Marine Heterobranchia (Gastropoda, Mollusca) in Bunaken National Park, North Sulawesi, Indonesia—A Follow-Up Diversity Study
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Animals Collected in 2016 (Figures 2–16)
3.1.1. Cephalaspidea and Runcinacea (16 species in eight genera belonging to five families, Figures 2A–4A)
3.1.2. Anaspidea (two species in two genera belonging to one family, Figure 4B,C)
3.1.3. Sacoglossa (16 species in four genera belonging to three families, Figures 4D–6D)
3.1.4. Pleurobranchomorpha (one species in one genus belonging to one family, Figure 6E)
3.1.5. Nudibranchia, Anthobranchia (40 species in 21 genera belonging to seven families, Figures 6F–11H)
3.1.6. Nudibranchia, Cladobranchia (36 species in 20 genera belonging to 11 families, Figures 12A–16B)
3.2. Species Collected in 2017 (Figures 17–20)
3.2.1. Cephalaspidea (six species in five genera belonging to four families, Figure 17A,B)
3.2.2. Anaspidea (four species in four genera belonging to one family, Figure 17C,D)
3.2.3. Sacoglossa (11 species in seven genera belonging to four families, Figures 17E–18C)
3.2.4. Pleurobranchomorpha (one species in one genus belonging to one family, Figure 18D)
3.2.5. Nudibranchia, Anthobranchia (24 species in 16 genera belonging to seven families, Figures 18E–20B)
3.2.6. Nudibranchia, Cladobranchia (11 species in nine genera belonging to five families, Figure 20C–F)
3.3. A Comparison of the Bunaken NP Studies
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kaligis, F.; Eisenbarth, J.-H.; Schillo, D.; Dialalo, J.; Schäberle, T.F.; Böhringer, N.; Bara, R.; Reumschuessel, S.; König, G.M.; Wägele, H. Second survey of heterobranch sea slugs (Mollusca, Gastropoda, Heterobranchia) from Bunaken National Park, North Sulawesi, Indonesia—How much do we know after 12 years? Mar. Biodivers. Rec. 2018, 11, 1–20. [Google Scholar] [CrossRef]
- Naturalis Biodiversity Center, Repository, Rumphius Biohistorical. Available online: http://www.repository.naturalis.nl/cgi/b/bib/bib-idx?type=simple;c=naturalis;rgn1=entire%20record;q1=Rumphius%20Biohistorical;Submit.x=14;Submit.y=11;lang=en;sort=year;cc=naturalis;view=reslist;fmt=short;page=reslist;size=10;start=1;sid= (accessed on 23 October 2018).
- Burghardt, I.; Carvalho, R.; Eheberg, D.; Gerung, G.; Kaligis, F.; Mamangkey, G.; Schrödl, M.; Schwabe, E.; Vonnemann, V.; Wägele, H. Molluscan Diversity at Bunaken National Park, Sulawesi. J. Zool. Soc. Wallacea 2006, 2, 29–43. [Google Scholar]
- Jensen, K.R. Sea slugs—Divers‘ favorites, taxonomists’ problems. Aquat. Sci. Manag. 2013, 1, 100–110. [Google Scholar]
- Fisch, K.M.; Hertzer, C.; Böhringer, N.; Wuisan, Z.G.; Schillo, D.; Bara, R.; Kaligis, F.; Wägele, H.; König, G.M.; Schäberle, T.F. The Potential of Indonesian Gastropods Found Around Bunaken Island for Production of Bioactive Compounds. Mar. Drugs 2017, 15, 384. [Google Scholar] [CrossRef] [PubMed]
- Böhringer, N.; Fisch, K.; Schillo, D.; Bara, R.; Hertzer, C.; Greins, F.; Eisenbarth, J.-H.; Kaligis, F.; Schneider, T.; Wägele, H.; et al. Antimicrobial Potential of Bacteria Associated with Marine Sea Slugs from North Sulawesi, Indonesia. Front. Microbiol. 2017, 8, 1092. [Google Scholar] [CrossRef] [PubMed]
- Setiawan, F.; Kusen, J.A.; Kaligis, G.J.F. Community changes of coral reef fishes in Bunaken National Park, North Sulawesi, Indonesia (Perubahan struktur komunitas ikan karang di Taman Nasional Bunaken, Sulawesi Utara). Aquat. Sci. Manag. 2013, 1, 117–123. [Google Scholar]
- Kenchington, R.; Hutchings, P. Some Implications of High Biodiversity for Management of Tropical Marine Ecosystems—An Australian Perspective. Diversity 2018, 10, 1. [Google Scholar] [CrossRef]
- Kimbal, J.D.; Soemarno; Yanuwiadi, B.; Sudarto. Mangrove Vegetation Structure and Its Carbon Stock Potency in Bunaken National Park, North Sulawesi. IOSR J. Environ. Sci. Toxic Food Technol. 2014, 8, 33–38. [Google Scholar] [CrossRef]
- Debelius, H.; Kuiter, R. Nudibranchs of the World; IKAN-Unterwasserarchiv: Frankfurt, Deutschland, 2007. [Google Scholar]
- Gosliner, T.M.; Valdés, A.; Behrens, D.W. Nudibranch & Sea Slug Identification Indo-Pacific; New World Publications: Jacksonville, FL, USA, 2015. [Google Scholar]
- Yonow, N. Results from the Rumphius Biohistorical Expedition to Ambon (1990). Part 11. Doridacea of the families Chromodorididae and Hexabranchidae (Mollusca, Gastropoda, Opisthobranchia, Nudibranchia). Zool. Meded. 2001, 75, 1–50. [Google Scholar]
- Yonow, N. Results from the Rumphius Biohistorical Expedition to Ambon (1990). Part 15. The suborder Doridina (Mollusca, Gastropoda, Opisthobranchia, Nudibranchia). Zool. Meded. 2011, 85, 905–956. [Google Scholar]
- Yonow, N. Results from the Rumphius Biohistorical Expedition to Ambon (1990). Part 16. Nudibranchia—Dendronotina, Arminina, Aeolidina, and Doridina (Mollusca: Gastropoda: Heterobranchia). Arch. Molluskenkd. 2017, 146, 135–172. [Google Scholar] [CrossRef]
- Stoffels, B.E.M.W.; van der Meij, S.E.T.; Hoeksema, B.W.; van Alphen, J.; van Alen, T.; Meyers-Munoz, M.A.; de Voogd, N.J.; Tuti, Y.; van der Velde, G. Phylogenetic relationships within the Phyllidiidae (Opisthobranchia, Nudibanchia). ZooKeys 2016, 605, 1–35. [Google Scholar]
- Martynov, A.V.; Korshunova, T.A. Opisthobranch molluscs of Vietnam (Gastropoda: Opisthobranchia). In Benthic Fauna of the Bay of Nhatrang, Southern Vietnam; Britayev, T.Z., Pavlov, D.S., Eds.; KMK Scientific Press: Moscow, Russia, 2012; Volume 2, pp. 142–257. [Google Scholar]
- Yonow, N.; Jensen, K.R. Results of the Rumphius Biohistorical Expedition to Ambon (1990). Part 17. The Cephalaspidea, Anaspidea, Pleurobranchida, and Sacoglossa (Mollusca: Gastropoda: Heterobranchia). Arch. Molluskenkd. 2018, 147, 1–48. [Google Scholar] [CrossRef]
- World Register of Marine Species. Available online: http://www.marinespecies.org/ (accessed on 23 October 2018).
- Korshunova, T.; Martynov, A.; Bakken, T.; Evertsen, J.; Fletcher, K.; Mudianta, I.W.; Saito, H.; Lundin, K.; Schrödl, M.; Picton, B. Polyphyly of the traditional family Flabellinidae affects a major group of Nudibranchia: Aeolidacean taxonomic reassessment with descriptions of several new families, genera, and species (Mollusca, Gastropoda). ZooKeys 2017, 717, 1–139. [Google Scholar] [CrossRef] [PubMed]
- Triebel, D.; Hagedorn, G.; Jablonski, S.; Rambold, G. (Eds.) Diversity Workbench—A Virtual Research Environment for Building and Accessing Biodiversity and Environmental Data. 1999 Onwards. Available online: http://www.diversityworkbench.net (accessed on 27 November 2018).
- Wägele, H.; Klussmann-Kolb, A.; Verbeek, E.; Schrödl, M. Flashback and foreshadowing—A review of the taxon Opisthobranchia. Org. Divers. Evol. 2014, 14, 133–149. [Google Scholar] [CrossRef]
- Gosliner, T. Three New Species of Aglajid cephalaspidean mollusks from the tropical Indo-pacific of the Verde Island Passage. Proc. Calif. Acad. Sci. 2015, 62, 191–205. [Google Scholar]
- Medina, M.; Collins, T.; Walsh, P.J. Phylogeny of sea hares in the Aplysia clade based on mitochondrial DNA sequence data. Bull. Mar. Sci. 2005, 76, 691–698. [Google Scholar]
- Yonow, N. Opisthobranchs from the western Indian Ocean, with descriptions of two new species and ten new records (Mollusca, Gastropoda). ZooKeys 2012, 197, 1–129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jensen, K.R.; Krug, P.J.; Dupont, A.; Nishina, M. A review of taxonomy and phylogenetic relationships in the genus Costasiella (Heterobranchia: Sacoglossa), with a description of a new species. J. Moll. Stud. 2014, 80, 562–574. [Google Scholar] [CrossRef]
- Vallès, Y.; Gosliner, T.M. Shedding light onto the genera (Mollusca: Nudibranchia) Kaloplocamus and Plocamopherus with description of new species belonging to these unique bioluminescent dorids. Veliger 2006, 48, 178–205. [Google Scholar]
- Fahey, S.; Gosliner, T.M. A phylogenetic analysis of the Aegiridae Fischer, 1883 (Mollusca, Nudibranchia, Phanerobranchia) with descriptions of eight new species and a reassessment of phanerobranch relationships. Proc. Calif. Acad. Sci. 2004, 55, 613–689. [Google Scholar]
- Layton, K.S.; Gosliner, T.M. Flexible colour patterns obscure identification and mimicry in Indo-Pacific Chromodoris nudibranchs (Gastropoda: Chromodorididae). Mol. Phyl. Evol. 2018, 124, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Gosliner, T.M.; Behrens, D.W. Five New Species of Chromodoris (Mollusca: Nudibranchia: Chromodorididae) from the Tropical Indo-Pacific Ocean. Proc. Calif. Acad. Sci. 1998, 50, 139–165. [Google Scholar]
- Rudman, W.B. The Chromodorididae (Opisthobranchia, Mollusca) of the Indo-West Pacific: Chromodoris quadricolor, C. lineolata and Hypselodoris nigrolineata colour groups. Zool. J. Linn. Soc. 1982, 76, 183–241. [Google Scholar] [CrossRef]
- Gosliner, T.M.; Johnson, R.F. Phylogeny of Hypselodoris (Nudibranchia: Chromodorididae) with a review of the monophyletic clade of Indo-Pacific species, including descriptions of twelve new species. Zool. J. Linn. Soc. 1999, 125, 1–125. [Google Scholar] [CrossRef]
- Epstein, H.E.; Hallas, J.M.; Johnson, R.F.; Lopez, A.; Gosliner, T.M. Reading between the lines: Revealing cryptic species diversity and colour patterns in Hypselodoris nudibranchs (Mollusca: Heterobranchia: Chromodorididae). Zool. J. Linn. Soc. 2018. [Google Scholar] [CrossRef]
- Poorman, L.H.; Mulliner, D.K. A new species of Crosslandia (Nudibranchia, Dendronotacea) from the Gulf of California. Nautilus 1981, 95, 96–99. [Google Scholar]
- Wild Singapore, Sargassum nudibranch, Crosslandia daedali. Available online: http://www.wildsingapore.com/wildfacts/mollusca/slugs/nudibranchia/crosslandia.html (accessed on 23 October 2018).
- Silva, F.; Azevedo, V.M.; Matthews-Cascon, H. A new species of Tritonia (Opisthobranchia: Nudibranchia: Tritoniidae) from the tropical South Atlantic Ocean. J. Mar. Biol. Assoc. UK 2014, 94, 579–585. [Google Scholar] [CrossRef]
- Meyers-Muñoz, M.A.; van der Velde, G.; van der Meij, S.E.T.; Stoffels, B.E.M.W.; van Alen, T.; Tuti, Y.; Hoeksema, B.W. The phylogenetic position of a new species of Plakobranchus from West Papua, Indonesia (Mollusca, Opisthobranchia, Sacoglossa). ZooKeys 2016, 594, 73–98. [Google Scholar]
- Tonozuka, T. Opisthobranchs of Bali and Indonesia; Hankyu Communications Co., Ltd.: Tokyo, Japan, 2003. [Google Scholar]
- Yonow, N. Red Sea Opisthobranchia 5: New species and new records of chromodorids from the Red Sea (Heterobranchia, Nudibranchia, Chromodorididae). ZooKeys 2018, 770, 9–42. [Google Scholar] [CrossRef]
- Gosliner, T.M. Biodiversity of tropical opisthobranch gastropod faunas. In Proceedings of the Seventh International Coral Reef Symposium, Guam, Micronesia, 22–27 June 1992; Volume 2, pp. 702–709. [Google Scholar]
- Rudman, W.B. The Chromodorididae (Opisthobranchia: Mollusca) of the Indo-West Pacific: Noumea purpurea and Chromodoris decora colour groups. Zool. J. Linn. Soc. 1986, 86, 309–353. [Google Scholar] [CrossRef]
- Rudman, W.B. Chromodoris tinctoria (Rüppell & Leuckart, 1828). In Sea Slug Forum; Australian Museum: Sydney, Australia, 1999; Available online: http://www.seaslugforum.net/factsheet/chrotinc (accessed on 27 November 2018).
- Burghardt, I.; Wägele, H. A new solar powered species of the genus Phyllodesmium Ehrenberg, (Mollusca: Nudibranchia: Aeolidoidea) from Indonesia with analysis of its photosynthetic activity and notes on biology. Zootaxa 2004, 596, 1–18. [Google Scholar] [CrossRef]
- Burghardt, I.; Gosliner, T.M. Phyllodesmium rudmani (Mollusca: Nudibranchia: Aeolidoidea), a new solar powered species from the Indo-West Pacific with data on its symbiosis with zooxanthellae. Zootaxa 2008, 1308, 31–47. [Google Scholar]
- Towoliu, R. Kondisi terumbu karang pada beberapa pusat penyelamatan di Pulau Bunaken, Sulawesi Utara [Coral reef condition in several dive points around coral Bunaken Island, North Sulawesi]. Aquat. Sci. Manag. 2014, 2, 44–48. [Google Scholar]
- Lahamendu, V. Analisis Kesesuaian Pemanfaatan Lahan yang Berkelanjutan di Pulau Bunaken Manado. Sabua 2015, 7, 383–388. [Google Scholar]
- Larat, R.V.; Lasut, M.T.; Bara, R.A. Kondisi Kualitas Air (Aspek Mikroorganisme) di Perairan Sekitar Pulau Bunaken, Sulawesi Utara (Water quality condition (microorganism aspect) in the water arround Bunaken Island, North Sulawesi). J. Pesisir Laut Trop. 2017, 3, 21–25. [Google Scholar]
- Lumi, V.N.; Manoppo, V.E.N.; Wasak, M.P. Dampak Pariwisata erhadap Kesejahteraan Masyarakat di Pulau Bunaken Kecamatan Bunaken Kepulauan Kota Manado. J. Akult. 2016, 4, 199–203. [Google Scholar]
- Kamagi, J.W.A.; Schaduw, J.N.W.; Lasut, M.T. Management strategies for dive site in Bunaken Island (North Sulawesi, Indonesia), based on stakeholder’s perceptions. Aquat. Sci. Manag. 2016, 4, 47–51. [Google Scholar]
- Kumaat, S.; Dundu, A.K.T.; Mandagi, R.J.M. Pemilihan Tipe Bangunan Pengamatan Pantai dengan Kearifan Lokal di Pulau Bunaken. J. Ilmiah Med. Eng. 2016, 6, 519–528. [Google Scholar]
- Ramirez Merida, E. Can Opisthobranchs Be a Useful Bioindicator of Cadmium Pollution? 2015. Available online: https://ddd.uab.cat/pub/tfg/2015/145754/TFG_estefaniaramirezmerida.pdf (accessed on 27 October 2018).
- Sánchez-Moyano, J.E.; Estacio, F.J.; García-Adiego, E.M.; García-Gómez, J.C. The Molluscan Epifauna of the Alga Halopteris Scoparia in Southern Spain as a Bioindicator of Coastal Environmental Conditions. J. Moll. Stud. 2000, 66, 431–448. [Google Scholar] [CrossRef]
- Guerra-García, J.M.; García-Gómez, J.C. Soft bottom mollusc assemblages and pollution in a harbour with two opposing entrances. Estuar. Coast. Shelf Sci. 2004, 60, 273–283. [Google Scholar] [CrossRef]
- Nimbs, M.J.; Larkin, M.; Davis, T.R.; Harasti, D.; Willan, R.C.; Smith, D.A. Southern range extensions for twelve heterobranch sea slugs (Gastropoda: Heterobranchia) on the eastern coast of Australia. Mar. Biodivers. Rec. 2016, 9, 27. [Google Scholar] [CrossRef]
- Pungus, F.; Kaligis, G.J.F.; Ompi, M. Status nudibranchia di perairan pantai desa Teep Minahasa Selatan dan Selat Lembeh Bitung. J. Pesisir Laut Trop. 2017, 1, 139–146. [Google Scholar]
- Aunurohim; Saptarini, D.; Raraswati, I. Keanekaragaman Nudibranchia di Perairan Pasir Putih Situbondo. Berk. Penel. Hayati 2010, 4, 1–7. [Google Scholar]
- Sari, L.N. and Aunorohim. Korelasi Komunitas Nudibranchia dengan Komunitas Porifera di perairan Pasir Putih Situbondo. J. Sains Seni Mopits 2013, 2, 224–229. [Google Scholar]
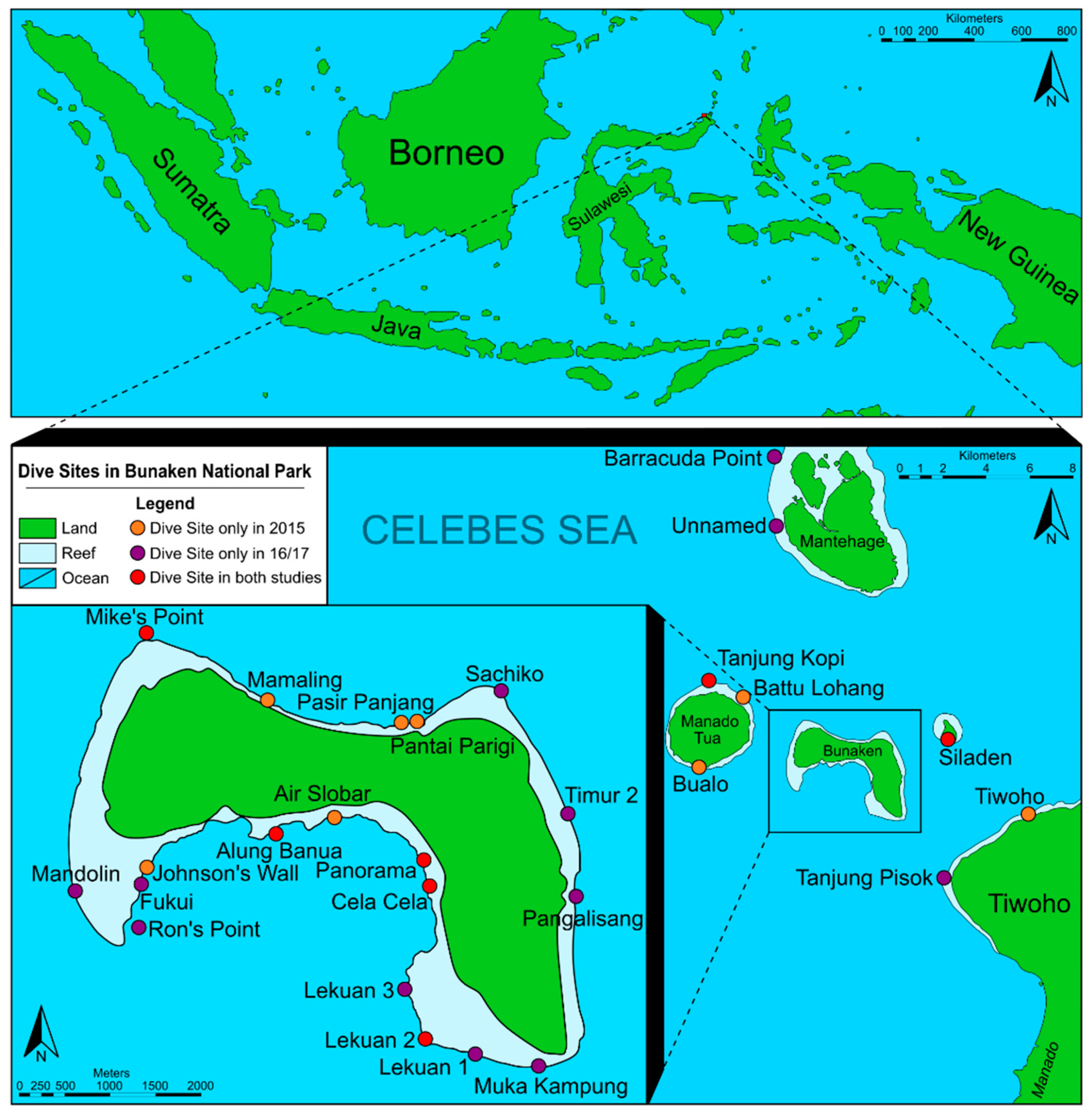




















| Area and Name of Collection Site | Abbreviation | Geographic Location | Characterisation of the Habitat | Date of Collection |
|---|---|---|---|---|
| Bunaken South | ||||
| Alung Banua | AB | 1°36′60.0″ N 124°45′11.5″ E | Wall-like coral reef structure with canyons and caves covered by a high diversity of sponges as well as soft and hard corals. | 18 + 19 Oct 2016 02 + 03 Nov 2016 |
| Cela Cela | CC | 1°36′42.4″ N 124°46′04.7″ E | Wall-like coral reef structure with deep canyons, covered by a high diversity of soft and hard corals, hydrozoans, and tunicates. | 20 + 25 Oct 2016 05 Sept 2017 |
| Fukui | Fu | 1°36′42.9″ N 124°44′21.0″ E | Large reef flat with large coral rubble fields, as well as large fields covered by Acropora and Tubastraea at 5 m depths; continuing into a slope down to 60 m with large sponges, Acropora, and several Tridacna. Larger sandy areas down to 12 m. | 19 + 30 Oct 2016 02 Nov 2016 |
| Lekuan 1 | Le1 | 1°35′46.4″ N 124°46′03.4″ E | Steep winding coral wall with deep canyons down to 57 m; covered by large sponges, soft corals, gorgonians, hydrozoans, and ascidians. Overhanging areas with dense colonies of Parazoanthus. | 20 + 27 Oct 2016 30 + 31 Oct 2016 06 Sept 2017 |
| Lekuan 2 | Le2 | 1°36′04.4″ N 124°45′54.4″ E | Coral and sand slopes with wall-like coral reef structures in-between. | 18 Oct 2016 02 Nov 2016 |
| Lekuan 3 | Le3 | 1°36′19.2″ N 124°46′01.5″ E | Slope with white coral sand and large coral rocks continuing into a slope with corals covered by Acropora, soft corals, hydrozoans, and ascidians. | 15 + 27 Oct 2016 |
| Mandolin | Ma | 1°36′39.5″ N 124°43′57.2″ E | Large winding coral wall with drop-offs down to 45 and then 60 m; with a high diversity including large black corals, soft corals, gorgonians, hydrozoans, ascidians, sponges. Overhanging areas with dense colonies of Parazoanthus. | 17 Oct 2016 |
| Muka Kampung | MK | 1°35′35.9″ N 124°46′44.1″ E | Partially with coral wall areas with overhangs covered by black corals, hydrozoans, soft corals, ascidians; large colonies of Parazoanthus and one area with Tubastraea. Some areas with sandy slopes. | 15 + 28 Oct 2016 03 Nov 2016 |
| Panorama | Pa | 1°36′50.0″ N 124°46′03.4″ E | Wall-like coral reef structure with deep canyons covered in sponges, soft and hard corals. Upper area dominated by larger ascidians. Upper reef structure partly with coral rubble and coral blocks. | 16 Oct 2016 02 Nov 2016 03 Sept 2017 05–09 Sept 2017 11 Sept 2017 |
| Ron’s Point | RP | 1°36′25.6″ N 124°44′21.0″ E | Highly diverse area with large coral blocks covered by algae, sponges, gorgonians; others with more soft corals and hydrozoans. Coral rubble in-between, as well as a few sandy areas. Reef flat with healthy living coral coverage. | 17 + 29 Oct 2016 10 Sept 2017 |
| Bunaken North | ||||
| Mike’s Point | MP | 1°38′12.6″ N 124°44′23.0″ E | Slope with terraces and many tiny caves. Less sponge-dominated than many other areas. | 23 Oct 2016 |
| Pangalisang | Pg | 1°36′38.4″ N 124°46′57.5″ E | Wall-like coral reef structure with terraces and many tiny caves; with hard and soft corals, gorgonians, black corals, colonies of Parazoanthus, hydrozoans, and ascidians. | 05 Sept 2017 12 Sept 2017 |
| Sachiko | Sa | 1°37′41.7″ N 124°45′60.0″ E | Sloping coral wall structure with sediment slope in the deeper part as well as some vertical walls. Very diverse fauna, including large sponges, hard and soft corals; gorgonians and hydrozoans also in deeper areas. | 22 + 28 Oct 2016 01 Nov 2016 |
| Timur 2 | Ti2 | 1°37′07.9″ N 124°46′52.5″ E | Steep coral wall with small caves and terraces with coral rubble or white sandy patches. Reef flat with living corals. | 20 + 26 Oct 2016 03 Nov 2016 |
| Manado Tua | ||||
| Tanjung Kopi | TK | 1°39′07.1″ N 124°41′49.6″ E | Slope until 30 m and then a steep drop-off (wall-like). | 23 Oct 2016 |
| Mantehage | ||||
| Barracuda Point | BP | 1°44′55.0″ N 124°43′33.5″ E | Destroyed reef structures on top with overgrowing turf algae; continuing into a large winding coral wall. | 24 Oct 2016 |
| Unnamed | Un | 1°43′11.7″ N 124°43′33.7″ E | Destroyed reef structures on top, continuing to a coral wall (no further information collected). | 24 Oct 2016 |
| Siladen | ||||
| Siladen | Si | 1°37′35.7″ N 124°48′03.6″ E | Wall with many tiny caves, dominated by soft corals, as well as whip gorgonians. | 22 Oct 2016 01 Nov 2016 12 Sept 2017 |
| Mainland | ||||
| Tanjung Pisok | TP | 1°34′04.0″ N 124°47′55.0″ E | Highly diverse structured area, partly with walls, partly with slope-like coral structures, with some sandy slopes in-between. Some areas dominated by Halimeda algae and others with many hydrozoans; soft and hard corals, gorgonians, one area with many large Tubastraea colonies. | 31 Oct 2016 |
| Higher Taxon Affiliation | Identifier | Species Name | Localities of Expedition 2016/2017 | Expedition 2016 | Expedition 2017 | Expedition 2015 [1] | Expedition 2003 [3] | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bunaken South | Bunaken North | Manado Tua | Mantehage | Siladen | Tiwoho | Number of Specimens | Size in mm | Depths in m | Number of Specimens | Size in mm | Depths in m | Number of Specimens | Size in mm | Depths in m | ||||
| Cephalaspidea | ||||||||||||||||||
| Haminoeidae Pilsbry, 1895 | Hasp15Bu-1 Hasp16Bu-1 | Haminoea spec. (Haminoea sp. 2 in Gosliner et al. [11]: 30) | AB | - | - | - | - | - | 1 | 7 | 11.3 | - | - | - | 4 | 5–8 | 3–13 | - |
| Hasp3_16Bu-1 | Haminoea spec. (Haminoeid sp. 2 in Gosliner et al. [11]: 34) | CC | - | - | - | - | - | 1 | 2 | 2 | - | - | - | - | - | - | - | |
| Hasp2_15Bu-1 | Haminoea spec.* | - | - | - | - | - | - | - | - | - | - | - | - | 2 | 4 | 5 | - | |
| Hasp2_16Bu-1 | Haminoea spec. * | AB | - | - | - | - | - | 1 | 7 | 10 | - | - | - | - | - | - | - | |
| Phanerophthalmus cf. albocollaris Heller and Thompson, 1983 | - | Ti2 | - | - | - | - | 1 | 35 | 2 | - | - | - | - | - | - | X | ||
| Phsp3_16Bu-1 | Phanerophthalmus spec. (Phanerophthalmus sp. 3 in Gosliner et al. [11]: 33) | - | - | - | - | - | TP | 1 | 10 | 10 | - | - | - | - | - | - | - | |
| Philinidae Gray, 1850 (1815) | Ilsp17Bu-1 | Philine spec. * | Pa | - | - | - | - | - | - | - | - | 1 | 4 | - | - | - | - | - |
| Colpodaspididae Oskars, Bouchet and Malaquias, 2015 | Colpodaspis thompsoni Brown, 1979 | AB, CC, Fu, Le1, Le2, Ma | MP, Sa, Ti2 | TK | BP, Un | Si | - | 20 | 2–5 | 2–21.4 | 1 | 2 | 4 | 15 | 1–6 | 4–11 | X | |
| Aglajidae Pilsbry, 1895 (1847) | Agsp15Bu-1 | Aglajidae spec. * | - | - | - | - | - | - | - | - | - | - | - | - | 1 | 5 | 7 | - |
| Chelidonura amoena Bergh, 1905 | Le2, Pa | - | - | - | Si | - | 2 | 8 | 6–12 | 4 | 25–30 | 1–2 | 1 | 30 | 1 | X | ||
| Chelidonura hirundinina (Quoy and Gaimard, 1833) | - | - | - | - | - | - | - | - | - | - | - | - | 2 | 15–25 | 5–6 | X | ||
| Odontoglaja guamensis Rudman, 1978 | - | MP, Ti2 | - | Un | Si | - | 4 | 4–10 | 2–7 | - | - | - | 12 | 6–13 | 3–19 | - | ||
| Phisp16Bu-1 | Tubulophilinopsis spec. * | AB | - | - | - | - | - | 1 | 3 | 16.8 | - | - | - | - | - | - | - | |
| Gastropteridae Swainson, 1840 | Gasp5_16Bu-1 | Gastropteron spec. (Gastropteron sp. 5 in Gosliner et al. [11]: 56) | - | - | - | Un | - | - | 1 | 3 | 7 | - | - | - | - | - | - | - |
| Sagaminopteron psychedelicum Carlson and Hoff, 1974 | AB, Ma | MP | TK | BP | Si | - | 6 | 3–10 | 2–11.7 | 2 | 5-6 | 14 | 7 | 3–8 | 4–15 | - | ||
| Sasp17Bu-1 | Sagaminopteron spec. * | - | - | - | - | Si | - | - | - | - | 1 | 4 | 6 | - | - | - | - | |
| Siphopteron brunneomarginatum (Carlson & Hoff, 1974) | Fu, Le1 | - | - | - | - | - | 3 | 4–5 | 5–6.5 | - | - | - | 5 | 3–5 | 4–10 | - | ||
| Siphopteron ladrones (Carlson and Hoff, 1974) | Fu, Pa | - | - | BP | - | - | 4 | 3–6 | 3-4 | 2 | 4 | - | 1 | 4 | 5 | - | ||
| Siphopteron nigromarginatum Gosliner, 1989 | Le1 | Ti2 | - | - | - | - | 3 | 3–7 | 2–5 | - | - | - | 2 | 4–5 | 5 | - | ||
| Siphopteron tigrinum Gosliner, 1989 | - | - | - | - | - | - | - | - | - | - | - | - | 2 | 5–5 | 5–6 | X | ||
| Sini15Bu-19+20 | Siphopteron spec. * | - | - | - | - | - | - | - | - | - | - | - | - | 2 | 4 | 4–5 | - | |
| Runcinacea | ||||||||||||||||||
| Runcinidae H. Adams and A. Adams, 1854 | Rusp15Bu-1 | Runcina spec. * | AB | - | - | - | - | - | - | - | - | - | - | - | 1 | 2 | 5 | - |
| Rusp16Bu-1 | Runcina spec. * | - | Sa | - | - | - | - | 1 | 3 | 2 | - | - | - | - | - | - | - | |
| Rusp2_16Bu-1 Rusp3_16Bu-1 | Runcina spec. * | - | - | TK | - | - | - | 2 | 4–7 | 10–15.8 | - | - | - | - | - | - | - | |
| Anaspidea | ||||||||||||||||||
| Aplysiidae Lamarck, 1809 | Aplysia parvula Guilding in Mörch, 1863 | Le2, Pa | - | - | Un | - | - | 5 | 4–10 | 1.5–7 | 9 | 4–9 | 1.2–6 | - | - | - | - | |
| Stylocheilus striatus (Quoy and Gaimard, 1832) | CC, RP | - | - | - | - | - | 2 | 13–15 | 7.3 | 4 | 6–20 | 5 | 1 | 7 | 10 | X | ||
| Dolabella auricularia (Lightfoot, 1786) | Pa | - | - | - | - | - | - | - | - | 1 | 85 | 0.1 | - | - | - | X | ||
| Dolabrifera dolabrifera (Rang, 1828) | Pa | - | - | - | - | - | - | - | - | 2 | ~6 | - | - | - | - | - | ||
| Sacoglossa | ||||||||||||||||||
| Cylindrobullidae Thiele, 1931 | Assp1_17Bu-1-4 | Cylindrobulla spec. * | Pa | - | - | - | - | - | - | - | - | 4 | ~12 | 0.1 | - | - | - | - |
| Oxynoidae Stoliczka, 1868 (1847) | Lobiger nevilli Pilsbry, 1896 | - | - | - | - | - | - | - | - | - | - | - | - | 1 | 8 | 8 | - | |
| Lovi15Bu-1 | Lobiger spec. (Lobiger sp. 1 in Gosliner et al. [11]: 70) | CC | - | - | - | - | - | 1 | 8 | 14.3 | - | - | - | 1 | 20 | 7 | - | |
| Caliphyllidae Tiberi, 1881 | Cysp4_Bu-1-4 Cysp17Bu-1-8 | Cyerce cf. bourbonica Yonow, 2012 | Pa | - | - | - | - | - | - | - | - | 8 | 2–7 | ? | 4 | 4–6 | 3–10 | X |
| Cysp2_15Bu-5 | Cyerce spec. * | - | - | - | - | - | - | - | - | - | - | - | - | 5 | 4–6 | 3–7 | - | |
| Sohgenia palauensis Hamatani, 1991 | Pa | - | - | - | - | - | - | - | - | 1 | 3 | - | - | - | - | - | ||
| Costasiellidae Clarke, 1984 | Cosp17Bu-1 | Costasiella kuroshimae Ichikawa, 1993 | Pa | - | - | - | - | - | - | - | - | 1 | 1 | - | - | - | - | - |
| Cosp1_17Bu-1-2 | Costasiella spec. (Costasiella sp. 1 in Gosliner et al. [11]: 79) | CC | - | - | - | - | - | - | - | - | 2 | 2.8–3.5 | 12 | - | - | - | - | |
| Cosp8_17Bu-1 | Costasiella spec. (Costasiella sp. 8 in Gosliner et al. [11]: 81) | - | Pg | - | - | - | - | - | - | - | 1 | 4 | 13 | - | - | - | - | |
| Cosp3_16Bu-1-5 | Costasiella spec. * | - | - | - | BP | - | - | 5 | 3–4 | 13.8 | - | - | - | - | - | - | - | |
| Plakobranchidae Gray, 1840 | Elysia asbecki Wägele, Stemmer, Burghardt and Händeler, 2010 | Ma | Ti2 | - | - | - | - | 2 | 5–10 | 6–13 | - | - | - | 9 | 5–13 | 4–15 | - | |
| Elysia marginata (Pease, 1871) | CC | - | - | - | - | - | 1 | 10 | 2 | - | - | - | - | - | - | |||
| Elysia mercieri (Pruvot-Fol, 1930) | Fu | Ti2 | - | BP | - | - | 5 | 4–10 | 2–13 | - | - | - | - | - | - | - | ||
| Elysia pusilla (Bergh, 1871) | Ma, Pa | - | - | - | - | - | 1 | 6 | 5 | 1 | 3 | 1 | - | - | - | X | ||
| Elsp19_15Bu-2 Elsp30_16Bu-3 Elsp30_16Bu-4 | Elysia spec. (Elysia sp. 25 in Gosliner et al. [11]: 89) | Le1 | MP | - | - | - | TP | 2 | 4–12 | 7–18.3 | - | - | - | 3 | 5–10 | 5–9 | - | |
| Elsp1_16Bu-1 | Elysia spec. * | AB | - | - | - | - | - | 1 | 3 | 3 | - | - | - | - | - | - | - | |
| Elsp16Bu-1 | Elysia spec. * | AB | - | - | - | - | - | 1 | 8 | 23 | - | - | - | - | - | - | - | |
| Elsp4_16Bu-1 | Elysia spec. * | - | - | - | BP | - | - | 1 | 6 | 4 | - | - | - | - | - | - | - | |
| Plakobranchus ocellatus van Hasselt, 1824 | Pa | - | - | - | - | - | - | - | - | 1 | 23 | 0.1 | - | - | - | - | ||
| Thuridilla albopustulosa Gosliner, 1995 | - | - | - | - | Si | - | 1 | 12 | 4 | - | - | - | 1 | 7 | 6 | - | ||
| Thuridilla flavomaculata Gosliner, 1995 | - | MP | - | Un | - | - | 3 | 6–10 | 5.8–7.5 | - | - | - | 2 | 10–13 | 4–7 | - | ||
| Thuridilla gracilis (Risbec, 1928) | AB, CC, Le1, Pa | Ti2 | - | BP, Un | Si | - | 12 | 5–25 | 1.5–12.5 | 3 | 6–20 | 0.1–5 | 6 | 15–25 | 3–8 | X | ||
| Thuridilla lineolata (Bergh, 1905) | CC, Pa | MP | - | BP, Un | - | - | 34 | 1.5–7.7 | 6–30 | 4 | 15–20 | 1.1–2 | >50 | 15–17 | 1–9 | X | ||
| Thuridilla livida (Baba, 1955) | Pa | MP | - | - | - | - | 1 | 10 | 6 | 3 | 5–7 | 0.1 | - | - | - | - | ||
| Thuridilla undula Gosliner, 1995 | CC | - | - | - | - | - | 1 | 2 | 10 | - | - | - | - | - | - | - | ||
| Thuridilla vataae (Risbec, 1928) | - | MP | - | - | - | - | 1 | 7 | 11 | - | - | - | - | - | - | - | ||
| Pleurobranchomorpha | ||||||||||||||||||
| Pleurobranchidae Gray, 1827 | Berthellina citrina (Pease, 1861) | Pa | - | - | - | - | - | - | - | - | 1 | 7 | ? | - | - | - | - | |
| Pleurobranchus forskalii Rüppell and Leuckart, 1828 | - | - | - | Un | - | - | 1 | 22 | 7.3 | - | - | - | 5 | 100–150 | 4-8 | - | ||
| Nudibranchia, Doridina | ||||||||||||||||||
| Hexabranchidae Bergh, 1891 | Hexabranchus sanguineus (Rüppell and Leuckart, 1830); egg mass | - | - | - | - | - | - | - | - | - | - | - | - | 2 | - | 2 | X | |
| Polyceridae Alder and Hancock, 1845 | Kaloplocamus dokte Vallès and Gosliner, 2006 | - | - | - | - | - | - | - | - | - | - | - | - | 1 | 10 | 7 | - | |
| Kalsp8_16Bu-1 | Kaloplocamus spec. (Kaloplocamus sp. 8 in Gosliner et al. [11]: 116) | - | - | TK | - | - | - | 1 | 4 | 14.2 | - | - | - | - | - | - | - | |
| Kalsp9_16Bu-1 | Kaloplocamus spec. (Kaloplocamus sp. 9 in Gosliner et al. [11]: 116) | CC | - | - | - | - | - | 1 | 5 | 8 | - | - | - | - | - | - | - | |
| Nembrotha cristata Bergh, 1877 | - | - | - | - | - | - | - | - | - | - | - | - | 2 | 50–80 | 4–15 | - | ||
| Nembrotha kubaryana Bergh, 1877 | Ma | Pg | - | - | - | - | 1 | 35 | 15 | 1 | 13 | - | 1 | 55 | 6 | - | ||
| Polycera japonica Baba, 1949 | - | - | - | BP | - | - | 1 | 5 | 2 | - | - | - | 3 | 5–8 | 7–8 | - | ||
| Polycera risbeci Odhner, 1941 | - | - | - | - | - | - | - | - | - | - | - | - | 2 | 8 | 7–8 | - | ||
| Posp516Bu-1 | Polycera spec. (Polycera sp. 5 in Gosliner et al. [11]: 113) | Ma | - | - | - | - | - | 1 | 7 | 4 | - | - | - | - | - | - | - | |
| Roboastra gracilis (Bergh, 1877) | CC | MP | - | - | - | - | 2 | 12–15 | 2 | - | - | - | - | - | - | X | ||
| Goniodorididae H. Adams and A. Adams, 1854 | Trapania euryeia Gosliner and Fahey, 2008 | - | - | - | - | - | - | - | - | - | - | - | - | 1 | 7 | 6 | - | |
| Aegiridae P. Fischer, 1883 | Aegires citrinus Pruvot-Fol, 1930 | Fu, Le2 | - | - | - | - | - | 2 | 2–11 | 6–10 | - | - | - | - | - | - | - | |
| Aegires malinus Fahey and Gosliner, 2004 | - | - | - | - | - | TP | 2 | 10 | 10 | - | - | - | - | - | - | - | ||
| Notodoris serenae Gosliner and Behrens, 1997 | - | - | - | - | - | - | - | - | - | - | - | - | 1 | 100 | 13 | - | ||
| Gymnodorididae Odhner, 1941 | Gymnodoris tuberculosa Knutson and Gosliner, 2014 | Ma | - | - | - | - | - | 1 | 11 | 4 | - | - | - | - | - | - | - | |
| Gysp2_16Bu-1 | Gymnodoris spec. (Gymnodoris sp. 2 in Gosliner et al. [11]: 152) | - | - | TK | - | - | - | 1 | 10 | 14.2 | - | - | - | - | - | - | - | |
| Gysp16Bu-1 | Gymnodoris spec. (Gymnodoris cf. sp. 35 in Gosliner et al. [11]: 159) | CC | - | - | - | - | - | 1 | 5 | 2 | - | - | - | - | - | - | - | |
| Gysp1_17Bu-1 Gysp22_17Bu-1 | Gymnodoris spec. (Gymnodoris cf. sp. 46 in Gosliner et al. [11]: 161) | Pa | - | - | - | - | - | - | - | - | 2 | 6 | - | - | - | - | - | |
| Gysp1_15Bu-2 | Gymnodoris spec. * | - | - | - | - | - | - | - | - | - | - | - | - | 3 | 6–13 | 5–7 | - | |
| Doridoidea Rafinesque, 1815 | Dosp17Bu-3 | Doridoidea spec. * | Pa | - | - | - | - | - | - | - | - | 1 | 3 | - | - | - | - | - |
| Scsp1_17Bu-1 | Doridoidea spec. * | - | Pg | - | - | - | - | - | - | - | 1 | 12 | - | - | - | - | - | |
| Discodorididae Bergh, 1891 | Asteronotus cespitosus (van Hasselt, 1824) | Pa | - | - | - | - | - | - | - | - | 1 | 50 | 12 | - | - | - | - | |
| Asteronotus mimeticus Gosliner and Valdés, 2002 | CC | - | - | - | - | - | 7 | 7–18 | 2.3–6.3 | - | - | - | - | - | - | - | ||
| Disp1_Bu-1 | Diaulula spec. (Diaulula sp. 1 in Gosliner et al. [11]: 197) | Pa | - | - | - | - | - | - | - | - | 1 | 10 | 1 | - | - | - | - | |
| Halgerda batangas Carlson and Hoff, 2000 | CC | - | - | - | - | - | 1 | 40 | 10.4 | - | - | - | - | - | - | X | ||
| Halgerda carlsoni Rudman, 1978 | Pa | - | - | - | - | - | - | - | - | 1 | 50 | 8 | 1 | 15 | 5 | - | ||
| Halgerda tessellata (Bergh, 1880) | - | MP, Pg | - | - | - | - | 1 | 10 | 6 | 1 | 16 | - | 1 | 8 | 5 | - | ||
| Platydoris sanguinea Bergh, 1905 | - | - | - | Un | - | - | 1 | 17 | 7 | - | - | - | - | - | - | - | ||
| Scsp2_16Bu-1 | Sclerodoris spec. (Sclerodoris sp. 2 in Gosliner et al. [11]: 195) | - | MP | - | - | - | - | 1 | 10 | 2 | - | - | - | - | - | - | - | |
| Taringa halgerda Gosliner and Behrens, 1998 | - | - | - | - | - | - | - | - | - | - | - | - | 1 | 10 | 6 | - | ||
| Dosp17Bu-1 | Discodorididae spec. * | - | Pg | - | - | - | - | - | - | - | 1 | 12 | - | - | - | - | - | |
| Dosp17Bu-2 | Discodorididae spec. * | Pa | - | - | - | - | - | - | - | - | 1 | 6 | 1.2 | - | - | - | - | |
| Chromodorididae Bergh, 1891 | Cesp2_15Bu-3 Cesp1_17Bu-1 | Ceratosoma spec. (Ceratosoma sp. 1 in Gosliner et al. [11]: 266) | Le1 | - | - | - | - | - | - | - | - | 1 | 12 | 5 | 2 | 4–8 | 5–8 | - |
| Chromodoris annae Bergh, 1877 | AB, CC, Ma, Pa | MP | - | - | Si | - | 17 | 23–42 | 2–28 | 4 | 4–20 | 1.5–19 | 62 | 6–50 | 4–23 | X | ||
| Chromodoris cf. boucheti Rudman, 1982 | - | - | - | - | - | - | - | - | - | - | - | - | 1 | 20 | 8 | - | ||
| Chromodoris dianae Gosliner and Behrens, 1998 | AB, CC, Pa | MP | - | - | Si | - | 7 | 18–45 | 5-20 | 2 | 17-19 | ?–22.9 | 64 | 10–50 | 4–21 | X | ||
| Chsp30-15Bu-5 | Chromodoris lochi Rudman, 1982 | CC | - | - | - | Si | - | 6 | 25–40 | 5.3–21.8 | - | - | - | 32 | 15–50 | 5–21 | X | |
| Chromodoris cf. michaeli Gosliner and Behrens, 1998 | - | - | - | BP | - | - | 1 | 34 | 12 | - | - | - | - | - | - | - | ||
| Chromodoris strigata Rudman, 1982 | - | - | - | - | - | - | - | - | - | - | - | - | 1 | 25 | 11 | X | ||
| Chromodoris willani Rudman, 1982 | CC | - | - | - | Si | - | 4 | 18–50 | 10.5–17 | - | - | - | 36 | 20–70 | 7–21 | X | ||
| Doriprismatica stellata (Rudman, 1986) | CC, Pa | - | - | - | - | - | 8 | 2.3–65 | 6.5–10 | 3 | 13–65 | 13.9 | 5 | 50–60 | 4–21 | - | ||
| Glossodoris cincta (Bergh, 1888) | Le1, RP | Sa, Pg | TK | Un | - | - | 5 | 17–50 | 3–13 | 2 | 20–52 | 5 | 1 | 30 | 6 | - | ||
| Glossodoris hikuerensis (Pruvot-Fol, 1954) | - | - | - | - | - | - | - | - | - | 1 | 65 | 2 | - | - | - | - | ||
| Goniobranchus fidelis (Kelaart, 1858) | Le1 | - | - | - | - | - | 1 | 15 | 16 | - | - | - | - | - | - | - | ||
| Goniobranchus geometricus (Risbec, 1928) | Fu, Ma | MP | - | - | - | - | 3 | 6–25 | 2–15 | - | - | - | 3 | 10–40 | 4–8 | X | ||
| Goniobranchus reticulatus (Quoy and Gaimard, 1832) | - | - | - | - | - | - | - | - | - | - | - | - | 1 | 75 | 15 | - | ||
| Gosp40_16Bu-1 | Goniobranchus spec. (Goniobranchus sp. 40 in Gosliner et al. [11]: 230) | Le1 | - | - | - | - | - | 1 | 20 | 8.5 | - | - | - | - | - | - | - | |
| Hypselodoris apolegma (Yonow, 2001) | - | Ti2 | - | - | - | - | 1 | 70 | 16 | - | - | - | - | - | - | X | ||
| Hypselodoris maculosa (Pease, 1871) | - | - | - | Un | - | - | 1 | 11 | 4.8 | - | - | - | 2 | 4–13 | 4–6 | - | ||
| Hypselodoris tryoni (Garrett, 1873) | Ma | - | - | - | - | - | 2 | 55–60 | 10 | - | - | - | - | - | - | - | ||
| Hysp16Bu-1 | Hypselodoris spec. * | Ma | - | - | - | - | - | 1 | 5 | 4 | - | - | - | - | - | - | - | |
| Hysp2_16Bu-1 | Hypselodoris spec. * | - | - | - | BP | - | - | 1 | 6 | 16 | - | - | - | - | - | - | - | |
| Miamira sinuata (van Hasselt, 1824) | - | - | - | - | Si | - | 1 | 12 | 13 | - | - | - | - | - | - | - | ||
| Thorunna australis (Risbec, 1928) | - | - | - | - | - | - | - | - | - | - | - | - | 1 | 17 | 2 | - | ||
| Thorunna furtiva Bergh, 1878 | - | - | - | Un | - | - | 1 | 10 | 7 | - | - | - | - | - | - | - | ||
| Dosp17Bu-4 | Verconia spec. * | - | - | - | - | Si | - | - | - | - | 1 | 4 | 4 | - | - | - | - | |
| Dendrodorididae O’Donoghue, 1924 (1864) | Dendrodoris albobrunnea Allan, 1933 | - | - | - | - | - | - | - | - | - | - | - | - | 1 | 40 | 4 | - | |
| Dendrodoris nigra (Stimpson, 1855) | Pa | - | - | - | Si | - | 1 | 20 | 3 | 2 | 4–5 | ? | 1 | 30 | 4 | - | ||
| Rosp17Bu-1 | Dendrodoris spec. * | Pa | - | - | - | - | - | - | - | - | 1 | 5 | - | - | - | - | - | |
| Phyllidiidae Rafinesque, 1814 | Phyllidia coelestis Bergh, 1905 | Le3, Pa | Sa, Pg | - | - | Si | - | 3 | 13–38 | 1–17.2 | 2 | 26–35 | 6 | 20 | 10–40 | 2–15 | X | |
| Phyllidia elegans Bergh, 1869 | Pa | MP | - | - | Si | - | 1 | 30 | 2 | 1 | 23 | 1.7 | 13 | 10–40 | 2–19 | X | ||
| Phyllidia ocellata Cuvier, 1804 | - | - | - | - | - | - | - | - | - | - | - | - | 1 | 30 | 5 | X | ||
| Phyllidia varicosa Lamarck, 1801 | AB, Pa | Sa | - | Un | Si | - | 5 | 30–70 | 2–15.6 | 2 | 55–56 | 1,7 | 1,7–2 | 30–80 | 4–21 | X | ||
| Phsp17Bu-1 | Phyllidia spec. * | Pa | - | - | - | - | - | - | - | - | 1 | 16 | 25.3 | - | - | - | - | |
| Phyllidiella cf. annulata (Gray, 1853) | AB | - | - | - | - | - | 1 | 40 | 4 | - | - | - | 3 | 15 | 11–13 | - | ||
| Phyllidiella pustulosa (Cuvier, 1804) | AB, Pa | MP, Sa | TK | BP | Si | - | 11 | 20–63 | 1.5–14.8 | 4 | 29–44 | 1.7–20 | 44 | 13–80 | 5–19 | X | ||
| Phyllidiopsis pipeki Brunckhorst, 1993 | - | - | - | - | - | - | - | - | - | - | - | - | 3 | 25–40 | 14–15 | - | ||
| Phyllidiopsis sphingis Brunckhorst, 1993 | - | - | - | - | - | - | - | - | - | - | - | - | 1 | 5 | 19 | - | ||
| Phyllidiopsis xishaensis (Lin, 1983) | Le2 | - | TK | - | - | - | 2 | 12 | 6.8–21.9 | - | - | - | 1 | 13 | 15 | X | ||
| Nudibranchia, Cladobranchia | ||||||||||||||||||
| Arminidae Iredale and O’Donoghue, 1923 | Dermatobranchus diagonalis Gosliner and Fahey, 2011 | - | - | - | - | - | TP | 1 | 20 | 8.8 | - | - | - | - | - | - | - | |
| Dermatobranchus fasciatus Gosliner and Fahey, 2011 | - | - | - | - | - | - | - | - | - | - | - | - | 1 | 12 | 7 | - | ||
| Dermatobranchus cf. kokonas Gosliner and Fahey, 2011 | - | - | TK | - | Si | - | 2 | 7–8 | 3–14 | - | - | - | - | - | - | - | ||
| Dermatobranchus cf. piperoides Gosliner and Fahey, 2011 | Le1 | - | - | - | - | - | 1 | 7 | 8 | - | - | - | - | - | - | - | ||
| Desp8_17Bu-1 | Dermatobranchus spec. (Dermatobranchus sp. 8 in Gosliner et al. [11]: 302) | - | - | - | - | Si | - | - | - | - | 1 | 8 | 8 | - | - | - | - | |
| Dest15Bu-1 | Dermatobranchus spec. * | - | - | - | - | - | - | - | - | - | - | - | - | 1 | 30 | 7 | - | |
| Desp1_16Bu-1 | Dermatobranchus spec. * | - | MP | - | - | - | - | 4 | 1 | 7 | - | - | - | - | - | - | - | |
| Proctonotidae Gray, 1853 | Janolus cf. mirabilis Baba and Abe, 1970 | Le1 | - | - | - | - | - | 1 | 7 | 5 | - | - | - | - | - | - | X | |
| Cysp15Bu-1 | Janolus spec. (Janolus sp. 11 in Gosliner et al. [11]: 308) | - | - | - | - | - | - | - | - | - | - | - | - | 1 | 10 | 7 | - | |
| Scyllaeidae Alder and Hancock, 1855 | Cross16Bu-1-8 | Crosslandia daedali Poorman and Mulliner, 1981 | Pa | - | - | - | Si | - | 8 | 8–20 | ~0 | - | - | - | - | - | - | - |
| Dotidae Gray, 1853 | Doto ussi Ortea, 1982 | Le1 | Sa | - | BP | Si | TP | 21 | 5–20 | 3–10 | - | - | - | - | - | - | - | |
| Kabeiro rubroreticulata Shipman and Gosliner, 2015 | Le1 | - | - | - | - | TP | 3 | 8 | 10–16 | - | - | - | - | - | - | - | ||
| Dotosp15Bu-1 | Kabeiro spec. * | - | - | - | - | - | - | - | - | - | - | - | - | 1 | 5 | 19 | - | |
| Kasp16Bu-1-15+17 Kasp17Bu-1 | Kabeiro spec. * | Fu, Le2, Ma, RP | - | - | - | Si | - | 16 | 2–15 | 10–20 | 1 | 4 | 13 | - | - | - | - | |
| Kasp16Bu-16 | Kabeiro spec. * | - | - | - | - | Si | - | 1 | 8 | 10 | - | - | - | - | - | - | - | |
| Tritoniidae Lamarck, 1809 | Marianina rosea (Pruvot-Fol, 1930) | - | - | - | BP | - | - | 1 | 10 | 2 | - | - | - | - | - | - | - | |
| Trsp8_16Bu-1 | Tritonia spec. (Tritonia sp. 9 in Gosliner et al. [11]: 321) | - | MP | - | - | - | - | 1 | 8 | 7.1 | - | - | - | - | - | - | - | |
| Trisp10_16Bu-1 | Tritonia spec. (Tritonia sp. 10 in Gosliner et al. [11]: 321) | AB | - | - | - | - | - | 1 | 20 | 14 | - | - | - | - | - | - | - | |
| Trsp16Bu-1 | Tritonia spec. * | Ti2 | - | - | - | - | 1 | 4 | 6 | - | - | - | - | - | - | - | ||
| Flabellinidae Bergh, 1889 | Coryphellina delicata (Gosliner and Willan, 1991) | Fu | - | - | - | - | - | 1 | 15 | - | - | - | - | - | - | - | - | |
| Coryphellina exoptata (Gosliner and Willan, 1991) | Le2, Ma | Sa, Pg | - | BP, Un | - | - | 6 | 22–30 | 2–8.1 | 1 | 20 | - | 5 | 20 | 5–8 | X | ||
| Coryphellina rubrolineata O’Donoghue, 1929 | RP | - | TK | - | - | - | 2 | 15–25 | 7.3–8 | - | - | - | 1 | 30 | 6 | - | ||
| Samlidae Korshunova, Martynov, Bakken, Evertsen, Fletcher, Mudianta, Saito, Lundin, Schrödl and Picton, 2017 | Samla bicolor (Kelaart, 1858) | AB, Le1, Ma | MP, Ti2 | TK | BP, Un | - | - | 11 | 6–23 | 1.5–17.5 | 1 | 12 | 5 | 3 | 8–13 | 4–8 | - | |
| Samla riwo (Gosliner and Willan, 1991) | CC | Ti2 | TK | - | - | - | 3 | 8–17 | 2–17.5 | - | - | - | - | - | - | - | ||
| Eubranchidae Odhner, 1934 | Eusp22_16Bu-1 | Eubranchus spec. (Eubranchus sp. 22 in Gosliner et al. [11]: 341) | RP | - | - | - | - | - | 1 | 3 | 10 | - | - | - | - | - | - | - |
| Tergipedidae Bergh, 1889 | Cusp4_16Bu-1 | Cuthona spec. (Cuthona sp. 4 in Gosliner et al. [11]: 343) | - | Ti2 | - | - | - | - | 1 | 3 | 2 | - | - | - | - | - | - | - |
| Cusp54_16Bu-1 | Cuthona spec. (Cuthona sp. 54 in Gosliner et al. [11]: 353) | Ma | - | - | - | - | - | 1 | 8 | 6 | - | - | - | - | - | - | - | |
| Cusp65_16Bu-1 | Cuthona spec. (Cuthona cf. sp. 65 in Gosliner et al. [11]: 343) | - | - | - | Un | - | - | 1 | 2 | 2 | - | - | - | - | - | - | - | |
| Facelinidae Bergh, 1889 | Caloria indica (Bergh, 1896) | Le1, Le2 | Sa, Ti2 | - | Un | Si | - | 8 | 6–25 | 2–7.3 | - | - | - | 6 | 7–40 | 3–6 | - | |
| Casp15Bu-1 | Caloria spec. (Caloria sp. 1 in Gosliner et al. [11]: 362) | - | - | - | - | - | - | - | - | - | - | - | - | 1 | 5 | 17 | - | |
| Crsp5_16Bu-1 | Cratena spec. (Cratena sp. 5 in Gosliner et al. [11]: 383) | - | - | - | Un | - | - | 1 | 5 | 7.3 | - | - | - | - | - | - | - | |
| Facelina rhodopos Yonow, 2000 | - | - | - | - | - | - | - | - | - | - | - | - | 1 | 30 | 15 | - | ||
| Fasp3_16Bu-1+3-5 | Facelina spec. (Facelina sp. 3 in Gosliner et al. [11]: 359) | - | Sa | TK | BP | - | - | 4 | 3–4 | 5–15.8 | - | - | - | - | - | - | - | |
| Fasp3_16Bu-2 | Facelina spec. (Facelina sp. 4 in Gosliner et al. [11]: 359) | - | - | - | - | Si | - | 1 | 5 | 17 | - | - | - | - | - | - | - | |
| Fasp8_16Bu-1 | Facelina spec. (Facelina sp. 8 in Gosliner et al. [11]: 360) | - | MP | - | - | - | - | 1 | 8 | 6 | - | - | - | - | - | - | - | |
| Favorinus japonicus Baba, 1949 | - | - | - | - | - | - | - | - | - | - | - | - | 4 | 5–8 | 5–10 | - | ||
| Favorinus mirabilis Baba, 1955 | AB | - | - | - | - | - | 1 | 9 | 11.5 | 1 | 9 | 10 | 1 | 12 | 23 | - | ||
| Favorinus tsuruganus Baba and Abe, 1964 | Fu | - | - | - | - | - | 1 | 6 | 8.3 | - | - | - | 7 | 8–20 | 6–23 | - | ||
| Nosp13_16Bu-1-6 Nosp3_17Bu-1 | Noumeaella spec. (Noumeaella sp. 3 in Gosliner et al. [11]: 367) | AB, MK, Pa | MP | - | - | Si | - | 9 | 3–13 | 2–13 | 1 | 6 | 5 | - | - | - | - | |
| Nosp6_17Bu-1-2 | Noumeaella spec. (Noumeaella cf. sp. 6 in Gosliner et al. [11]: 368) | Pa | - | - | - | - | - | - | - | - | 2 | 7–9 | 1.2 | - | - | - | - | |
| Nosp12_16Bu-1 | Noumeaella spec. (Noumeaella sp. 12 in Gosliner et al. [11]: 367) | - | MP | - | - | Si | - | 1 | 4 | 2 | - | - | - | - | - | - | - | |
| Nosp2_15Bu 1 Nosp2_16Bu-1-6 | Noumeaella spec. * | CC, Le2 | - | - | - | - | - | 6 | 12–40 | 8–11.2 | - | - | - | 7 | 12–30 | 4–12 | - | |
| Phyllodesmium briareum (Bergh, 1896) | - | Pg | TK | - | - | - | 7 | 12–15 | 18.4 | 1 | 25 | - | Ca. 50 | 10–30 | 2–7 | X | ||
| Phyllodesmium poindimiei (Risbec, 1928) | - | Pg | - | - | - | - | - | - | - | 1 | 4 | - | 2 | 4–8 | 17 | - | ||
| Pteraeolidia semperi (Bergh, 1870) | Ma | MP, Ti2 | TK | Un | Si | - | 11 | 4–70 | 2–17.5 | 1 | 30 | - | 20 | 6–50 | 4–15 | X (as P. ianthina) | ||
| Fasp17Bu-1 | Facelinidae spec. * | Le1 | - | - | - | - | - | - | - | - | 1 | 7 | - | - | - | - | - | |
| Aeolidiidae Gray, 1827 | Bulbaeolidia alba (Risbec, 1928) | Le2 | - | - | - | - | - | 1 | 5 | 4.3 | - | - | - | - | - | - | - | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eisenbarth, J.-H.; Undap, N.; Papu, A.; Schillo, D.; Dialao, J.; Reumschüssel, S.; Kaligis, F.; Bara, R.; Schäberle, T.F.; König, G.M.; et al. Marine Heterobranchia (Gastropoda, Mollusca) in Bunaken National Park, North Sulawesi, Indonesia—A Follow-Up Diversity Study. Diversity 2018, 10, 127. https://doi.org/10.3390/d10040127
Eisenbarth J-H, Undap N, Papu A, Schillo D, Dialao J, Reumschüssel S, Kaligis F, Bara R, Schäberle TF, König GM, et al. Marine Heterobranchia (Gastropoda, Mollusca) in Bunaken National Park, North Sulawesi, Indonesia—A Follow-Up Diversity Study. Diversity. 2018; 10(4):127. https://doi.org/10.3390/d10040127
Chicago/Turabian StyleEisenbarth, Jan-Hendrik, Nani Undap, Adelfia Papu, Dorothee Schillo, Jobel Dialao, Sven Reumschüssel, Fontje Kaligis, Robert Bara, Till F. Schäberle, Gabriele M. König, and et al. 2018. "Marine Heterobranchia (Gastropoda, Mollusca) in Bunaken National Park, North Sulawesi, Indonesia—A Follow-Up Diversity Study" Diversity 10, no. 4: 127. https://doi.org/10.3390/d10040127
APA StyleEisenbarth, J. -H., Undap, N., Papu, A., Schillo, D., Dialao, J., Reumschüssel, S., Kaligis, F., Bara, R., Schäberle, T. F., König, G. M., Yonow, N., & Wägele, H. (2018). Marine Heterobranchia (Gastropoda, Mollusca) in Bunaken National Park, North Sulawesi, Indonesia—A Follow-Up Diversity Study. Diversity, 10(4), 127. https://doi.org/10.3390/d10040127







