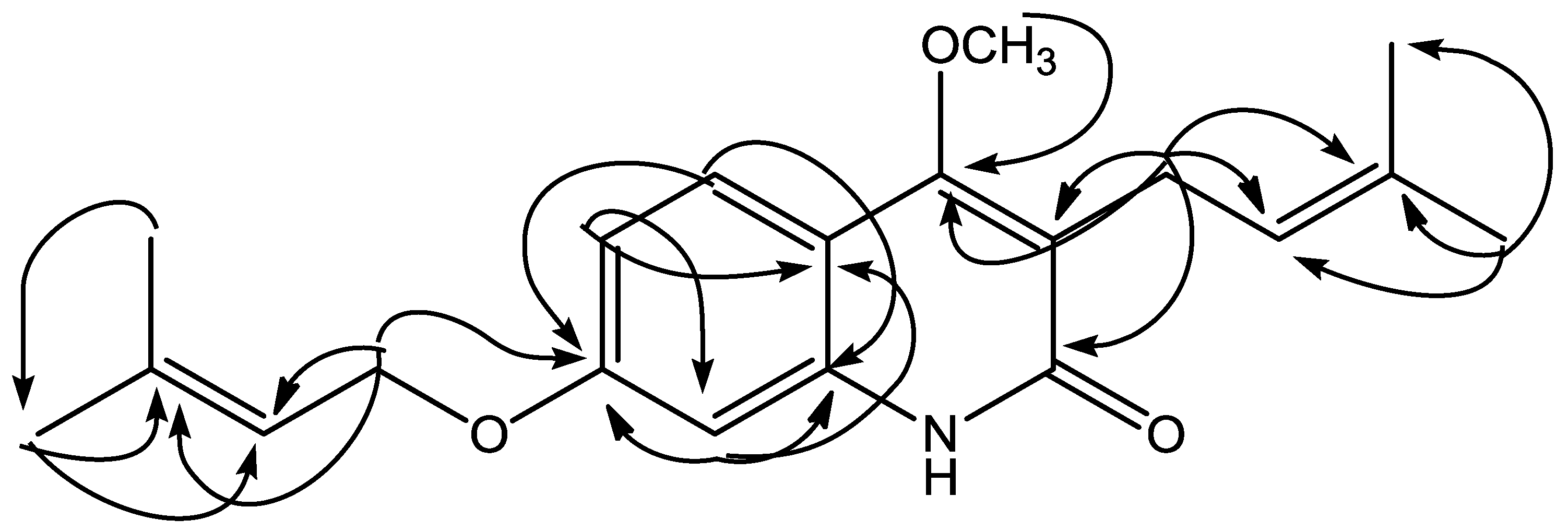
4-Methoxy-3-(3-methylbut-2-en-1-yl)-7-[(3-methylbut-2-en-1-yl)oxy]quinolin-2(1H)-one (1) was isolated as yellow solid, reacted positively with Dragendorff reagent to give reddish brown. The HRESIMS displayed a positive molecular ion peak at m/z 328.1928, indicating a molecular formula of C20H26NO3 (see Figure S6, supporting material). The UV spectrum exhibited absorption maxima λmax (nm) (log ε): 227 (4.57), 269 (3.86), 282 (3.85), 322 (4.09), and 336 (3.98) typical for a 2-quinolone skeleton [11]. The IR spectrum (see Figure S7, supporting information) indicated absorptions for hydroxyl (3421 cm−1), conjugated carbonyl (1645 cm−1), aromatic (1562, 1512, 1461 cm−1) and ether (1093 cm−1) groups [13]. The 1H NMR spectrum of 1 (Table 1, Figures S1 and S2, supporting material)) showed the presence of ABX coupling aromatic systems at δH 7.65 (d, J = 8.8 Hz; H-5), 7.65 (dd, J = 8.8; 2.4 Hz; H-6), and 7.05 (d, J = 2.4 Hz; H-8) characteristic for 2-quinolone with three substituents. Furthermore, the 1H NMR spectrum also showed an isoprenyl (3-methyl-2-butenyl) group [δH 1.83 (3H, s, H-4′), 1.79 (3H, s, H-5′), 3.39 (2H, d, J = 6.8 Hz, H-1′), 5.29 (1H, t, J = 6.8 Hz, H-2′)], an oxyisoprenyl (3-methylbut-2-en-1-yl)oxy) group [δH 1.83 (3H, s, H-4′′), 1.70 (3H, s, H-5′′), 4.59 (2H, d, J = 6.8 Hz, H-1′′), 5.52 (1H, t, J = 6.8 Hz, H-2′′)], and a singlet proton of methoxy group at δH 3.93. The 13C-NMR spectrum (Table 1) of 1 showed 20 carbon signals. The assignment of 13C-NMR spectrum was confirmed by HMQC and HMBC spectra. The placement of isoprenyl, oxyisoprenyl, and methoxy groups in 2-quinolone skeleton was established by HMQC and HMBC spectra. Long-range correlation was observed in the HMBC spectrum of 1 between the proton signal of a methoxy group at δH 3.93 with an oxyaryl atom (δC 162.5). The methylene proton signal of an isoprenyl group at δH 3.39 (H-1′) showed long-range correlations with a carbonyl carbon (δC 166.0; C-2), a oxyaryl carbon (δC 162.5), two quaternary carbons (δC 132.2, 119.3), and a methine carbon signal (δC 121.8), showing a methoxy group attached at C-4. The proton signal of an aromatic region at δH 7.65 (H-5) showed long-range correlations with an oxyaryl carbon signals (δC 160.6) and a quarternary carbon (δC 139.1). The result revealed δC 160.6 has position at C-7 and δC 139.1 at C-8a. Furthermore, the methylene proton signal at 4.59 (H-1′′) showed long-range correlations with an oxyaryl carbon (δC 160.6), a quarternary carbon (δC 139.0), and a methine carbon signal (δC 118.9), showing that the oxyisoprenyl group attached at C-7. See also Figure S3-S5, supporting information)

Table 1.
NMR spectroscopic data of 4-methoxy-3-(3-methylbut-2-en-1-yl)-7-[(3-methylbut-2-en-1-yl)oxy]quinolin-2(1H)-one in CDCl3.
On the basis of above spectral evidence, the structure of 1 was elucidated as 4-methoxy-3-(3-methylbut-2-en-1-yl)-7-[(3-methylbut-2-en-1-yl)oxy]quinolin-2(1H)-one. Other HMBC correlations consistent with structure 1 are shown in Table 1 and Figure 2. The structure of 1 has not been previously reported, and it is a novel compound. On cytotoxic evaluation against P-388 cells, compound 1 exhibited IC50 values of 0.63 μg/mL. That cytotoxic data suggested that compound 1 has high activity. The antiplasmodial activity of 1 showed IC50 values of 4.28 μg/mL, which categorized as moderate activity. The data on cytotoxic and antiplasmodial activity are presented in Table 2.
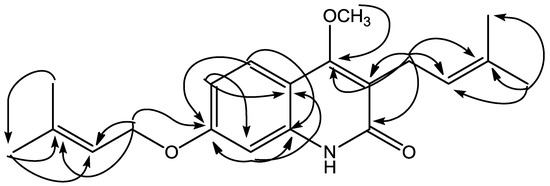
Figure 2.
Selected HMBC correlations for 1.

Table 2.
Cytotoxic analysis against P-388 cells and antiplasmodial activity of compound 1.
3. Experimental Section
3.1. General
The UV and IR spectra were measured with a Shimadzu 1800 spectrophotometer (Kyoto, Japan) and Perkin Elmer Spectrum One FTIR spectrophotometer (Waltham, MA, USA). NMR spectra were recorded on a JEOL ECA 400 spectrometer (Tokyo, Japan) in CDCl3 at 400 (1H) and 100 (13C) MHz using TMS as the internal standard. The mass spectra were recorded using a Waters LCT Premier XE (Santa Clara, CA, USA). Column chromatography and radial chromatography were carried out using Si gel 60 G 1.07734.1000 and Si gel 60 PF254 1.07749.1000 (Merck, Darmstadt, Germany).
3.2. Plant Material
The leaves of M. moluccana T.G. Hartley were collected in July 2015 from the conserved forest of Weda, Central Halmahera, North Maluku, Indonesia. The plant was identified at the Herbarium Bogoriense, and a voucher specimen number 46889 was deposited in the Bogor Botanical Garden, Bogor, Indonesia.
3.3. Extraction and Isolation
The dried and powdered leaves of M. moluccana T.G. Hartley (2.0 kg) were macerated in methanol at room temperature for 24 h two times, and the methanol extract was evaporated under reduced pressure to give methanol extract with dark brown residue (108 g). The methanol extract was partitioned with n-hexane (1:1 v/v) and then evaporated by rotavapor to give n-hexane extract (15 g). To the methanol extract was then added 3% sulfuric acid and adjusted to pH 3–4. Furthermore, the acid extracts were partitioned with ethyl acetate and then evaporated by rotavapor, yielding non-alkaloid (phenolic compound) extract (8 g). The acid extracts were basified with ammonia solution and adjusted to pH 8–9 and then partitioned with ethyl acetate to give crude alkaloid. The crude alkaloid (5 g) of M. moluccana was separated by column chromatography on silica gel. Elution with n-hexane-ethyl acetate mixture by increasing amount of ethyl acetate (9:1 to 1:1) to give four major fractions A–D. On thin layer chromatographic (TLC) analysis, fraction A (325 mg) showed two major spots. Separation of fraction A using planar radial chromatography and eluted with n-hexane-acetone mixture (from 9:1 to 7:3) to give two subfractions (A1, A2). Purification of subfraction A1 by planar radial chromatography with eluent n-hexane-chloroform (from 1:1 to 3:7) yielded compound 1 (14 mg).
3.4. Cytotoxic Assay
Preliminary cytotoxic evaluation of compound 1 was carried out against murine leukemia P-388 cells according to the MTT assay, as previously described [14,15].
3.5. Antiplasmodial Analysis
The antiplasmodial activity of compound 1 was expressed by the 50% inhibitory concentrations (IC50), representing the concentration of chloroquine that induced a 50% parasitaemia decrease compared to the positive control culture referred as 100% parasitaemia [16,17].
Supplementary Materials
1H-NMR, 13C-NMR, HMQC, HMBC, and HRESIMS spectra are reported in the supplementary materials as Figure S1–S7 and structure refinement parameters as Table S1.
Supplementary File 1Supplementary File 2Supplementary File 3Supplementary File 4Acknowledgments
This research was funded by Universitas Airlangga, Ministry of Research, Technology and Higher Education, 2017.
Author Contributions
M.T. and T.S.T. designed the whole experiment. R.D.S. and R.A.W. executed the experiment. All authors interpreted data and prepare the manuscript in the same contribution.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hartley, T.G. The genus Melicope (Rutaceae) in Borneo Sandakan. Sandakanian 1994, 47–74. [Google Scholar]
- Nakashima, K.; Oyama, M.; Ito, T.; Witono, J.R.; Darnaedi, D.; Tanaka, T.; Murata, T.; Iinuma, M. Melicodenines A and B, novel Diels-Alder type adduct isolated from Melicope denhamii. Tetrahedron. Lett. 2011, 52, 4694–4696. [Google Scholar] [CrossRef]
- Nakashima, K.; Oyama, M.; Ito, T.; Akao, Y.; Witono, J.R.; Darnaedi, D.; Tanaka, T.; Murata, T.; Iinuma, M. Novel quinolinone alkaloids bearing a lignoid moiety and related constituents in the leaves of Melicope denhamii. Tetrahedron 2012, 68, 2421–2428. [Google Scholar] [CrossRef]
- Sultana, N.; Hartley, T.G.; Waterman, P.G. Two novel prenylated flavanones from the aerial parts of Melicope micrococca. Phytochemistry 1999, 50, 1249–1253. [Google Scholar] [CrossRef]
- Chung, L.Y.; Yap, K.F.; Goh, S.H.; Mustafa, M.R.; Imiyabir, Z. Muscarinic receptor binding activity of polyoxygenated flavanones from Melicope subunifoliolata. Phytochemistry 2008, 69, 1548–1554. [Google Scholar] [CrossRef] [PubMed]
- O′Donnell, F.; Ramachandran, V.N.; Smyth, T.J.P.; Brooks, P. An investigation of bioactive phytochemicals in the leaves Melicope vitiflora by electrospray ionisation trap mass spectrometry. Anal. Chim. Acta 2009, 634, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Simonsen, H.T. Four novel geminaly dialkylated, non-aromatic acetophenone derivatives from Melicope coodeana. Phytochem. Lett. 2012, 5, 371–375. [Google Scholar] [CrossRef]
- Kassim, N.K.; Rahmani, M.; Ismail, A.; Sukari, M.A.; Ee, G.C.L.; Nasir, N.M.; Awang, K. Antioxidant activity-guided separation of coumarins and lignan from Melicope glabra (Rutaceae). Food Chem. 2013, 139, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Oyama, M.; Nakashima, K.; Kamiya, T.; Haba, M.; Ito, T. Flavonoids isolated from the leaves of Melicope triphylla and their extracellular-superoxide dismutase-inducing activity. Phytochem. Lett. 2013, 6, 215–218. [Google Scholar] [CrossRef]
- Komala, I.; Rahmani, M.; Sukari, M.A.; Ismail, H.B.M.; Ee, G.C.L.; Rahmat, A. Furoquinoline alkaloids from Melicope bonwickii (F.Muell) T. Hartley. Nat. Prod. Res. 2006, 4, 355–360. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.-J.; Chang, Y.-L.; Teng, C.-M.; Su, C.-C.; Chen, I.-S. Quinoline alkaloids and anti-platelet aggregation constituents from the leaves of Melicope semecarpifolia. Planta Med. 2002, 68, 790–793. [Google Scholar]
- Chen, J.; Cho, J.; Hwang, T.; Chen, I. Benzoic acid derivatives, acetophenones, and anti-inflammatory constituents from Melicope semecarpifolia. J. Nat. Prod. 2008, 71, 71–75. [Google Scholar] [CrossRef] [PubMed]
- Tanjung, M.; Saputri, R.D.; Tjahjandarie, T.S. Antioxidant activity of two isomeric benzoxepin derivatives from the stem bark of Bauhinia acuelata L. J. Chem. Pharm. Res. 2014, 6, 705–708. [Google Scholar]
- Tanjung, M.; Tjahjandarie, T.S.; Sentosa, M.H. Antioxidant and cytotoxic agent from the rhizomes of Kaempferia pandurata. Asian Pac. J. Trop. Dis. 2013, 3, 401–404. [Google Scholar] [CrossRef]
- Tanjung, M.; Mujahidin, D.; Hakim, E.H.; Mujahidin, D.; Darmawan, A.; Syah, Y.M. Geranylated flavonols from Macaranga rhizinoides. Nat. Prod. Commun. 2012, 5, 1209–1211. [Google Scholar]
- Tjahjandarie, T.S.; Pudjiastuti, P.; Saputri, R.D.; Tanjung, M. Antimalaria and antioxidant activity of phenolic compounds isolated from Erythrina crysta-galli L. J. Chem. Pharm. Res. 2014, 6, 786–790. [Google Scholar]
- Tanjung, M.; Saputri, R.D.; Tjahjandarie, T.S. 5,9,11-Trihydroxy-2,2-dimethyl-10-(3′-methyl-2′-butenyl)-3-(2′′-methyl-3′′-butenyl)pyrano[2,3-a]xanthen-12(2H)-one from the stem bark of Calophyllum pseudomole. Molbank 2016, 3, M906. [Google Scholar] [CrossRef]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).