Abstract
Fluorinated aza-heterocycles are important in organic and medicinal chemistry. Currently, a quarter of the drugs on the global market contain fluorine. We report the synthesis of the title compound and its single-crystal XRD structure.
1. Introduction
Fluorine is massively involved in chemistry and biomedicine with special emphasis on drug design. Replacing hydrogen atoms in organic compounds with fluorine atoms often produces positive pharmacological and physicochemical effects, particularly by increasing compounds’ metabolic stability, membrane permeability, and binding affinity towards biological targets. At present, a quarter of the drugs on the global market contain fluorine. Of these, heterocyclics are essentially important [1,2,3,4,5,6,7,8,9,10].
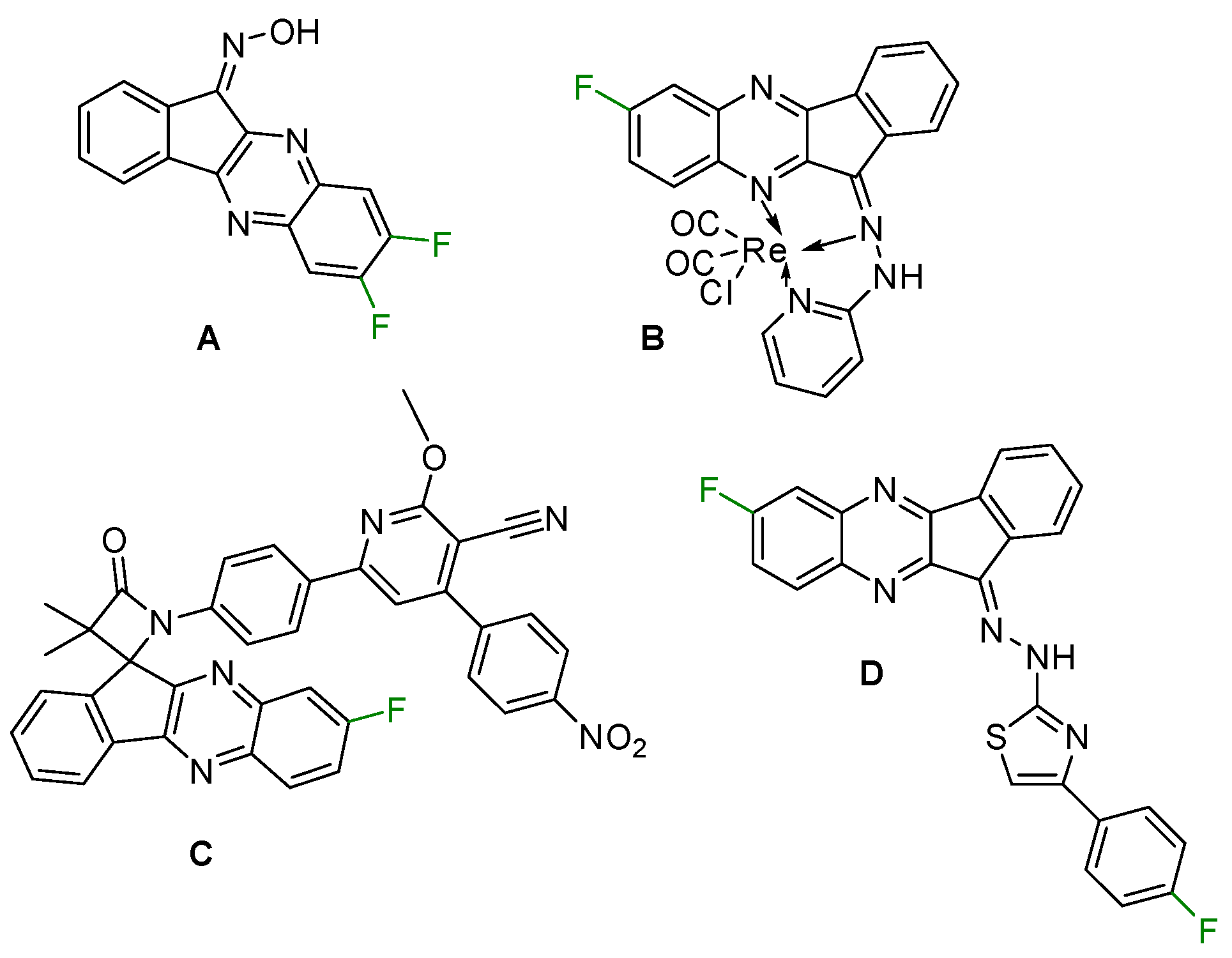
Similarly to many other aza-heterocyclics, quinoxalines are promising for the design and synthesis of new bioactive substances [11]. They exhibit a wide range of pharmacological activities encompassing, for example, anti-HIV [12], anti-inflammatory, anti-tumor [13] and anti-depressant [14] effects. Indenoquinoxalines are currently of interest as antioxidants and anticancer agents [15]. Low-fluorinated indenoquinoxalines, e.g., compound A, display exciting antitumor properties and c-Jun N-terminal kinase (JNK) inhibitory activity [16] (note that JNK plays a key role in the pathogenesis of serious diseases such as Alzheimer’s, Parkinson’s, cardiovascular diseases, and ischemic damage). Re(I) complexes that contain fluorinated indenoquinoxaline ligand B are effective against MCF-7 cancer cells [17]. Compound C is able to effectively intercalate with DNA [18], whereas compound D is a hepatocellular carcinoma (HCC) drug targeting the tyrosine kinase [19] (for A–D, see Scheme 1).
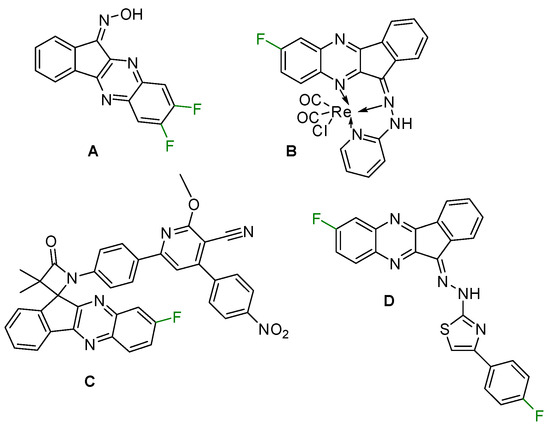
Scheme 1.
Selected bioactive fluorinated indenoquinoxalines.
Alongside this, there is an emerging interest in highly fluorinated chemotypes [20,21,22], which include still-unknown highly fluorinated indenoquinoxalinones. Here, we report on synthesis of 6,7,8,9-tetrafluoro-11H-indeno[1,2-b]quinoxalin-11-one and elucidate its structure by means of single-crystal XRD.
2. Results and Discussion
Synthesis and Molecular and Crystal Structure
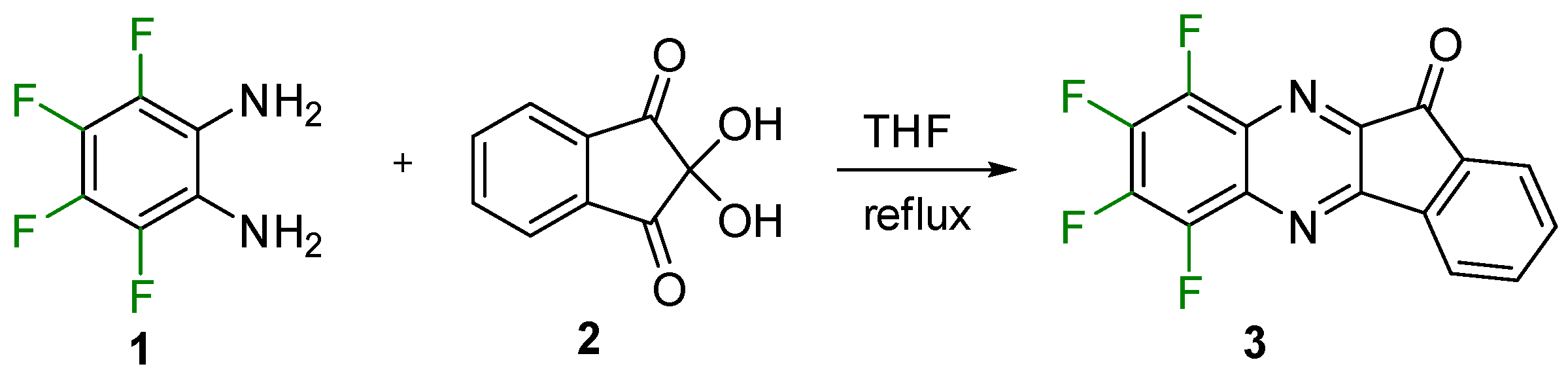
The target indenoquinoxalinone (compound 3, Scheme 2) was synthesized by the Körner–Hinsberg-type condensation of 1,2-diamino-3,4,5,6-tetrafluorobenzene [23] with ninhydrin (compounds 1 and 2, respectively; Scheme 2) in THF. The structure of 3 was confirmed by single-crystal XRD (Figure 1), multinuclear NMR, LC-MS, and elemental analysis. The compound is a fluorophore emitting at 490 nm in CHCl3 solution.
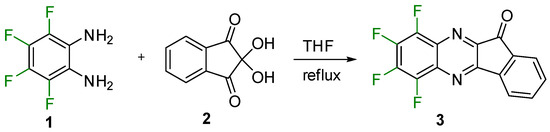
Scheme 2.
Synthesis of indenoquinoxalinone 3.
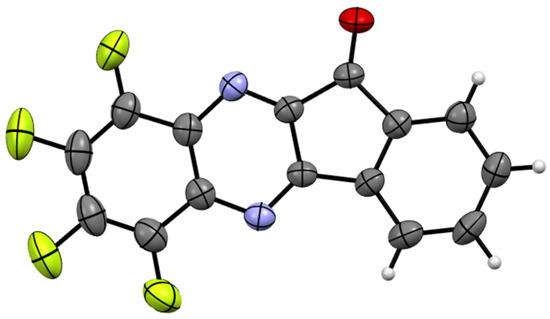
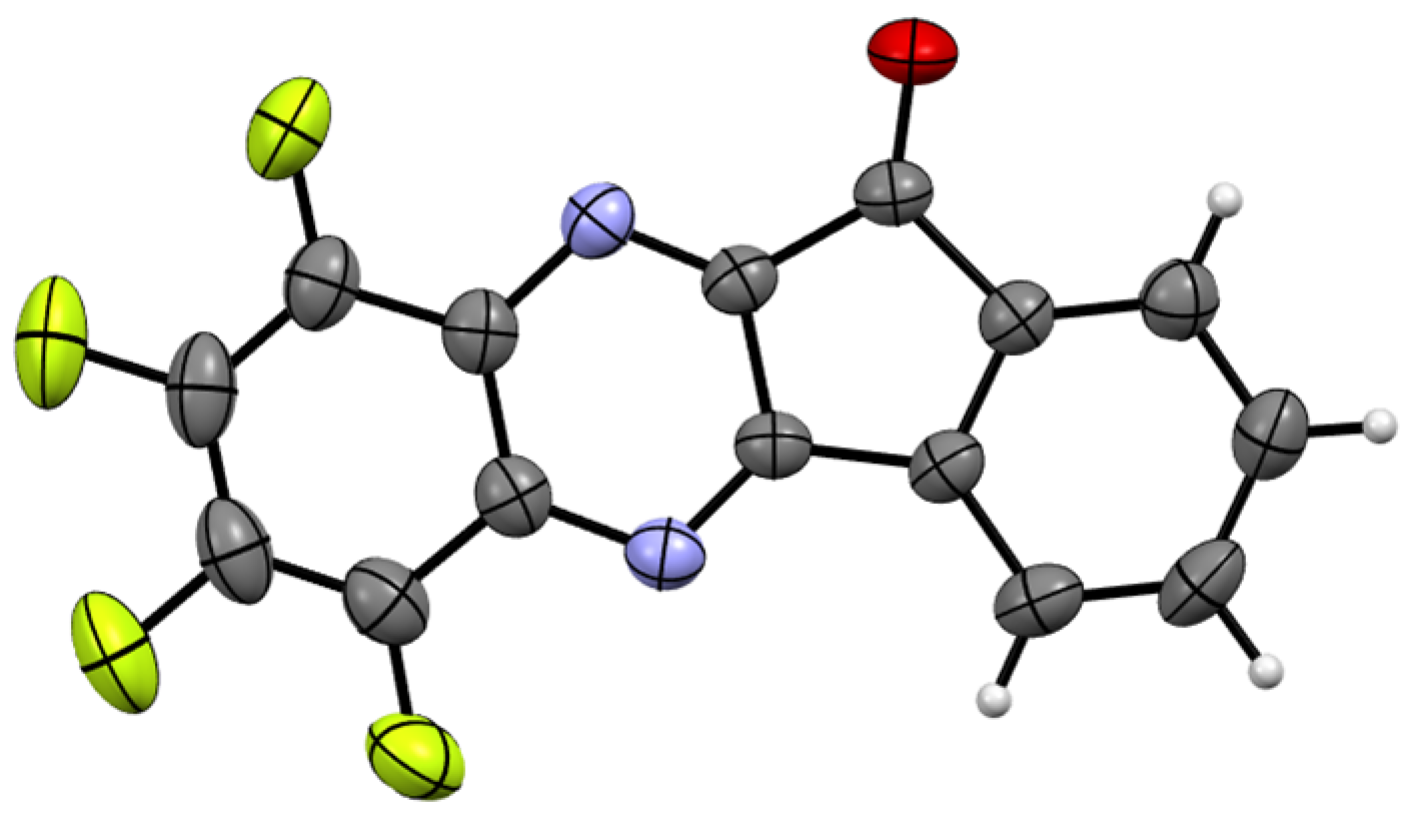
Figure 1.
XRD molecular structure of 3 (CCDC 2433446; thermal ellipsoids at 50% probability). The molecule is perfectly planar; the standard deviations from the mean plane are 0.055 Å. The bond distances and bond angles correspond to the statistical means [24]. Color code: C, gray; H, light gray; F, green; N, blue; O, red.
In its crystal form, 3 exhibits infinite π-stacks with an interplanar separation of ~3.36 Å; the sums of the van der Waals (VdW) radii of the C and E atoms are ~3.54 and 3.43 Å for E = C and N, respectively [25]. The stacks are connected by C–H···O hydrogen bonds featuring a H···O distance of ~2.32 Å (the sum of the VdW radii is ~2.70 Å [25]) and a C–H···O angle of 168°. The F atoms reveal F···π orientations; however, the atom-to-plane F···π distances of ~3.21–3.63 Å are close to or exceed the sum of the VdW radii of the C and F atoms, which are equal to 3.23 Å [25] (see CCDC 2433446 for 3D visualization and details). Such secondary bonding interactions in the crystalline state are typical of fluoroorganics [26].
3. Materials and Methods
3.1. General
XRD was carried out with a Bruker Kappa Apex CCD diffractometer. The 1H (400 MHz), 13C (100 MHz), and 19F (376 MHz) NMR spectra were collected using a Bruker AVANCE III HD spectrometer. The chemical shifts, δ, were measured with respect to TMS (1H, 13C) and C6F6 (δ = −162.9 ppm with respect to CFCl3). LC-MS analysis was performed with a DFS Thermo Electron Corporation instrument. Electronic absorption (UV-Vis) and emission (FL) spectra were measured on Varian Cary 5000 and Varian Cary Eclipse spectrophotometers, respectively. The elemental analysis for C, H, N, and F was performed with a Carlo Erba Model 1106 instrument. The melting point of 3 was measured using a Melting Point Apparatus SMP30 at a heating rate of 2.5 °C min−1.
3.2. Synthesis
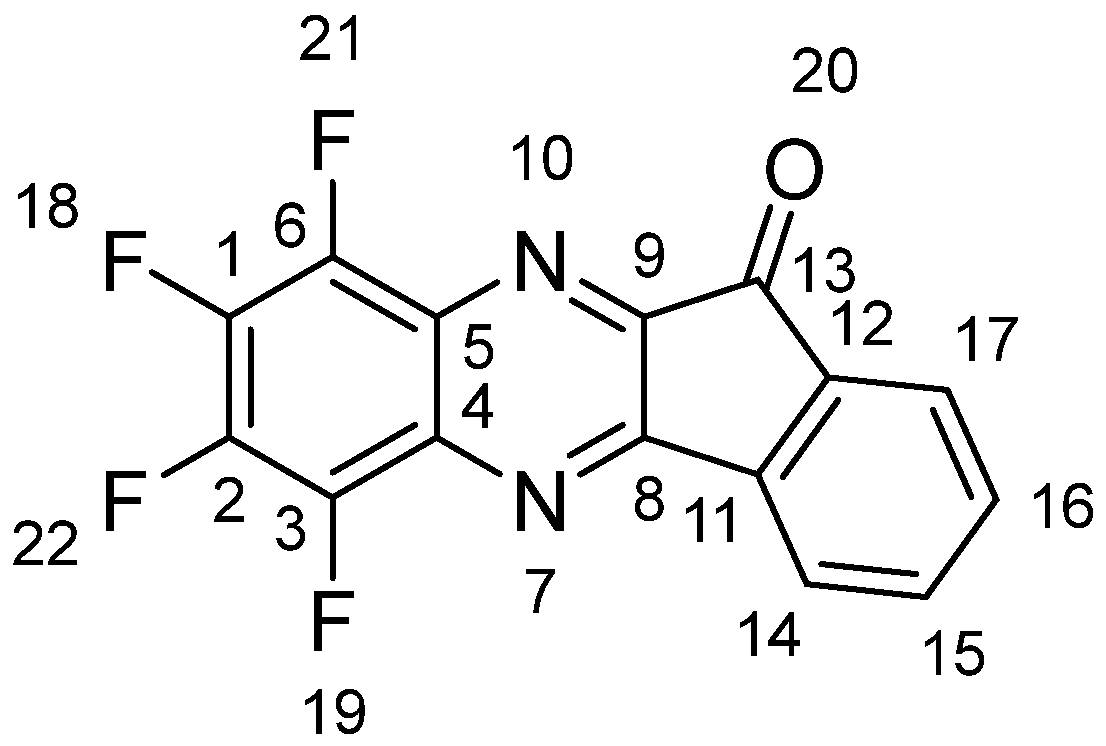
A stirring solution of 786 mg (4.4 mmol) of 1 and 776 mg (4.4 mmol) of 2 in 50 mL of dried THF was refluxed for 5 h. The solvent was distilled off under reduced pressure, and a dark red solid was chromatographed on a silica column with chloroform. The bright yellow zone collected was evaporated, and the residue was recrystallized using acetone. Compound 3 was obtained in the form of yellow plates (907 mg, 69%), m. p. 276–279 °C (from ethanol). Single crystals suitable for XRD were obtained by recrystallization using chloroform. The following ratios were found/calculated for C15H4F4N2O, in %: C 58.91/59.23, H 1.07/1.33, F 25.80/24.98, N 9.08/9.21. NMR (CDCl3; ESI, Figures S1–S3), δ, ppm (J, Hz): 1H (for atom numbering, see Figure 2)—7.68 (dt, J = 7.6, J = 1, 1H, H-16), 7.82 (dt, J = 7.5, J = 1, 1H, H-17), 7.95 (d, J = 7.6, 1H, H-15), 8.17 (d, J = 7.6, 1H, H-14). 13C—187.7, 157.3, 150.3, 143.4, 142.9, 142.4, 141.1, 140.4, 137.3, 136.9, 133.7, 129.9, 129.4, 125.2, 123.4. 19F—13.9, 13.5, 10.9, 9.8; ESI LC-MS: M+, m/z: measured 304.0256 [C15H4F4N2O + H]+, calculated 304.0254. UV-Vis (CHCl3) λmax, nm (log ε) 298 (4.49); FL (CHCl3) λmax em/ex, nm: 490/400.
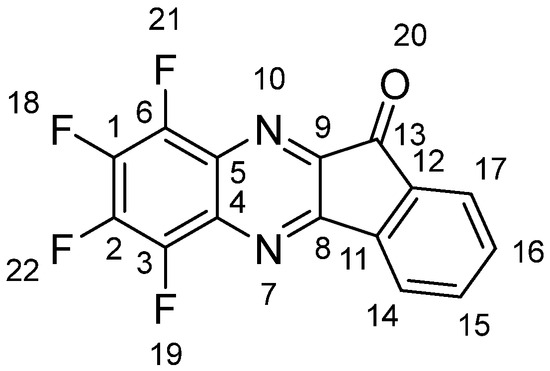
Figure 2.
Atom numbering to assign 1H NMR spectrum of 3.
3.3. X-Ray Diffraction
XRD data for 3 (CCDC 2433446) were obtained at 296 K with a Bruker Kappa Apex II CCD diffractometer using φ and ω scans of narrow (0.5°) frames with Mo Kα radiation (λ = 0.71073 Å) and a graphite monochromator. The structure was solved by direct methods using the SHELXT 2014/5 programs set [27] and refined by the full-matrix least-squares method against all F2 in anisotropic approximation using the SHELXL-2018/3 programs set [27]. The H atoms’ positions were calculated with the riding model. Absorption corrections were applied empirically using SADABS programs [28]. Compound 3 crystallizes in an orthorhombic system with the space group P212121, a = 5.5581(3), b = 6.2028(4), c = 34.309(2) Å, V = 1182.8(1) Å3, Z = 4, C15H4F4N2O, Dc = 1.708 g cm−3, μ = 0.153 mm−1, F(000) = 608, crystal size 0.90 × 0.50 × 0.10 mm3, 2252 independent reflections (Rint. = 0.020), θmax = 27.5°, wR2 = 0.0845, and S = 1.03 for all reflections (R = 0.0332 for 1996 F > 4σ). The obtained crystal structures were analyzed for shortened contacts between non-bonded atoms using the PLATON program [29].
4. Conclusions
The new highly fluorinated title compound 3 was synthesized and unambiguously structurally characterized for further biomed studies.
Supplementary Materials
Figures S1–S3 (1H, 13C, and 19F NMR spectra of 3).
Author Contributions
Conceptualization was conducted by A.R.K., A.V.Z. and A.I.K.; experiments were carried out by A.R.K. and I.Y.B.; data analysis and the writing of the initial manuscript were performed by A.R.K., I.Y.B. and A.I.K.; editing of the final version of the manuscript was performed by A.R.K., A.I.K., A.V.Z. and I.Y.B.; project administration and supervision were conducted by A.I.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Russian Science Foundation (project no. 24-73-00202).
Data Availability Statement
Experimental data associated with this research are available from the authors. Crystallographic data for compound 3 were deposited at the Cambridge Crystallographic Data Centre, CCDC 2433446. Copies of the data can be obtained free of charge from the Cambridge Crystallographic Data Centre, 12 Union Road, Cambridge CB2 1EZ, UK (Fax: +44-1223-336-033; e-mail: deposit@ccdc.cam.ac.uk).
Acknowledgments
The authors are grateful to Arkady G. Makarov for his participation in preliminary experiments and his helpful discussions.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Dehnen, S.; Schafer, L.; Lectka, T.; Togni, A. Fluorine: A very special element and its very special impacts on chemistry. J. Org. Chem. 2021, 86, 16213–16219. [Google Scholar] [CrossRef] [PubMed]
- O’Hagan, D. Understanding organofluorine chemistry. An introduction to the C–F bond. Chem. Soc. Rev. 2008, 37, 308–319. [Google Scholar] [CrossRef] [PubMed]
- Henary, E.; Casa, S.; Dost, T.L.; Sloop, J.C.; Henary, M. The role of small molecules containing fluorine atoms in medicine and imaging applications. Pharmaceuticals 2024, 17, 218. [Google Scholar] [CrossRef] [PubMed]
- O’Hagan, D.; Young, R.J. Future challenges and opportunities with fluorine in drugs? Med. Chem. Res. 2023, 32, 1231–1234. [Google Scholar] [CrossRef]
- Meanwell, N.A. Fluorine and fluorinated motifs in the design and application of bioisosteres for drug design. J. Med. Chem. 2018, 61, 5822–5880. [Google Scholar] [CrossRef]
- Petterson, M.; Hou, X.; Kuhn, M.; Wager, T.T.; Kauffman, G.M.; Verhoest, P.R. Quantitative assessment of the impact of fluorine substitution on P-glycoprotein (P-gp) mediated efflux, permitiability, lipophilicity, and metabolic stability. J. Med. Chem. 2016, 59, 5284–5296. [Google Scholar] [CrossRef]
- Gillis, E.P.; Eastman, K.J.; Hill, M.D.; Donelly, D.J.; Meanwell, N.A. Applications of fluorine in medicinal chemistry. J. Med. Chem. 2015, 58, 8315–8359. [Google Scholar] [CrossRef]
- Rizzo, C.; Amata, S.; Pibiri, I.; Pace, A.; Buscemi, S.; Piccionello, A.P. FDA-approved fluorinated heterocyclic drugs from 2016 to 2022. Int. J. Mol. Sci. 2023, 24, 7728. [Google Scholar] [CrossRef]
- Inoue, M.; Sumii, Y.; Shibata, N. Contribution of organofluorine compounds in pharmaceuticals. ACS Omega 2020, 5, 10633–10640. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, J.; Gu, Z.; Zhu, W.; Aceña, J.L.; Soloshonok, V.A.; Iwata, K.; Liu, H. Next generation of fluorine-containing pharmaceuticals, compounds currently in phase II-III clinical trials of major pharmaceutical companies: New structural trends and therapeutic areas. Chem. Rev. 2014, 114, 2432–2506. [Google Scholar] [CrossRef]
- Suthar, S.K.; Chundawat, N.S.; Singh, G.P.; Pedrón, J.M.; Jhala, Y.K. Quinoxaline: A comprehension of current pharmacological advancements in medicinal chemistry. Eur. J. Med. Chem. Rep. 2022, 5, 100040. [Google Scholar] [CrossRef]
- Selvam, P.; Lakra, D.R.; Pannecouque, C.; De Clercq, E. Synthesis, anti-viral and cytotoxicity studies of novel N-substituted indophenazine derivatives. Indian J. Pharm. Sci. 2012, 74, 22–25. [Google Scholar] [CrossRef] [PubMed]
- Ghalib, R.M.; Hashim, R.; Mehdi, S.H.; Sulaiman, O.; Silva, P.S.P.; Jassbi, A.R.; Firuzi, O.; Kawamura, F.; Chan, K.-L.; Murugaiyah, V. Synthesis of ninhydrin derivatives and their anticancer, antimicrobial and cholinesterase enzymes inhibitory activities. Lett. Drug Des. Discov. 2012, 9, 767–774. [Google Scholar] [CrossRef]
- Magesh, R.; Devadoss, T.; Pandey, D.K.; Bhatt, S. Discovery of new antidepressants from structurally novel 5-HT3 receptor antagonists: Design, synthesis and pharmacological evaluation of 3-ethoxyquinoxaline-2-carboxamides. Bioorg. Med. Chem. Lett. 2011, 21, 1253–1256. [Google Scholar] [CrossRef]
- Mani, K.S.; Kaminski, W.; Rajendron, S.P. A facile atom-economic one pot multicomponent synthesis of bioactive spiro-indenoquinoxaline pyrrolizines as potent antioxidants and anti-cancer agents. New J. Chem. 2018, 42, 301–310. [Google Scholar] [CrossRef]
- Liakhov, S.A.; Schepetkin, I.A.; Karpenko, O.S.; Duma, H.I.; Haidarzhy, N.M.; Kirpotina, L.N.; Kovrizhina, A.R.; Khlebnikov, A.I.; Bagryanskaya, I.Y.; Quinn, M.T. Novel c-Jun N-terminal kinase (JNK) inhibitors with an 11H-indeno[1,2-b]quinoxalin-11-one scaffold. Molecules 2021, 26, 5688. [Google Scholar] [CrossRef]
- Padariya, A.D.; Savaliya, N.K.; Dhaduk, M.P.; Dabhi, P.A.; Bhatt, B.S.; Bhatt, V.D.; Patel, M.N. Synthesis and characterization of quinoxaline-based rhenium(I) organometallic compounds: Biological and computational applications. J. Mol. Struct. 2024, 1302, 137477. [Google Scholar] [CrossRef]
- Dabhi, R.A.; Daduk, M.P.; Savaliya, N.K.; Padariya, A.D.; Patil, A.P.; Desai, R.A.; Bhatt, V.D.; Bhatt, B.S. Influence of pyridine entangled novel hybrid quinoxaline spirane on the fluorescence and absorption spectra of biomolecules: Molecular docking, pharmacokinetics and in-vitro biological investigations. J. Fluoresc. 2024, 1–18. [Google Scholar] [CrossRef]
- Abusaif, M.S.; Ragab, A.; Fayed, E.A.; Ammar, Y.A.; Gowitel, A.M.H.; Hassanin, S.O.; Ahmed, G.E.; Gohar, N.A. Exploring a novel triazole derivatives hybrid with fluorinated indenoquinoxaline as dual inhibitors targeting VEGFR2/AKT and apoptosis inducers against hepatocellular carcinoma with docking simulation. Bioorg. Chem. 2025, 154, 108023. [Google Scholar] [CrossRef]
- Basseto, M.; Ferla, S.; Pertusati, F. Polyfluorinated groups in medicinal chemistry. Future Med. Chem. 2015, 7, 527–546. [Google Scholar] [CrossRef]
- Prima, D.O.; Vorontsova, E.V.; Makarov, A.G.; Makarov, A.Y.; Bagryanskaya, I.Y.; Mikhailovskaya, T.F.; Slizhov, Y.G.; Zibarev, A.V. Halogenated (F, Cl) 1,3-benzodiazoles, 1,2,3-benzotriazoles, 2,1,3-benzothia/selenadiazoles and 1,4-benzodiazines inducing Hep2 cell apoptosis. Mendeleev Commun. 2017, 27, 439–442. [Google Scholar] [CrossRef]
- Shchegolkov, E.V.; Burgart, Y.V.; Shchur, I.V.; Saloutin, V.I. Polyfluorinated benzoic acids as promising reagents for organic synthesis and medicinal chemistry. Russ. Chem. Rev. 2024, 93, RCR5131. [Google Scholar] [CrossRef]
- Prima, D.O.; Makarov, A.G.; Bagryanskaya, I.Y.; Kolesnikov, A.E.; Zargarova, L.V.; Baev, D.S.; Eliseeva, T.F.; Politanskaya, L.V.; Makarov, A.Y.; Slizhov, Y.G.; et al. Fluorine-containing n-6 and angular and linear n-6-n′ (n, n′ = 5, 6, 7) diaza-heterocyclic scaffolds assembled on benzene core in unified way. ChemistrySelect 2019, 4, 2383–2386. [Google Scholar] [CrossRef]
- Allen, F.H.; Kennard, O.; Watson, D.G.; Brammer, L.; Orpen, A.G.; Taylor, R. Tables of bond lengths determined by X-ray and neutron diffraction. Part 1. Bond lengths in organic compounds. J. Chem. Soc. Perkin Trans. 1987, 2, S1–S19. [Google Scholar] [CrossRef]
- Alvarez, S. A cartography of the van der Waals territories. Dalton Trans. 2013, 42, 8617–8636. [Google Scholar] [CrossRef]
- Rybalova, T.V.; Bagryanskaya, I.Y. C–F···π, F···H, and F···F intermolecular interactions and F-aggregations: Role in crystal engineering of fluoroorganic compounds. J. Struct. Chem. 2009, 50, 741–753. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure determination with SHELXL. Acta Crystallogr. C 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SADABS, v. 2008-1; Bruker AXS: Madison, WI, USA, 2008.
- Spek, A.L. Single-crystal structure validation with the program PLATON. J. Appl. Crystallogr. 2003, 36, 7–13. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).