Trophic Diversity of Plankton in the Epipelagic and Mesopelagic Layers of the Tropical and Equatorial Atlantic Determined with Stable Isotopes
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Oceanographic Zones
3.2. Zonal Variability of Carbon and Nitrogen Sources
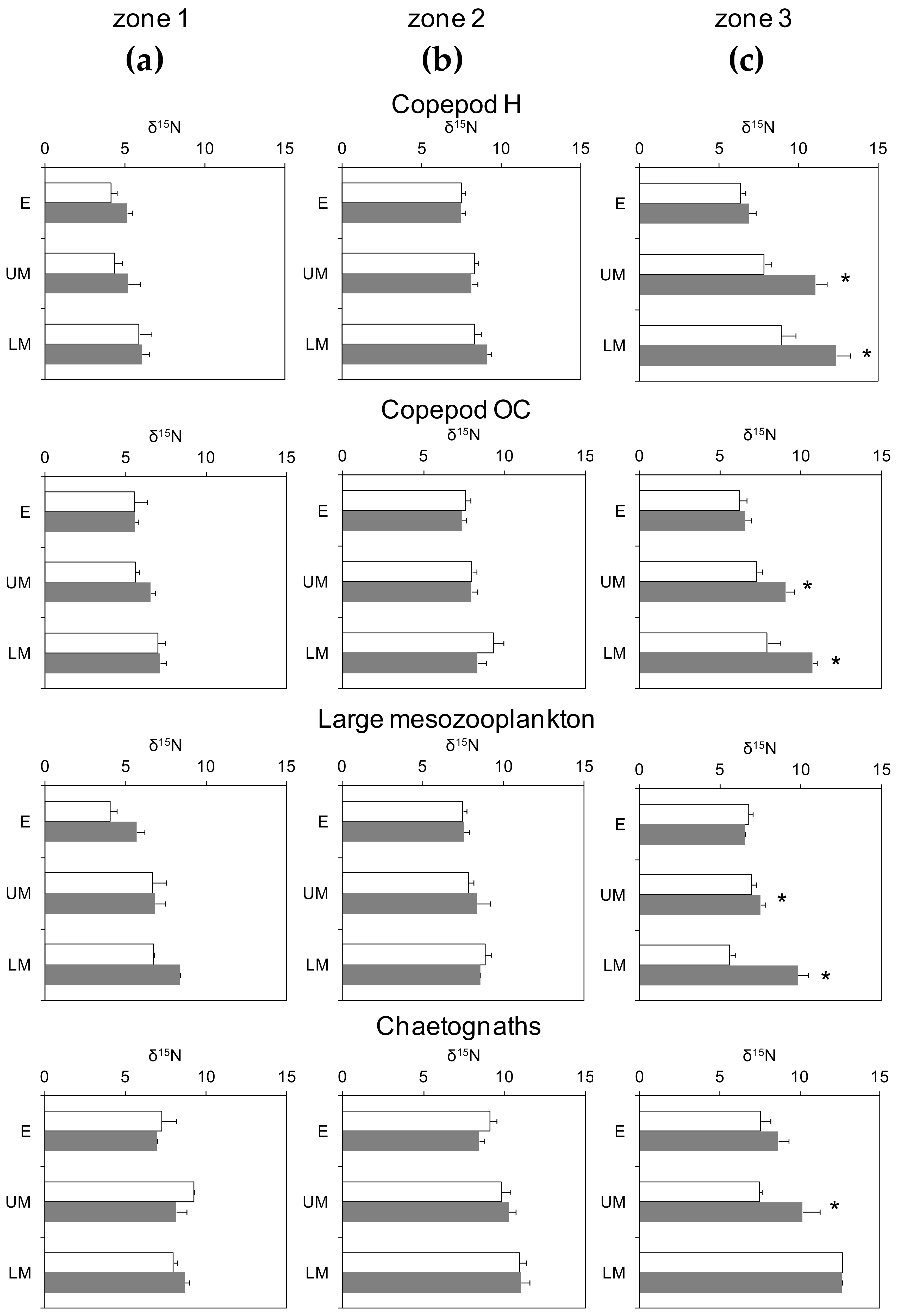
3.3. Day-Night Variability of Nitrogen Isotopes Among Plankton Guilds
3.4. Isotopic Niche Estimations
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sanders, R.; Henson, S.A.; Koski, M.; De La Rocha, C.L.; Painter, S.C.; Poulton, A.J.; Riley, J.; Salihoglu, B.; Visser, A.; Yool, A.; et al. The biological pump in the North Atlantic. Prog. Oceanogr. 2014, 129, 200–218. [Google Scholar] [CrossRef]
- Angel, M.V. Does mesopelagic biology affect the vertical flux. In Productivity of the Ocean: Present and Past; Berger, W.H., Smetacek, V.S., Wefer, G., Eds.; Wiley: New York, NY, USA, 1989; pp. 155–173. [Google Scholar]
- Longhurst, A.R.; Bedo, A.W.; Harrison, W.G.; Head, E.J.H.; Sameoto, D.D. Vertical flux of respiratory carbon by oceanic diel migrant biota. Deep Sea Res. 1990, 37, 685–694. [Google Scholar] [CrossRef]
- Steinberg, D.K.; Goldwaith, S.A.; Hansell, D.A. Zooplankton vertical migration and the active transport of dissolved organic and inorganic nitrogen in the Sargasso Sea. Deep Sea Res. 2002, 49, 1445–1461. [Google Scholar] [CrossRef]
- Siegel, D.A.; Buesseler, K.O.; Behrenfeld, M.J.; Benitez-Nelson, C.R.; Boss, E.; Brzezinski, M.A.; Burd, A.; Carlson, C.A.; D’Asaro, E.A.; Doney, S.C.; et al. Prediction of the export and fate of global ocean net primary production: The EXPORTS science plan. Front. Mar. Sci. 2016, 3, 22. [Google Scholar] [CrossRef]
- Aristegui, J.; Duarte, C.M.; Agusti, S.; Doval, M.; Alvarez-Salgado, X.A.; Hansell, D.A. Dissolved organic carbon support of respiration in the dark ocean. Science 2002, 298, 1967. [Google Scholar] [CrossRef] [PubMed]
- Vinogradov, M.E. Feeding of the deep sea zooplankton. Rapp. Cons. Int. Expl. de la Mer. 1962, 153, 114–120. [Google Scholar]
- Bollens, S.M.; Rollwagen-Bollens, G.; Quenette, J.A.; Bochdansky, A.B. Cascading migrations and implications for vertical fluxes in pelagic ecosystems. J. Plankton Res. 2011, 33, 349–355. [Google Scholar] [CrossRef]
- Pearre, S., Jr. Eat and run? The hunger/satiation hypothesis in vertical migration: History, evidence and consequences. Biol. Rev. 2003, 78, 1–79. [Google Scholar] [CrossRef] [PubMed]
- Olivar, M.P.; González-Gordillo, J.I.; Salat, J.; Chust, G.; Cózar, A.; Hernández León, S.; Fernández de Puelles, M.L.; Irigoien, X. The contribution of migratory mesopelagic fishes to neuston fish assemblages across the Atlantic, Indian and Pacific oceans. Mar. Freshw. Res. 2016, 67, 1114–1127. [Google Scholar] [CrossRef]
- Hays, G.C. A review of the adaptive significance and ecosystem consequences of zooplankton diel vertical migrations. Hydrobiologia 2003, 503, 163–170. [Google Scholar] [CrossRef]
- Hernández-León, S.; Franchy, G.; Moyano, M.; Menéndez, I.; Schmoker, C.; Putzeys, S. Carbon sequestration and zooplankton lunar cycles: Could we be missing a major component of the biological pump? Limnol. Oceanogr. 2010, 55, 2503–2512. [Google Scholar] [CrossRef]
- Visser, A.W.; Grønning, J.; Jónasdóttir, S.H. Calanus hyperboreus and the lipid pump. Limnol. Oceanogr. 2017, 62, 1155–1165. [Google Scholar] [CrossRef]
- Fry, B.; Wainright, S.C. Diatom sources of 13C-rich carbon in marine food webs. Mar. Ecol. Prog. Ser. 1991, 76, 149–157. [Google Scholar] [CrossRef]
- Sigman, D.M.; Karsh, K.L.; Casciotti, K.L. Ocean process tracers: Nitrogen isotopes in the ocean. In Encyclopedia of Ocean Sciences (Second Edition); Steele, J.H., Ed.; Academic Press: Oxford, UK, 2009; pp. 40–54. [Google Scholar]
- Layman, C.A.; Arrington, D.A.; Montaña, C.G.; Post, D.M. Can stable isotope ratios provide for community–wide measurements of trophic structure? Ecology 2007, 88, 42–48. [Google Scholar] [CrossRef]
- Layman, C.A.; Araujo, M.S.; Boucek, R.; Hammerschlag-Peyer, C.M.; Harrison, E.; Jud, Z.R.; Matich, P.; Rosenblatt, A.E.; Vaudo, J.J.; Yeager, L.A.; et al. Applying stable isotopes to examine food-web structure: An overview of analytical tools. Biol. Rev. 2012, 87, 545–562. [Google Scholar] [CrossRef] [PubMed]
- Jackson, A.L.; Inger, R.; Parnell, A.C.; Bearhop, S. Comparing isotopic niche widths among and within communities: SIBER—Stable isotope bayesian ellipses in R. J. Anim. Ecol. 2011, 80, 595–602. [Google Scholar] [CrossRef] [PubMed]
- Koppelmann, R.; Boöttger-Schnack, R.; Möbius, J.; Weickert, H. Trophic relationships of zooplankton in the Eastern Mediterranean based on stable isotope measurements. J. Plankton Res. 2009, 31, 669–686. [Google Scholar] [CrossRef]
- Polunin, N.V.C.; Morales-Nin, B.; Pawsey, W.E.; Cartes, J.E.; Pinnegar, J.K.; Moranta, J. Feeding relationships in Mediterranean bathyal assemblages elucidated by stable nitrogen and carbon isotope data. Mar. Ecol. Prog. Ser. 2001, 220, 13–23. [Google Scholar] [CrossRef]
- Bode, M.; Hagen, W.; Schukat, A.; Teuber, L.; Fonseca-Batista, D.; Dehairs, F.; Auel, H. Feeding strategies of tropical and subtropical calanoid copepods throughout the Eastern Atlantic Ocean: Latitudinal and bathymetric aspects. Prog. Oceanogr. 2015, 138, 268–282. [Google Scholar] [CrossRef]
- Gartner, J.V.; Hopkins, T.L.; Baird, R.C.; Milliken, D.M. The lanternfishes (pisces, myctophidae) of the Eastern Gulf of Mexico. Fish. Bull. 1987, 85, 81–98. [Google Scholar]
- Morel, A.; Claustre, H.; Gentili, B. The most oligotrophic subtropical zones of the global ocean: Similarities and differences in terms of chlorophyll and yellow substance. Biogeosciences 2010, 7, 3139–3151. [Google Scholar] [CrossRef]
- Arístegui, J.; Alvarez-Salgado, X.A.; Barton, E.D.; Figueiras, F.G.; Hernández-León, S.; Roy, C.; Santos, A.M.P. Oceanography and fisheries of the Canary Current/Iberian region of the Eastern North Atlantic (18a, e). In The Global Coastal Ocean: Interdisciplinary Regional Studies and Syntheses; Robinson, A.R., Brink, K., Eds.; Harvard University Press: Boston, MA, USA, 2006; pp. 877–931. [Google Scholar]
- Hernández-León, S.; Gómez, M.; Arístegui, J. Mesozooplankton in the Canary Current System: The coastal–ocean transition zone. Prog. Oceanogr. 2007, 74, 397–421. [Google Scholar] [CrossRef]
- Mouriño-Carballido, B.; Graña, R.; Fernández, A.; Bode, A.; Varela, M.; Domínguez, J.F.; Escánez, J.; De Armas, D.; Marañón, E. Importance of N2 fixation vs. nitrate eddy diffusion along a latitudinal transect in the Atlantic Ocean. Limnol. Oceanogr. 2011, 56, 999–1007. [Google Scholar] [CrossRef]
- Mompeán, C.; Bode, A.; Benítez-Barrios, V.M.; Domínguez-Yanes, J.F.; Escánez, J.; Fraile-Nuez, E. Spatial patterns of plankton biomass and stable isotopes reflect the influence of the nitrogen-fixer Trichodesmium along the subtropical North Atlantic. J. Plankton Res. 2013, 35, 513–525. [Google Scholar] [CrossRef]
- Fernández, A.; Marañón, E.; Bode, A. Large-scale meridional and zonal variability in the nitrogen isotopic composition of plankton in the Atlantic Ocean. J. Plankton Res. 2014, 36, 1060–1073. [Google Scholar] [CrossRef]
- Ariza, A.; Garijo, J.C.; Landeira, J.M.; Bordes, F.; Hernández-León, S. Migrant biomass and respiratory carbon flux by zooplankton and micronekton in the subtropical Northeast Atlantic Ocean (Canary Islands). Prog. Oceanogr. 2015, 134, 330–342. [Google Scholar] [CrossRef]
- Olivar, M.P.; Bode, A.; López-Pérez, C.; Hulley, P.A.; Hernández-León, S. Trophic position of lanternfishes (pisces: Myctophidae) of the tropical and equatorial Atlantic estimated using stable isotopes. ICES J. Mar. Sci. 2018. [Google Scholar] [CrossRef]
- Karstensen, J.; Stramma, L.; Visbeck, M. Oxygen minimum zones in the Eastern Tropical Atlantic and Pacific Oceans. Prog. Oceanogr. 2008, 77, 331–350. [Google Scholar] [CrossRef]
- Olivar, M.P.; Hulley, P.A.; Castellón, A.; Emelianov, M.; López, C.; Tuset, V.M.; Contreras, T.; Molí, B. Mesopelagic fishes across the tropical and equatorial Atlantic: Biogeographical and vertical patterns. Prog. Oceanogr. 2017, 151, 116–137. [Google Scholar] [CrossRef]
- Benedetti, F.; Gasparini, S.; Ayata, S.-D. Identifying copepod functional groups from species functional traits. J. Plankton Res. 2016, 38, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Coplen, T.B. Guidelines and recommended terms for expression of stable isotope-ratio and gas-ratio measurement results. Rapid Commun. Mass Spectrom. 2011, 25, 2538–2560. [Google Scholar] [CrossRef] [PubMed]
- Post, D.M.; Layman, C.A.; Arrington, D.A.; Takimoto, G.; Quattrochi, J.; Montaña, C.G. Getting to the fat of the matter: Models, methods and assumptions for dealing with lipids in stable isotope analyses. Oecologia 2007, 152, 179–189. [Google Scholar] [CrossRef] [PubMed]
- Estrada, M.; Delgado, M.; Blasco, D.; Latasa, M.; Cabello, A.M.; Benítez-Barrios, V.; Fraile-Nuez, E.; Mozetic, P.; Vidal, M. Phytoplankton across tropical and subtropical regions of the Atlantic, Indian and Pacific oceans. PLoS ONE 2016, 11, e0151699. [Google Scholar] [CrossRef] [PubMed]
- Hunt, B.P.V.; Carlotti, F.; Donoso, K.; Pagano, M.; D’Ortenzio, F.; Taillandier, V.; Conan, P. Trophic pathways of phytoplankton size classes through the zooplankton food web over the spring transition period in the north-west Mediterranean Sea. J. Geophys. Res. 2017, 122, 6309–6324. [Google Scholar] [CrossRef]
- McClelland, J.W.; Holl, C.M.; Montoya, J.P. Relating low δ15N values of zooplankton to N2-fixation in the tropical North Atlantic: Insights provided by stable isotope ratios of amino acids. Deep Sea Res. 2003, 50, 849–861. [Google Scholar] [CrossRef]
- Mompeán, C.; Bode, A.; Gier, E.; McCarthy, M.D. Bulk vs. aminoacid stable N isotope estimations of metabolic status and contributions of nitrogen fixation to size-fractionated zooplankton biomass in the subtropical N Atlantic. Deep Sea Res. 2016, 114, 137–148. [Google Scholar] [CrossRef]
- Agawin, N.S.R.; Tovar-Sanchez, A.; Knoth de Zarruck, K.; Duarte, C.M.; Agusti, S. Variability in the abundance of Trichodesmium and nitrogen fixation activities in the subtropical NE Atlantic. J. Plankton Res. 2013, 35, 1126–1140. [Google Scholar] [CrossRef]
- Waser, N.A.D.; Harrison, W.G.; Head, E.J.H.; Nielsen, B.; Lutz, V.A.; Calvert, S.E. Geographic variations in the nitrogen isotope composition of surface particulate nitrogen and new production across the North Atlantic Ocean. Deep Sea Res. 2000, 47, 1207–1226. [Google Scholar] [CrossRef]
- Jennings, S.; Maxwell, T.A.D.; Schratzberger, M.; Milligan, S.P. Body-size dependent temporal variations in nitrogen stable isotope ratios in food webs. Mar. Ecol. Prog. Ser. 2008, 370, 199–206. [Google Scholar] [CrossRef]
- Bode, A.; Barquero, S.; Varela, M.; Braun, J.G.; de Armas, D. Pelagic bacteria and phytoplankton in oceanic waters near the Canary Islands in summer. Mar. Ecol. Prog. Ser. 2001, 209, 1–17. [Google Scholar] [CrossRef]
- Hirst, A.G.; Bunker, A.J. Growth of marine planktonic copepods: Global rates and patterns in relation to chlorophyll a, temperature, and body weight. Limnol. Oceanogr. 2003, 48, 1988–2010. [Google Scholar] [CrossRef]
- Fernández-Castro, B.; Mouriño-Carballido, B.; Marañón, E.; Chouciño, P.; Gago, J.; Ramírez, T.; Vidal, M.; Bode, A.; Blasco, D.; Royer, S.J.; et al. Importance of salt fingering for new nitrogen supply in the oligotrophic ocean. Nat. Commun. 2015, 6, 8002. [Google Scholar] [CrossRef] [PubMed]
- Teuber, L.; Schukat, A.; Hagen, W.; Auel, H. Trophic interactions and life strategies of epi- to bathypelagic calanoid copepods in the tropical Atlantic Ocean. J. Plankton Res. 2014, 36, 1109–1123. [Google Scholar] [CrossRef]
- Agersted, M.D.; Bode, A.; Nielsen, T.G. Trophic position of coexisting krill species: A stable isotope approach. Mar. Ecol. Prog. Ser. 2014, 516, 136–151. [Google Scholar] [CrossRef]
- Cartes, J.E.; Huguet, C.; Parra, S.; Sanchez, F. Trophic relationships in deep-water decapods of le Danois Bank (Cantabrian Sea, NE Atlantic): Trends related with depth and seasonal changes in food quality and availability. Deep Sea Res. 2007, 54, 1091–1110. [Google Scholar] [CrossRef]
- Pearre, S., Jr. Feeding by chaetognatha: Aspects of inter- and intra-specific predation. Mar. Ecol. Prog. Ser. 1982, 7, 33–45. [Google Scholar]
- Kehayias, G.; Lykakis, J.; Fragopoulu, N. The diets of the chaetognaths Sagitta enflata, S. serratodentata atlantica and S. bipunctata at different seasons in Eastern Mediterranean coastal waters. ICES J. Mar. Sci. 1996, 53, 837–846. [Google Scholar] [CrossRef]
- Baier, C.T.; Purcell, J.E. Trophic interactions of chaetognaths, larval fish, and zooplankton in the South Atlantic Bight. Mar. Ecol. Prog. Ser. 1997, 146, 43–53. [Google Scholar] [CrossRef]
- Madigan, D.J.; Carlisle, A.B.; Dewar, H.; Snodgrass, O.E.; Litvin, S.Y.; Micheli, F.; Block, B.A. Stable isotope analysis challenges wasp-waist food web assumptions in an upwelling pelagic ecosystem. Sci. Rep. 2012, 2, 654. [Google Scholar] [CrossRef] [PubMed]
- Svanback, R.; Quevedo, M.; Olsson, J.; Eklov, P. Individuals in food webs: The relationships between trophic position, omnivory and among-individual diet variation. Oecologia 2015, 178, 103–114. [Google Scholar] [CrossRef] [PubMed]
- Altabet, M.A.; François, R. Sedimentary nitrogen isotopic ratio as a recorder for surface ocean nitrate utilization. Glob. Biogeochem. Cycles 1994, 8, 103–116. [Google Scholar] [CrossRef]
- Holmes, E.; Lavik, G.; Fischer, G.; Segl, M.; Ruhland, G.; Wefer, G. Seasonal variability of δ15N in sinking particles in the Benguela upwelling region. Deep Sea Res. 2002, 49, 377–394. [Google Scholar] [CrossRef]
- Hernández-León, S.; Almeida, C.; Portillo-Hahnefeld, A.; Gómez, M.; Rodríguez, J.M.; Arístegui, J. Zooplankton biomass and indices of feeding and metabolism in relation to an upwelling filament off Northwest Africa. J. Mar. Res. 2002, 60, 327–346. [Google Scholar] [CrossRef]
- Fernández-Urruzola, I.; Osma, N.; Packard, T.T.; Gómez, M.; Postel, L. Distribution of zooplankton biomass and potential metabolic activities across the northern Benguela upwelling system. Deep Sea Res. 2014, 140, 138–139. [Google Scholar] [CrossRef]
- Sommer, F.; Hansen, T.; Sommer, U. Transfer of diazotrophic nitrogen to mesozooplankton in kiel fjord, western baltic sea: A mesocosm study. Mar. Ecol. Prog. Ser. 2007, 324, 105–112. [Google Scholar] [CrossRef]
- Williams, R.L.; Wakeham, S.; McKinney, R.; Wishner, K.F. Trophic ecology and vertical patterns of carbon and nitrogen stable isotopes in zooplankton from oxygen minimum zone regions. Deep Sea Res. 2014, 90, 36–47. [Google Scholar] [CrossRef]
- Harris, B.P.; Young, J.W.; Revill, A.T.; Taylor, M.D. Understanding diel-vertical feeding migrations in zooplankton using bulk carbon and nitrogen stable isotopes. J. Plankton Res. 2014, 36, 1159–1163. [Google Scholar] [CrossRef]
- Layman, C.A.; Allgeier, J.E. Characterizing trophic ecology of generalist consumers: A case study of the invasive lionfish in the Bahamas. Mar. Ecol. Prog. Ser. 2012, 448, 131–141. [Google Scholar] [CrossRef]





| Zone 1 | Zone 2 | Zone 3 | ||||
|---|---|---|---|---|---|---|
| Metric | Mean | SE | Mean | SE | Mean | SE |
| NR | 1.716 | 0.004 | 1.706 | 0.003 | 2.504 | 0.003 |
| CR | 1.095 | 0.003 | 1.703 | 0.003 | 0.714 | 0.003 |
| TA | 0.711 | 0.003 | 0.155 | 0.002 | 0.154 | 0.002 |
| CD | 0.819 | 0.001 | 0.854 | 0.001 | 0.914 | 0.001 |
| MNND | 1.161 | 0.003 | 1.170 | 0.002 | 1.271 | 0.002 |
| SDNND | 0.220 | 0.002 | 0.169 | 0.002 | 0.148 | 0.002 |
| E | UM | ||||
|---|---|---|---|---|---|
| Zone | Layer | Mean | SE | Mean | SE |
| zone 1 | UM | 27.94 | 0.33 | --- | --- |
| LM | 47.17 | 0.45 | 54.46 | 0.54 | |
| zone 2 | UM | 38.70 | 0.38 | --- | --- |
| LM | 43.93 | 0.39 | 70.98 | 0.71 | |
| zone 3 | UM | 43.88 | 0.52 | --- | --- |
| LM | 33.94 | 0.57 | 52.16 | 1.01 | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bode, A.; Hernández-León, S. Trophic Diversity of Plankton in the Epipelagic and Mesopelagic Layers of the Tropical and Equatorial Atlantic Determined with Stable Isotopes. Diversity 2018, 10, 48. https://doi.org/10.3390/d10020048
Bode A, Hernández-León S. Trophic Diversity of Plankton in the Epipelagic and Mesopelagic Layers of the Tropical and Equatorial Atlantic Determined with Stable Isotopes. Diversity. 2018; 10(2):48. https://doi.org/10.3390/d10020048
Chicago/Turabian StyleBode, Antonio, and Santiago Hernández-León. 2018. "Trophic Diversity of Plankton in the Epipelagic and Mesopelagic Layers of the Tropical and Equatorial Atlantic Determined with Stable Isotopes" Diversity 10, no. 2: 48. https://doi.org/10.3390/d10020048
APA StyleBode, A., & Hernández-León, S. (2018). Trophic Diversity of Plankton in the Epipelagic and Mesopelagic Layers of the Tropical and Equatorial Atlantic Determined with Stable Isotopes. Diversity, 10(2), 48. https://doi.org/10.3390/d10020048






